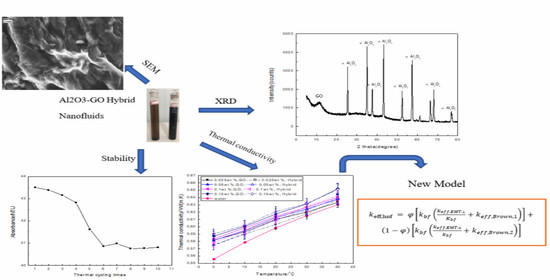
Thermal Conductivity and Stability of Novel Aqueous Graphene Oxide–Al2O3 Hybrid Nanofluids for Cold Energy Storage
Abstract
:Featured Application
Abstract
1. Introduction
2. Experimental Apparatus and Procedure
3. Results and Discussion
3.1. Effect of Different Factors on the Stability of the Hybrid Nanofluids
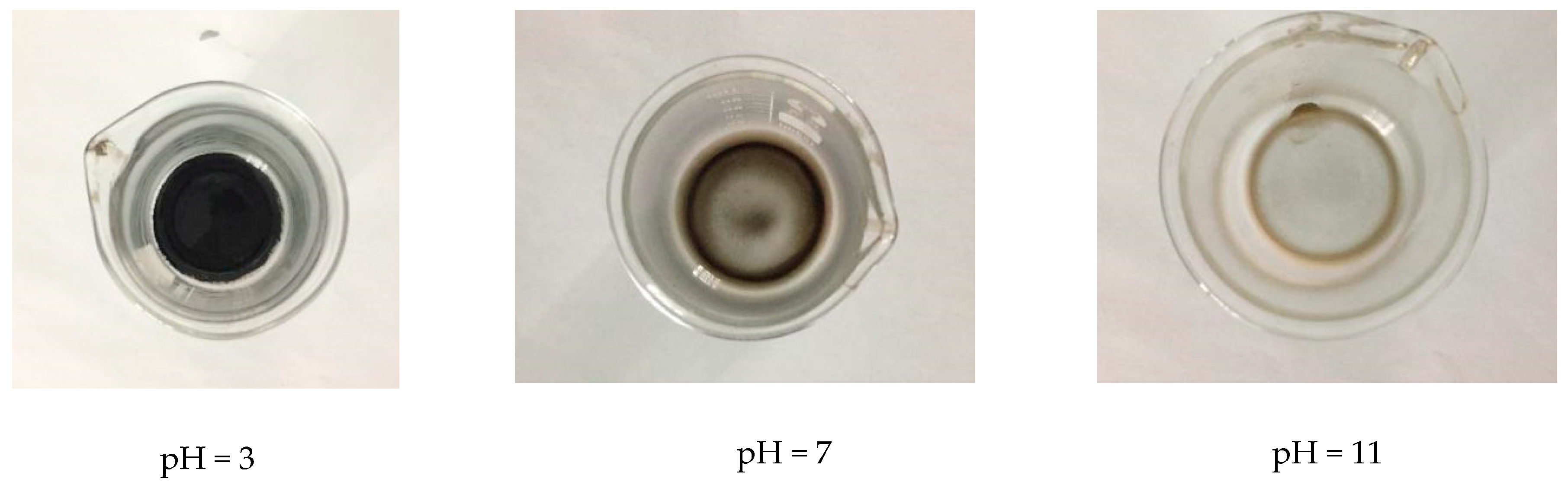
3.1.1. Influence of pH
3.1.2. Influence of Dispersant
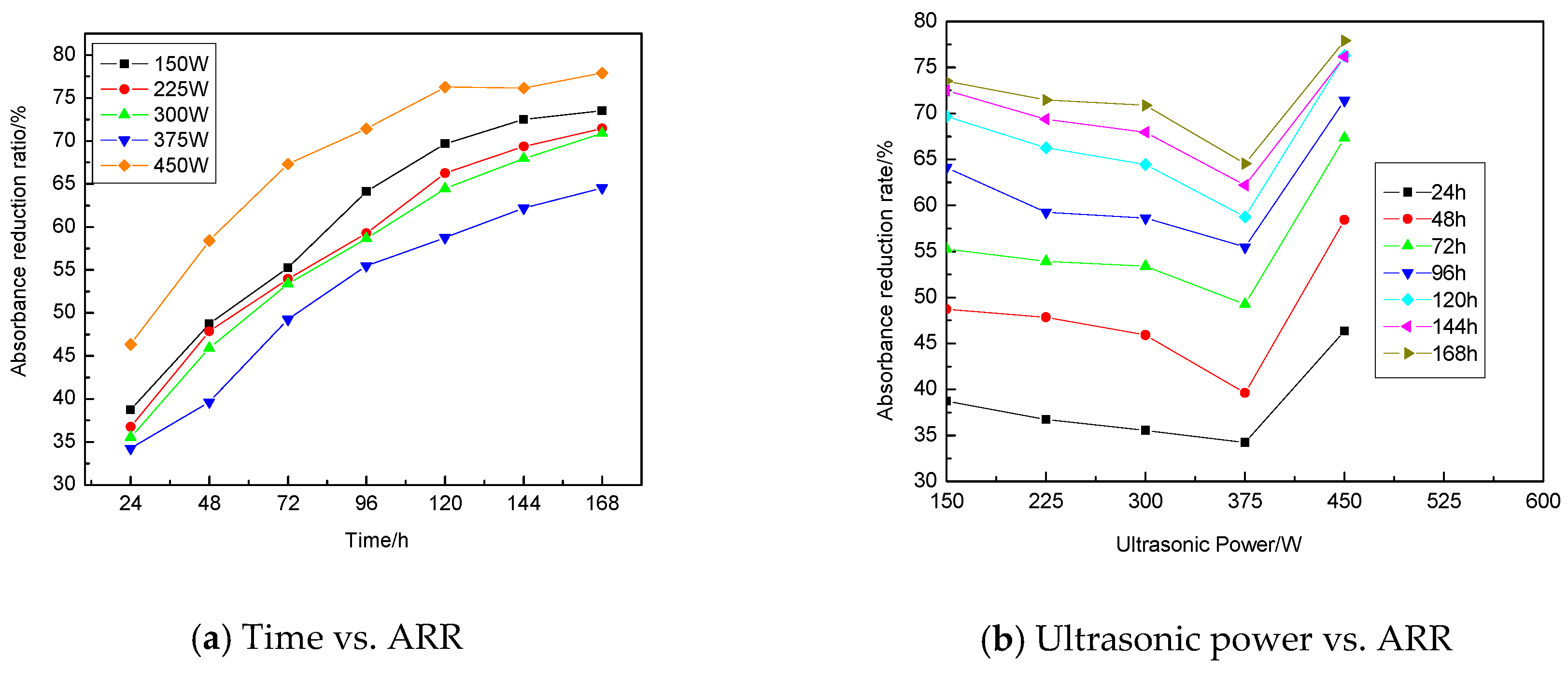
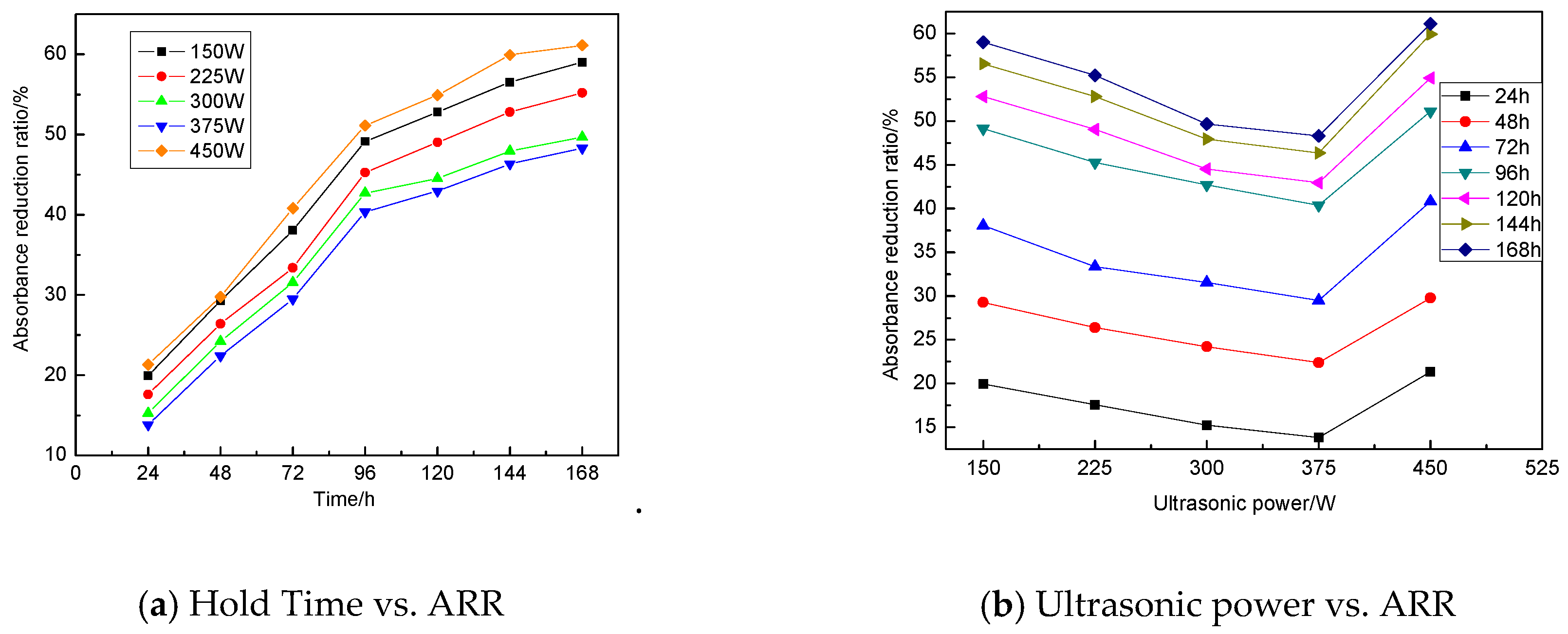
3.1.3. Influence of Ultrasonic Power
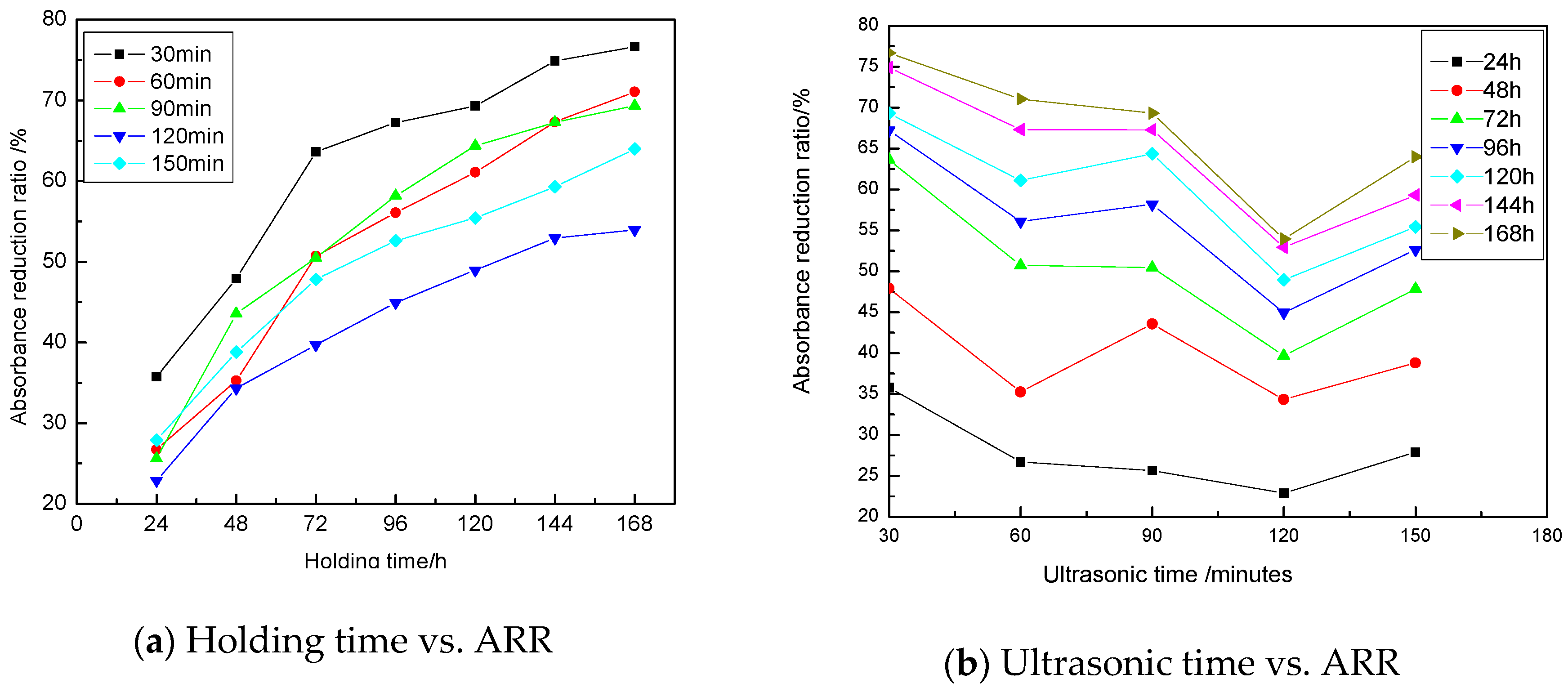
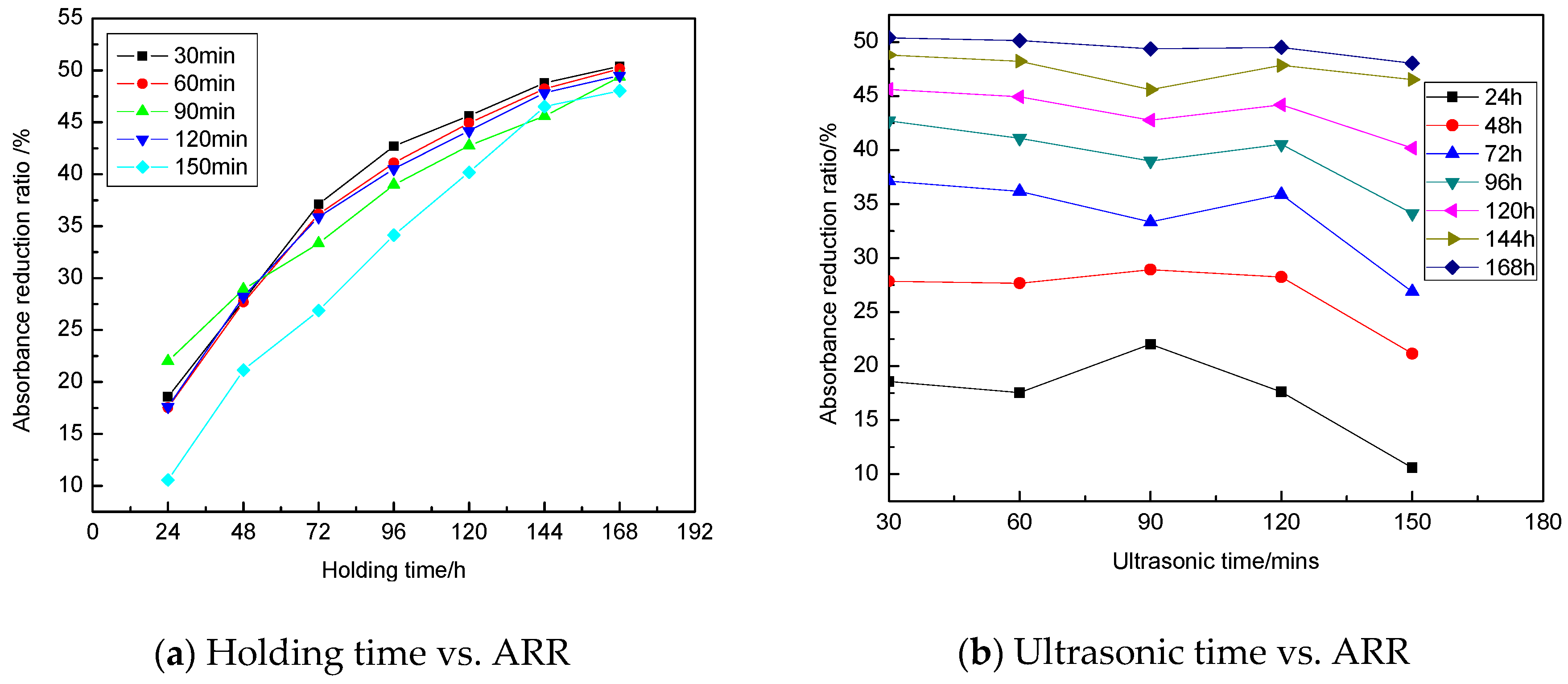
3.1.4. Influence of Ultrasonic Time
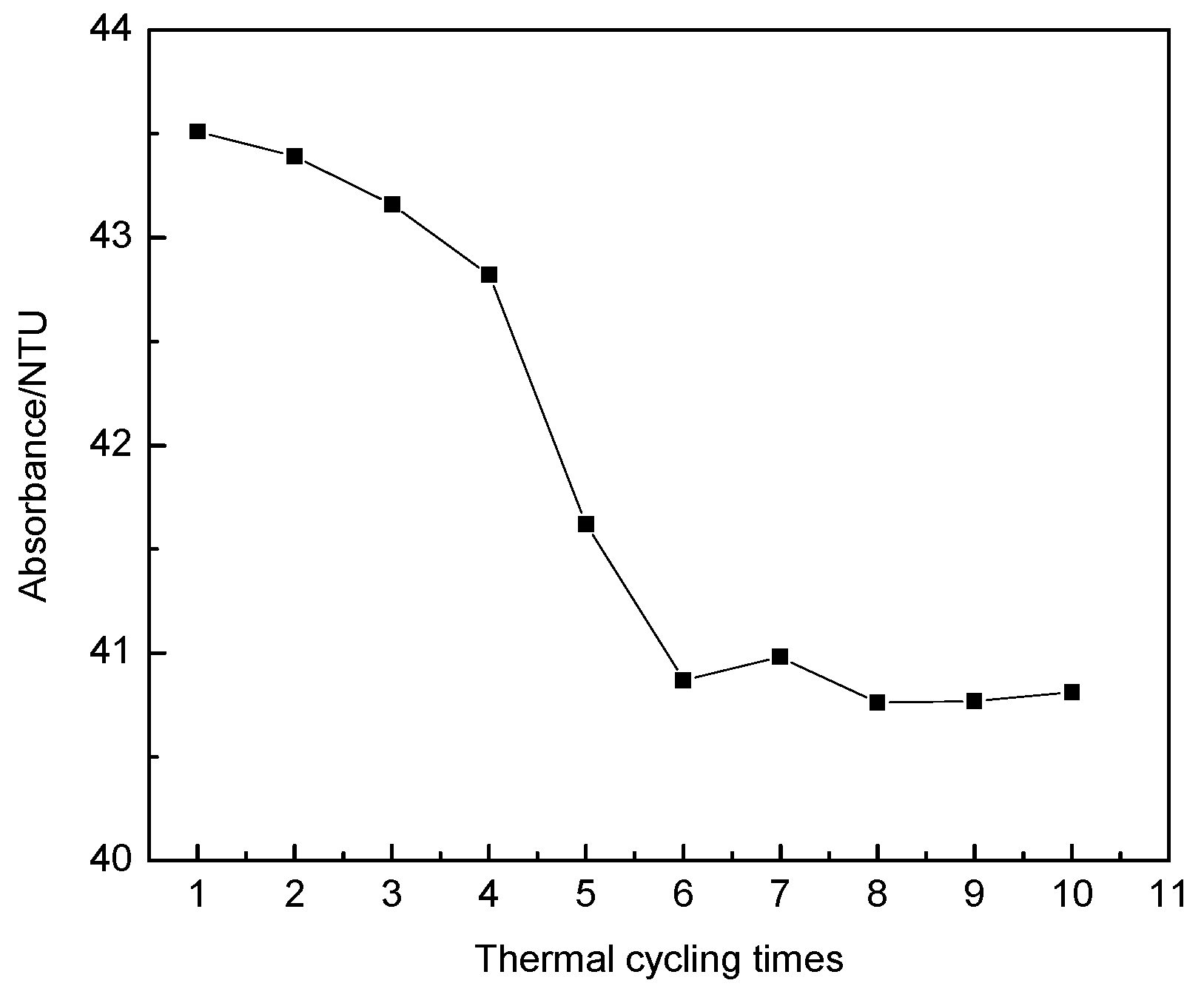
3.1.5. Influence of Thermal Cycling
3.2. Thermal Conductivity of the Hybrid Nanofluids
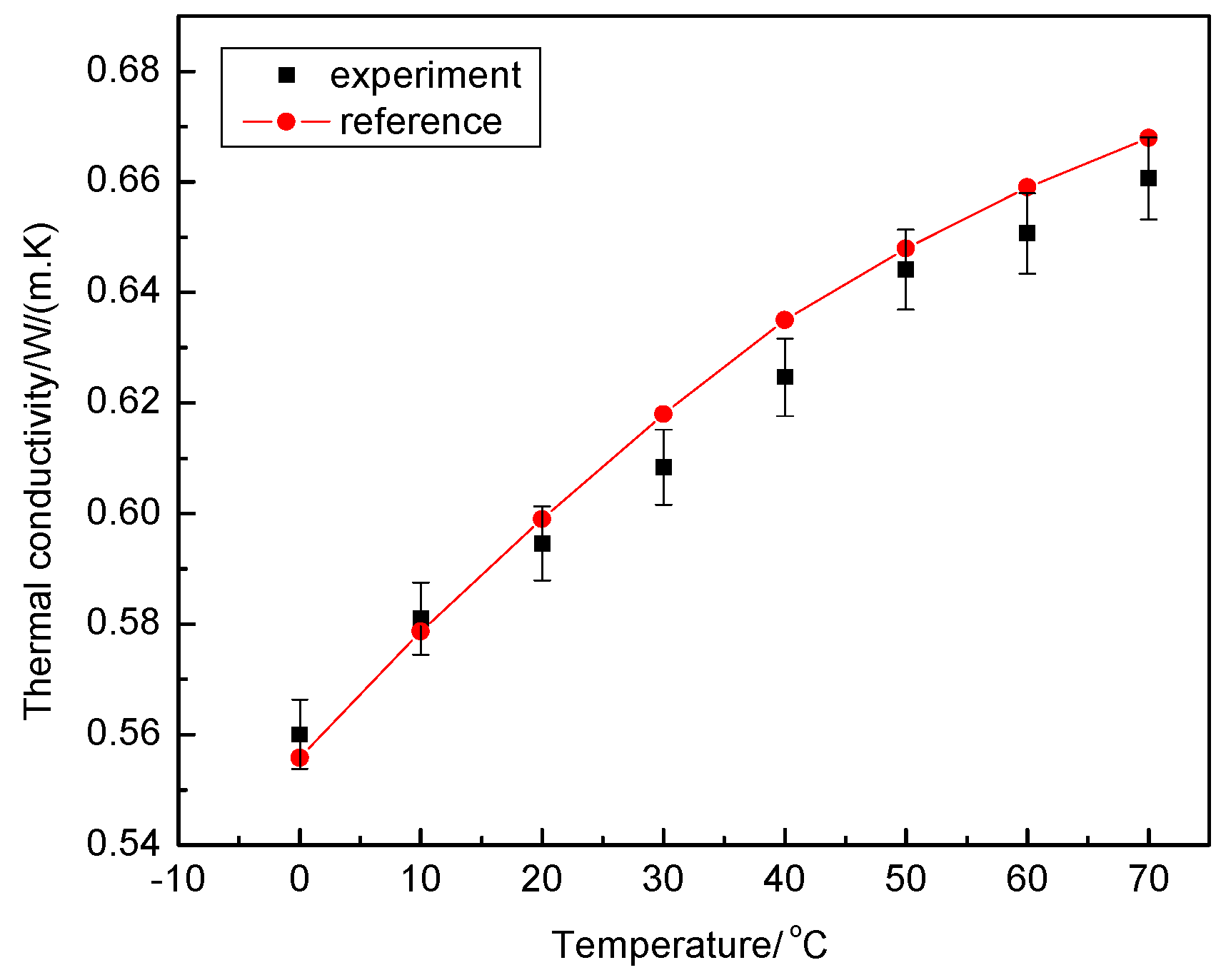
3.2.1. Reliability Verification
3.2.2. Effect of Mass Fraction of the Nanoparticle
3.2.3. Effect of Temperature
3.2.4. Comparison of Thermal Conductivity of GO Nanofluid with the Hybrid Nanofluid
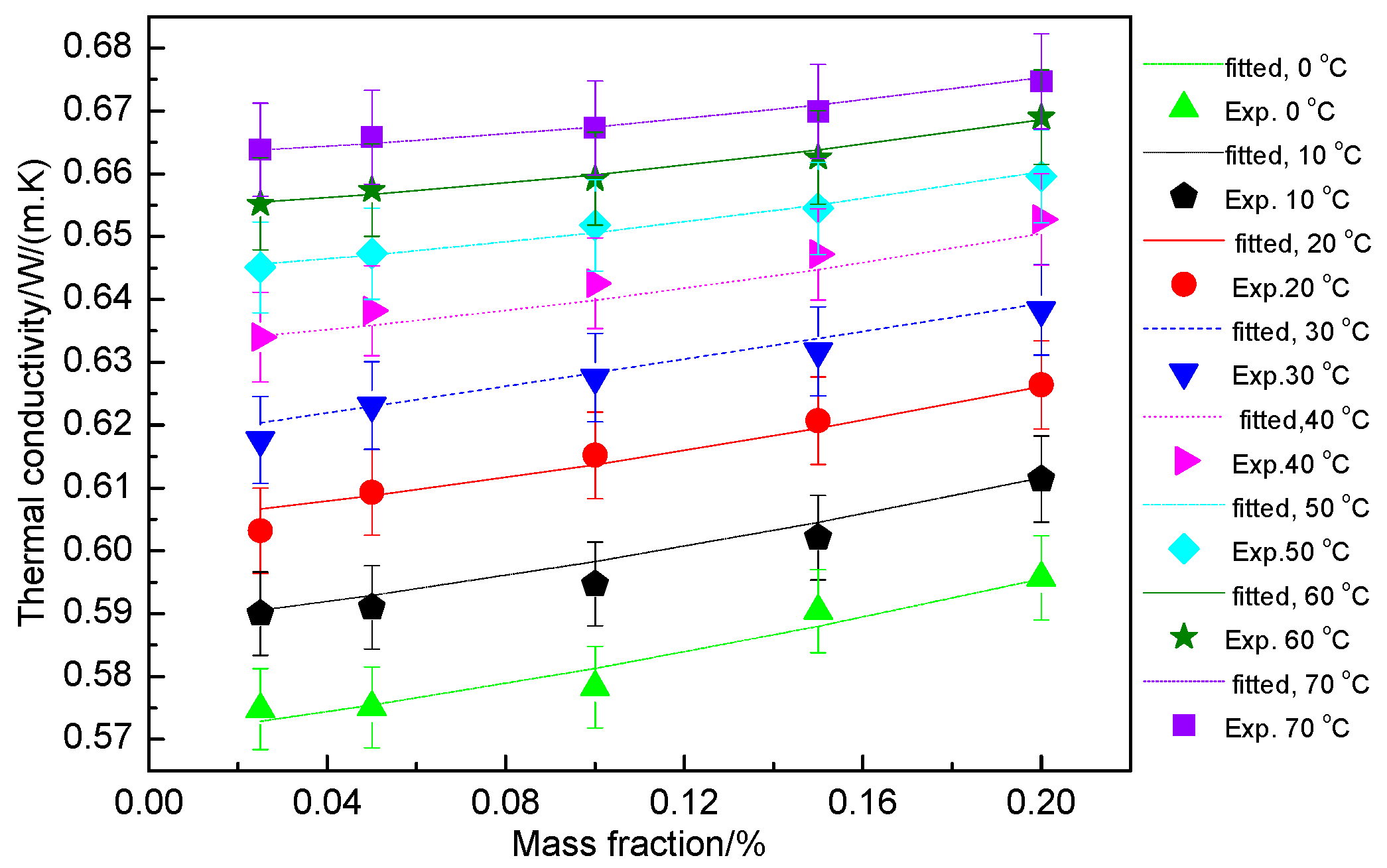
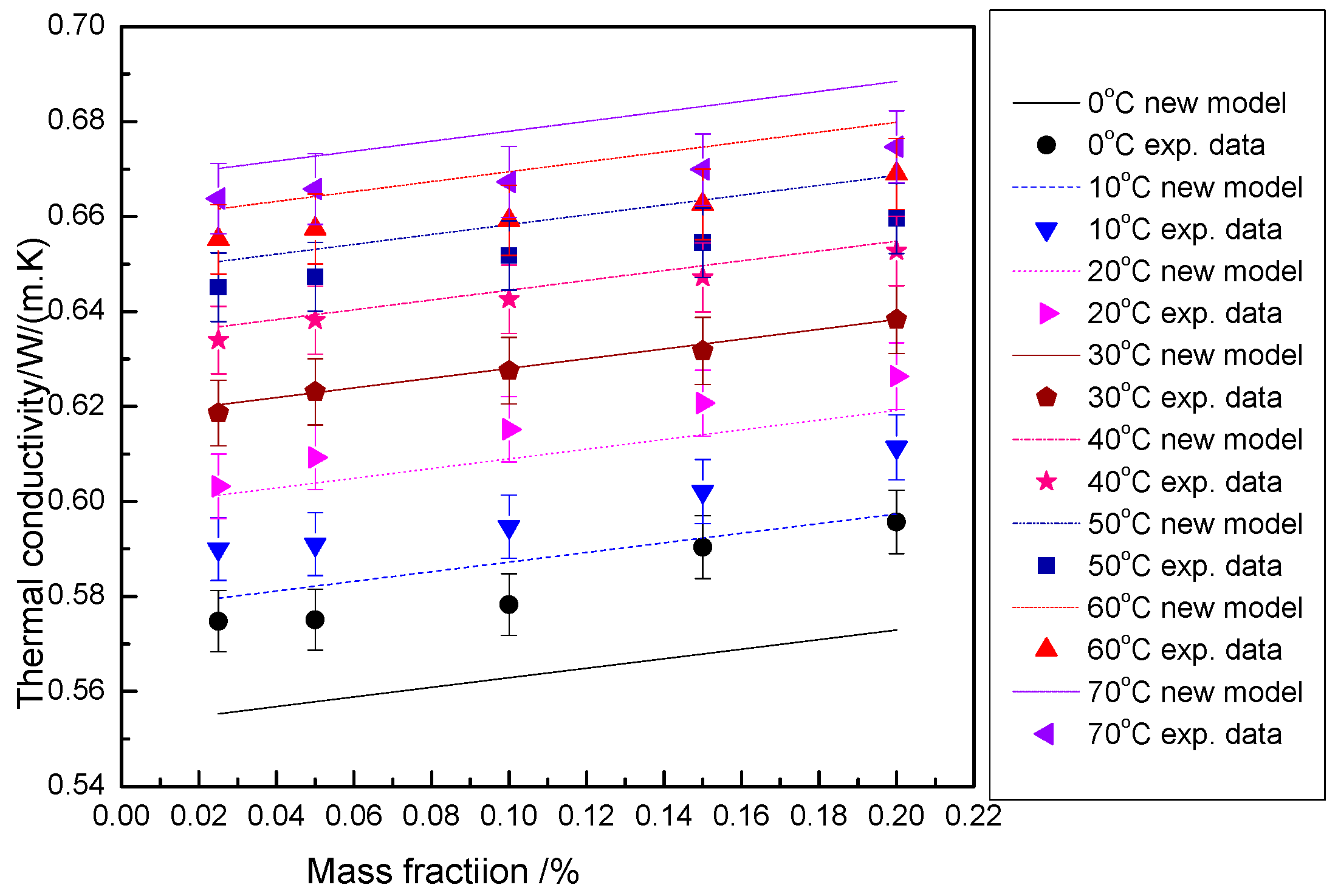
3.2.5. Fitting of Thermal Conductivity of the Hybrid Nanofluid
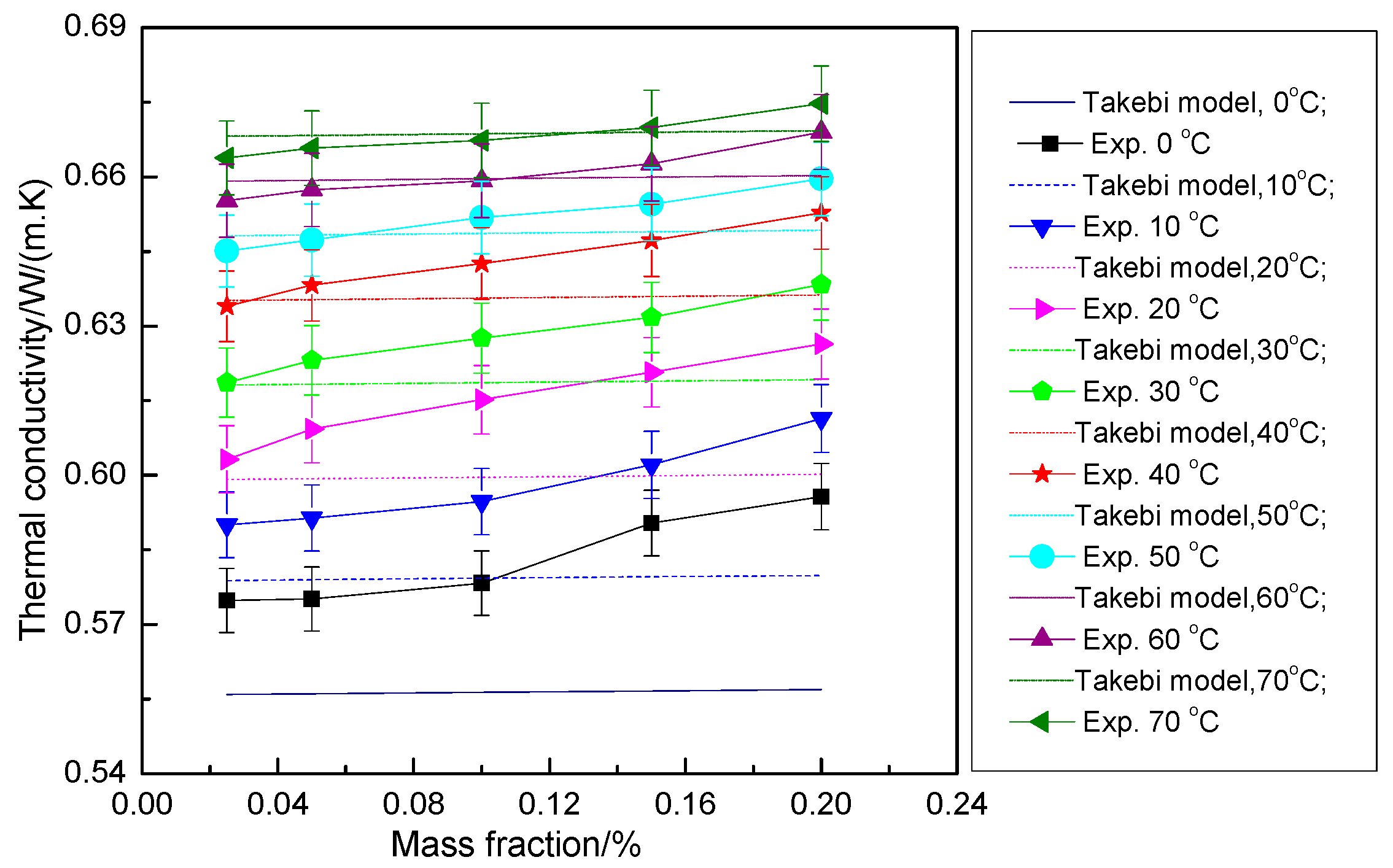
