Abstract
Plants are often exposed to unfavorable environmental conditions, for instance abiotic stresses, which dramatically alter distribution of plant species among ecological niches and limit the yields of crop species. Among these, drought stress is one of the most impacting factors which alter seriously the plant physiology, finally leading to the decline of the crop productivity. Drought stress causes in plants a set of morpho-anatomical, physiological and biochemical changes, mainly addressed to limit the loss of water by transpiration with the attempt to increase the plant water use efficiency. The stomata closure, one of the first consistent reactions observed under drought, results in a series of consequent physiological/biochemical adjustments aimed at balancing the photosynthetic process as well as at enhancing the plant defense barriers against drought-promoted stress (e.g., stimulation of antioxidant systems, accumulation of osmolytes and stimulation of aquaporin synthesis), all representing an attempt by the plant to overcome the unfavorable period of limited water availability. In view of the severe changes in water availability imposed by climate change factors and considering the increasing human population, it is therefore of outmost importance to highlight: (i) how plants react to drought; (ii) the mechanisms of tolerance exhibited by some species/cultivars; and (iii) the techniques aimed at increasing the tolerance of crop species against limited water availability. All these aspects are necessary to respond to the continuously increasing demand for food, which unfortunately parallels the loss of arable land due to changes in rainfall dynamics and prolonged period of drought provoked by climate change factors. This review summarizes the most updated findings on the impact of drought stress on plant morphological, biochemical and physiological features and highlights plant mechanisms of tolerance which could be exploited to increase the plant capability to survive under limited water availability. In addition, possible applicative strategies to help the plant in counteracting unfavorable drought periods are also discussed.
1. Introduction
Plants experience continuous fluctuations of environmental conditions and are often exposed to abiotic stresses, for instance shortage of available water, salinity, excess light, high/low temperatures and nutrient imbalance, all leading to impairment of plant performance [1]. The capability of plants to respond to abiotic stress is associated with their plasticity as well as the adaptableness of plant traits to the fluctuating conditions of water availability [2]. Amongst these limiting abiotic factors, drought (or water deficit) stress is extensively studied given that it is likely the main constraint for crop productivity in many arid and semi-arid areas worldwide [3].
Water deficit occurs when the plant water requirement cannot be fully satisfied and this situation takes place when the level of transpired water exceed the water taken up by the roots, which is caused by inadequate precipitation, decreased ground water level or the retention of water by soil particles [4,5]. As a result of water stress, plants respond with morpho-anatomical, physiological and biochemical adjustments aimed at counteracting the loss of water with the attempt to preserve their hydric status [2].
Being sessile organisms, plants have to face several adverse factors in natural environments, and, for this reason, they possess numerous defense strategies and have evolved several resistance mechanisms through which they cope with abiotic stresses [6]. Enduring severe water deficit periods, which relies on plant-genotype-specific features, also depends upon stress intensity, duration, speed and recovery effectiveness to regulate plant performance [7,8]. In the case of water scarcity, plants need to respond quickly, thus virtually all biological functions are altered by water deficit conditions at whole plant level [9,10]. Plants have to stimulate different strategies that benefit them to absorb water through their roots and to uphold cell turgor, i.e., evade the water loss [11]. Declined frequency of cell division and cell enlargement, root differentiation, foliage dimensions, shoot length, altered stomatal movements, water and mineral nutrition association with decreased plant yield and water usage efficacy are major outcomes of drought in plants [12]. Photosynthesis activity is decreased primarily by closing of stomata, membrane injury and altered functioning of several enzymes, specifically those which are associated with ATP synthesis [12,13]. Drought stress conditions also result in increased generation of reactive oxygen species (ROS) and reactive nitrogen species (RNS), which disturb the cell redox regulatory functioning [8,14].
Plants that are able to tolerate drought stress for extended periods and sustain their vigor and yield represent one of the foremost exploration fields in agriculture studies [15]. As detailed below, tolerant plants may benefit from different features which allow them to tolerate better than others the effect of water scarcity. For example, among morpho-anatomical features, a well-developed root apparatus ensures the plant a deeper exploration of the soil thereby increasing the capability of water uptake [16]. Other physiological (e.g., rapid stomata closure and water use efficiency) and/or biochemical responses (e.g., synthesis of osmolytes, aquaporins and a powerful antioxidant apparatus) may contribute in increasing the drought tolerance of some plant individuals [17], thereby supporting the use of those drought-tolerant genotypes/varieties.
Besides the exploitation of plant tolerant genotypes/varieties based on classic breeding selection, some applicative strategies have also been applied to attempt to overcome drought effects in crop species. For example, under controlled circumstances, regulated deficit irrigation may allow to obtain positive results in plant growth, likely due to a significant overproduction of advantageous moieties such as sugars, organic acids and antioxidant compounds [18,19]. In addition, foliar application of some compounds (including those produced by drought-tolerant genotypes, which are supposed to contribute to plant drought tolerance) may help plants better tolerate a condition of limited water availability. Among these, brassinosteroids [20,21], salicylic acid [22], amino acids [22,23], polyamines [24] and micronutrients (e.g., potassium and phosphorous) [25] are certainly the most efficient with consistent results in different plant species. Knowledge of the morpho-anatomical, physiological and biochemical mechanisms underlying drought tolerance (as discussed in the next sections) is crucial for conferring drought tolerance to major crops, in order to valorize marginal areas (e.g., semi-arid environments) in which water availability is the major constraint for the plant growth.
2. Influence of Drought Stress on Plant Performances: From Morpho-Anatomy to Biochemical Changes
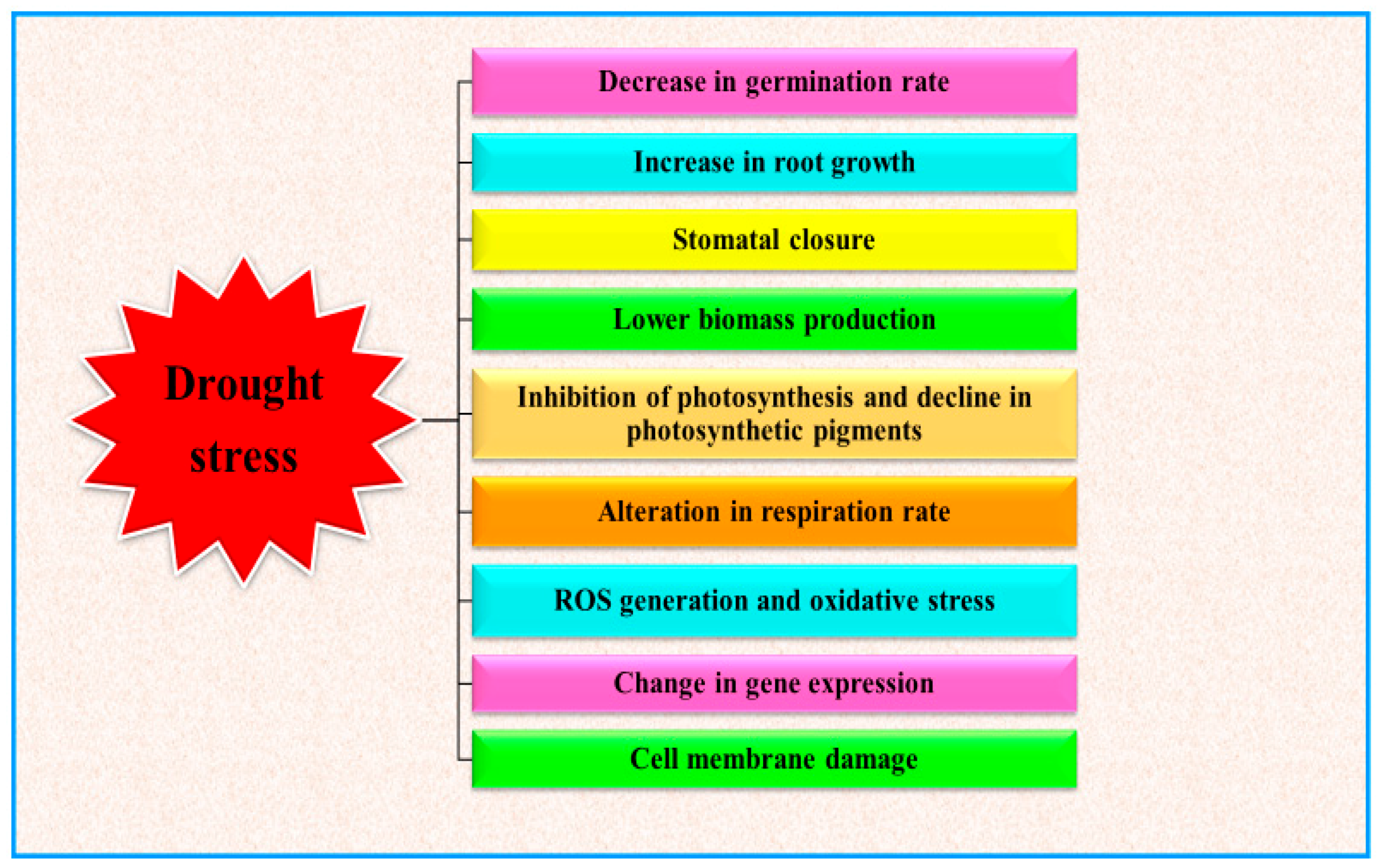
Water deficit conditions stimulate several plant responses, such as morphological, physiological, biochemical and molecular alterations, which ultimately result in disturbing plant functioning [26] (Figure 1). As depicted in Figure 1, drought events limit plant performances in different developmental stages. Limited water availability can indeed reduce the germination rate and the development of young plants [27]. During the progression of plant growth, drought basically influences the plant water relations, which in turn cause severe perturbation to the whole plant metabolism (at physiological, biochemical and molecular levels), depending to the stress severity and duration [14,28]. Water deficit conditions alter several activities of plant, but one of the main effects is the decline of photosynthetic activity [29,30] and finally the plant yield [31,32]. During drought stress conditions, oxidative stress, directly or indirectly generated in plants, is one of the main drivers of plant responses and results in damage to cell membrane, altering membrane integrity, physiological and biochemical alterations which lead to acute metabolic disorders and eventually alter the plant productivity [33,34].
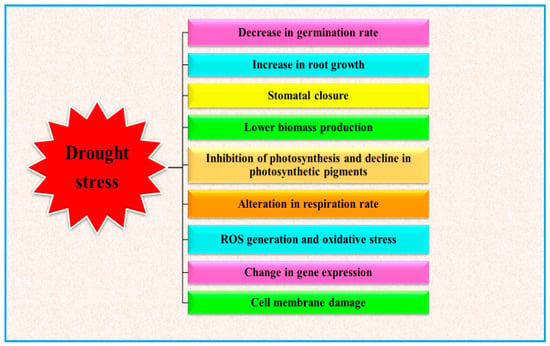
Figure 1.
Effect of drought stress on plant growth and development (modified from Ghatak et al. [35]).
3. Drought Stress and Plant Growth
Drought stress is well recognized as a limiting factor which alters multiple aspects of plant growth and development. Germination of seeds, health and coleoptile length are foremost for the plant progression [36]. Seed germination is the primary aspect of growth which is sensitive to drought stress. Noteworthy alterations are observed in the seed germination of a plethora of plant species, including some of the most widely cultivated crops such as maize [37], sorghum [38] and wheat [39].
