Featured Application
The Ca-SZ fabricated in the current study may be used as framework for construction of dental crown and bridgework.
Abstract
Various oxides are used to stabilize zirconium oxide (ZrO2), but their superior hardness causes wear of the machining tool. Calcia-doped zirconia has been studied but reports on properties suitable for dental application are lacking. Therefore, this study aimed to fabricate and characterize zirconia stabilized by calcium oxide (CaO) derived from cockle shells and compare it with zirconia stabilized by commercial CaO, sintered at different temperatures. In this study, 176 pressed pellets of zirconia mixed with CaO either derived from cockle shells or commercial CaO were sintered between 1200 and 1500 °C to produce calcia-doped zirconia. Characterizations were made with SEM and XRD. Specimens were subjected to density, compressive and flexural strength, and Vickers hardness testing. Data were analyzed using the independent t-test and one-way ANOVA. XRD revealed the zirconia was stabilized into tetragonal and cubic phases (Ca-SZ). Ca-SZ cockle shells (CS) and Ca-SZ commercial (CC) have average particle sizes of 267 nm and 272 nm, respectively, with similar surface roughness. At 1400 °C sintering temperature, flexural strengths were 1165 and 1152 MPa, compressive strengths were 4914 and 4913 MPa, and Vickers hardness were 977 and 960 MPa for Ca-SZ(CS) and Ca-SZ(CC), respectively. Both Ca-SZ materials showed no significant difference in most properties (p < 0.05) when sintered at different temperatures. The fully sintered Ca-SZ is less hard compared to the ceria-stabilized tetragonal zirconia polycrystal (Ce-TZP) available on the market. Thus, Ca-SZ may be used as an alternative to the current zirconia available on the market for dental application.
1. Introduction
Zirconia-based ceramics have been used as dental implants [1] in addition to framework underneath the brittle veneering porcelain in the fabrication of fixed dental prostheses, such as crowns and bridges [2]. As the aesthetic properties of zirconia-based ceramics improve, they have also been used to construct the whole crown and bridge works in combination with computer-aided design/computer-aided manufacturing (CAD/CAM) [3].
Pure zirconia has three polymorphic forms at atmospheric pressure: monoclinic from room temperature until 1170 °C, tetragonal (1170–2370 °C) and cubic (2370–2680 °C) [4]. However, pure zirconia encounters a setback whereby upon cooling of the polymorphs, large volume change occurs, which would result in the cracking of the brittle products. Incorporation of a small percentage of cubic oxide stabilizers such as yttria (Y2O3), ceria (CeO2), magnesia (MgO), and calcia (CaO) to zirconia can delay the creation of the transformation phase [5,6]. Subsequently, the zirconia crystals in their tetragonal or cubic shape at room temperature remain stable. Zirconia-containing ceramic systems currently used in dentistry are yttrium cation-doped tetragonal zirconium polycrystals (3Y-TZP), magnesium cation-doped partially stabilized zirconium (Mg-PSZ), zirconium-toughened alumina (ZTA) and ceria-stabilized zirconia-alumia (CeTZP/A) [3,7,8].
Yttrium partially stabilized tetragonal zirconia polycrystalline (Y-TZP) bridge framework with a high fracture toughness, from 5 to 10 MPa m1/2, and a flexural strength of 900–1400 MPa [9,10] can be fabricated by milling either fully sintered blocks or partially sintered blocks/green blocks using computer-aided design/computer-aided manufacture (CAD/CAM) procedures [11]. However, yttrium-stabilized zirconia has the disadvantage of inferior machining associated with wear of the tool due to its superior hardness. Partially sintered Y-TZP produces extensive sintering shrinkage during the post-sintering process, which might affect the final fit of the zirconia framework [12]. Y-TZP also suffers from low-temperature aging degradation (LTAD) caused by phase transformation [13]. Ce-TZP/A is the toughest dental ceramic material available, with a fracture toughness of 19 MPa m1/2, and a flexural strength of 1400 MPa [8]. Although alumina and ceria, as stabilizing oxides, reduce the susceptibility of zirconia to LTAD [14], they can cause wear of the milling machine if milled fully sintered, due to their superior hardness. Therefore, other partially and fully stabilized zirconia, for example Ca- PSZ, may be used as suitable replacement materials for dental application.
Studies have been conducted to produce calcia-doped zirconia in biomedical application such as for hip and knee replacement. Nath in 2008 sintered Ca-PSZ for a hip replacement implant. The material exhibits modest fracture toughness (6 MPa m1/2). This property is better than commercial grade alumina, but lower than Y-TZP and Ce-TZP/A [15]. Therefore, Ca-PSZ is a possible material that has a better combination of mechanical properties than other commercially available bioceramics, as it can give optimum strength but at the same time does not wear the CAD/CAM milling machine.
Drazin and Castro in 2016 reported that calcia-doped zirconia exhibits all the polymorphism seen in the yttria-doped zirconia ceramics, but can be produced at lowered costs and in greater abundance due to the accessibility of calcium precursors in comparison to yttrium [16]. In most cases, the particles sizes of stabilizers are not clearly specified. Moreover, the reports have mostly focused on the synthesis of the calcia-doped zirconia, but the use of nano-CaO derived from cockle shells to stabilize zirconia for dental application has not been reported.
In our previous study, spherical nano-CaO was successfully extracted from cockle shells [17]. Cockle shells are a natural source of nano-CaO and are readily available as waste. CaO is non-toxic and biocompatible in many applications and is one of the compositions of hydroxyapatite in human bone. In the current study, nano-CaO derived from cockle shells was used to stabilize zirconia. It is therefore important to investigate whether the incorporation of nano-CaO derived from cockle shells into zirconia will produce a better microstructure of CaO-stabilized zirconia with good material properties, as often obtained by the commercial CaO. One of the major aims of the present work is to address this issue. The other issue to be addressed is whether the nano-CaO derived from cockle shells stabilized zirconia is less hard than alumina- and yttria-stabilized zirconia.
If the nano-CaO stabilized zirconia derived from cockle shells and from commercial source are comparable, then it may be used as a stabilizer in the fabrication of zirconia for dental application. The cockle shells are readily available as waste. Therefore, using nano-CaO from cockle shells not only will have the benefit of waste removal (recycling), but at the same time produce added value to the cockle shell waste.
Therefore, the purpose of this study was to synthesize and investigate the effect of different sintering temperatures on the physical and mechanical properties of novel calcia-stabilized zirconia (Ca-SZ) with the CaO derived from cockle shells and compare it to the Ca-SZ stabilized by commercial CaO.
2. Materials and Methods
2.1. Materials
Commercially available zirconium oxide (ZrO2) nanopowder (<40 nm) and commercial CaO nanopowder (<160 nm) CaO (CC) were purchased from Sigma, Ronkonkoma, NY, USA. Calcium oxide nanopowder (22–70 nm) derived from cockle shells CaO (CS) was prepared according to protocol as described in a previous study [17].
2.2. Extraction of Calcium Oxide Cockle Shells
Local cockle shells were collected from a local beach, Pantai Bachok. The cockle shells were washed, pulverized and sieved to form a uniform sized cockle shell powder of 63 µm. The cockle shell powder was dissolved in hydrochloric acid to produce calcium chloride (CaCl2), which was then titrated with ethanol and potassium carbonate to form insoluble calcium carbonate (CaCO3). This was followed by calcination of CaCO3 at 750 °C for 30 min to produce spherical, nano-CaO powder, which was used in the current study.
2.3. Fabrication of CaO Stabilized Zirconia Pellet
To prepare the zirconia specimen pellet, 9.2 g of zirconium oxide (92%) was mixed with 0.8 g of calcium oxide (8%), either CaO(CS) or CaO(CC) (8%). The mixed powder was placed in a plastic bottle containing ceramic balls, which was then placed on an electric mixer for 30 min to allow for proper mixing. Then, the inner surface of a stainless steel mold with the dimension of 32 mm in diameter and 6 mm in height was isolated with silicone wax-based separating medium. The mixed powder was placed in the stainless steel mold, and then pressed using a hydraulic press (AtlasTM Manual 15 T and 25 T Hydraulic Press, Specac, Kent, Orpington, UK) under 5 tons (60 Mpa) pressure. A total of 176 pressed zirconia specimen pellets were then divided into four groups according to the different sintering temperatures. Each group was sintered in a furnace (Linn High Therm Furnace VMK-1800) for two hours at four different temperatures (1200, 1300, 1400, and 1500 °C) to produce pellet blanks of calcia-stabilized zirconia (Ca-SZ) with either CaO derived from cockle shells (CS) or commercial CaO (CC), which from here on will be called Ca-SZ(CS) and Ca-SZ(CC), respectively. An example of the sintered zirconia pellet blank is shown in Figure 1.

Figure 1.
Sintered calcium oxide (CaO)-stabilized zirconia pellet blank.
2.4. Physical and Mechanical Tests of Ca-SZ(CS) and Ca-SZ(CC) Sintered at Different Temperatures
Eight specimens from both Ca-SZ(CS) and Ca-SZ(CC) groups sintered at different temperatures were analyzed with an X-ray diffractometer (XRD) (Shimadzu XRD-6000 powder diffractometer) using Cu Kα radiation (λ = 1.5406 Å, 40 kV at 160 mA) at a scan rate of 0.05° 2θ s−1.
Another eight specimens, from each group sintered at four different temperatures, were analyzed using a scanning electron microscope (SEM) (LEICA EM SCD005, Bannockburn, IL, USA). Specimens were then polished using an electric polishing machine (EXAKT 400CS, AW110, Oklahoma City, OK, USA) with 300 mm Schleifscheiben sandpaper discs (Hermes Schleifmittel GmbH & Co. KG, Hamburg, Germany) at different grit sizes (P500, P800, P1000 and P1200) for 15 min for each grit, at 5000 rpm. Water was used to prevent the overheating of the samples. The sandpaper was changed for every sample. After that, the polished specimens were then re-analyzed using the SEM machine.