3.2.6. Mechanism Model of Thermal Conductivity of the Hybrid Nanofluid
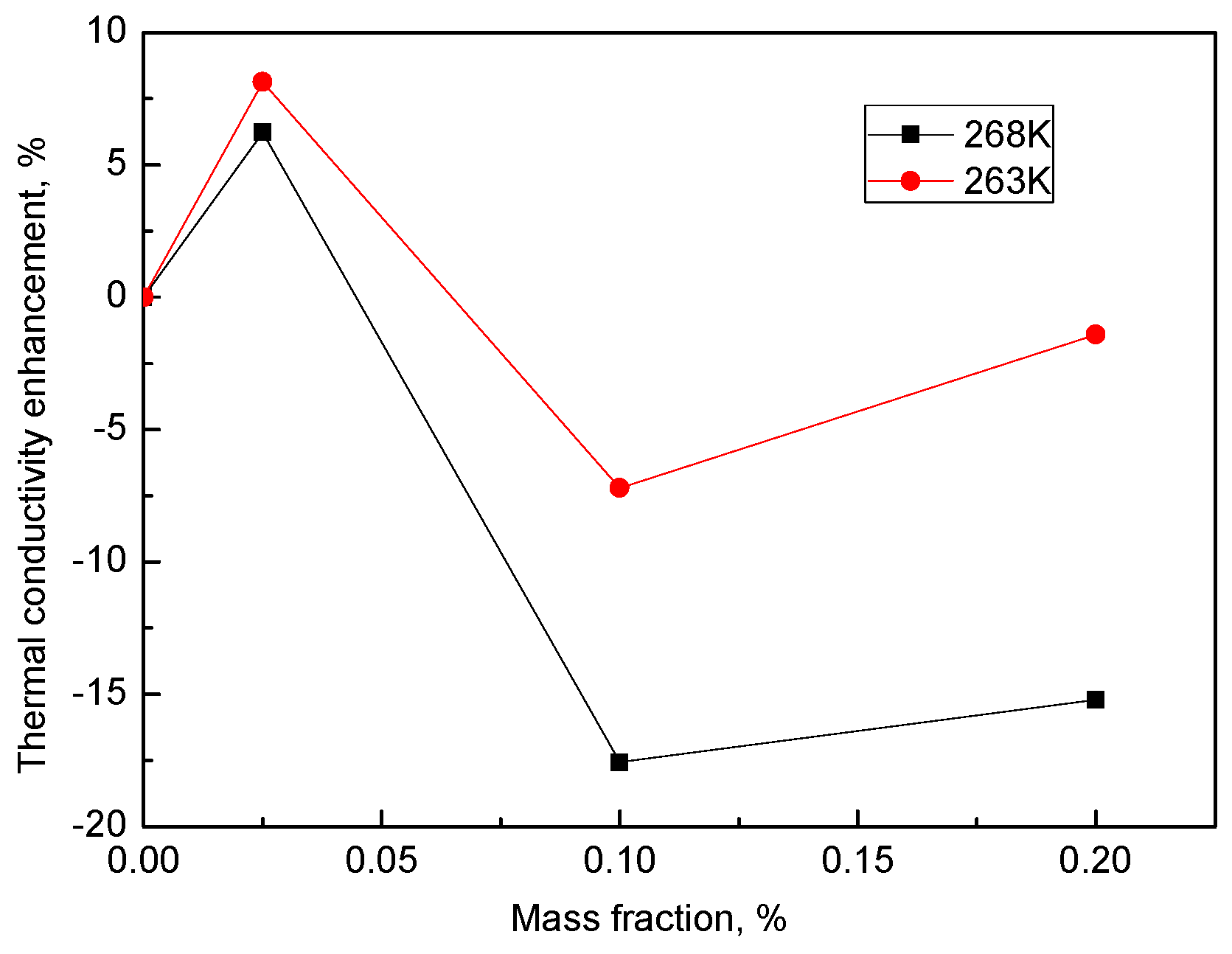
3.3. Thermal Conductivity of Ice Containing the Hybrid Nanoparticles
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| A0 | initial absorbance |
| An | nth hour absorbance |
| ARR | absorbance reduction ratio |
| Cp | specific thermal capacity |
| equivalent diameter | |
| k | thermal conductivity |
| kB | Boltzmann constant |
| L | length |
| n | empirical shape factor |
| T | temperature |
| t | thickness |
| RK | interfacial thermal resistance |
| rc | mean radius of gyration of the cluster |
| vnon-sph | non-spherical particles volume |
Greek Symbols
| η | average flatness ratio |
| μ | viscosity of base fluid |
| ρ | density |
| σ | uncertainty |
| volume fraction | |
| mass fraction |
Subscripts
| Brown | Brownian motion |
| bf | base fluid |
| E | enhancement |
| EMT | effective medium theory |
| eff | effective |
| hnf | hybrid nanofluid |
| nf | nanofluid |
| np1 | No.1 nanoparticle |
| np2 | No.2 nanoparticle |
| p | particle |
References
- Siddhartha, B.M. Ice based energy storage integration with solar PV power plants for cooling energy. J. CPRI 2016, 12, 297–316. [Google Scholar]