Visible symptoms of plant exposed to water scarcity in the initial vegetative stage are leaf wilting, decline in plant height and interruption in establishment of buds and flowers [40]. Drought conditions also limit the uptake of nutrients by the plants due to limited soil moisture, leading to decreased stem length [41]. Shoot length was also reduced under water deficit conditions in Lathyrus sativus L. [42]. In conditions of water deficit, plants seek to extract water from deeper soil layers by boosting their root architecture [43]. Moreover, water availability is primarily recognized by roots, which in turn regulates its growth and organization characteristics such as root length, spread, number and length of lateral roots [44]. Roots are crucial for different biological activities and plant yield, for instance nutrient accumulation and water absorption, and they are also involved in rhizosphere symbiotic associations with other microorganisms. Drought stress escalated root length in Crocus sativus L. [45]. Thus, a healthy root apparatus provides the benefit for sustenance of the escalation of plant growth, especially in the course of primary plant growth phase [46]. Escalation in root length is recognized as a useful strategy to increase soil water retention and nutrient accumulation to enhance plant biomass production [47]. Under water deficit, the plant root to shoot proportion generally improves, and, subsequently, the plant biomass decreases substantially [48].
The leaf is the chief part of the plant where most of the photosynthetic products are synthetized. The number of leaves decreased when subjected to water stress in Andrographis paniculate [49]. Optimal leaf development and the maintenance of an adequate leaf area is vital for photosynthesis, which in turn is the main driver of plant growth. Water stress causes reduction in leaf area, which results in decreased photosynthesis, hence reducing the crop yield. Leaf area declined under water stress conditions in Petroselinum crispum L. and in Stevia rabaudiana plants to achieve stability among the water absorbed by roots and the water status of various plant parts [50,51]. Reduction in leaf area is a drought avoidance strategy because declining leaf area results in a decreased water loss by the process of transpiration and this reduction in leaf area is attributable to the inhibition of leaf expansion by declined rate of cell division, which results in loss of cell turgidity [52]. Decrease in soil moisture causes a parallel reduction of leaf water content, which, in turn, induces a decline of turgor pressure of guard cells due to stomata closure [53]. Of note, the rate of premature leaf senescence is enhanced in drought environments [17].
4. Drought Stress and Photosynthesis
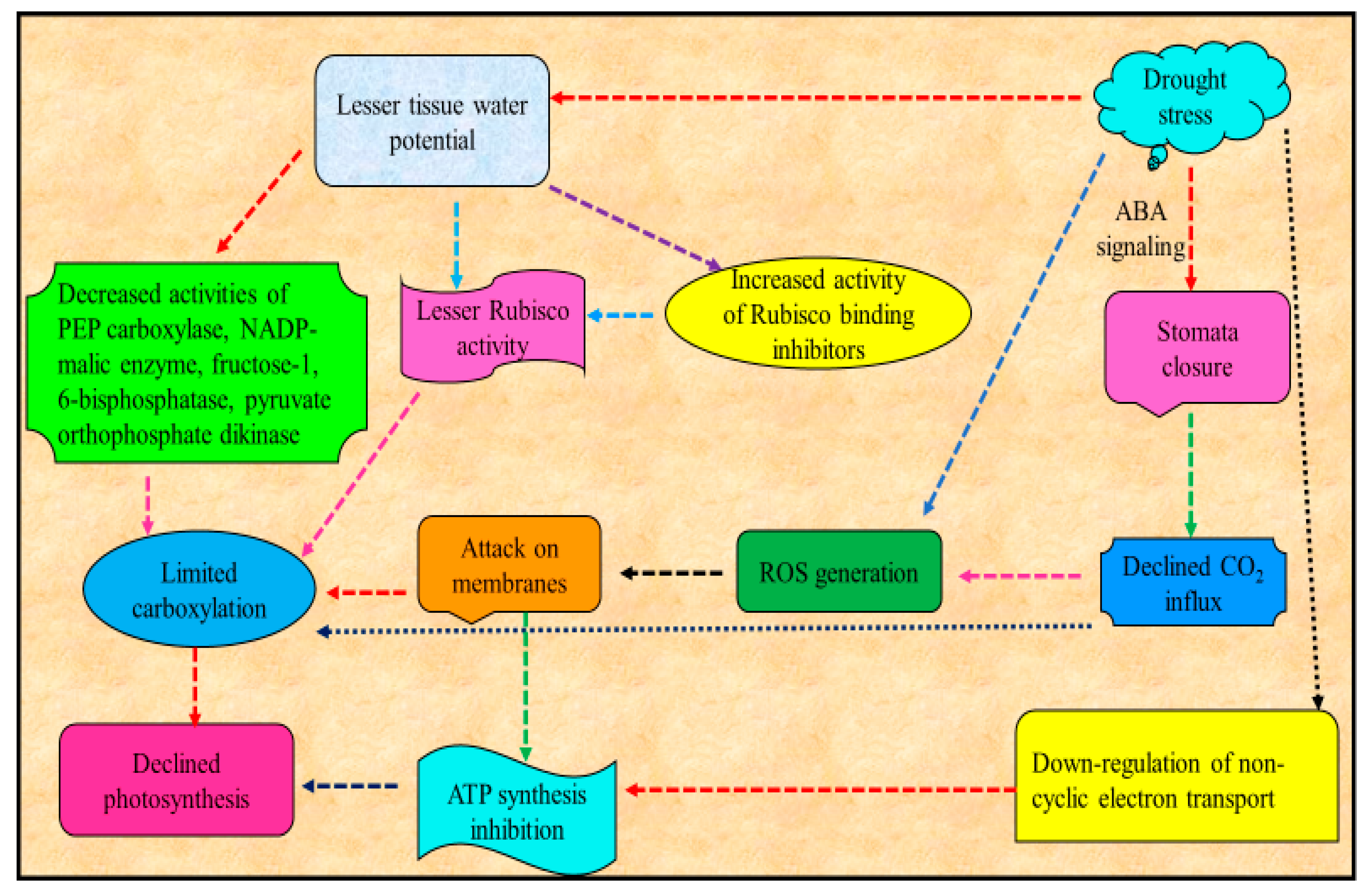
Major consequence of water deficit in plants is the decrease or suppression of photosynthesis [54] (Figure 2). Reduced leaf area, increased stomata closure and consequent reduced leaf cooling by evapotranspiration increases osmotic stress leading to damages to the photosynthetic apparatus are among the major constraints for photosynthesis [55,56]. Among these, the decrease in photosynthetic process in plants under drought is mainly attributable to the decline in CO2 conductance via stomata and mesophyll limitations [57]. Decrease in photosynthetic activity due to drought may also be due to reduced ability of stomatal movement [58,59]. Declined activity of photosynthesis is triggered by the loss of CO2 [60] uptake, whose drop has been shown to affect Rubisco activity and decrease the function of nitrate reductase and sucrose phosphate synthase and the ability for ribulose bisphosphate (RuBP) production. Supportively, CO2 enrichment eliminated many early responses of maize metabolites and transcripts attributable to drought stress [61].
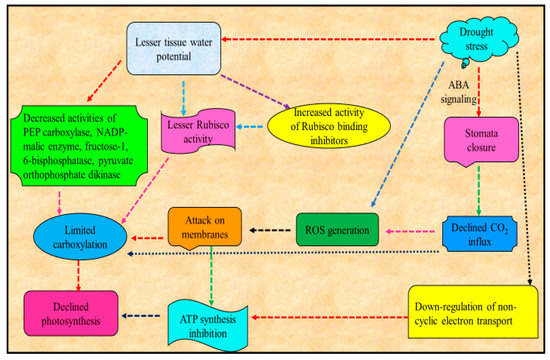
Figure 2.
Schematic representation of effect of drought stress on photosynthesis (modified from Farooq et al. [17]).
Water deficit also resulted in decreased leaf area per shoot, and, thus, modification in canopy architecture, and this feature can alter gas exchange, water relations, vegetative growth and sink development (e.g., fruits or grains) [62], altering, for example, berry sugar concentration in grape [63] and biomass partition in maize (i.e., kernel number and 100-kernel dry weight decreased with increasing water stress duration) [64].
Chlorophyll content, which is of outmost importance for photosynthesis [65], is another photosynthetic attribute strongly influenced by water deficit that has been recognized as a distinctive indication of photo oxidation and degradation of chlorophylls [66]. For example, leaf chlorophyll synthesis and chlorophyll a/b proportion in soybean is altered by drought stress [67]. Decline in photosynthetic activity, amount of chlorophylls, loss of photosystem II photochemical efficiency, alteration in stomatal movement and disturbance in water status of plants resulted in declined plant productivity [68]. Among others, a major cause for decline in amount of chlorophyll due to drought stress is the drought-promoted O2− and H2O2, which results in lipid peroxidation and ultimately chlorophyll degradation [69]. The decrease of plant development and yield in several plant species under water deficit is often associated with decline in photosynthetic action and chlorophyll content impairment [70]. Water deficit alters the action of photosynthetic moieties and chlorophyll pigments, which ultimately resulted in reduced photosynthetic activities in Vigna mungo [71].
Drought stress induces a decreased net photosynthesis and also changes the plant carbon allocation and metabolism, which ultimately results in energy dissipation and declined yield [72]. For example, drought stress decreased the physiological metabolic disorders by suppressing the photosynthetic products production and disrupting the carbon balance in soybean [16]. Drought stress also caused a reduction in the abundance of several Calvin cycle proteins, including Rubisco downregulation in olive [73]. Acute drought stress conditions also cause the damage to Rubisco enzyme and other enzymes associated with photosynthesis and are responsible for the loss of photosynthetic pigment content [74].
5. Drought Stress and Antioxidant Defense System
Most of the plant defensive system is devoted to contrast the adverse consequences of drought-triggered ROS. In this context, a prompt, powerful and efficient antioxidant system is of pivotal importance to provide drought tolerance [75]. This machinery involves enzymatic and non-enzymatic detoxification moieties, which lessen and repair injury triggered by ROS. Enhancement of the antioxidant apparatus helps in ROS scavenging that decreases electrolyte leakage and lipid peroxidation, therefore maintaining the vitality and integrity of organelles and cell membrane [76].
It is well recognized that drought induces oxidative stress by generating ROS, for instance O2•−, hydroxyl radicals (OH•), singlet oxygen (1O2) and H2O2 [77]. The proportion of ROS generation and antioxidant enzyme activities regulates the cell redox state, thereby resulting in ROS control or cell injury and cell death when ROS exceed the physiological levels [78]. Numerous studies conducted under water deficit conditions found enhanced activities of pivotal antioxidant enzymes, namely CAT, SOD, POD and APX [79]. Usually, tolerant species/varieties/genotypes have an enhanced antioxidant enzymes activity in comparison to non-tolerant plants, which is supportive for their essential role in drought tolerance, especially to control H2O2 and O2•− production and diffusion in leaf tissues [80].
Production of O2•− and H2O2 were controlled by superoxide dismutase (SOD), peroxidase (POX) and catalase (CAT) action, whose activity was enhanced for example in drought-tolerant potato genotypes [81]. Ascorbate peroxidase (APX) also participates as excess ROS scavenger (APX uses ascorbate as a substrate to stimulate the conversion of H2O2 to H2O), and its activity is usually elevated under stress conditions [82]. Alteration in APX activity in leaves is more common than in fibrous roots because APX mainly occurs in the chloroplast and cytoplasm and is a crucial enzyme for scavenging H2O2 in chloroplasts [83]. Activities of SOD, POD, CAT and APX were altered and played a key role in protecting peony plants against acute water deficit [84]. The amount of non-enzymatic antioxidants (ascorbic acid, reduced glutathione and α- tocopherol) and antioxidant enzymes (SOD, CAT and APX) activities were simultaneously enhanced in Coleus plectranthus in drought stress conditions [85]. SOD, CAT and POX enzymes activities were stimulated by limited water availability in Vicia faba [70]. Increase of SOD, POX and CAT activities was observed in drought-tolerant genotype, in comparison to the drought sensitive plants of faba bean [86]. The amount of enzymatic and non-enzymatic antioxidants improved in drought tolerant plants under mild and moderate water deficit conditions [87]. CAT, SOD, POD and APX activities increased in Adonis amurensis and Adonis pseudoamurensis subjected to drought, indicating that improved functioning of these enzymes helps to lower the level of ROS and mitigate the drought generated oxidative stress [88]. Water deficit boosted the levels of SOD and POD in Vigna mungo and the authors concluded that increased levels of these enzymes stimulate tolerance against drought stress and are vital to reduce its adverse effects [71]. Water deficit increased the CAT, POX and SOD levels in leaves of Glycyrrhiza glabra L., which aimed at counteracting the spread of H2O2 [89].