Forty specimens from both groups, sintered at four different temperatures (n = 5), were weighed on a precision balance using Mettler Toledo DA110 toploading electrical balance (Tokyo, Japan), before and after immersion in double distilled water (DDW), for actual and suspended weights, respectively. Archimedes’ principle was used to measure the density of sintered specimens. The following formula was used to calculate the density [18].
where, ρ is the calculated density (g/cm3) and ρw is the density of the water (g/cm3).
Forty specimens with dimensions of 25 mm × 2 mm × 2 mm were measured with vernier calipers and cut using a micro motor handpiece driven at 30,000 rpm for a flexural strength test (n = 5). Another 40 specimens with dimensions of 6 mm height × 4 mm diameter (International Standards Organization (ISO) no. 9917 (2000)) were prepared for a compressive strength test (n = 5). Another 40 specimens measuring 30 mm × 10 mm × 2.5 mm (International Standards Organization (ISO) Specification no. 1567) were prepared for the Vickers hardness test (n = 5). The cut specimens were polished using the same protocol mentioned above. The flexural strength and compressive strength of the specimens were tested using the universal testing machine (Shimadzu, Model: AGX plus 20 kN, Kyoto, Japan) at the crosshead speed of 0.75 mm/min. The formula used for calculating the flexural strength and compressive strength was: Flexural strength = 3PL/2bd2 where, P is the maximum force applied, L is the span length, b is the width, d is the thickness; Compressive strength = 4P/πd2 where P stands for maximum force applied and d is the specimen diameter. For measuring hardness, a load of 1000 g = 9.81 newtons earth was placed on the top surface of the polished surface of the specimens by the Vickers hardness indenter.
Statistical Analyses
The one-way ANOVA test was used to analyze the means of flexural, compressive, Vickers hardness and density of the materials sintered at different temperatures, with p < 0.05 considered as significant. After checking the equal variance, the post-hoc Tukey test was used for multiple comparisons between the groups. The independent t test was used to analyze the mechanical and physical strengths between Ca-SZ(CS) and Ca-SZ(CC), with p < 0.05 considered significant.
3. Results
3.1. XRD Analyses
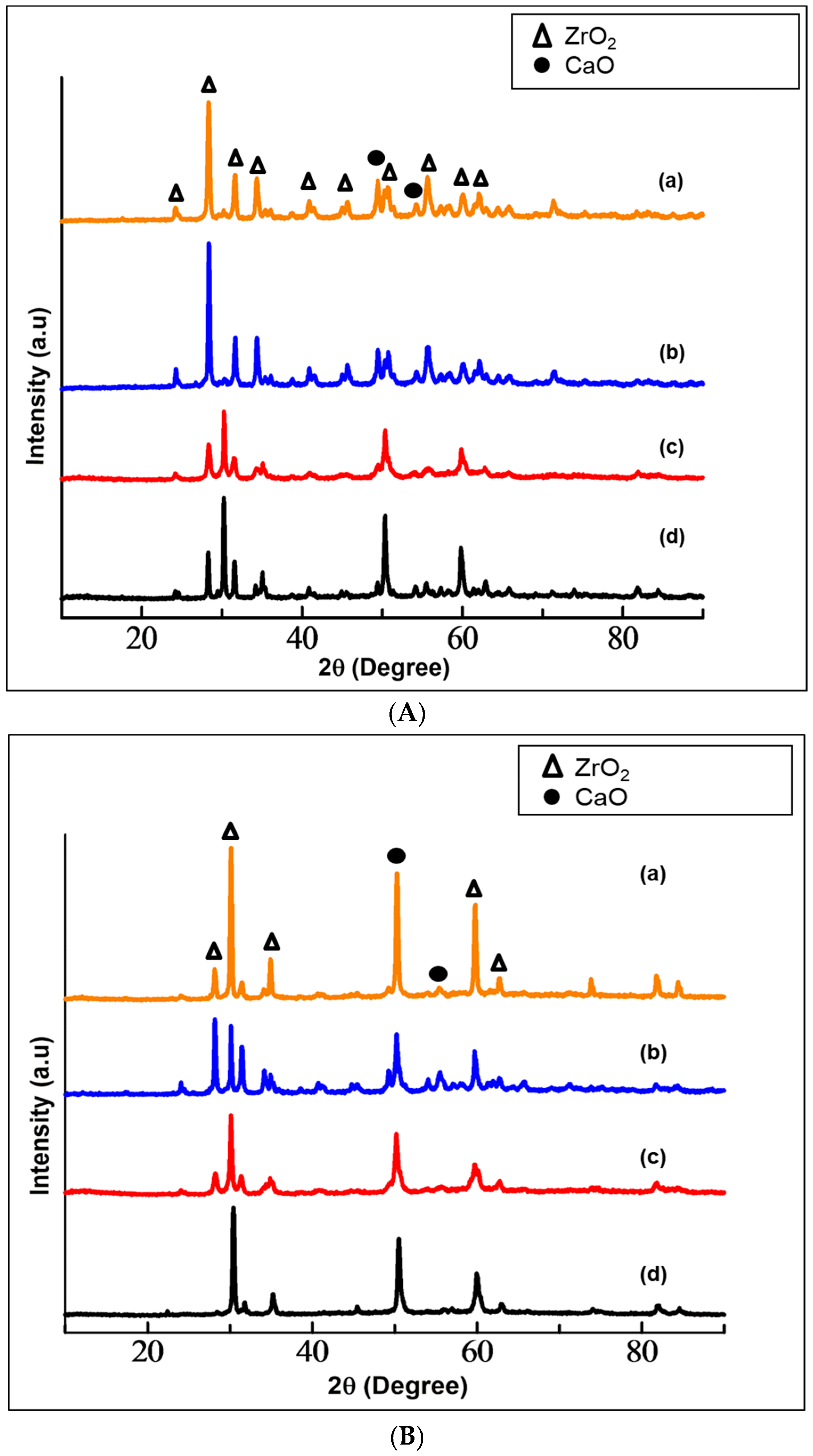
The phase identification of the prepared Ca-SZ using XRD are shown in Figure 2A,B. The diffraction patterns of cubic ZrO2 were obtained at 2θ = 28°, 41° and 47°, corresponding to the Inorganic Crystal Structure Database (ICDS) no. 98-007-2021. Moreover, the tetragonal ZrO2 was obtained at 2θ = 30°, 31.5°, 34°, 50°, 60° and 62.7°, corresponding to ICDS no. 98-010-8644. The zirconia was successfully stabilized with nano-CaO derived from cockle shells and from commercial source into tetragonal and cubic phases. The peaks at an angle 2θ = 49° and 54° are assigned to the CaO structure corresponding to ICDS no. 01-073-5492. The same patterns were obtained for all samples at different sintering temperatures, which diverged only in the peak intensities. The increase in sintering temperature results in increasing the intensity of the cubic phase.
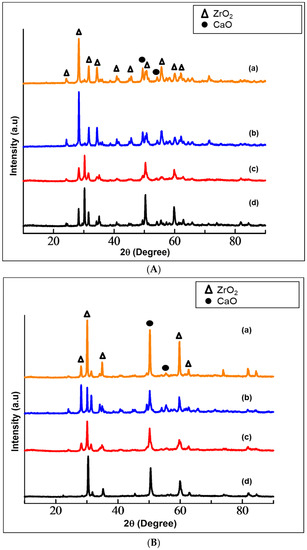
Figure 2.
(A) XRD patterns of calcia-stabilized zirconia with CaO derived from cockle shells (Ca-SZ(CS)) sintered for 2 h at (a) 1500 °C; (b) 1400 °C; (c) 1300 °C; (d) 1200 °C. (B) XRD patterns of calcia-stabilized zirconia with CaO derived from commercial CaO (Ca-SZ(CC)) sintered for 2 h at (a) 1500 °C; (b) 1400 °C; (b) 1300 °C; (d) 1200 °C.
3.2. SEM Analyses
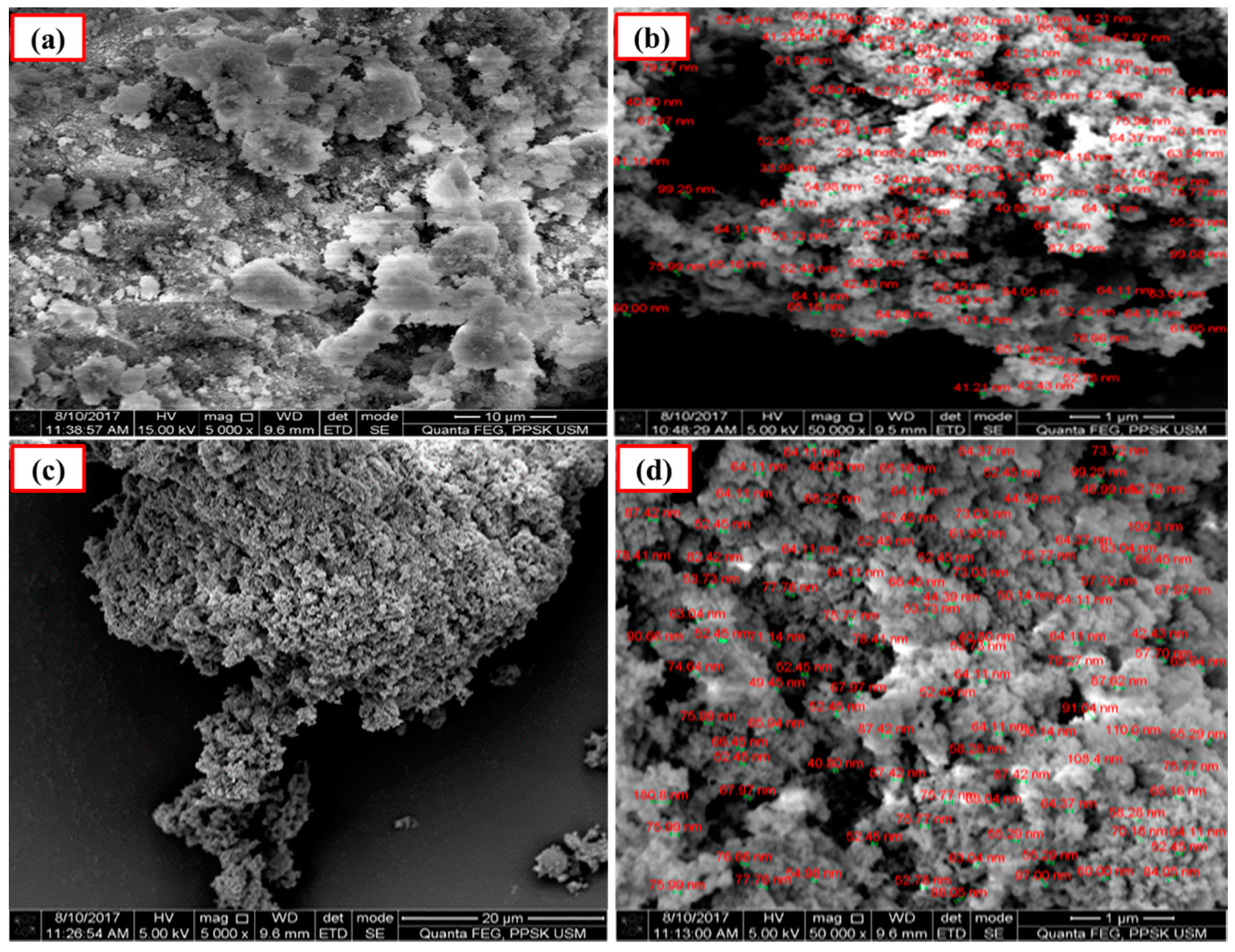
The SEM analyses indicated that the average particle size of Ca-SZ derived from cockle shells and commercial nano-CaO are 64 nm and 110 nm, respectively, before sintering (Figure 3). The SEM also show the uniform and even distribution of the particles.