- Li, S.-F.; Liu, Z.-H.; Wang, X.-J. A comprehensive review on positive cold energy storage technologies and applications in air conditioning with phase change materials. Appl. Energy 2019, 255, 113667. [Google Scholar] [CrossRef]
- Philip, N.; Dheep, G.R.; Sreekumar, A. Cold thermal energy storage with lauryl alcohol and cetyl alcohol eutectic mixture: Thermophysical studies and experimental investigation. J. Energy Storage 2020, 27, 101060. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, Y.; Wang, J.; Geng, S.; Su, C.; Peng, Q. Nucleation mechanism of nanofluid drops under acoustic levitation. Appl. Therm. Eng. 2018, 130, 40–48. [Google Scholar]
- Tayyab, R.S.; Hafiz, M.A. Applications of hybrid nanofluids in solar energy, practical limitations and challenges: A critical review. Sol. Energy 2019, 183, 173–203. [Google Scholar]
- Radzi, A.R.; Nor, A.C.; Syahrullail, S. Recent progress on concentrating direct absorption solar collector using nanofluids. J. Therm. Anal. Calorim. 2019, 137, 903–922. [Google Scholar]
- He, Y.; Hu, Y.; Li, H. An Ag@TiO2/ethylene glycol/water solution as a nanofluid-based beam splitter for photovoltaic/thermal applications in cold regions. Energy Convers. Manag. 2019, 198, 111838. [Google Scholar] [CrossRef]