6. Drought Stress and Secondary Metabolites
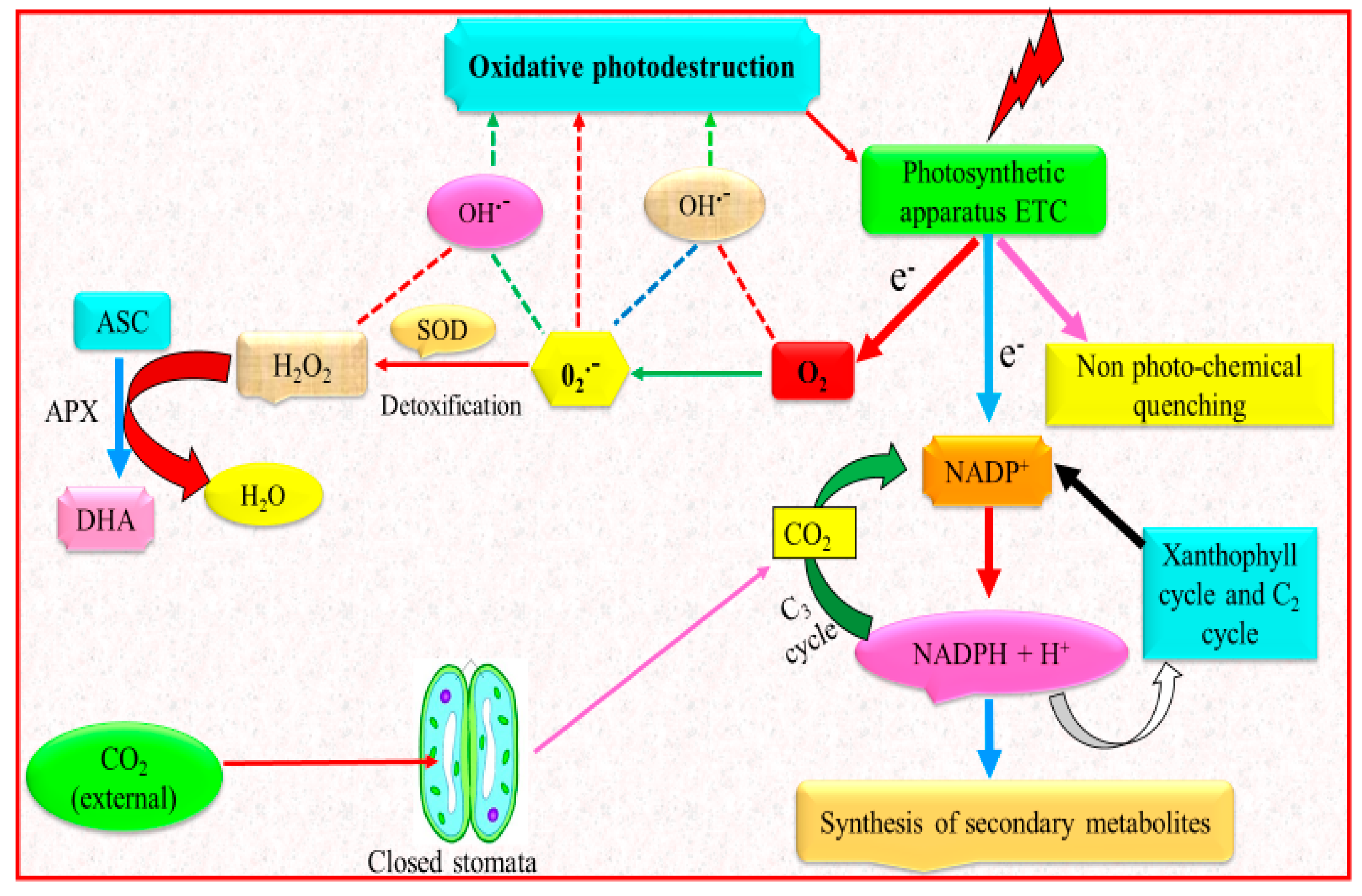
Secondary metabolites are produced by plants in the attempt to respond to various environmental stresses [90,91]. It is recognized that the biosynthesis of secondary metabolites is regulated by environmental factors, for instance temperature, light regime and nutrient availability [92]. Improved production of secondary metabolites is usually observed under water deficit conditions, which is caused by reduction in biomass formation and destination of assimilated CO2 to C-based secondary metabolites to avoid sugar-promoted feedback of photosynthesis (Figure 3) [93].
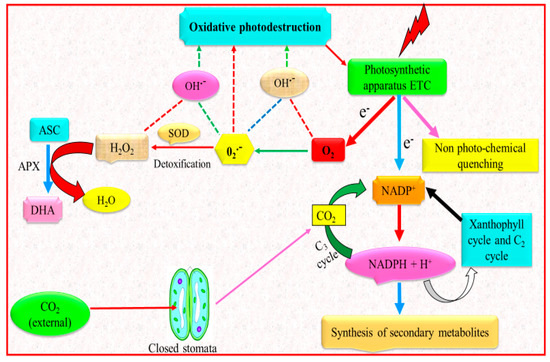
Figure 3.
Enhanced synthesis of secondary metabolites under drought stress. Light energy captured by the photosynthetic machinery is considerably greater than the energy essential for the CO2 fixation. Energy dissipation takes place by non-photochemical quenching and re-oxidation of NADPH + H+, i.e., via xanthophyll cycle and C2 cycle. Endogenous CO2 level is low because of the escalated diffusion resistance caused by closing of stomata. Hence, a smaller amount of NADPH + H+ is utilized in the C3 cycle for the fixation and reduction of CO2, and, ultimately, a greater amount of energy has to be dissipated. Protective activities such as non-photochemical quenching, C2 cycle and xanthophyll cycle are boosted by feedback mechanisms; a number of e− is transported to O2 (Mehler reaction). Generation of O2•− ions further produce various ROS. Due to the stress-associated stimulation of SOD and APX, detoxification of the O2•− ions occurs and therefore results in reduction of generation of ROS. Greater enhancement in the reduction potential, i.e., the ratio of NADPH + H+ to NADP+, elevates the plants secondary metabolites synthesis (modified from Kleinwächter and Selmar [90]).
In Hypericum brasiliense, concentration of phenolic acids is considerably enhanced when grown in water deficit conditions [94]. In two native sub species of Iranian Origanum vulgare, i.e., subsp. gracile and subsp. Virens, the content of sesquiterpene (E) β-caryophyllene strongly increased by water limitation [95]. Under mild and mild/severe drought, the content of oleanolic acid and betulin increased in Betula platyphylla [96] and level of triterpenoid glycyrrhizin in Glycyrrhiza glabra [97]. The lignin content was increased in bermudagrass Tifton-85, which is a variety of Cynodon dactylon L., under drought conditions [98]. The flavonoids content was enhanced under stress conditions and high-water deficit conditions improved the medicinal properties of Labisia pumila [99]. Phaseolus lunatus under water deficit condition had an elevated level of cyanogenic glucosides [92]. In Lamiaceae family, the content of essential oils declined in Lavandula latifolia and Salvia sclarea, whereas, in Mentha piperita, Salvia lavandulifolia, Thymus capitatus and Thymus mastichina, the essential oil amount was enhanced under drought conditions and the increase was attributable to a higher concentration oil glands due to decrease in leaf area [100]. The amount of phenolics and flavonoids increased in Achillea species against drought stress [76]. The content of phenolic acids simultaneously improved, while the level of flavonoids declined in Achillea pachycephala [101].
7. Drought Stress and Mineral Nutrition
Water deficit situations usually reduce the overall soil nutrient accessibility, root nutrient translocation and ultimately lessen the ion content in various plant tissues [102]. Water deficit conditions decreased plant potassium (K) uptake [103]. This decline in K was attributable to reduced K mobility, declined transpiration rate and weakened action of root membrane transporters [103,104]. Decreased K amount was also found in drought-stressed plants of Malus hupehensis [105]. Resistant genotypes of Triticum durum had the maximum amount of K and susceptible genotypes had the maximum amount of sodium (Na) [69]. Genes encoding K transporters were inhibited by water deficit [106] and inner K channels are stimulated by a protein kinase, CIPK23, which in turn cooperates with calcineurin B-like calcium sensors. This K channel was inhibited in roots but activated in leaves of grapevine [107]. Leaf nitrogen (N) level did not change in drought-stressed Mentha piperita, Salvia lavandulifolia, Salvia sclarea and Thymus capitatus, whereas, in Lavandula latifolia and Thymus mastichina plants, N content decreased while leaf phosphorus (P) level reduced in all species except S. sclarea whose concentration remained the same [100]. This reduction in N was considered as the main responsible factor for photosynthesis decline and leaf senescence [108]. There was a significant reduction in leaf P amount in Ocimum gratissimum [109] and decline in K level in Thymus daenensis under water deficit conditions [110]. K level also decreased in Ocimum basilicum and Ocimum americanum plants subjected to limited water availability [111]. Principally, decrease of K amount occurs in leaves because water scarcity disturbs stomata movement and guard cell turgidity, which results in decreased photosynthesis and, finally, the plant biomass production [112]. Drought-stress conditions increased the accumulation of manganese (Mn), molybdenum (Mo), P, K, copper (Cu), calcium (Ca) and zinc (Zn) in soybean [113].
8. Plant Tolerance Mechanisms Against Drought Stress to Increase Crop Tolerance: How to Exploit These Mechanisms to Increase Crop Tolerance
The intimal meaning of drought tolerance or drought resistance is still under debate. It is conceivable that water-saving plants mainly refer to the effective use of water resource in the process of growth and development of plants, thereby increasing crop water use efficiency (WUE) [114]. WUE is defined as the economic production per unit water consumption and it may or may not be related to drought resistance [115]. On the other hand, the main accepted definition of drought resistance is the ability of an individual to survive or grow in a water-stressed environment due to dehydration avoidance, dehydration tolerance or drought recovery, where dehydration is considered as the progressive loss of water content in plant tissue [115]. Discerning between drought tolerance or drought resistance can be very complex and is out of the scope of the present review, as there are already excellent papers dealing with this topic [116,117]. Therefore, in the next paragraphs of the present review, plants able to tolerate drought stress conditions better than others are referred to as “tolerant” without any distinctions between drought tolerant or drought resistant.