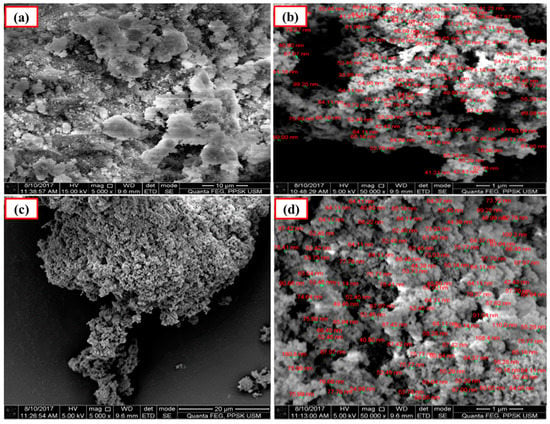
Figure 3.
SEM images of calcia-stabilized zirconia (Ca-SZ) pellets before sintering. (a,b): CaO derived from cockle shells; (c,d): commercial nano-CaO.
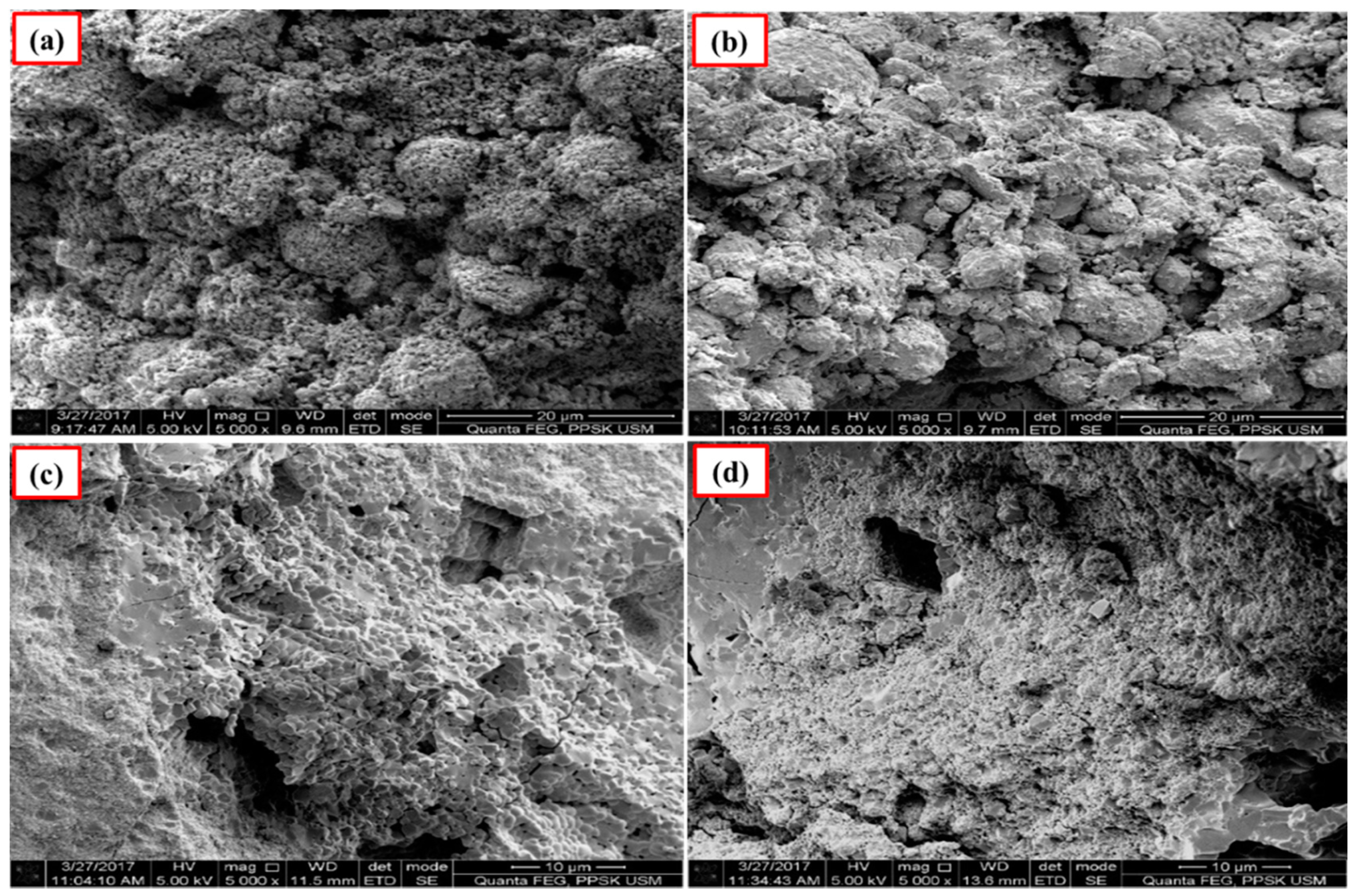
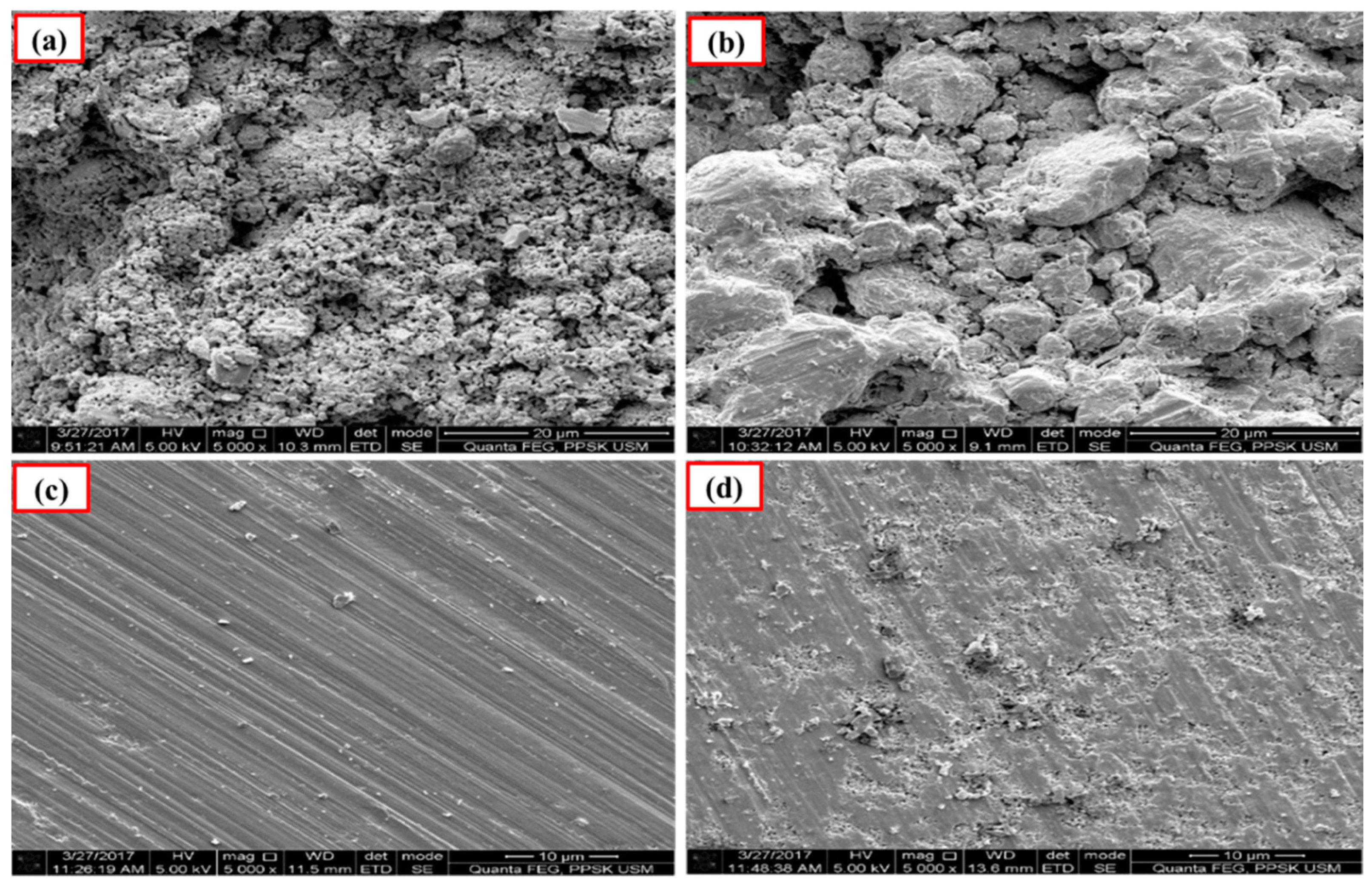
Figure 4 and Figure 5 show the images of unpolished Ca-SZ(CS) and Ca-SZ(CC) pellets, respectively, when sintered for two hours at 1200, 1300, 1400 and 1500 °C. Unpolished Ca-SZ pellets from both groups show irregular and rough surfaces at all four different sintering temperatures, with the roughest surface at 1200 °C.