- Evangelos, B.; Christos, T.; Nikolaos, N. Nanofluid-Based hybrid PV. Energy Convers. Manag. 2019, 198, 111831. [Google Scholar]
- Seyed, R.M.; Meysam, K.; Ehsan, E.B.; David, W. Coupled thermal-optical numerical modeling of PV/T module-Combining CFD approach and two-band radiation DO model. Energy Convers. Manag. 2019, 198, 111781. [Google Scholar]
- Mohammad, H.; Ali, S.; Mohammad, S.; Mohammad, P.F. Optimization and parametric analysis of a nanofluid based photovoltaic thermal system: 3D numerical model with experimental validation. Energy Convers. Manag. 2018, 160, 93–108. [Google Scholar]
- Omar, Z.S.; Ashraf, N.A.; Dimitrios, C.K.; Eiyad, A.N. Four-Way coupling of particle-wall and colloidal particle-particle interactions in direct absorption solar collectors. Energy Convers. Manag. 2019, 195, 7–20. [Google Scholar]
- Shi, L.; He, Y.; Wang, X.; Hu, Y. Recyclable photo-thermal conversion and purification systems via Fe3O4@TiO2 nanoparticles. Energy Convers. Manag. 2018, 171, 272–278. [Google Scholar] [CrossRef]