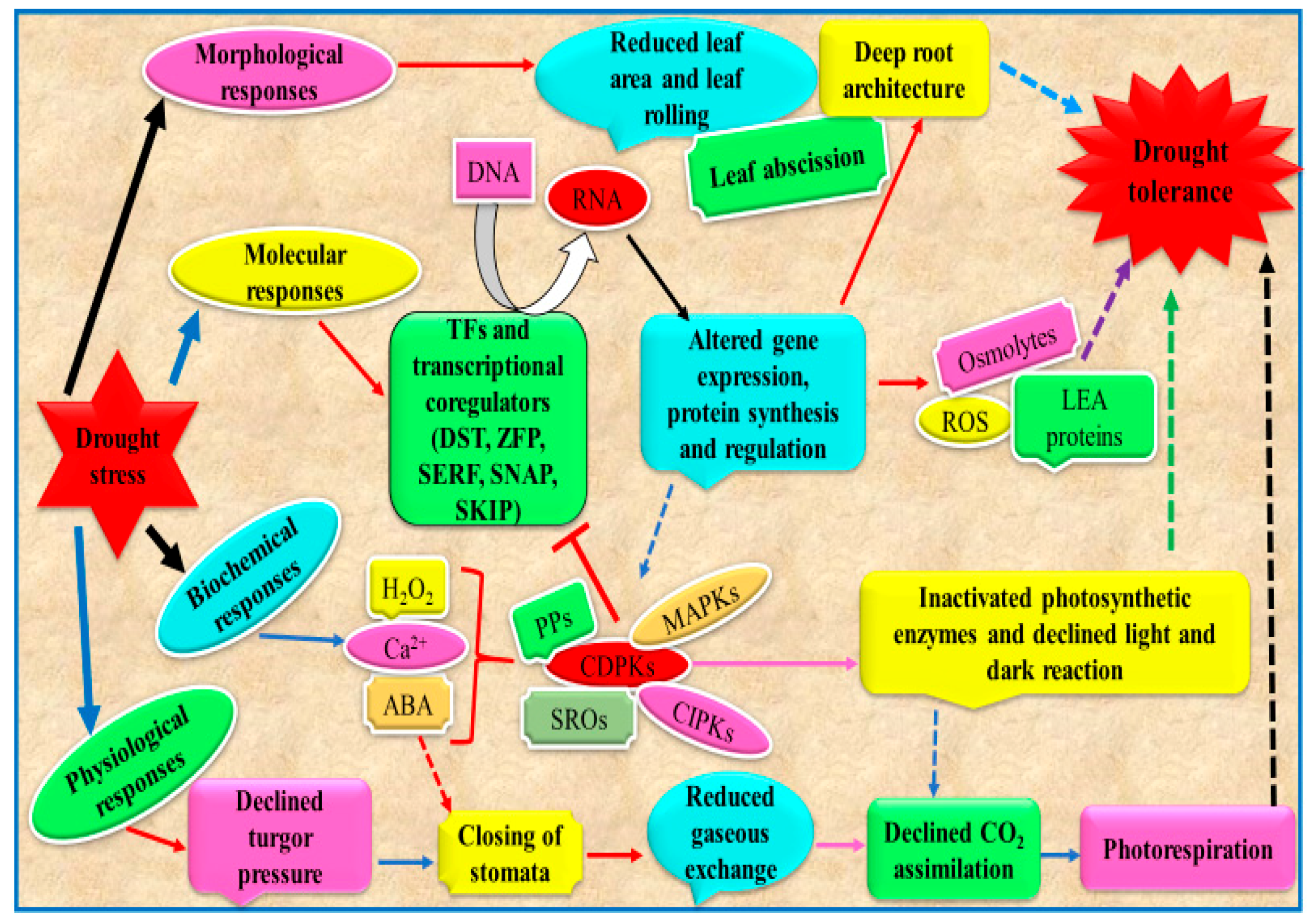
Plant drought tolerance encompasses alterations at morphological, biochemical and molecular levels (Figure 4). Exhibition of single or multiple tolerance factors governs the plant capability to survive under adverse drought conditions. From an applicative point of view, an in-depth knowledge of these mechanism can be exploited to select crop species/varieties/genotypes with a lower degree of sensitivity to limited water availability. Below, physiological, biochemical and molecular mechanisms which allow tolerant plants to tolerate better drought conditions are described with the attempt to propose some of them as suitable features for crop selection in the context of reduced water availability.
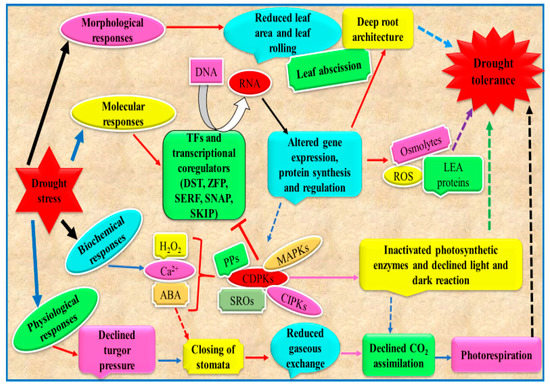
Figure 4.
Diagram showing plants drought tolerance mechanism. TFs, transcription factors; DST, drought and salt tolerance; SERF, serum response factor; SKIP, ski-interacting protein; ZFP, zinc finger TF; SNAC, stress responsive NAC TF; LEA, late embryogenesis abundant; ABA, abscisic acid; SROs, similar to RCD-ONE; CDPKs, Ca2+ dependent protein kinases; MAPKs, mitogen activated protein kinases; PPs, protein phosphatases; CIPKs, CBL interacting protein kinases (modified from Zargar et al. [118]).
8.1. Morphological and Biochemical Mechanisms Involved in Drought Tolerance
Plants survival to drought encompasses two main strategies: drought avoidance and drought tolerance [53]. Plants have adopted several strategies to increase their drought tolerance at different levels, morphological, physiological, biochemical, and molecular. Conversely, some plant species avoid water deficit situations by accomplishing, for example, their life cycle before or after a drought period while some other plants displayed adaptations to escalate water absorption and decrease water loss to circumvent its adverse consequences [1].
At the morphological level, root is one the major drivers of water; therefore, the root size, its progression rate and density and root proliferation are important features which prompt plant responses to drought stress [5]. Plants with a deep root organization and a perennial development system showed more ability to cope with drought in comparison to plants with shallow-root system [119]. In view of the above, the selection of genotypes with a more developed root apparatus resulted in increased plant yield, as demonstrated for example in rice seedlings [120] and tobacco [121].
When drought stress occurs at initial phases of plant growth, drought-avoidance plants gradually change to succulent types or develop advanced drought tolerance strategies such as generation of compatible solutes, enhancement of antioxidant apparatus and other physiological responses aimed at increase the water use efficiency [122]. Satisha et al. [123] demonstrated indeed, that selection of grape varieties with drought tolerance should follow the analyses of water use efficiency increased for example by the proper selection of rootstocks. Plants avoid water loss by stomata closure, thus decreasing evapotranspiration and increasing water use efficiency [124], therefore stomata regulation is of outmost importance in increasing WUE. Drought tolerance, water use efficiency and K+ content have close associations in plants as a sufficient level of K+ can improve the plant total dry mass and photosynthetic rate; K+ also regulates the SOD enzyme activity to mitigate the cell membrane injury which is caused by drought-triggered ROS [125]. Besides stomatal movement, drought stress may promote changes of leaf morpho-anatomical traits including vascular bundle per unit leaf area [126], stomata density and leaf thickness [127]. For leaf thickness, especially palisade parenchyma could contain larger numbers of CO2-fixation sites [128]. On the other hand, increases of epidermis thickness represent a way to contrast water loss under water limitation and both palisade and epidermis thicknesses can be used to select more tolerant olive genotypes [128]. However, despite drought-promoted morpho-anatomical traits, biochemical limitations might have a greater impact on plant performances [129].
At the biochemical level, plant hormones, secondary metabolites and other key molecules such as carbohydrate, amino acid and polyamines play crucial roles in stress tolerance mechanism and improving the capability of plant adaptation by altering their membrane stabilization, osmoregulation, ROS scavenging, lessening leaf area and its abscission, promoting root development and reducing ion leakage [130]. Osmolytes accumulation is essential for osmo-protection and osmotic adjustment against water deficit conditions which can lead to loss of cell turgor and dehydration. Among others, proline acts as an important signaling moiety against drought stress to stimulate mitochondria functioning and alter cell proliferation, stimulating particular drought stress recovery genes [131]. Proline accumulation helps to maintain membrane integrity by diminishing lipids peroxidation by defending cell redox potential and declining ROS level [132]. It has been shown that plants which accumulate higher levels of proline exhibit higher rates of plant survival (Triticum aestivum) [133], biomass production [134] and grain yield [135]. Similarly, genotypes which accumulate higher level of glycine betaine [136], mannitol and other non-structural carbohydrates [137] have greater drought tolerance. Likewise, trehalose under drought stress aids to stabilize macromolecules such as lipids, protein and other biological moieties to enhance photosynthetic functioning, thereby conferring drought tolerance [138,139]. Besides the selection of osmolite-overproducing genotypes/varieties, another promising strategy is the exogenous supplementation of these compatible solutes, which have exerted positive results in different crop species (for a review, see [140]).
Increased antioxidant defenses also assist to increase drought tolerance by defending plants from oxidative stress triggered by limited water availability (see Section 5). Therefore, selection of varieties/individuals with an enhanced antioxidant apparatus allow to select individual with greater possibility to survive and perform better in water-limiting conditions, e.g., in peanut [141] for which the enhanced activities of superoxide dismutase, ascorbate peroxidase and glutathione reductase were essential to plant drought tolerance. Shamin et al. [142] also observed that higher antioxidant capacity protects photosynthetic activities in drought tolerant tomato genotypes. In sugarcane, the tolerant genotype RB867515 exhibited a powerful antioxidant apparatus when compared to the more sensitive RB855536 [143], which was essential to tolerate prolonged drought.
8.2. Molecular and Phytohormone-Mediated Signaling Mechanisms of Drought Tolerance
Molecular responses to adverse stress conditions involve highly regulated genes and signal transduction processes that aid plants to confront the stress conditions. C-repeat/dehydration-responsive element binding factors (CBF/DREB), mitogen-activated protein (MYB), cup-shaped cotyledon CUC, no apical meristem NAC TFs and zinc-finger proteins (ZFPs) are recognized as significant moieties in conferring plant drought tolerance [144]. GsZFP1 gene improved Medicago sativa drought tolerance, suggesting that the GsZFP1 is effective to promote drought tolerant plants in genetic engineering breeding practices [145]. The overexpression of SNAC1 in Gossypium hirsutum elevates its ability to cope with water deficit and also escalates its root growth, which shows that bigger roots are useful in drought resistance breeding [146]. BdWRKY36 gene stimulated transcription of stress-related genes, reduced electrolyte leakage, decreased ROS level and elevated chlorophyll amount, plant water status and antioxidant enzyme activities to enhance the drought tolerance [147]. MpCYS4 boosted closing of stomata, triggered the transcription activity of abscisic acid (ABA) and water-deficit-associated genes to confer drought tolerance and was associated with ABA induced stress signal transduction [148]. Late embryogenesis associate (LEA) gene expression declined photosynthetic activity and boosted the plant antioxidant defense system to improve drought stress tolerance in three Linderniaceae species differing in desiccation tolerance [149]. In drought-tolerant Malus domestica, the foremost stimulatory strategy for high water use efficiency involves maintenance of C3 cycle activity by enhancing the function of photosynthetic enzymes, alleviating e− transfer, diminishing ROS amount by controlling the photosynthetic e− transport chain, C2 cycle and ROS mitigation ability to inhibit photoinhibition and improving photosynthetic activity [150].
Against water deficit stress, resulting signal transduction induced the generation of different constituents including phytohormones to respond and adapt to drought stress. ABA is useful in plant drought tolerance by triggering diverse signaling mechanisms [151]. Beside stimulating stomatal movement, root architecture and regulating photosynthesis, ABA-induced genes encoding drought-related proteins such as dehydrins, ROS-detoxifying enzymes, regulatory proteins and phospholipid signaling enzymes can improve drought stress tolerance [152]. Improved amount of ABA induced a signaling pathway in guard cells which results in outflow of guard cells K+ and reduced turgor pressure, ultimately causing stomata closure [44,153]. ABA mitigated drought stress and increased the wheat tolerance ability by improving stem lengths and plant biomass, declining the level of H2O2 and malondialdehyde (MDA) [154]. Increased level of cytokinin amount in xylem sap induced stomata opening by diminishing its sensitivity to ABA [155]. Jasmonic acid (JA) synthesis-related genes were stimulated in the overexpressing lines of VaNAC26 which increased ROS scavenging and stimulated stomata closure and root growth, thereby promoting higher drought tolerance [156]. JA enhances plants drought tolerance by stimulating root growth, decreasing level of ROS and promoting stomatal closure [157]. Auxin regulates root development, functioning of ABA related genes and ROS metabolism to improve drought-tolerance [158]. Ethylene mediates synthesis of guard cell antioxidant flavanols in an EIN2 dependent manner and adversely affects stomata closing by suppressing drought mediated ROS formation [159], thereby resulting in another possible target for genetically engineered plants tolerant to drought.