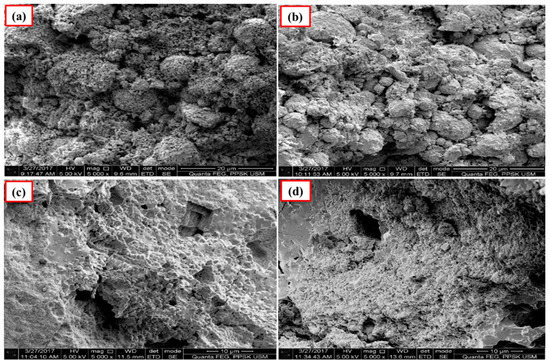
Figure 4.
SEM images of the unpolished Ca-SZ(CS) sintered for 2 h at (a) 1200 °C; (b) 1300 °C; (c) 1400 °C and (d) 1500 °C.
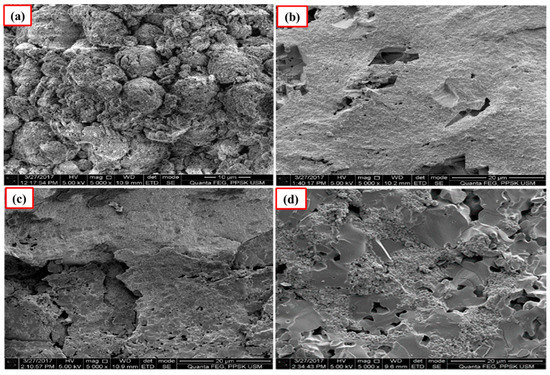
Figure 5.
SEM images of the unpolished Ca-SZ(CC) sintered for 2 h at (a) 1200 °C; (b) 1300 °C; (c) 1400 °C and (d) 1500 °C.
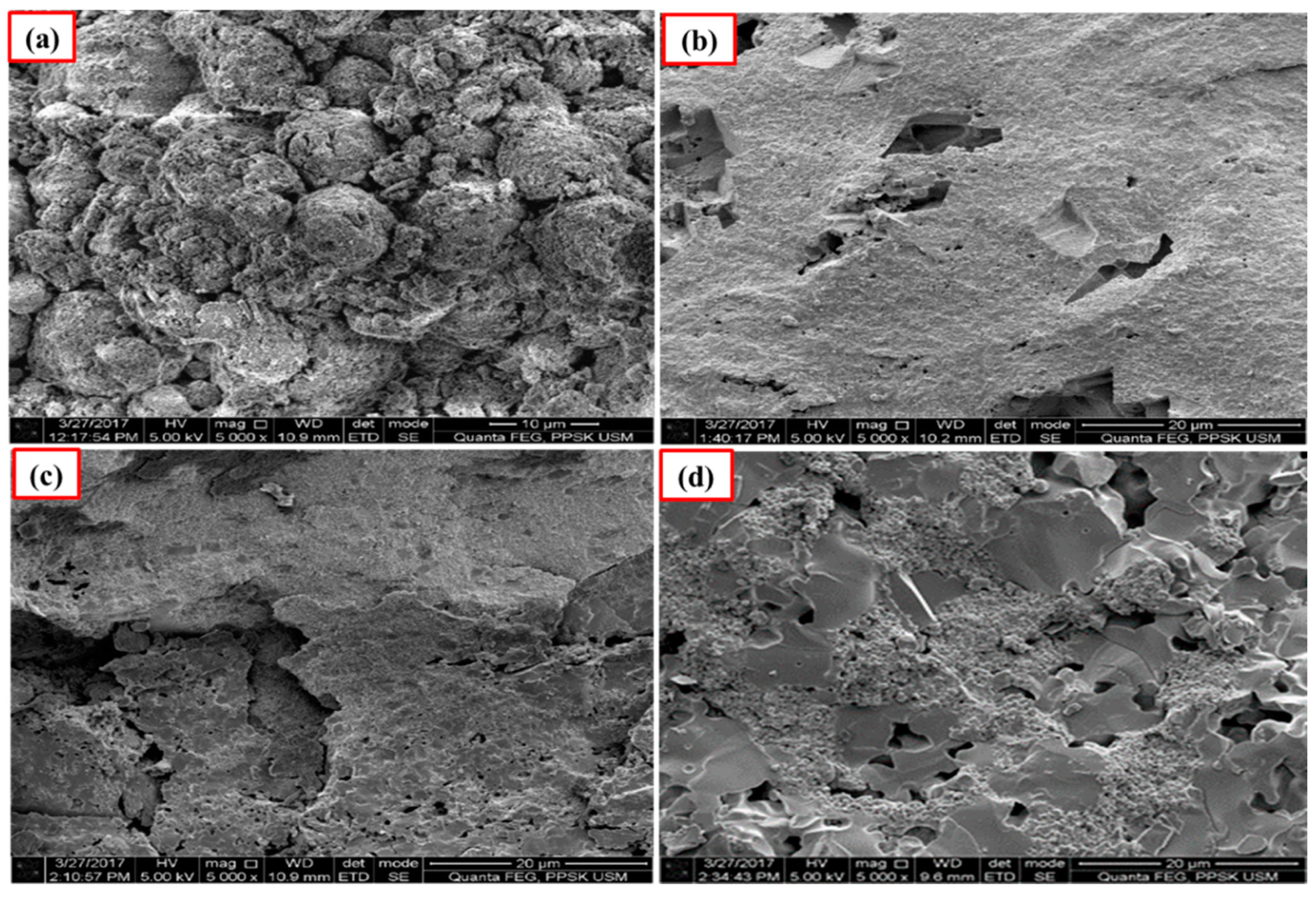
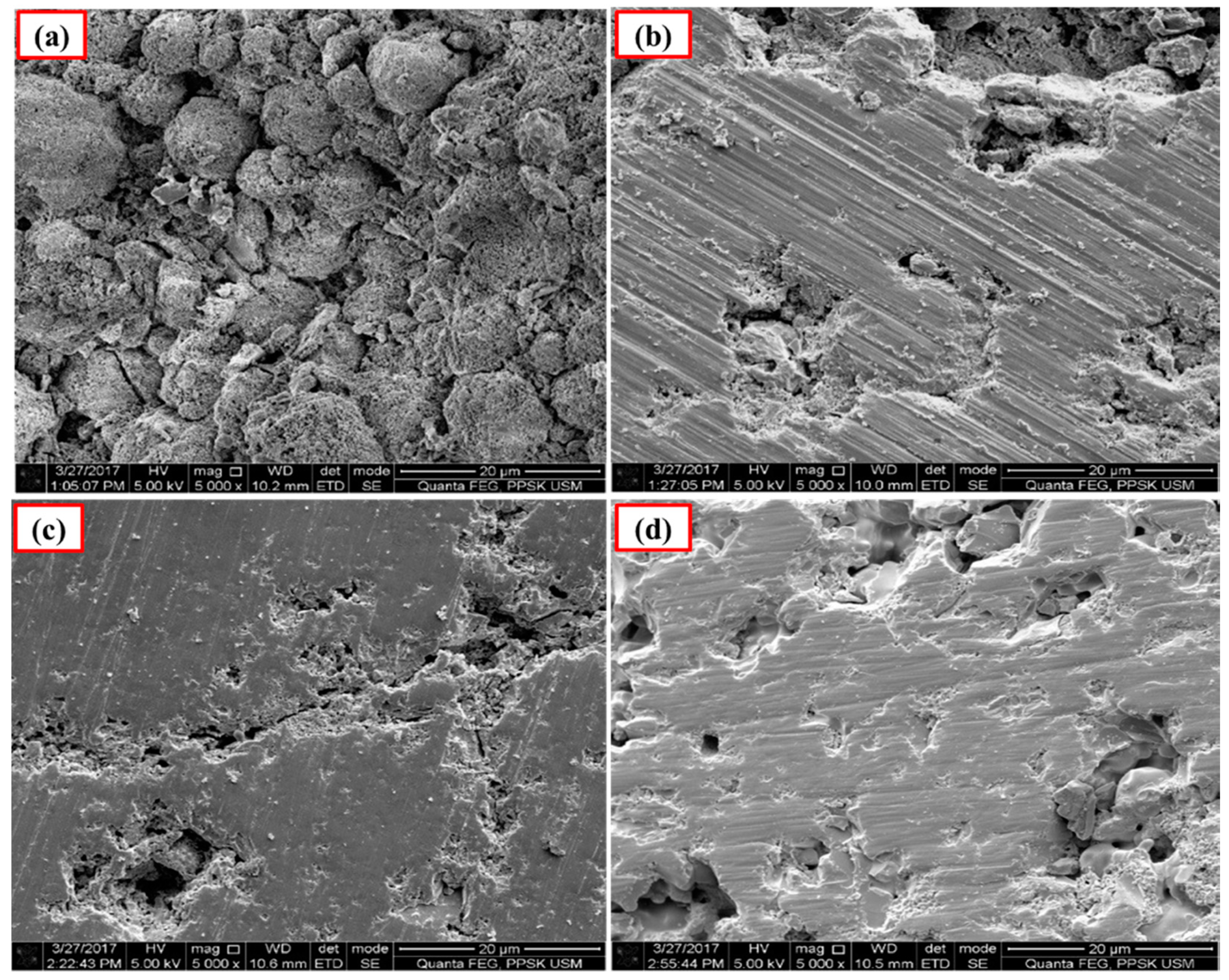
The polished surface of Ca-SZ(CS) pellets (Figure 6), show voids and spaces when sintered at 1200 °C and 1300 °C, similar to the polished surface of Ca-SZ(CC) when sintered at 1200 °C (Figure 7). The polished surface of Ca-SZ(CC) was relatively smooth when sintered at 1300 °C, 1400 °C and 1500 °C. Ca-SZ(CS) sintered at 1400 °C showed comparatively the glossiest appearance with minimum surface crack in addition to the smooth texture. Meanwhile, the particle sizes of Ca-SZ derived from both sources post-sintering showed no significant difference, with the value of 267 nm and 272 nm for Ca-SZ(CS) and Ca-SZ(CC), respectively.