- Carlos, C.; Diego, V.; Carolina, A.; Patricio, A.B.; José, C.; Humberto, P. About the relevance of particle shape and graphene oxide on the behavior of direct absorption solar collectors using metal based nanofluids under different radiation intensities. Energy Convers. Manag. 2019, 181, 247–257. [Google Scholar]
- Nazir, H.; Batool, M.; Osorio, F.J.B.; Isaza-Ruiz, M.; Xu, X.; Vignarooban, K.; Phelan, P.; Inamuddin; Kannan, A.N.M. Recent developments in phase change materials for energy storage applications: A review. Int. J. Heat Mass Transf. 2019, 129, 491–523. [Google Scholar] [CrossRef]
- Abdelrazik, A.S.; Al-Sulaiman, F.A.; Saidur, R.; Ben-Mansour, R. Evaluation of the effects of optical filtration and nanoPCM on the performance of a hybrid photovoltaic-thermal solar collector. Energy Convers. Manag. 2019, 195, 139–156. [Google Scholar] [CrossRef]
- Mehdi, B.; Saeed, H. Electronics cooling with nanofluids: A critical review. Energy Convers. Manag. 2018, 172, 438–456. [Google Scholar]
- Rodrigues, C.; Dias, M.M.; Martins, L.; Silva, D.J.; Araújo, J.P.; Oliveira, J.C.; Pereira, A.M.; Ventura, J. A magnetically-activated thermal switch without moving parts. Energy Convers. Manag. 2019, 197, 111881. [Google Scholar] [CrossRef]
- Ibai, M.; Steven, R.; Sébastien, P.; Jonathan, B.; Hakim, N. Exergy Analysis of a Parallel-Plate Active Magnetic Regenerator with Nanofluids. Entropy 2017, 19, 464. [Google Scholar]