In view of the above, obtaining transgenic plants is a promising approach to improving drought tolerance traits in a shorter time as compared to classical breeding programs. However, in view of the legal limitations which exist to cultivate transgenic plants in field, it remains arguable whether or not transgenic plants produced under controlled conditions to enhance drought tolerance really perform in field experiments in which other confounding variables may occur. Thus, much more has to be done from this point of view to establish the real value of the transgenic approach in conferring drought tolerance. For this goal, it is essential for environmentally-controlled experiments to be validated in long-term field experiments, thereby reducing the real advantage between the genetic approaches over the classical breeding.
9. Conclusions
Drought is a widespread adverse limiting factor which alters various characteristics of plant growth, physiology and metabolism. Timing, duration, severity and speed of growth are important factors to be considered in the attempt to select drought-tolerant species in particular environments. Drought stress negatively affects various biological processes of plants, from the embryo phase to the reproductive and maturity phases. Drought stress affects plants morphological, physiological, biochemical and metabolic pathways, ultimately declining plant productivity. The drought tolerance strategies adopted by plants include several biological mechanisms at cell, organ and entire plant levels, when stimulated at various phases of plant growth. Water loss declined by improving stomatal functioning, elevated water transport by emerging bigger and deeper rooting structures and production of compatible solutes. ROS scavenging by antioxidant defense system, maintenance of membrane integrity, usage of precise plant genotypes, treatment with plant growth regulators, production of compatible solutes, stress-related proteins and aquaporins activity are also helpful in generating drought tolerance in plants. Selection of individuals with increased water use efficiency, enhanced antioxidant apparatus and production of key osmolites and secondary metabolites represent some possible promising strategies to obtain higher drought tolerance plants. In addition, exogenous supply of compounds which are able to promote the drought tolerance in plants could be exploited in water-limiting environments. Biotechnological strategies should also be taken into consideration to generate transgenic plants able to tolerate water scarcity, although their validation cannot precede real field experiments.
Author Contributions
All the authors contributed in writing the original draft and revising the final version. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Verslues, P.E.; Agarwal, M.; Katiyar-Agarwal, S.; Zhu, J.; Zhu, J.K. Methods and concepts in quantifying resistance to drought, salt and freezing, abiotic stresses that affect plant water status. Plant J. 2006, 45, 523–539. [Google Scholar] [CrossRef] [PubMed]
- Chaves, M.M.; Costa, J.M.; Saibo, N.J.M. Recent advances in photosynthesis under drought and salinity. Adv. Bot. Res. 2011, 57, 49–104. [Google Scholar]
- Kabiri, R.; Nasibi, F.; Farahbakhsh, H. Effect of exogenous salicylic acid on some physiological parameters and alleviation of drought stress in Nigella sativa plant under hydroponic culture. Plant Protect. Sci. 2014, 50, 43–51. [Google Scholar] [CrossRef]
- Lambers, H.; Chapin, F.S., III; Pons, T.L. Plant Physiological Ecology; Springer: New York, NY, USA, 2008. [Google Scholar]
- Salehi-Lisar, S.Y.; Bakhshayeshan-Agdam, H. Drought stress in plants: Causes, consequences, and tolerance. In Drought Stress Tolerance in Plants; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Ballhorn, D.J.; Kautz, S.; Heil, M.; Hegeman, A.D. Cyanogenesis of wild lima bean (Phaseolus lunatus L.) is an efficient direct defence in nature. PLoS ONE 2009, 4, e5450. [Google Scholar] [CrossRef]
- Hazrati, S.; Tahmasebi-Sarvestani, Z.; Mokhtassi-Bidgoli, A.; Modarres-Sanavy, S.A.M.; Mohammadi, H.; Nicola, S. Effects of zeolite and water stress on growth, yield and chemical compositions of Aloe vera L. Agric. Water Manag. 2017, 181, 66–72. [Google Scholar] [CrossRef]
- Laxa, M.; Liebthal, M.; Telman, W.; Chibani, K.; Dietz, K.-J. The role of the plant antioxidant system in drought tolerance. Antioxidants 2019, 8, 94. [Google Scholar] [CrossRef]
- Kögler, F.; Söffker, D. Water (stress) models and deficit irrigation: System-theoretical description and causality mapping. Ecol. Model. 2017, 361, 135–156. [Google Scholar] [CrossRef]
- Wang, J.; Chen, J.; Sharma, A.; Tao, S.; Zheng, B.; Landi, M.; Yuan, H.; Yan, D. Melatonin Stimulates Activities and Expression Level of Antioxidant Enzymes and Preserves Functionality of Photosynthetic Apparatus in Hickory Plants (Carya cathayensis Sarg.) under PEG-Promoted Drought. Agronomy 2019, 9, 702. [Google Scholar] [CrossRef]
- Geilfus, C.M. Drought Stress. In Controlled Environment Horticulture; Springer: Cham, Switzerland, 2019; pp. 81–97. [Google Scholar]
- Kumawat, K.R.; Sharma, N.K. Effect of Drought Stress on Plants Growth. Popular Kheti 2018, 6, 239–241. [Google Scholar]
- Sharma, A.; Kumar, V.; Shahzad, B.; Ramakrishnan, M.; Sidhu, G.P.S.; Bali, A.S.; Handa, N.; Kapoor, D.; Yadav, P.; Khanna, K. Photosynthetic response of plants under different abiotic stresses: A review. J. Plant Growth Regul. 2020, 39, 509–531. [Google Scholar] [CrossRef]
- Sharma, A.; Wang, J.; Xu, D.; Tao, S.; Chong, S.; Yan, D.; Li, Z.; Yuan, H.; Zheng, B. Melatonin regulates the functional components of photosynthesis, antioxidant system, gene expression, and metabolic pathways to induce drought resistance in grafted Carya cathayensis plants. Sci. Total Environ. 2020, 713, 136675. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.F.; Hussain, S.; Ahmad, S.; Ejaz, S.; Zakir, I.; Ali, M.A.; Ahmed, N.; Anjum, M.A. Impacts of Abiotic Stresses on Growth and Development of Plants. In Plant Tolerance to Environmental Stress; CRC Press: Boca Raton, FL, USA, 2019; pp. 1–8. [Google Scholar]
- Du, Y.; Zhao, Q.; Chen, L.; Yao, X.; Zhang, W.; Zhang, B.; Xie, F. Effect of drought stress on sugar metabolism in leaves and roots of soybean seedlings. Plant Physioly Biochem. PPB 2020, 146, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S. Plant drought stress: Effects, mechanisms and management. In Sustainable Agriculture; Springer: Dordrecht, The Netherlands, 2009; pp. 153–188. [Google Scholar]
- Bogale, A.; Nagle, M.; Latif, S.; Aguila, M.; Müller, J. Regulated deficit irrigation and partial root-zone drying irrigation impact bioactive compounds and antioxidant activity in two select tomato cultivars. Sci. Hortic. 2016, 213, 115–124. [Google Scholar] [CrossRef]
- Coyago-Cruz, E.; Corell, M.; Moriana, A.; Hernanz, D.; Stinco, C.M.; Meléndez-Martínez, A.J. Effect of the fruit position on the cluster on fruit quality, carotenoids, phenolics and sugars in cherry tomatoes (Solanum lycopersicum L.). Food Res. Int. 2017, 100, 804–813. [Google Scholar] [CrossRef] [PubMed]
- Anjum, S.A.; Wang, L.C.; Farooq, M.; Hussain, M.; Xue, L.L.; Zou, C.M. Brassinolide application improves the drought tolerance in maize through modulation of enzymatic antioxidants and leaf gas exchange. J. Agron. Crop Sci. 2011, 197, 177–185. [Google Scholar] [CrossRef]
- Tanveer, M.; Shahzad, B.; Sharma, A.; Khan, E.A. 24-Epibrassinolide application in plants: An implication for improving drought stress tolerance in plants. Plant Physiol. Biochem. 2019, 135, 295–303. [Google Scholar] [CrossRef]
- Rao, S.; Qayyum, A.; Razzaq, A.; Ahmad, M.; Mahmood, I.; Sher, A. Role of foliar application of salicylic acid and l-tryptophan in drought tolerance of maize. J. Anim. Plant Sci. 2012, 22, 768–772. [Google Scholar]
- Hammad, S.A.R.; Ali, O.A.M. Physiological and biochemical studies on drought tolerance of wheat plants by application of amino acids and yeast extract. Ann. Agric. Sci. 2014, 59, 133–145. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Lee, D.-J. Exogenously applied polyamines increase drought tolerance of rice by improving leaf water status, photosynthesis and membrane properties. Acta Physiol. Plant. 2009, 31, 937–945. [Google Scholar] [CrossRef]
- Santos, M.G.D.; Ribeiro, R.V.; Oliveira, R.F.D.; Pimentel, C. Gas exchange and yield response to foliar phosphorus application in Phaseolus vulgaris L. under drought. Braz. J. Plant Physiol. 2004, 16, 171–179. [Google Scholar] [CrossRef]
- Zlatev, Z.; Lidon, F.C. An overview on drought induced changes in plant growth, water relationsand photosynthesis. Emir J. Food Agr. 2012, 24, 57–72. [Google Scholar]
- Yigit, N.; Sevik, H.; Cetin, M.; Kaya, N. Determination of the effect of drought stress on the seed germination in some plant species. In Water Stress in Plants; Intech Open: London, UK, 2016; pp. 43–62. [Google Scholar]
- Osakabe, Y.; Osakabe, K.; Shinozaki, K.; Tran, L.-S.P. Response of plants to water stress. Front. Plant Sci. 2014, 5, 86. [Google Scholar] [CrossRef] [PubMed]
- Bota, J.; Medrano, H.; Flexas, J. Is photosynthesis limited by decreased Rubisco activity and RuBP content under progressive water stress? New Phytol. 2004, 162, 671–681. [Google Scholar] [CrossRef]
- Flexas, J.; Bota, J.; Galmes, J.; Medrano, H.; Ribas-Carbó, M. Keeping a positive carbon balance under adverse conditions: Responses of photosynthesis and respiration to water stress. Physiol. Planta 2006, 127, 343–352. [Google Scholar] [CrossRef]
- Vurayai, R.; Emongor, V.; Moseki, B. Effect of water stress imposed at different growth and development stages on morphological traits and yield of bambara groundnuts (Vigna subterranea L. Verdc). Am. J. Plant Physiol. 2011, 6, 17–27. [Google Scholar] [CrossRef]
- Mafakheri, A.; Siosemardeh, A.; Bahramnejad, B.; Struik, P.; Sohrabi, Y. Effect of drought stress on yield, proline and chlorophyll contents in three chickpea cultivars. Aus. J. Crop Sci. 2010, 4, 580. [Google Scholar]
- Wang, X.; Liu, H.; Yu, F.; Hu, B.; Jia, Y.; Sha, H.; Zhao, H. Differential activity of the antioxidant defence system and alterations in the accumulation of osmolyte and reactive oxygen species under drought stress and recovery in rice (Oryza sativa L.) tillering. Sci. Rep. 2019, 9, 8543. [Google Scholar] [CrossRef]
- Sharma, A.; Zheng, B. Melatonin mediated regulation of drought stress: Physiological and molecular aspects. Plants 2019, 8, 190. [Google Scholar] [CrossRef]
- Ghatak, A.; Chaturvedi, P.; Weckwerth, W. Cereal Crop Proteomics: Systemic Analysis of Crop Drought Stress Responses Towards Marker-Assisted Selection Breeding. Front Plant Sci 2017, 8, 757. [Google Scholar] [CrossRef]
- Sourour, A.; Afef, O.; Mounir, R.; Mongi, B.Y. A review: Morphological, physiological, biochemical and molecular plant responses to water deficit stress. Int. J. Eng. Sci. 2017, 6, 1–4. [Google Scholar] [CrossRef]
- Queiroz, M.S.; Oliveira, C.E.S.; Steiner, F.; Zuffo, A.M.; Zoz, T.; Vendruscolo, E.P.; Menis, V.S.; Mello, B.F.F.R.; Cabral, R.C.; Menis, T.F. Drought stresses on seed germination and early growth of maize and sorghum. J. Agric. Sci. 2019, 11, 310–318. [Google Scholar] [CrossRef]
- Patanè, C.; Saita, A.; Sortino, O. Comparative effects of salt and water stress on seed germination and early embryo growth in two cultivars of sweet sorghum. J. Agron. Crop Sci. 2013, 199, 30–37. [Google Scholar] [CrossRef]
- Qayyum, A.; Razzaq, A.; Ahmad, M.; Jenks, M.A. Water stress causes differential effects on germination indices, total soluble sugar and proline content in wheat (Triticum aestivum L.) genotypes. Afr. J. Biotechnol. 2011, 10, 14038–14045. [Google Scholar]
- Bhatt, R.M.; Rao, N.K.S. Influence of pod load on response of okra to water stress. Indian J. Plant Physiol. 2005, 10, 54–59. [Google Scholar]
- Razmjoo, K.; Heydarizadeh, P.; Sabzalian, M.R. Effect of salinity and drought stresses on growth parameters and essential oil content of Matricaria chamomile. Int. J. Agric. Biol. 2008, 10, 451–454. [Google Scholar]
- Gheidary, S.; Akhzari, D.; Pessarakli, M. Effects of salinity, drought, and priming treatments on seed germination and growth parameters of Lathyrus sativus L. J. Plant Nutr. 2017, 40, 1507–1514. [Google Scholar] [CrossRef]
- Asadi, S.; Lebaschy, M.H.; Khourgami, A.; Rad, A.H.S. Effect of drought stress on the morphology of three Salvia sclarea populations. Ann. Biol. Res. 2012, 3, 4503–4507. [Google Scholar]
- Salazar, C.; Hernández, C.; Pino, M.T. Plant water stress: Associations between ethylene and abscisic acid response. Chil. J. Agric. Res. 2015, 75, 71–79. [Google Scholar] [CrossRef]
- Maleki, M.; Ebrahimzade, H.; Gholami, M.; Niknam, V. The effect of drought stress and exogenous abscisic acid on growth, protein content and antioxidative enzyme activity in saffron (Crocus sativus L.). Afr. J. Biotechnol. 2011, 10, 9068–9075. [Google Scholar]
- Smith, S.; De Smet, I. Root system architecture: Insights from Arabidopsis and cereal crops. Philos. Trans. R. Soc. B 2012, 367, 1441–1452. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Younis, A.; Riaz, A.; Mansoor, F.; Hameed, M.; Akram, N.A.; Abideen, Z. Morpho-anatomical adaptations of two Tagetes Erecta L. cultivars with contrasting response to drought stress. Pak. J. Bot. 2020, 52, 801–810. [Google Scholar] [CrossRef]
- Akhtar, I.; Nazir, N. Effect of waterlogging and drought stress in plants. Int. J. Water Res. Environ. Eng. 2013, 2, 34–40. [Google Scholar]
- Bhargavi, B.; Kalpana, K.; Reddy, J.K. Influence of Water Stress on Morphological and Physiological Changes in Andrographis paniculata. Int. J. Pure Appl. Biosci. 2017, 5, 1550–1556. [Google Scholar]
- Najla, S.; Sanoubar, R.; Murshed, R. Morphological and biochemical changes in two parsley varieties upon water stress. Physiol. Mol. Biol. Plants 2012, 18, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Srivastava, M. Morphological changes and antioxidant activity of Stevia rebaudiana under water stress. Am. J. Plant Sci. 2014, 5, 3417. [Google Scholar] [CrossRef]
- Bangar, P.; Chaudhury, A.; Tiwari, B.; Kumar, S.; Kumari, R.; Bhat, K.V. Morphophysiological and biochemical response of mungbean [Vigna radiata (L.) Wilczek] varieties at different developmental stages under drought stress. Turk. J. Biol. 2019, 43, 58–69. [Google Scholar] [CrossRef]
- Deka, D.; Singh, A.K.; Singh, A.K. Effect of Drought Stress on Crop Plants with Special Reference to Drought Avoidance and Tolerance Mechanisms: A Review. Int. J. Curr. Microbiol. App. Sci. 2018, 7, 2703–2721. [Google Scholar] [CrossRef]
- Nezhadahmadi, A.; Prodhan, Z.H.; Faruq, G. Drought tolerance in wheat. Sci. World J. 2013, 610721. [Google Scholar] [CrossRef]
- Zare, M.; Azizi, M.H.; Bazrafshan, F. Effect of drought stress on some agronomic traits in ten barley (Hordeum vulgare L.) cultivars. Tech. J. Eng. Appl. Sci. 2011, 1, 57–62. [Google Scholar]
- Bhargava, S.; Sawant, K. Drought stress adaptation: Metabolic adjustment and regulation of gene expression. Plant Breed. 2013, 132, 21–32. [Google Scholar] [CrossRef]
- Singh, J.; Thakur, J.K. Photosynthesis and abiotic stress in plants. In Biotic and Abiotic Stress Tolerance in Plants; Springer: Singapore, 2018; pp. 27–46. [Google Scholar]
- Marcińska, I.; Czyczyło-Mysza, I.; Skrzypek, E.; Filek, M.; Grzesiak, S.; Grzesiak, M.T.; Janowiak, F.; Hura, T.; Dziurka, M.; Dziurka, K. Impact of osmotic stress on physiological and biochemical characteristics in drought-susceptible and drought-resistant wheat genotypes. Acta Physiol. Plant. 2013, 35, 451–461. [Google Scholar] [CrossRef]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Deepak, S.B.; Thakur, A.; Singh, S.; Bakshi, M.; Bansal, S. Changes in crop physiology under drought stress: A review. J. Pharmacogn. Phytochem. 2019, 8, 1251–1253. [Google Scholar]
- Sicher, R.C.; Barnaby, J.Y. Impact of carbon dioxide enrichment on the responses of maize leaf transcripts and metabolites to water stress. Physiol. Planta 2012, 144, 238–253. [Google Scholar] [CrossRef]
- Rahmati, M.; Mirás-Avalos, J.M.; Valsesia, P.; Lescourret, F.; Génard, M.; Davarynejad, G.H.; Bannayan, M.; Azizi, M.; Vercambre, G. Disentangling the effects of water stress on carbon acquisition, vegetative growth, and fruit quality of peach trees by means of the QualiTree model. Front. Plant Sci. 2018, 9, 3. [Google Scholar] [CrossRef]
- Zsófi, Z.; Tóth, E.; Rusjan, D.; Bálo, B. Terroir aspects of grape quality in a cool climate wine region: Relationship between water deficit, vegetative growth and berry sugar concentration. Sci. Hortic. 2011, 127, 494–499. [Google Scholar] [CrossRef]
- Ge, T.; Sui, F.; Bai, L.; Tong, C.; Sun, N. Effects of water stress on growth, biomass partitioning, and water-use efficiency in summer maize (Zea mays L.) throughout the growth cycle. Acta Physiol. Planta 2012, 34, 1043–1053. [Google Scholar] [CrossRef]
- Rahdari, P.; Hosseini, S.M.; Tavakoli, S. The studying effect of drought stress on germination, proline, sugar, lipid, protein and chlorophyll content in purslane (Portulaca oleracea L.) leaves. J. Med. Plants Res. 2012, 6, 1539–1547. [Google Scholar]
- Anjum, S.A.; Xie, X.-y.; Wang, L.-c.; Saleem, M.F.; Man, C.; Lei, W. Morphological, physiological and biochemical responses of plants to drought stress. Afr. J. Agric. Res. 2011, 6, 2026–2032. [Google Scholar]
- Chowdhury, J.; Karim, M.; Khaliq, Q.; Ahmed, A. Effect of drought stress on bio-chemical change and cell membrane stability of soybean genotypes. Bangladesh J. Agric. Res. 2017, 42, 475–485. [Google Scholar] [CrossRef]
- Xiang, D.-B.; Peng, L.-X.; Zhao, J.-L.; Zou, L.; Zhao, G.; Song, C. Effect of drought stress on yield, chlorophyll contents and photosynthesis in tartary buckwheat (Fagopyrum tataricum). J. Food Agric. Environ. 2013, 11, 1358–1363. [Google Scholar]
- Karimpour, M. Effect of Drought Stress on RWC and Chlorophyll Content on Wheat (Triticum durum L.) Genotypes. World. Ess. J. 2019, 7, 52–56. [Google Scholar]
- Abid, G.; M’hamdi, M.; Mingeot, D.; Aouida, M.; Aroua, I.; Muhovski, Y.; Sassi, K.; Souissi, F.; Mannai, K.; Jebara, M. Effect of drought stress on chlorophyll fluorescence, antioxidant enzyme activities and gene expression patterns in faba bean (Vicia faba L.). Arch. Agron. Soil Sci. 2017, 63, 536–552. [Google Scholar] [CrossRef]
- Gurumurthy, S.; Sarkar, B.; Vanaja, M.; Lakshmi, J.; Yadav, S.; Maheswari, M. Morpho-physiological and biochemical changes in black gram (Vigna mungo L. Hepper) genotypes under drought stress at flowering stage. Acta Physiol. Plant. 2019, 41, 42. [Google Scholar] [CrossRef]
- Cuellar-Ortiz, S.M.; De La Paz Arrieta-Montiel, M.; Acosta-Gallegos, J.; Covarrubias, A.A. Relationship between carbohydrate partitioning and drought resistance in common bean. Plant Cell Environ. 2008, 31, 1399–1409. [Google Scholar] [CrossRef]
- Abdallah, M.B.; Trupiano, D.; Polzella, A.; De Zio, E.; Sassi, M.; Scaloni, A.; Zarrouk, M.; Youssef, N.B.; Scippa, G.S. Unraveling physiological, biochemical and molecular mechanisms involved in olive (Olea europaea L. cv. Chétoui) tolerance to drought and salt stresses. J. Plant Physiol. 2018, 220, 83–95. [Google Scholar] [CrossRef]
- Brito, C.; Dinis, L.-T.; Moutinho-Pereira, J.; Correia, C.M. Drought stress effects and olive tree acclimation under a changing climate. Plants 2019, 8, 232. [Google Scholar] [CrossRef]
- Hussain, S.; Rao, M.J.; Anjum, M.A.; Ejaz, S.; Zakir, I.; Ali, M.A.; Ahmad, N.; Ahmad, S. Oxidative Stress and Antioxidant Defense in Plants Under Drought Conditions. In Plant Abiotic Stress Tolerance: Agronomic, Molecular and Biotechnological Approaches; Hasanuzzaman, M., Hakeem, K.R., Nahar, K., Alharby, H.F., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 207–219. [Google Scholar] [CrossRef]
- Gharibi, S.; Tabatabaei, B.E.S.; Saeidi, G.; Goli, S.A.H. Effect of drought stress on total phenolic, lipid peroxidation, and antioxidant activity of Achillea species. Appl. Biochem. Biotechnol. 2016, 178, 796–809. [Google Scholar] [CrossRef]
- Impa, S.M.; Nadaradjan, S.; Jagadish, S.V.K. Drought stress induced reactive oxygen species and anti-oxidants in plants. In Abiotic Stress Responses in Plants; Springer: New York, NY, USA, 2012; pp. 131–147. [Google Scholar]
- Møller, I.M.; Jensen, P.E.; Hansson, A. Oxidative modifications to cellular components in plants. Ann. Rev. Plant Biol. 2007, 58, 459–481. [Google Scholar] [CrossRef]
- Cao, Y.; Luo, Q.; Tian, Y.; Meng, F. Physiological and proteomic analyses of the drought stress response in Amygdalus Mira (Koehne) Yü et Lu roots. BMC Plant Biol. 2017, 17, 53. [Google Scholar] [CrossRef]
- Moussa, H.R.; Abdel-Aziz, S.M. Comparative response of drought tolerant and drought sensitive maize genotypes to water stress. Aust. J. Crop Sci. 2008, 1, 31–36. [Google Scholar]
- Shi, S.; Fan, M.; Iwama, K.; Li, F.; Zhang, Z.; Jia, L. Physiological basis of drought tolerance in potato grown under long-term water deficiency. Int. J. Plant Prod. 2015, 9, 305–320. [Google Scholar]
- Hossain, Z.; Nouri, M.Z.; Komatsu, S. Plant cell organelle proteomics in response to abiotic stress. J. Proteome Res. 2012, 11, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Caverzan, A.; Casassola, A.; Brammer, S.P. Reactive oxygen species and antioxidant enzymes involved in plant tolerance to stress. In Abiotic and Biotic Stress in Plants-Recent Advances and Future Perspectives; Shanker, A., Shanker, C., Eds.; Publisher InTech Open: London, UK, 2016; pp. 463–480. [Google Scholar]
- Wang, Q.; Zhao, R.; Chen, Q.; da Silva, J.A.T.; Chen, L.; Yu, X. Physiological and Biochemical Responses of Two Herbaceous Peony Cultivars to Drought Stress. HortSci. 2019, 54, 492–498. [Google Scholar] [CrossRef]
- Prathyusha, I.V.S.N.; Chaitanya, K.V. Effect of water stress on the physiological and biochemical responses of two different Coleus (Plectranthus) species. Biol. Fut. 2019, 70, 312–322. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Al-Khaishany, M.Y.; Al-Qutami, M.A.; Al-Whaibi, M.H.; Grover, A.; Ali, H.M.; Al-Wahibi, M.S.; Bukhari, N.A. Response of different genotypes of faba bean plant to drought stress. Int. J. Mol. Sci. 2015, 16, 10214–10227. [Google Scholar] [CrossRef]
- Flexas, J.; Gallé, A.; Galmés, J.; Ribas-Carbo, M.; Medrano, H. The response of photosynthesis to soil water stress. In Plant Responses to Drought Stress; Springer: Berlin/Heidelberg, Germany, 2012; pp. 129–144. [Google Scholar]
- Gao, S.; Wang, Y.; Yu, S.; Huang, Y.; Liu, H.; Chen, W.; He, X. Effects of drought stress on growth, physiology and secondary metabolites of Two Adonis species in Northeast China. Sci. Hortic. 2020, 259, 108795. [Google Scholar] [CrossRef]
- Hosseini, M.S.; Samsampour, D.; Ebrahimi, M.; Abadía, J.; Khanahmadi, M. Effect of drought stress on growth parameters, osmolyte contents, antioxidant enzymes and glycyrrhizin synthesis in licorice (Glycyrrhiza glabra L.) grown in the field. Phytochemistry 2018, 156, 124–134. [Google Scholar] [CrossRef]
- Kleinwächter, M.; Selmar, D. New insights explain that drought stress enhances the quality of spice and medicinal plants: Potential applications. Agron. Sustain. Dev. 2015, 35, 121–131. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef]
- Ballhorn, D.J.; Kautz, S.; Jensen, M.; Schmitt, I.; Heil, M.; Hegeman, A.D. Genetic and environmental interactions determine plant defences against herbivores. J. Ecol. 2011, 99, 313–326. [Google Scholar] [CrossRef]
- Selmar, D.; Kleinwächter, M. Stress enhances the synthesis of secondary plant products: The impact of stress-related over-reduction on the accumulation of natural products. Plant Cell Physiol. 2013, 54, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Nacif de Abreu, I.; Mazzafera, P. Effect of water and temperature stress on the content of active constituents of Hypericum brasiliense Choisy. Plant Physiol. Biochem. 2005, 43, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Morshedloo, M.R.; Craker, L.E.; Salami, A.; Nazeri, V.; Sang, H.; Maggi, F. Effect of prolonged water stress on essential oil content, compositions and gene expression patterns of mono- and sesquiterpene synthesis in two oregano (Origanum vulgare L.) subspecies. Plant Physiol. Biochem. 2017, 111, 119–128. [Google Scholar] [CrossRef]
- Yin, J.; Liang, T.; Wang, S.; Zhang, M.; Xiao, J.; Zhan, Y.; Li, C. Effect of Drought and Nitrogen on Betulin and Oleanolic Acid Accumulation and OSC Gene Expression in White Birch Saplings. Plant Mol. Biol Rep. 2015, 33, 705–715. [Google Scholar] [CrossRef]
- Nasrollahi, V.; Mirzaie-Asl, A.; Piri, K.; Nazeri, S.; Mehrabi, R. The effect of drought stress on the expression of key genes involved in the biosynthesis of triterpenoid saponins in liquorice (Glycyrrhiza glabra). Phytochemistry 2014, 103, 32–37. [Google Scholar] [CrossRef]
- Saha, U.; Hancock, D.; Stewart, L.; Kissel, D.; Sonon, L. The Effect of Drought on Lignin Content and Digestibility of Tifton-85 and Coastal Bermudagrass (Cynodon dactylon L.) Hays Produced in Georgia. Int. J. Appl. Agric. Sci. 2016, 2, 69. [Google Scholar] [CrossRef]
- Jaafar, H.Z.; Ibrahim, M.H.; Fakri, M.; Farhana, N. Impact of soil field water capacity on secondary metabolites, phenylalanine ammonia-lyase (PAL), maliondialdehyde (MDA) and photosynthetic responses of Malaysian Kacip Fatimah (Labisia pumila Benth). Molecules 2012, 17, 7305–7322. [Google Scholar] [CrossRef]
- García-Caparrós, P.; Romero, M.J.; Llanderal, A.; Cermeño, P.; Lao, M.T.; Segura, M.L. Effects of drought stress on biomass, essential oil content, nutritional parameters, and costs of production in six Lamiaceae species. Water 2019, 11, 573. [Google Scholar] [CrossRef]
- Gharibi, S.; Sayed Tabatabaei, B.E.; Saeidi, G.; Talebi, M.; Matkowski, A. The effect of drought stress on polyphenolic compounds and expression of flavonoid biosynthesis related genes in Achillea pachycephala Rech.f. Phytochemistry 2019, 162, 90–98. [Google Scholar] [CrossRef]
- Kheradmand, M.A.; Fahraji, S.S.; Fatahi, E.; Raoofi, M.M. Effect of water stress on oil yield and some characteristics of Brassica napus. Int. Res. J. Basic Appl. Sci. 2014, 8, 1447–1453. [Google Scholar]
- Hu, Y.; Schmidhalter, U. Drought and salinity: A comparison of their effects on mineral nutrition of plants. J. Plant Nutr. Soil. Sc. 2005, 168, 541–549. [Google Scholar] [CrossRef]
- Hu, L.; Wang, Z.; Huang, B. Effects of cytokinin and potassium on stomatal and photosynthetic recovery of Kentucky bluegrass from drought stress. Crop Sci. 2013, 53, 221–231. [Google Scholar] [CrossRef]
- Qi, J.; Sun, S.; Yang, L.; Li, M.; Ma, F.; Zou, Y. Potassium uptake and transport in apple roots under drought stress. Hortic. Plant J. 2019, 5, 10–16. [Google Scholar] [CrossRef]
- Li, Y.; Sun, C.; Huang, Z.; Pan, J.; Wang, L.; Fan, X. Mechanisms of progressive water deficit tolerance and growth recovery of Chinese maize foundation genotypes Huangzao 4 and Chang 7-2, which are proposed on the basis of comparison of physiological and transcriptomic responses. Plant Cell Physiol. 2009, 50, 2092–2111. [Google Scholar] [CrossRef]
- Cuéllar, T.; Pascaud, F.; Verdeil, J.L.; Torregrosa, L.; Adam-Blondon, A.F.; Thibaud, J.B.; Sentenac, H.; Gaillard, I. A grapevine Shaker inward K(+) channel activated by the calcineurin B-like calcium sensor 1-protein kinase CIPK23 network is expressed in grape berries under drought stress conditions. Plant J. 2010, 61, 58–69. [Google Scholar] [CrossRef]
- Da Silva, E.C.; Nogueira, R.J.M.C.; da Silva, M.A.; de Albuquerque, M.B. Drought stress and plant nutrition. Plant Stress 2011, 5, 32–41. [Google Scholar]
- Osuagwu, G.G.E.; Edeoga, H.O.; Osuagwu, A.N. The influence of water stress (drought) on the mineral and vitamin potential of the leaves of Ocimum gratissimum (L). Recent Res. Sci. Technol. 2010, 2, 27–33. [Google Scholar]
- Bahreininejad, B.; Razmjou, J.; Mirza, M. Influence of water stress on morpho-physiological and phytochemical traits in Thymus daenensis. Int. J. Plant Prod. 2013, 7, 151–166. [Google Scholar]
- Khalid, K.A. Influence of water stress on growth, essential oil, and chemical composition of herbs (Ocimum sp.). Int. Agrophys. 2006, 20, 289–296. [Google Scholar]
- Sarani, M.; Namrudi, M.; Hashemi, S.M.; Raoofi, M.M. The effect of drought stress on chlorophyll content, root growth, glucosinolate and proline in crop plants. Intl. J. Farm. Alli. Sci. 2014, 3, 994–997. [Google Scholar]
- Samarah, N.; Mullen, R.; Cianzio, S. Size distribution and mineral nutrients of soybean seeds in response to drought stress. J. Plant Nutr. 2004, 27, 815–835. [Google Scholar] [CrossRef]
- Zhang, Z. Fundamentals of Physiology and Genetics and Breeding in Crop Drought Resistance and Water Saving; Science Press: Beijing, China, 2003. (In Chinese) [Google Scholar]
- Luo, L.J. Breeding for water-saving and drought-resistance rice (WDR) in China. J. Exp. Bot. 2010, 61, 3509–3517. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Zhang, Q. The status and strategies on studying drought resistance of rice. Chin. J. Rice. Sci. 2001, 15, 209–214. [Google Scholar]
- Zhang, M.; Chen, R. Molecular Physiology and Genetic Improvement on Drought Resistance in Crop; Science Press: Beijing, China, 2005. [Google Scholar]
- Zargar, S.M.; Gupta, N.; Nazir, M.; Mahajan, R.; Malik, F.A.; Sofi, N.R.; Shikari, A.B.; Salgotra, R.K. Impact of drought on photosynthesis: Molecular perspective. Plant Gene 2017, 11, 154–159. [Google Scholar] [CrossRef]
- Chowdhury, J.A.; Karim, M.A.; Khaliq, Q.A.; Ahmed, A.U.; Khan, M.S.A. Effect of drought stress on gas exchange characteristics of four soybean genotypes. Bangladesh J. Agric. Res. 2016, 41, 195–205. [Google Scholar] [CrossRef]
- Steele, K.A.; Price, A.H.; Shashidhar, H.E.; Witcombe, J.R. Marker-assisted selection to introgress rice QTLs controlling root traits into an Indian upland rice variety. Theor. App. Genet. 2006, 112, 208–221. [Google Scholar] [CrossRef]
- Karim, S.; Aronsson, H.; Ericson, H.; Pirhonen, M.; Leyman, B.; Welin, B.; Mäntylä, E.; Palva, E.T.; Van Dijck, P.; Holmström, K.O. Improved drought tolerance without undesired side effects in transgenic plants producing trehalose. Plant Mol. Biol. 2007, 64, 371–386. [Google Scholar] [CrossRef]
- Waraich, E.A.; Ahmad, R.; Ashraf, M.Y. Role of mineral nutrition in alleviation of drought stress in plants. Aust. J. Crop Sci. 2011, 5, 764. [Google Scholar]
- Satisha, J.; Prakash, G.; Venugopalan, R. Statistical modeling of the effect of physio-biochemical parameters on water use efficiency of grape varieties, rootstocks and their stionic combinations under moisture stress conditions. Turk. J. Agric. For. 2006, 30, 261–271. [Google Scholar]
- Fathi, A.; Tari, D.B. Effect of drought stress and its mechanism in plants. Int. J. Life Sci. 2016, 10, 1–6. [Google Scholar] [CrossRef]
- Mitra, G. Molecular Approaches to Nutrient Uptake and Cellular Homeostasis in Plants Under Abiotic Stress. In Plant Nutrients and Abiotic Stress Tolerance; Springer: Singapore, 2018; pp. 525–590. [Google Scholar]
- Gong, C.-M.; Bai, J.; Deng, J.-M.; Wang, G.-X.; Liu, X.-P. Leaf anatomy and photosynthetic carbon metabolic characteristics in Phragmites communis in different soil water availability. Plant Ecol. 2011, 212, 675–687. [Google Scholar] [CrossRef]
- Wang, J.H.; Li, S.C.; Sun, M.; Huang, W.; Cao, H.; Xu, F.; Zhou, N.N.; Zhang, S.B. Differences in the stimulation of cyclic electron flow in two tropical ferns under water stress are related to leaf anatomy. Physiol. Planta 2013, 147, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Ennajeh, M.; Vadel, A.; Cochard, H.; Khemira, H. Comparative impacts of water stress on the leaf anatomy of a drought-resistant and a drought-sensitive olive cultivar. J. Hortic. Sci. Biotechnol. 2010, 85, 289–294. [Google Scholar] [CrossRef]
- Tomás, M.; Medrano, H.; Brugnoli, E.; Escalona, J.; Martorell, S.; Pou, A.; Ribas-Carbó, M.; Flexas, J. Variability of mesophyll conductance in grapevine cultivars under water stress conditions in relation to leaf anatomy and water use efficiency. Aus. J. Grape Wine Res. 2014, 20, 272–280. [Google Scholar] [CrossRef]
- El Sabagh, A.; Hossain, A.; Barutcular, C.; Gormus, O.; Ahmad, Z.; Hussain, S.; Islam, M.; Alharby, H.; Bamagoos, A.; Kumar, N.; et al. Effects of drought stress on the quality of major oilseed crops: Implications and possible mitigation strategies—A review. Appl. Ecol. Environ. Res. 2019, 17, 4019–4043. [Google Scholar] [CrossRef]
- Jayant, K.S.; Sarangi, S.K. Effect of drought stress on proline accumulation in peanut genotypes. Int. J. Adv. Res. 2014, 2, 301–309. [Google Scholar]
- Shinde, S.; Villamor, J.G.; Lin, W.; Sharma, S.; Verslues, P.E. Proline Coordination with Fatty Acid Synthesis and Redox Metabolism of Chloroplast and Mitochondria. Plant Physiol. 2016, 172, 1074–1088. [Google Scholar] [CrossRef]
- Zhou, W.; Li, Y.; Zhao, B.C.; Ge, R.C.; Shen, Y.Z.; Wang, G.; Huang, Z.J. Overexpression of TaSTRG gene improves salt and drought tolerance in rice. J. Plant Physiol. 2009, 166, 1660–1671. [Google Scholar] [CrossRef]
- Yamada, M.; Morishita, H.; Urano, K.; Shiozaki, N.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Yoshiba, Y. Effects of free proline accumulation in petunias under drought stress. J. Exp. Bot. 2005, 56, 1975–1981. [Google Scholar] [CrossRef]
- Maralian, H.; Ebadi, A.; Haji-Eghrari, B. Influence of water deficit stress on wheat grain yield and proline accumulation rate. Afr. J. Agric. Res. 2010, 5, 286–289. [Google Scholar]
- Zhang, G.H.; Su, Q.; An, L.J.; Wu, S. Characterization and expression of a vacuolar Na(+)/H(+) antiporter gene from the monocot halophyte Aeluropus littoralis. Plant Physiol. Biochem. 2008, 46, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Karakas, B.; Ozias-Akins, P.; Stushnoff, C.; Suefferheld, M.; Rieger, M. Salinity and drought tolerance of mannitol-accumulating transgenic tobacco. Plant Cell Environ. 1997, 20, 609–616. [Google Scholar] [CrossRef]
- Ibrahim, H.A.; Abdellatif, Y.M.R. Effect of maltose and trehalose on growth, yield and some biochemical components of wheat plant under water stress. Ann. Agric. Sci. 2016, 61, 267–274. [Google Scholar] [CrossRef]
- Khater, M.A.; Dawood, M.G.; Sadak, M.S.; Shalaby, M.A.; El-Awadi, M.E.; El-Din, K.G. Enhancement the performance of cowpea plants grown under drought conditions via trehalose application. Middle East J. 2018, 7, 782–800. [Google Scholar]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Bhatnagar-Mathur, P.; Devi, M.J.; Vadez, V.; Sharma, K.K. Differential antioxidative responses in transgenic peanut bear no relationship to their superior transpiration efficiency under drought stress. J. Plant Physiol. 2009, 166, 1207–1217. [Google Scholar] [CrossRef]
- Shamim, F.; Johnson, G.N.; Saqlan, S.; Waheed, A. Higher antioxidant capacity protects photosynthetic activities as revealed by Chl a fluorescence in drought tolerant tomato genotypes. Pak. J. Bot. 2013, 45, 1631–1642. [Google Scholar]
- Vilela, R.D.; Bezerra, B.K.L.; Froehlich, A.; Endres, L. Antioxidant system is essential to increase drought tolerance of sugarcane. Ann. Appl. Biol. 2017, 171, 451–463. [Google Scholar] [CrossRef]
- Cong, L.; Chai, T.Y.; Zhang, Y.X. Characterization of the novel gene BjDREB1B encoding a DRE-binding transcription factor from Brassica juncea L. Biochem. Biophy. Res. Commun. 2008, 371, 702–706. [Google Scholar] [CrossRef]
- Tang, L.; Cai, H.; Ji, W.; Luo, X.; Wang, Z.; Wu, J.; Wang, X.; Cui, L.; Wang, Y.; Zhu, Y.; et al. Overexpression of GsZFP1 enhances salt and drought tolerance in transgenic alfalfa (Medicago sativa L.). Plant Physiol. Biochem. 2013, 71, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Li, X.; Jin, S.; Liu, X.; Zhu, L.; Nie, Y.; Zhang, X. Overexpression of rice NAC gene SNAC1 improves drought and salt tolerance by enhancing root development and reducing transpiration rate in transgenic cotton. PLoS ONE 2014, 9, e86895. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Hu, W.; Zhou, R.; Wang, L.; Wang, X.; Wang, Q.; Feng, Z.; Li, Y.; Qiu, D.; He, G.; et al. The Brachypodium distachyon BdWRKY36 gene confers tolerance to drought stress in transgenic tobacco plants. Plant Cell Rep. 2015, 34, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Li, M.; Yang, Y.; Sun, X.; Wang, N.; Liang, B.; Ma, F. Overexpression of MpCYS4, A Phytocystatin Gene from Malus prunifolia (Willd.) Borkh., Enhances Stomatal Closure to Confer Drought Tolerance in Transgenic Arabidopsis and Apple. Front. Plant Sci. 2017, 8, 33. [Google Scholar] [CrossRef] [PubMed]
- Juszczak, I.; Bartels, D. LEA gene expression, RNA stability and pigment accumulation in three closely related Linderniaceae species differing in desiccation tolerance. Plant Sci. 2017, 255, 59–71. [Google Scholar] [CrossRef]
- Li, X.; Liu, F. Drought stress memory and drought stress tolerance in plants: Biochemical and molecular basis. In Drought Stress Tolerance in Plants; Springer: Cham, Switzerland, 2016; Volume 1, pp. 17–44. [Google Scholar]
- Bücker-Neto, L.; Paiva, A.L.S.; Machado, R.D.; Arenhart, R.A.; Margis-Pinheiro, M. Interactions between plant hormones and heavy metals responses. Genet. Mol. Biol. 2017, 40, 373–386. [Google Scholar] [CrossRef]
- Fahad, S.; Ullah, A.; Ali, U.; Ali, E.; Saud, S.; Hakeem, K.; Alharby, H.; Sabagh, A.; Barutcular, C.; Kamran, M.; et al. Drought Tolerance in Plants Role of Phytohormones and Scavenging System of ROS. In Plant Tolerance to Environmental Stress Role of Phytoprotectants; Hasanuzzaman, M., Fujita, M., Oku, H., Tofazzal Islam, M., Eds.; CRC Press: Boca Raton, FL, USA, 2019; pp. 1–12. [Google Scholar]
- Lim, C.W.; Baek, W.; Jung, J.; Kim, J.H.; Lee, S.C. Function of ABA in Stomatal Defense against Biotic and Drought Stresses. Intern. J. Mol. Sci. 2015, 16, 15251–15270. [Google Scholar] [CrossRef]
- Wei, L.; Wang, L.; Yang, Y.; Wang, P.; Guo, T.; Kang, G. Abscisic acid enhances tolerance of wheat seedlings to drought and regulates transcript levels of genes encoding ascorbate-glutathione biosynthesis. Front. Plant Sci. 2015, 6, 458. [Google Scholar] [CrossRef]
- Bielach, A.; Hrtyan, M.; Tognetti, V.B. Plants under Stress: Involvement of Auxin and Cytokinin. Int. J. Mol. Sci. 2017, 18, 1427. [Google Scholar] [CrossRef]
- Fang, L.; Su, L.; Sun, X.; Li, X.; Sun, M.; Karungo, S.K.; Fang, S.; Chu, J.; Li, S.; Xin, H. Expression of Vitis amurensis NAC26 in Arabidopsis enhances drought tolerance by modulating jasmonic acid synthesis. J. Exp. Bot. 2016, 67, 2829–2845. [Google Scholar] [CrossRef]
- Ullah, A.; Manghwar, H.; Shaban, M.; Khan, A.H.; Akbar, A.; Ali, U.; Ali, E.; Fahad, S. Phytohormones enhanced drought tolerance in plants: A coping strategy. Environ. Sci. Pollut. Res. Int. 2018, 25, 33103–33118. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Chen, L.; Ye, T.; Liu, X.; Ding, K.; Chan, Z. Modulation of auxin content in Arabidopsis confers improved drought stress resistance. Plant Physiol. Biochem. PPB 2014, 82, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Watkins, J.M.; Hechler, P.J.; Muday, G.K. Ethylene-induced flavonol accumulation in guard cells suppresses reactive oxygen species and moderates stomatal aperture. Plant Physiol. 2014, 164, 1707–1717. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).