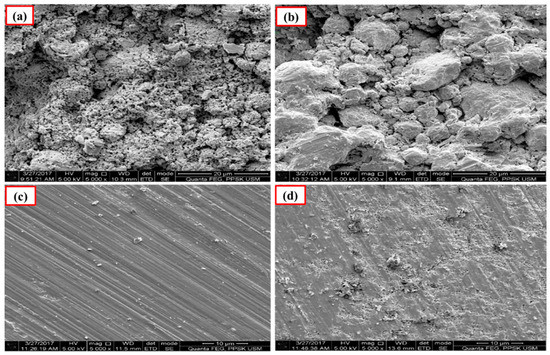
Figure 6.
SEM images of polished Ca-SZ(CS) sintered for 2 h at (a) 1200 °C; (b) 1300 °C; (c) 1400 °C and (d) 1500 °C.
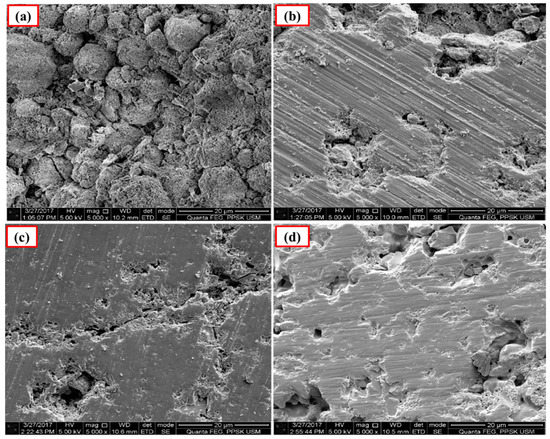
Figure 7.
SEM images of the polished Ca-SZ(CC) sintered for 2 h at (a) 1200 °C; (b) 1300 °C; (c) 1400 °C and (d) 1500 °C.
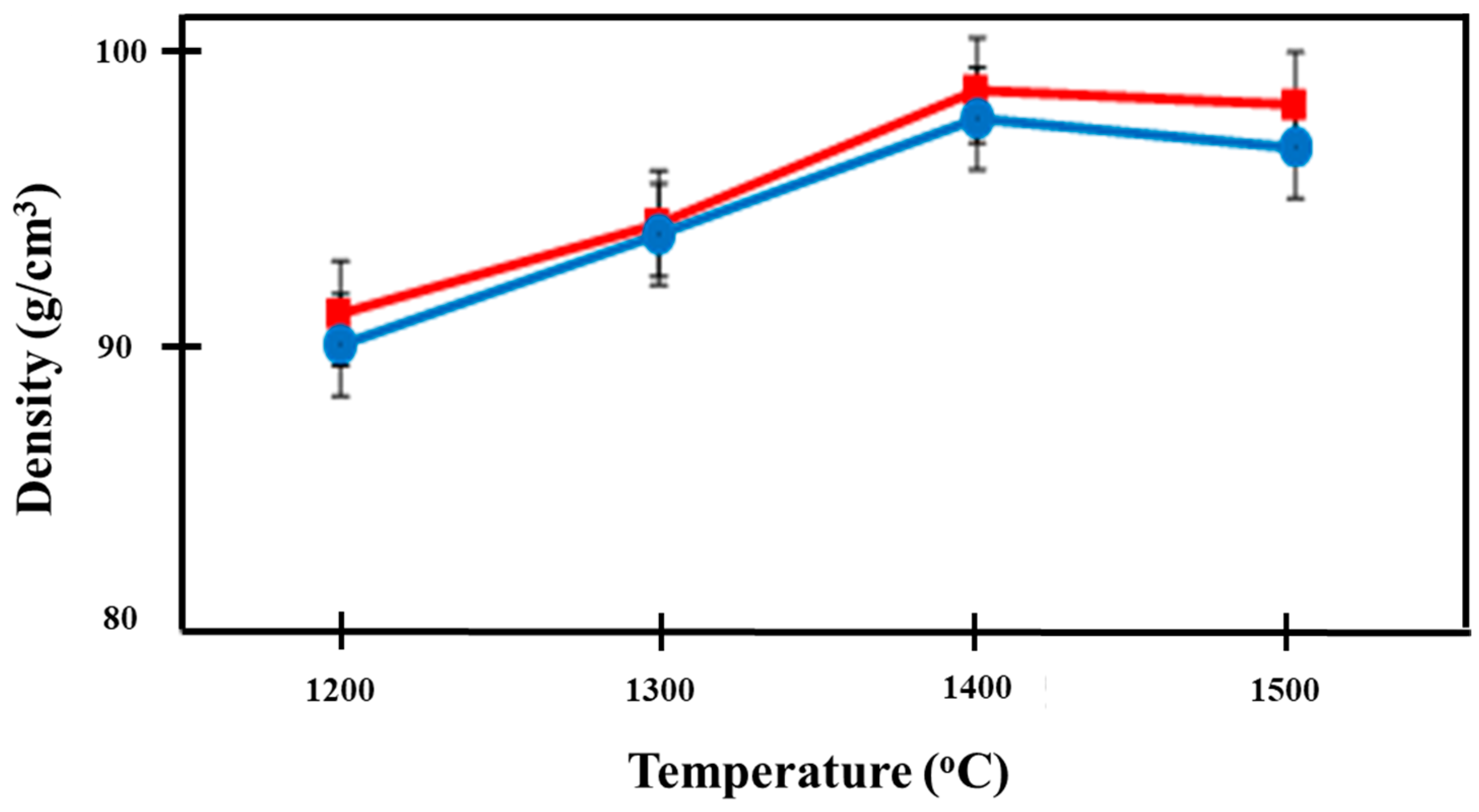
3.3. Density
The densities of the prepared Ca-SZ(CS) and Ca-SZ(CC) are shown in Figure 8. As the sintering temperature increased, the density of the Ca-SZ increased up to 1400 °C and levelled off as the sintering temperature further increased to 1500 °C. The independent t-test showed that the mean density of Ca-SZ(CS) was not significantly different from Ca-SZ(CC) when the samples were sintered at different temperatures with p > 0.05, except when samples were sintered at 1500 °C. One-way ANOVA analysis revealed that there was a significant difference in the density of the Ca-SZ from both groups between different sintering temperatures (p < 0.05). Further post-hoc Tukey analysis revealed that the density of both materials at 1200 °C were significantly lower compared to other temperatures with p < 0.05.
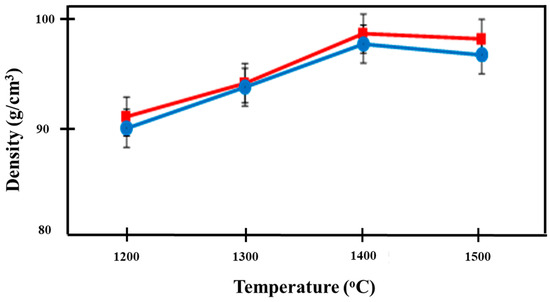
Figure 8.
Density of Ca-SZ(CS); red line and Ca-SZ(CC); blue line when sintered for 2 h at different temperatures of 1200, 1300, 1400 and 1500 °C.