- Manzoore, E.M.S.; Nik-Nazri, N.G.; Kalam, M.A.; Badruddin, I.A.; Banapurmath, N.R.; Naveed, A. The effect of nano-additives in diesel-biodiesel fuel blends: A comprehensive review on stability, engine performance and emission characteristics. Energy Convers. Manag. 2018, 178, 146–177. [Google Scholar] [CrossRef]
- Örs, I.; Sarıkoç, S.; Atabani, A.E.; Ünalan, S.; Akansu, S.O. The effects on performance, combustion and emission characteristics of DICI engine fuelled with TiO2 nanoparticles addition in diesel/biodiesel/n-butanol blends. Fuel 2018, 234, 177–188. [Google Scholar] [CrossRef]
- Karimi, A.; Afrand, M.J. Numerical study on thermal performance of an air-cooled heat exchanger: Effects of hybrid nanofluid, pipe arrangement and cross section. Energy Convers. Manag. 2018, 164, 615–628. [Google Scholar] [CrossRef]
- Hooman, Y.; Nurin, W.B.; Samira, G.; Seyed, F.S.S.; Abdullah, A.A.; Mohamad, A.B.A. Convective heat transfer enhancement with graphene nanoplatelet/platinum hybrid nanofluid. Int. Commun. Heat Mass Transf. 2017, 88, 120–125. [Google Scholar]
- Gupta, M.; Singh, V.; Kumar, S.; Kumar, S.; Dilbaghi, N.; Said, Z. Up to date review on the synthesis and thermophysical properties of hybrid nanofluids. J. Clean. Prod. 2018, 190, 169–192. [Google Scholar] [CrossRef]
- Hajatzadeh Pordanjani, A.; Aghaklani, S.; Afrand, M.; Mahmoudi, B.; Mahian, O.; Wongwises, S. An updated review on application of nanofluids in heat exchangers for saving energy. Energy Convers. Manag. 2019, 198, 111886. [Google Scholar] [CrossRef]
- Babu, J.R.; Kumar, K.K.; Rao, S.S. State-Of-Art review on hybrid nanofluids. Renew. Sustain. Energy Rev. 2017, 77, 551–565. [Google Scholar] [CrossRef]
- Sarkar, J.; Ghosh, P.; Adil, A. A review on hybrid nanofluids: Recent research, development and applications. Renew. Sustain. Energy Rev. 2015, 43, 164–177. [Google Scholar] [CrossRef]
- Sidik, N.A.C.; Adamu, I.M.; Jamil, M.M.; Kefayati, G.; Mamat, R.; Najafi, G. Recent progress on hybrid nanofluids in heat transfer applications: A comprehensive review. Int. Commun. Heat Mass Transf. 2016, 78, 68–79. [Google Scholar] [CrossRef]
- Ramezanizadeh, M.; Nazari, M.A.; Ahmadi, M.A.; Lorenzini, G.; Pop, I. A review on the applications of intelligence methods in predicting thermal conductivity of nanofluids. J. Therm. Anal. Calorim. 2019, 138, 827–843. [Google Scholar] [CrossRef]
- Minea, A.A.; El-Maghlany, W.M. Influence of hybrid nanofluids on the performance of parabolic trough collectors in solar thermal systems: Recent findings and numerical comparison. Renew. Energy 2018, 120, 350–364. [Google Scholar] [CrossRef]
- Sidik, N.A.C.; Jamil, M.M.; Japar, W.M.A.A.; Adamu, I.M. A review on preparation methods, stability and applications of hybrid nanofluids. Renew. Sustain. Energy Rev. 2017, 80, 1112–1122. [Google Scholar] [CrossRef]
- Sundar, L.S.; Sharma, K.V.; Singh, M.K.; Sousa, A. Hybrid nanofluids preparation, thermal properties, heat transfer and friction factor—A review. Renew. Sustain. Energy Rev. 2017, 68, 185–198. [Google Scholar] [CrossRef]
- Sajid, M.U.; Ali, H.M. Thermal conductivity of hybrid nanofluids: A critical review. Int. J. Heat Mass Transf. 2018, 126, 211–234. [Google Scholar] [CrossRef]
- Babar, H.; Sajid, M.U.; Ali, H.M. Viscosity of hybrid nanofluids: A critical review. Therm. Sci. 2019, 23, 1713–1754. [Google Scholar] [CrossRef] [Green Version]
- Sezer, N.; Atieh, M.A.; Koç, M. A comprehensive review on synthesis, stability, thermophysical properties, and characterization of nanofluids. Powder Technol. 2019, 344, 404–431. [Google Scholar] [CrossRef]
- