Follow-Up Study Evaluating the Long Term Outcome of ChondroMimetic in the Treatment of Osteochondral Defects in the Knee
Abstract
1. Introduction
2. Methods
2.1. Study Design and Participants
2.2. Interventional Details
2.3. Outcome Measures
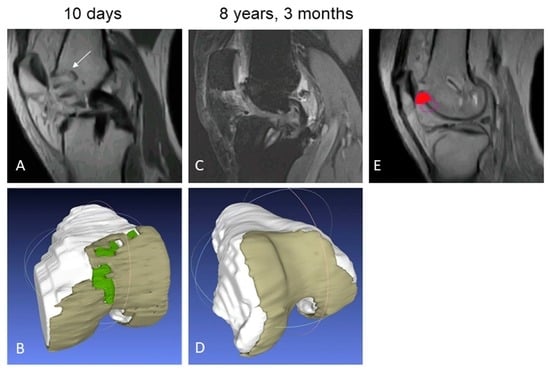
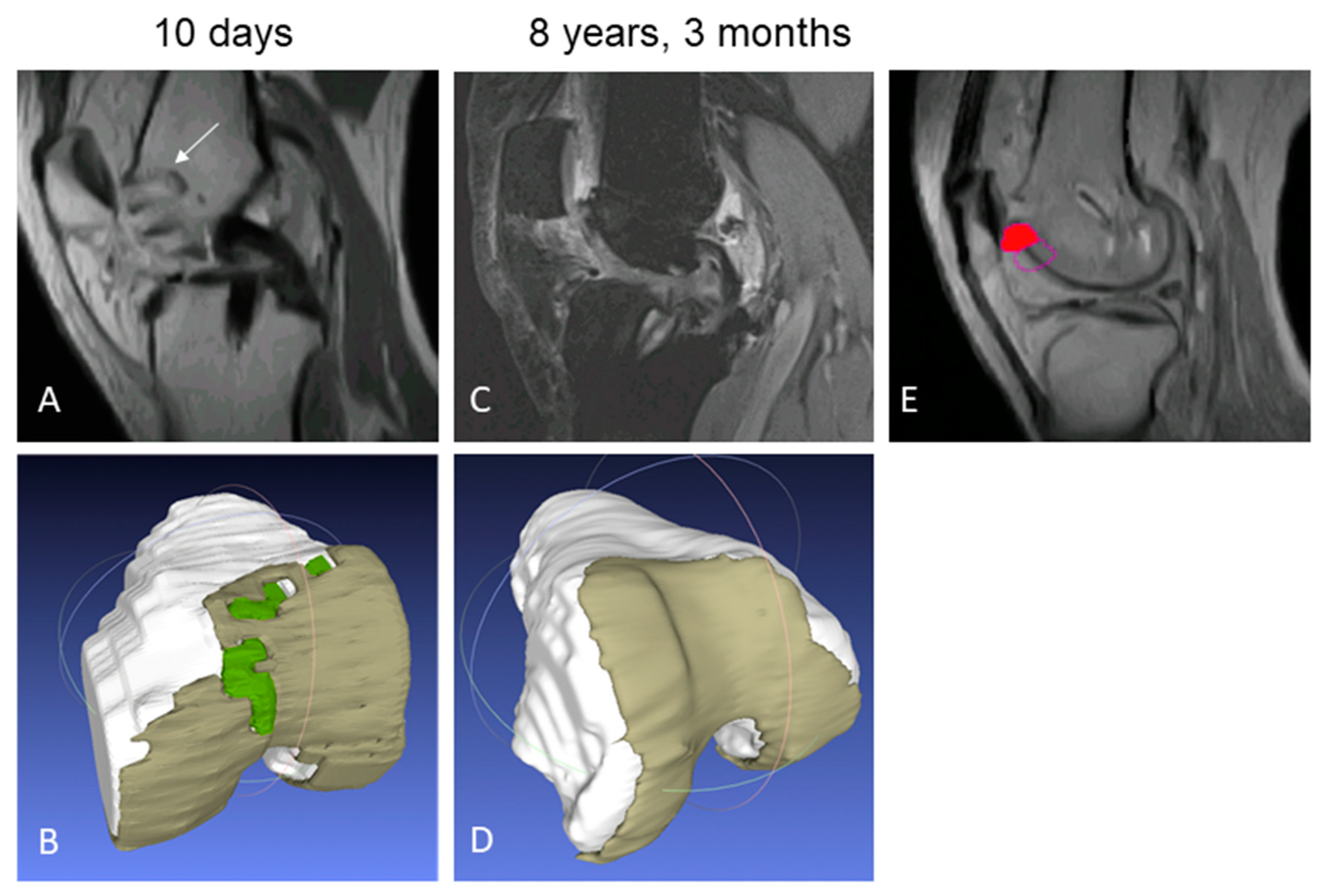
2.3.1. Structural Repair
2.3.2. Clinical Benefit
2.4. Statistical Considerations
3. Results
3.1. Enrolment and Subject Characteristics
3.2. Repair Outcomes
3.2.1. Structural Repair
3.2.2. Clinical Benefit
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Ethical Approval
List of Abbreviations
| 2D | two dimensional |
| 3D | three dimensional |
| ACI | Autologous chondrocyte implantation |
| AMIC | autologous matrix-induced chondrogenesis |
| CCI | characterized chondrocyte implantation |
| FS SPGR | fat-suppressed 3D spoiled gradient-echo |
| GAG | glycosaminoglycan |
| kg | kilogram |
| KOOS | Knee Injury and Osteoarthritis Outcome Score |
| m | meter |
| mm | millimeter |
| ms | millisecond |
| MRI | Magnetic Resonance Imaging |
| PRP | Platelet rich plasma |
| QoL | Quality of life |
| ROI | region-of-interest |
| T2 | Transverse relaxation time |
References
- Steadman, J.R.; Rodkey, W.G.; Briggs, K.K. Microfracture: Its History and Experience of the Developing Surgeon. Cartilage 2010, 1, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Peterson, L.; Vasiliadis, H.S.; Brittberg, M.; Lindahl, A. Autologous chondrocyte implantation: A long-term follow-up. Am. J. Sports Med. 2010, 38, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Bugbee, W.D.; Pallante-Kichura, A.L.; Gortz, S.; Amiel, D.; Sah, R. Osteochondral allograft transplantation in cartilage repair: Graft storage paradigm, translational models, and clinical applications. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2016, 34, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Hangody, L.; Duska, Z.; Karpati, Z. Autologous Osteochondral Mosaicplasty. Tech. Knee Surg. 2002, 1, 13–22. [Google Scholar] [CrossRef]
- Riboh, J.C.; Cvetanovich, G.L.; Cole, B.J.; Yanke, A.B. Comparative efficacy of cartilage repair procedures in the knee: A network meta-analysis. Knee Surg. sports Traumatol. Arthrosc. Off. J. ESSKA 2017, 25, 3786–3799. [Google Scholar] [CrossRef]
- Mundi, R.; Bedi, A.; Chow, L.; Crouch, S.; Simunovic, N.; Sibilsky Enselman, E.; Ayeni, O.R. Cartilage Restoration of the Knee: A Systematic Review and Meta-analysis of Level 1 Studies. Am. J. Sports Med. 2016, 44, 1888–1895. [Google Scholar] [CrossRef]
- Richter, D.L.; Schenck, R.C., Jr.; Wascher, D.C.; Treme, G. Knee Articular Cartilage Repair and Restoration Techniques: A Review of the Literature. Sports Health 2016, 8, 153–160. [Google Scholar] [CrossRef]
- Buckwalter, J.A.; Mankin, H.J. Articular Cartilage Part II: Degeneration and osteoarthrosis, repair, regeneration and transplantation. J. Bone Jt. Surg. 1997, 79, 612–632. [Google Scholar] [CrossRef]
- Davies-Tuck, M.L.; Wluka, A.E.; Wang, Y.; Teichtahl, A.J.; Jones, G.; Ding, C.; Cicuttini, F.M. The natural history of cartilage defects in people with knee osteoarthritis. OARS 2008, 16, 337–342. [Google Scholar] [CrossRef]
- Harley, B.A.; Lynn, A.K.; Wissner-Gross, Z.; Bonfield, W.; Yannas, I.V.; Gibson, L.J. Design of a multiphase osteochondral scaffold III: Fabrication of layered scaffolds with continuous interfaces. J. Biomed. Mater. Res. 2010, 92, 1078–1093. [Google Scholar] [CrossRef]
- Harley, B.A.; Lynn, A.K.; Wissner-Gross, Z.; Bonfield, W.; Yannas, I.V.; Gibson, L.J. Design of a multiphase osteochondral scaffold. II. Fabrication of a mineralized collagen-glycosaminoglycan scaffold. J. Biomed. Mater. Res. 2010, 92, 1066–1077. [Google Scholar] [CrossRef] [PubMed]
- Gille, J.; Behrens, P.; Volpi, P.; de Girolamo, L.; Reiss, E.; Zoch, W.; Anders, S. Outcome of Autologous Matrix Induced Chondrogenesis (AMIC) in cartilage knee surgery: Data of the AMIC Registry. Arch. Orthop. Trauma Surg. 2013, 133, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Shive, M.S.; Stanish, W.D.; McCormack, R.; Forriol, F.; Mohtadi, N.; Pelet, S.; Desnoyers, J.; Methot, S.; Vehik, K.; Restrepo, A. BST-CarGel(R) Treatment Maintains Cartilage Repair Superiority over Microfracture at 5 Years in a Multicenter Randomized Controlled Trial. Cartilage 2015, 6, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Getgood, A.M.; Kew, S.J.; Brooks, R.; Aberman, H.; Simon, T.; Lynn, A.K.; Rushton, N. Evaluation of early-stage osteochondral defect repair using a biphasic scaffold based on a collagen-glycosaminoglycan biopolymer in a caprine model. Knee 2012, 19, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Getgood, A.; Henson, F.; Skelton, C.; Herrera, E.; Brooks, R.; Fortier, L.A.; Rushton, N. The Augmentation of a Collagen/Glycosaminoglycan Biphasic Osteochondral Scaffold with Platelet-Rich Plasma and Concentrated Bone Marrow Aspirate for Osteochondral Defect Repair in Sheep: A Pilot Study. Cartilage 2012, 3, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Getgood, A.; Henson, F.; Skelton, C.; Brooks, R.; Guehring, H.; Fortier, L.A.; Rushton, N. Osteochondral tissue engineering using a biphasic collagen/GAG scaffold containing rhFGF18 or BMP-7 in an ovine model. J. Exp. Orthop. 2014, 1, 13. [Google Scholar] [CrossRef]
- Tamez-Pena, J.G.; Farber, J.; Gonzalez, P.C.; Schreyer, E.; Schneider, E.; Totterman, S. Unsupervised segmentation and quantification of anatomical knee features: Data from the Osteoarthritis Initiative. IEEE Trans. Biomed. Eng. 2012, 59, 1177–1186. [Google Scholar] [CrossRef]
- Tamez-Peña, J.; Shive, M.; Restrepo, A.; Gonzalez, P.; Schreyer, E.; Totterman, S. Validation of an Objective, Analyst-Independent, Non-Invasive Method for Assessing Effectiveness of Cartilage Repair Therapies in Multicenter RCTs. In Proceedings of the 6th International Workshop on Osteoarthritis Imaging, Hilton Head, SC, USA, 12–14 July 2012. [Google Scholar]
- Stanish, W.D.; McCormack, R.; Forriol, F.; Mohtadi, N.; Pelet, S.; Desnoyers, J.; Restrepo, A.; Shive, M.S. Novel scaffold-based BST-CarGel treatment results in superior cartilage repair compared with microfracture in a randomized controlled trial. J. Bone Jt. Surg. Am. Vol. 2013, 95, 1640–1650. [Google Scholar] [CrossRef]
- Gatti, A.A.; Noseworthy, M.D.; Stratford, P.W.; Brenneman, E.C.; Totterman, S.; Tamez-Pena, J.; Maly, M.R. Acute changes in knee cartilage transverse relaxation time after running and bicycling. J. Biomech. 2017, 53, 171–177. [Google Scholar] [CrossRef]
- Farber, J.M.; Totterman, S.M.; Martinez-Torteya, A.; Tamez-Pena, J.G. Scan-rescan precision of subchondral bone curvature maps from routine 3D DESS water excitation sequences: Data from the Osteoarthritis Initiative. Comput. Biol. Med. 2016, 69, 83–91. [Google Scholar] [CrossRef]
- Bentley, G.; Biant, L.C.; Carrington, R.W.; Akmal, M.; Goldberg, A.; Williams, A.M.; Skinner, J.A.; Pringle, J. A prospective, randomised comparison of autologous chondrocyte implantation versus mosaicplasty for osteochondral defects in the knee. J. Bone Jt. Surg. Br. Vol. 2003, 85, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Bekkers, J.E.; de Windt, T.S.; Raijmakers, N.J.; Dhert, W.J.; Saris, D.B. Validation of the Knee Injury and Osteoarthritis Outcome Score (KOOS) for the treatment of focal cartilage lesions. OARS 2009, 17, 1434–1439. [Google Scholar] [CrossRef] [PubMed]
- Grodzinsky, A.J.; Levenston, M.E.; Jin, M.; Frank, E.H. Cartilage tissue remodeling in response to mechanical forces. Annu. Rev. Biomed. Eng. 2000, 2, 691–713. [Google Scholar] [CrossRef] [PubMed]
- Mosher, T.J.; Dardzinski, B.J. Cartilage MRI T2 relaxation time mapping: Overview and applications. Semin. Musculoskelet. Radiol. 2004, 8, 355–368. [Google Scholar] [CrossRef] [PubMed]
- Nieminen, M.T.; Rieppo, J.; Toyras, J.; Hakumaki, J.M.; Silvennoinen, J.; Hyttinen, M.M.; Helminen, H.J.; Jurvelin, J.S. T2 relaxation reveals spatial collagen architecture in articular cartilage: A comparative quantitative MRI and polarized light microscopic study. Magn. Reson. Med. Off. J. Soc. Magn. Reson. Med. 2001, 46, 487–493. [Google Scholar] [CrossRef]
- Trattnig, S.; Mamisch, T.C.; Welsch, G.; Glaser, C. Quantitative T2 Mapping of Matrix-Associated Autologous Chondrocyte Transplantation at 3 Tesla: An In Vivo Cross-Sectional Study. Investig. Radiol. 2007, 42, 442–448. [Google Scholar] [CrossRef]
- Xia, Y.; Moody, J.B.; Alhadlaq, H. Orientational dependence of T2 relaxation in articular cartilage: A microscopic MRI (microMRI) study. Soc. Magn. Reson. Med. 2002, 48, 460–469. [Google Scholar] [CrossRef]
- White, L.M.; Sussman, M.S.; Hurtig, M.; Probyn, L.; Tomlinson, G.; Kandel, R. Cartilage T2 assessment: Differentiation of normal hyaline cartilage and reparative tissue after arthroscopic cartilage repair in equine subjects. Radiology 2006, 241, 407–414. [Google Scholar] [CrossRef]
- Kaipel, M.; Schreiner, M.; Kellner, R.; Klikovits, J.; Apprich, S.; Brix, M.; Boszotta, H.; Domayer, S.; Trattnig, S. Beneficial clinical effects but limited tissue quality following osteochondral repair with a cell-free multilayered nano-composite scaffold in the talus. Foot Ankle Surg. 2017, 23, 302–306. [Google Scholar] [CrossRef]
- Lansdown, D.A.; Wang, K.; Cotter, E.; Davey, A.; Cole, B.J. Relationship Between Quantitative MRI Biomarkers and Patient-Reported Outcome Measures After Cartilage Repair Surgery: A Systematic Review. Orthop. J. Sports Med. 2018, 6, 2325967118765448. [Google Scholar] [CrossRef]
- Welsch, G.H.; Mamisch, T.C.; Domayer, S.E.; Dorotka, R.; Kutscha-Lissberg, F. Cartilage T2 Assessment at 3-T MR Imaging: In Vivo Differentiation of Normal Hyaline Cartilage from Reparative Tissue after Two Cartilage Repair Procedures—Initial Experience1. Radiology 2008, 247, 154–161. [Google Scholar] [CrossRef]
- Eyre, D. Collagen of articular cartilage. Arthritis Res. 2002, 4, 30–35. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wu, J.P.; Kirk, T.B.; Zheng, M.H. Study of the collagen structure in the superficial zone and physiological state of articular cartilage using a 3D confocal imaging technique. J. Orthop. Surg. Res. 2008, 3, 29. [Google Scholar] [CrossRef] [PubMed]
- Hangody, L.; Feczko, P.; Bartha, L.; Bodo, G.; Kish, G. Mosaicplasty for the treatment of articular defects of the knee and ankle. Clin. Orthop. Relat. Res. 2001, 391, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Flynn, T.W.; Soutas-Little, R.W. Patellofemoral joint compressive forces in forward and backward running. J. Orthop. Sports Phys. Ther. 1995, 21, 277–282. [Google Scholar] [CrossRef]
- Andrade, R.; Vasta, S.; Pereira, R.; Pereira, H.; Papalia, R.; Karahan, M.; Oliveira, J.M.; Reis, R.L.; Espregueira-Mendes, J. Knee donor-site morbidity after mosaicplasty—A systematic review. J. Exp. Orthop. 2016, 3, 31. [Google Scholar] [CrossRef]
- US Food and Drug Administration. Guidance for Industry: Preparation of IDEs and INDs for Products Intended to Repair or Replace Knee Cartilage; US Food and Drug Administration: Washington, DC, USA, 2011.
- Abrams, G.; Mall, N.A.; Fortier, L.; Roller, B.L.; Cole, B.J. BioCartilage: Background and Operative Technique. Oper. Tech. Sports Med. 2013, 21, 116–124. [Google Scholar] [CrossRef]
- Brix, M.O.; Stelzeneder, D.; Chiari, C.; Koller, U.; Nehrer, S.; Dorotka, R.; Windhager, R.; Domayer, S.E. Treatment of Full-Thickness Chondral Defects With Hyalograft C in the Knee: Long-term Results. Am. J. Sports Med. 2014, 42, 1426–1432. [Google Scholar] [CrossRef]
- Brittberg, M.; Recker, D.; Ilgenfritz, J.; Saris, D.B.F.; Group, S.E.S. Matrix-Applied Characterized Autologous Cultured Chondrocytes Versus Microfracture: Five-Year Follow-up of a Prospective Randomized Trial. Am. J. Sports Med. 2018, 46, 1343–1351. [Google Scholar] [CrossRef]
- Frenkel, S.R.; Di Cesare, P.E. Scaffolds for articular cartilage repair. Ann. Biomed. Eng. 2004, 32, 26–34. [Google Scholar] [CrossRef]
- Kon, E.; Roffi, A.; Filardo, G.; Tesei, G.; Marcacci, M. Scaffold-based cartilage treatments: With or without cells? A systematic review of preclinical and clinical evidence. Arthrosc. J. Arthrosc. Relat. Surg. 2015, 31, 767–775. [Google Scholar] [CrossRef] [PubMed]


| Characteristic | Chondromimetic (n = 15) |
|---|---|
| Consented subjects from original study, n (%) | 15 (88) |
| Follow-up, years | 7.9 ± 0.3 |
| Age, years | 32.7 ± 9.3 |
| Gender, n (%) | |
| Male | 7 (46.7) |
| Female | 8 (53.3) |
| Body Mass Index, kg/m2 | 25.3 ± 4.2 |
| Smokers, n (%) | 2 (13.3) |
| Index defects and implants | |
| Mosaicplasty donor site(s), n (%) | 13 (86.7) |
| Medial femoral condyle defect, n (%) | 2 (13.3) |
| Defect volume including missing bone, mm3 ¶ | 1573 ± 1233 |
| ChondroMimetic Implants/knee, median (range) | 2 (1–5) |
| Follow-up Pain, n (%) * | |
| No knee pain | 11 (73.3) |
| Mild knee pain | 2 (13.3) |
| Moderate knee pain | 2 (13.3) |
| Follow-up Activity Level, n (%) * | |
| Unlimited | 8 (53) |
| Slightly limited | 4 (27) |
| Moderately limited | 3 (20) |
| Additional interventions since treatment | |
| Index knee related procedures ^ | 12 |
| Non-knee related procedures | 2 |
| Variable | Outcome |
|---|---|
| Total defect fill (%) | 95.2 ± 3.6 (89.8, 99.9) |
| T2 relaxation time (ms) | |
| Cartilage repair tissue | 52.5 ± 4.8 (44.4, 58.5) + |
| Ipsilateral native cartilage | 52.3 ± 9.2 (39.9, 78.5) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berta, A.; Shive, M.S.; Lynn, A.K.; Getgood, A.; Totterman, S.; Busby, G.; Hollenstein, J.; Vásárhelyi, G.; Kéki, I.; Hangody, L. Follow-Up Study Evaluating the Long Term Outcome of ChondroMimetic in the Treatment of Osteochondral Defects in the Knee. Appl. Sci. 2020, 10, 5642. https://doi.org/10.3390/app10165642
Berta A, Shive MS, Lynn AK, Getgood A, Totterman S, Busby G, Hollenstein J, Vásárhelyi G, Kéki I, Hangody L. Follow-Up Study Evaluating the Long Term Outcome of ChondroMimetic in the Treatment of Osteochondral Defects in the Knee. Applied Sciences. 2020; 10(16):5642. https://doi.org/10.3390/app10165642
Chicago/Turabian StyleBerta, Agnes, Matthew S. Shive, Andrew K. Lynn, Alan Getgood, Saara Totterman, Grahame Busby, Jerome Hollenstein, Gábor Vásárhelyi, Imre Kéki, and László Hangody. 2020. "Follow-Up Study Evaluating the Long Term Outcome of ChondroMimetic in the Treatment of Osteochondral Defects in the Knee" Applied Sciences 10, no. 16: 5642. https://doi.org/10.3390/app10165642
APA StyleBerta, A., Shive, M. S., Lynn, A. K., Getgood, A., Totterman, S., Busby, G., Hollenstein, J., Vásárhelyi, G., Kéki, I., & Hangody, L. (2020). Follow-Up Study Evaluating the Long Term Outcome of ChondroMimetic in the Treatment of Osteochondral Defects in the Knee. Applied Sciences, 10(16), 5642. https://doi.org/10.3390/app10165642