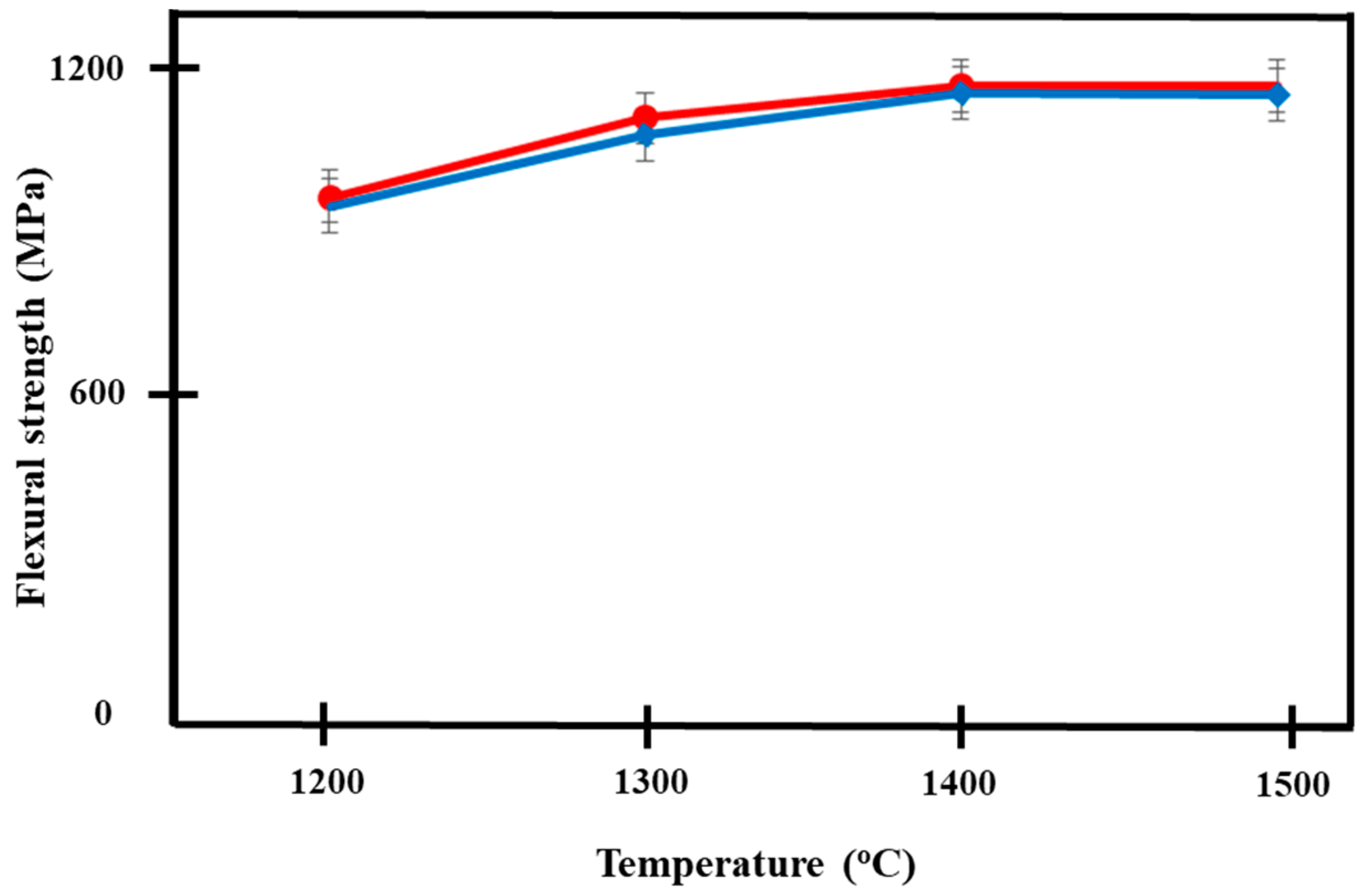
3.4. Flexural Strength
Figure 9 shows the increasing trend of flexural strength in both materials with increasing temperature. The independent t-test showed no significant difference in the flexural strengths between both groups of Ca-SZ when sintered at different temperatures with p < 0.05, apart from when the samples were sintered at 1300 °C. The highest flexural strength was observed in Ca-SZ sintered at 1400 °C for Ca-SZ(CS) and Ca-SZ(CC), with the values of 1165 and 1152 MPa, respectively. One-way ANOVA analysis revealed that there was a significant difference in the flexural strength of Ca-SZ(CS) and Ca-SZ(CC) between different sintering temperatures with p < 0.05. Further post-hoc Tukey test revealed that the flexural strength value of Ca-SZ sintered at 1200 °C was significantly lower compared to other temperatures with p < 0.05.
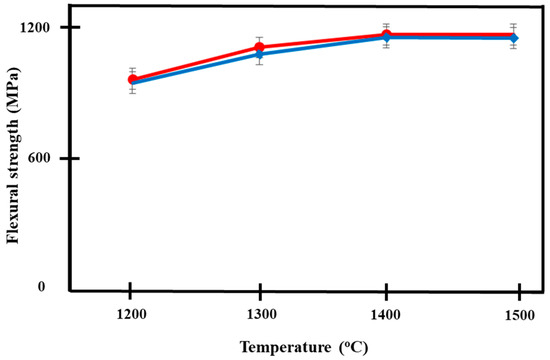
Figure 9.
Flexural strength of Ca-SZ(CS); red line and Ca-SZ(CC); blue line when sintered for 2 h at different temperatures of 1200, 1300, 1400 and 1500 °C.
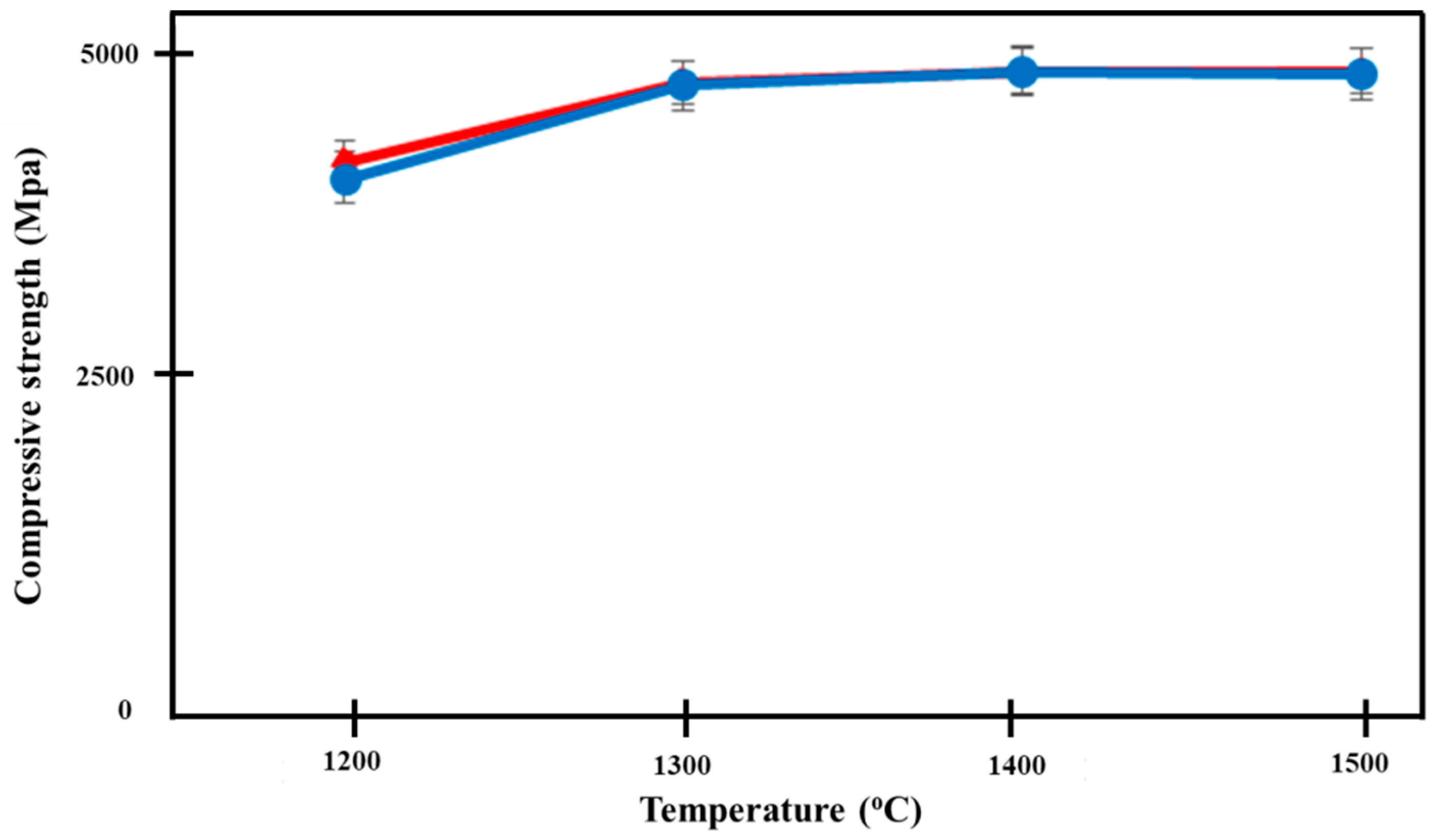
3.5. Compressive Strength
Figure 10 shows the increase in compressive strength of both groups of Ca-SZ, as the sintering temperature increases. One-way ANOVA analysis revealed that there was a significant difference in the compressive strength of Ca-SZ(CS) and Ca-SZ(CC) between different sintering temperatures (p < 0.05). Further post-hoc Tukey test revealed that the compressive strength of both Ca-SZ groups sintered at temperature 1200 °C was significantly lower compared to other temperature with p < 0.05. The independent t-test showed that there were no significant differences in the compressive strength of Ca-SZ(CS) and Ca-SZ(CC) sintered at different temperature p < 0.05, with the exception of 1200 °C.
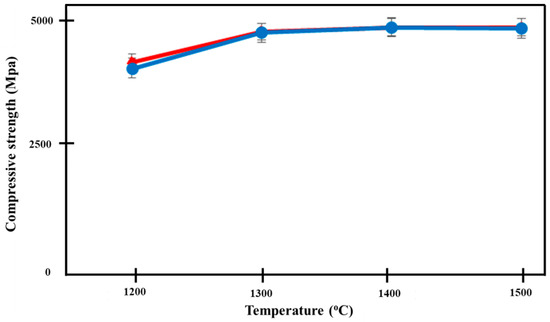
Figure 10.
Compressive strength of Ca-SZ(CS); red line and Ca-SZ(CC); blue line when sintered for 2 h at different temperatures of 1200, 1300, 1400 and 1500 °C.
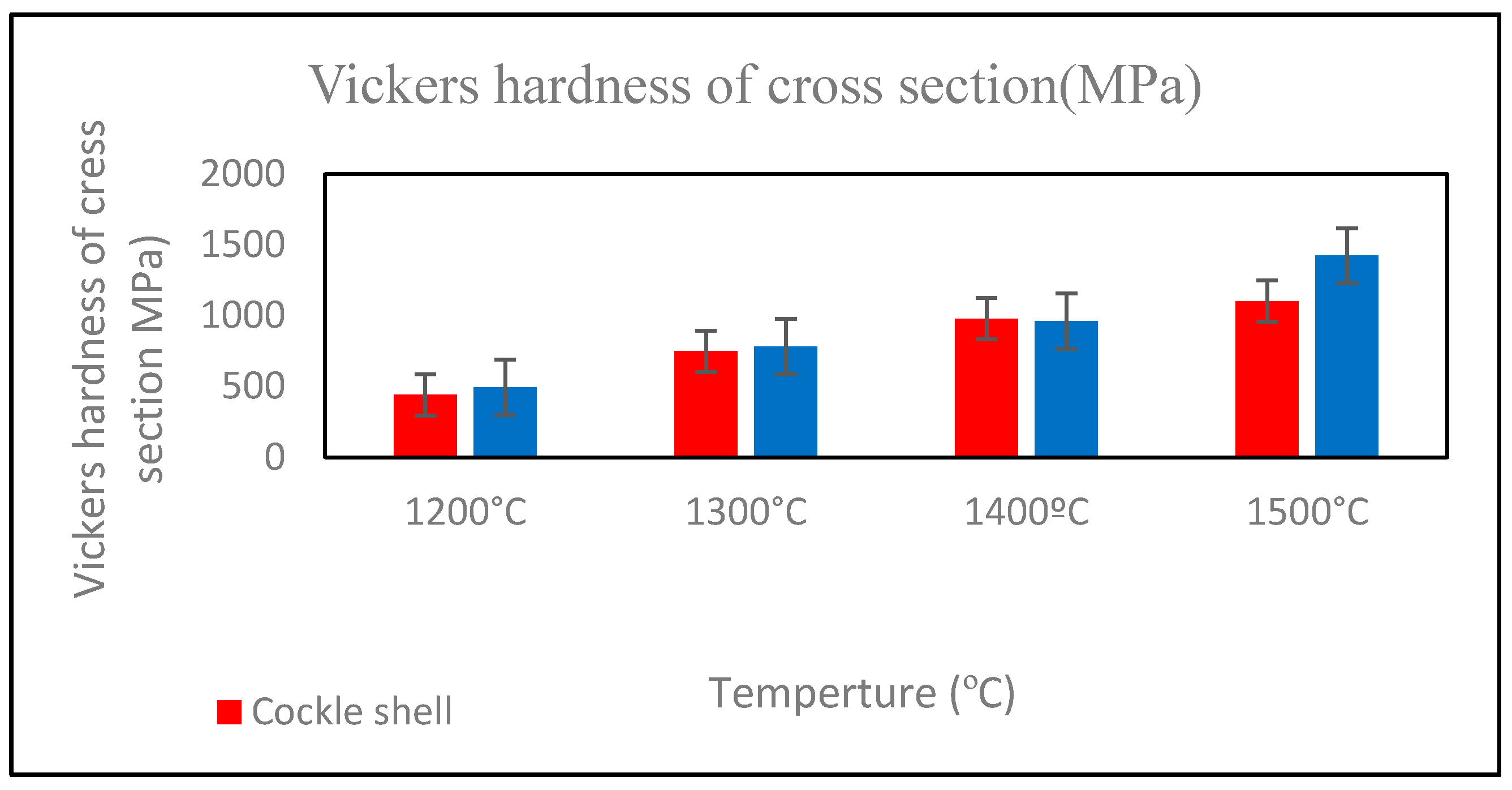
3.6. Vickers Hardness
Figure 11 illustrates the Vickers hardness values of both Ca-SZ groups. The hardness increased with the increase in sintering temperature, reaching their maximum at 1500 °C in both Ca-SZ(CS) and Ca-SZ(CC). One-way ANOVA analysis revealed that there was a significant difference in the Vickers hardness values of Ca-SZ(CS) and Ca-SZ(CC) between different sintering temperatures (p < 0.05). Further post-hoc Tukey test revealed that the hardness of the samples sintered at 1200 °C was significantly lower compared to other temperatures (p < 0.05). The independent t test revealed there was a significant difference in the Vickers hardness values between Ca-SZ(CS) and Ca-SZ(CC) at all temperatures (p < 0.05), except the group that was sintered at 1400 °C.
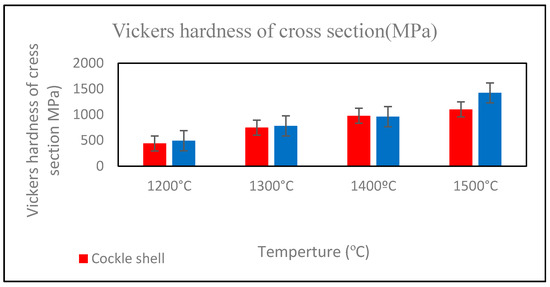
Figure 11.
Vickers hardness of cross-section of Ca-SZ(CS); red line and Ca-SZ(CC); blue line when sintered for 2 h at different temperatures of 1200, 1300, 1400 and 1500 °C.
4. Discussion
Several studies have worked on stabilizing zirconia with CaO [19,20,21,22] and on obtaining CaO from cockle shells [23,24,25,26,27]. However, to the authors’ knowledge, there has not been any work on the use of CaO obtained from cockle shells to stabilize zirconia for dental application.
In the current study, XRD analysis showed that the ZrO2 was successfully stabilized with both the nano-CaO derived from cockle shells and also commercial nano-CaO (Ca-SZ). Different sintering temperatures (1200 °C, 1300 °C, 1400 °C, and 1500 °C) were used to prepare Ca-SZ pellets. Physical and mechanical properties of Ca-SZ(CS) were compared with Ca-SZ(CC).
The morphological micrographs of Ca-SZ were characterized by SEM in order to deduce its surface structure and the average particle size. The particle sizes of Ca-PSZ(CS) and Ca-SZ(CC) obtained in the current study were 267 nm and 272 nm, respectively. The particle size of CaO derived from cockle shells ranged from 22 to 70 nm [17] and the particle size of the commercial CaO was <160 nm as compared to the 0.6-µm grain size of yttria used in a previous study [28]. The smaller particle size of the CaO derived from cockle shells might provide a larger surface area for CaO to be uniformly dispersed on the surface of ZrO2 and therefore may enhance the mechanical properties of the material [29], hence making the material more resistant to degradation or transformation [28]. The application of external heat during sintering enables the Ca-PSZ particle to be closely packed, with pores or voids removed, leading to shrinkage of the materials and forming more compact structures [18]. The voids and spaces shown on the polished surface of both Ca-PSZ pellets when sintered at lower temperatures suggests insufficient heat energy supplied in order to densely pack the materials, leading to an irregular surface upon polishing. Ca-PSZ(CS) sintered at 1400 °C showed the glossiest appearance with minimum surface crack, in addition to the smooth texture, which is indicative of sufficient and optimum heat energy during the sintering of the material.
The density of the materials is inter-correlated with the porosity of the microstructure. The incorporation of the Ca particle in ZrO2 solid solution was probably the main reason the density of the prepared material increased for both groups. The increase in density of the prepared materials as the sintering temperature increased was in agreement with other researchers [18,30,31]. The slight enhancement of the density of Ca-SZ(CS) was in accordance with the SEM image in Figure 6, showing a compact structure with smooth morphology. However, the decrement of the density of Ca-PSZ for both specimens over 1400 °C was probably due to surface diffusion phenomenon as previously reported [32,33].
The highest flexural strength was observed in Ca-SZ(CS) and Ca-SZ(CC) sintered at 1400 °C with the values of 1165 and 1152 MPa, respectively. These values were higher than another study, which reported the flexural strength value of 800–1000 MPa [3]. The higher flexural strength of the Ca-SZ in the present study was in accord with Stawarczyk et al. who reported that the optimal sintering temperature for the third generation of ZrO2 is in between 1400 °C and 1550 °C [34]. Therefore, the high flexural strength of Ca-SZ enables the material to afford restorations with less susceptibility to fracture, thus may be suitable for dental application.
The highest compressive strength was achieved when the Ca-SZ was sintered at 1400 °C, with similar values of 4914 and 4913 MPa for Ca-PSZ(CS) and Ca-SZ(CC), respectively. The current result is in agreement with a previous report by Hu et al. [35]. The shrinkage and distance of the particles depend on the strength of the cohesion between the particles of the material, as well as the shape and size of the particles. The more particles form close to a spherical shape, the more likely they are to bind the particles together leading to high compressive strength [35]. The higher compressive strength in the current study was probably due to more particles forming close to their spherical shape, which further enhanced the bonding, in addition to the densification of the material.
The mean Vickers hardness of Ca-SZ(CS) and Ca-SZ(CC) was 977 MPa and 960 MPa, respectively, which are lower in comparison to the Vickers hardness of Ce-TZP, which was around 1200 MPa [35,36]. Turon-Vinas et al. [29] developed pure 10 mol% ceria-stabilized tetragonal zirconia polycrystal (10Ce-TZP) with hardness value of 6.2 GPa. Tavor-Vargas et al. reinforced ceria-calcia stabilized zirconia with alumina, and reported hardness of 10 GPa–13 GPa [22]. Thus, it can be seen in the current study that the zirconia stabilized with calcia produced lower Vickers hardness when compared to the zirconia stabilized using other oxides, which suggests that it is easier to prepare using cutting tools with minimal wear.
In general, all mechanical properties show optimum values when the specimens were sintered at 1400 °C. At 1200 °C, the heat was not sufficient to fully sinter the zirconia and the presence of pores and voids lead to its lower density, flexural, compressive and hardness values. Whereas, at 1500 °C, the zirconia was overheated, showing lower mechanical properties.
Other properties that are important to dental zirconia should be tested in future research. These results are limited to in-vitro study. Further in-vivo animal studies are needed to test tissue response to the current material, followed by clinical trials. Currently the material is produced in a small quantity. More work is needed for upscaling to prepare for possibility of future commercialization.
5. Conclusions
The zirconia was successfully stabilized with nano-CaO derived from cockle shells and from commercial source into tetragonal and cubic phase (Ca-SZ) using a physical mixing technique. The physical and mechanical properties of the material were at their optimum when the pellets were sintered at 1400 °C. The physical and mechanical properties of the fabricated Ca-SZ with nano-CaO derived from cockle shell were comparable to the Ca-SZ stabilized by commercial nano-CaO. The flexural, compressive strength are also comparable with zirconia stabilized by other oxides, namely, Y-TZP. The fully sintered Ca-SZ is less hard compared to Ce-TZP, which makes it easier to mill. From the study, it can be concluded that the experimental Ca-PSZ with the CaO derived from cockle shells may be used as an alternative to the current zirconia available on the market for dental application.
Author Contributions
Conceptualization, Z.A.-G., I.A.R., A.H.; methodology, A.I.H., A.N.C.M., N.A.A.A.W.; data analysis, A.I.H.; investigation, A.I.H., A.N.C.M.; resources, Z.A.-G., I.A.R.; writing—original draft preparation, A.I.H., A.N.C.M.; writing—review and editing, Z.A.-G., I.A.R., A.N.C.M.; supervision, Z.A.-G., I.A.R., A.H.; project administration, Z.A.-G.; funding acquisition, Z.A.-G., I.A.R., A.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Science Fund from the Ministry of Science, Technology and Innovation (MOSTI) with the grant number: 07-01-05 SF0845 (305/PPSG/6113701).
Acknowledgments
The authors would also like to acknowledge all Multidisciplinary Laboratory (MDL) staff for their support in providing kind assistance.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Afrashtehfar, K.I.; Del Fabbro, M. Clinical performance of zirconia implants: A meta-review. J. Prosthet. Dent. 2020, 123, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Raigrodski, A.J. Contemporary materials and technologies for all-ceramic fixed partial dentures: A review of the literature. J. Prosthet. Dent. 2004, 92, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Denry, I.; Kelly, J.R. State of the art of zirconia for dental applications. Dent. Mater. 2008, 24, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Lazar, D.R.; Bottino, M.C.; Özcan, M.; Valandro, L.F.; Amaral, R.; Ussui, V.; Bressiani, A.H. Y-TZP ceramic processing from coprecipitated powders: A comparative study with three commercial dental ceramics. Dent. Mater. 2008, 24, 1676–1685. [Google Scholar] [CrossRef]
- Piconi, C.; Maccauro, G. Zirconia as a ceramic biomaterial. Biomaterials 1999, 20, 1–25. [Google Scholar] [CrossRef]
- Dion, I.; Rouais, F.; Baquey, C.; Lahaye, M.; Salmon, R.; Trut, L.; Cazorla, J.; Huong, P.; Monties, J.; Havlik, P. Physico-chemistry and cytotoxicity of ceramics: Part I–Characterization of ceramic powders. J. Mater. Sci. Mater. Med. 1997, 8, 325–332. [Google Scholar] [CrossRef]
- Nawa, M.; Nakamoto, S.; Sekino, T.; Niihara, K. Tough and strong Ce-TZP/alumina nanocomposites doped with titania. Ceram. Int. 1998, 24, 497–506. [Google Scholar] [CrossRef]
- Tanaka, K.; Tamura, J.; Kawanabe, K.; Nawa, M.; Oka, M.; Uchida, M.; Kokubo, T.; Nakamura, T. Ce-TZP/Al2O3 nanocomposite as a bearing material in total joint replacement. J. Biomed. Mater. Res. 2002, 63, 262–270. [Google Scholar] [CrossRef]
- Christel, P.; Meunier, A.; Heller, M.; Torre, J.; Peille, C. Mechanical properties and short-term in vivo evaluation of yttrium-oxide-partially-stabilized zirconia. J. Biomed. Mater. Res. 1989, 23, 45–61. [Google Scholar] [CrossRef]
- Guazzato, M.; Albakry, M.; Ringer, S.P.; Swain, M.V. Strength, fracture toughness and microstructure of a selection of all-ceramic materials. Part II. Zirconia-based dental ceramics. Dent. Mater. 2004, 20, 449–456. [Google Scholar] [CrossRef]
- Strub, J.R.; Rekow, E.D.; Witkowski, S. Computer-aided design and fabrication of dental restorations: Current systems and future possibilities. J. Am. Dent. Assoc. 2006, 137, 1289–1296. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T.; Nakamura, T.; Matsumura, H.; Ban, S.; Kobayashi, T. Current status of zirconia restoration. J. Prosthodont. Res. 2013, 57, 236–261. [Google Scholar] [CrossRef] [PubMed]
- Hannink, R.H.; Kelly, P.M.; Muddle, B.C. Transformation toughening in zirconia-containing ceramics. J. Am. Ceram. Soc. 2000, 83, 461–487. [Google Scholar] [CrossRef]
- Kohorst, P.; Borchers, L.; Strempel, J.; Stiesch, M.; Hassel, T.; Bach, F.-W.; Hübsch, C. Low-temperature degradation of different zirconia ceramics for dental applications. Acta Biomater. 2012, 8, 1213–1220. [Google Scholar] [CrossRef] [PubMed]
- Nath, S.; Sinha, N.; Basu, B. Microstructure, mechanical and tribological properties of microwave sintered calcia-doped zirconia for biomedical applications. Ceram. Int. 2008, 34, 1509–1520. [Google Scholar] [CrossRef]
- Drazin, J.W.; Castro, R.H. Phase stability in calcia-doped zirconia nanocrystals. J. Am. Ceram. Soc. 2016, 99, 1778–1785. [Google Scholar] [CrossRef]
- Hussein, A.I. Synthesis of Nano Calcium Oxide from Cockle Shells as Stabilizer for Fabrication of Zirconia. Ph.D. Thesis, Pusat Pengajian Sains Pergigian, Universiti Sains Malaysia, Penang, Malaysia, 2018. [Google Scholar]
- Wahi, A.; Muhamad, N.; Sulong, A.B.; Ahmad, R.N. Effect of sintering temperature on density, hardness and strength of MIM Co30Cr6Mo biomedical alloy. J. Jpn. Soc. Powder Powder Metall. 2016, 63, 434–437. [Google Scholar] [CrossRef]
- Radfarnia, H.R.; Iliuta, M.C. Development of zirconium-stabilized calcium oxide absorbent for cyclic high-temperature CO2 capture. Ind. Eng. Chem. Res. 2012, 51, 10390–10398. [Google Scholar] [CrossRef]
- Gempita, G.; Hasratiningsih, Z.; Subrata, G.; Purwasasmita, B.S. Synthesis of mesh-shaped calcia partially stabilized zirconia using eggshell membrane template as filler composite. Padjadjaran J. Dent. 2017, 29. [Google Scholar] [CrossRef]
- Kurapova, O.Y.; Glumov, O.; Pivovarov, M.; Golubev, S.; Konakov, V. Structure and conductivity of calcia stabilized zirconia ceramics, manufactured from freeze-dried nanopowder. Rev. Adv. Mater. Sci. 2017, 52, 134–141. [Google Scholar]
- Tovar-Vargas, D.; Turon-Vinas, M.; Anglada, M.; Jimenez-Pique, E. Enhancement of mechanical properties of ceria-calcia stabilized zirconia by alumina reinforcement. J. Eur. Ceram. Soc. 2020. [Google Scholar] [CrossRef]
- Jung, J.-H.; Yon, S.-J.; Seok, J.-W. Cubic zirconia single crystal growth using shell by skull melting method. J. Korean Cryst. Growth Cryst. Technol. 2013, 23, 124–128. [Google Scholar] [CrossRef]
- Buasri, A.; Chaiyut, N.; Loryuenyong, V.; Worawanitchaphong, P.; Trongyong, S. Calcium oxide derived from waste shells of mussel, cockle, and scallop as the heterogeneous catalyst for biodiesel production. Sci. World J. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Laonapakul, T.; Sutthi, R.; Chaikool, P.; Mutoh, Y.; Chindaprasirt, P. Optimum conditions for preparation of bio-calcium from blood cockle and golden apple snail shells and characterization. Sci. Asia 2019, 45, 10–20. [Google Scholar] [CrossRef]
- Ghazali, S.S.; Kem, K.L.; Jusoh, R.; Abdullah, S.; Shariffuddin, J.H. Evaluation of La-Doped CaO Derived from Cockle Shells for Photodegradation of POME. Bull. Chem. React. Eng. Catal. 2019, 14, 205. [Google Scholar] [CrossRef]
- Mohamad, S.F.S.; Mohamad, S.; Jemaat, Z. Study of calcination condition on decomposition of calcium carbonate in waste cockle shell to calcium oxide using thermal gravimetric analysis. ARPN J. Eng. Appl. Sci. 2016, 11, 9917–9921. [Google Scholar]
- Papanagiotou, H.P.; Morgano, S.M.; Giordano, R.A.; Pober, R. In vitro evaluation of low-temperature aging effects and finishing procedures on the flexural strength and structural stability of Y-TZP dental ceramics. J. Prosthet. Dent. 2006, 96, 154–164. [Google Scholar] [CrossRef]
- Turon-Vinas, M.; Zhang, F.; Vleugels, J.; Anglada, M. Effect of calcia co-doping on ceria-stabilized zirconia. J. Eur. Ceram. Soc. 2018, 38, 2621–2631. [Google Scholar] [CrossRef]
- Jaiswal, N.; Kumar, D.; Upadhyay, S.; Parkash, O. Ceria co-doped with calcium (Ca) and strontium (Sr): A potential candidate as a solid electrolyte for intermediate temperature solid oxide fuel cells. Ionics 2014, 20, 45–54. [Google Scholar] [CrossRef]
- Jaiswal, N.; Kumar, D.; Upadhyay, S.; Parkash, O. Effect of Mg and Sr co-doping on the electrical properties of ceria-based electrolyte materials for intermediate temperature solid oxide fuel cells. J. Alloys Compd. 2013, 577, 456–462. [Google Scholar] [CrossRef]
- Meng, F.; Liu, C.; Zhang, F.; Tian, Z.; Huang, W. Densification and mechanical properties of fine-grained Al2O3–ZrO2 composites consolidated by spark plasma sintering. J. Alloys Compd. 2012, 512, 63–67. [Google Scholar] [CrossRef]
- Wang, C.-J.; Huang, C.-Y.; Wu, Y.-C. Two-step sintering of fine alumina–zirconia ceramics. Ceram. Int. 2009, 35, 1467–1472. [Google Scholar] [CrossRef]
- Stawarczyk, B.; Özcan, M.; Hallmann, L.; Ender, A.; Mehl, A.; Hämmerlet, C.H. The effect of zirconia sintering temperature on flexural strength, grain size, and contrast ratio. Clin. Oral Investig. 2013, 17, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Wang, C.-A. Effect of sintering temperature on compressive strength of porous yttria-stabilized zirconia ceramics. Ceram. Int. 2010, 36, 1697–1701. [Google Scholar] [CrossRef]
- Benzaid, R.; Chevalier, J.; Saâdaoui, M.; Fantozzi, G.; Nawa, M.; Diaz, L.A.; Torrecillas, R. Fracture toughness, strength and slow crack growth in a ceria stabilized zirconia–alumina nanocomposite for medical applications. Biomaterials 2008, 29, 3636–3641. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).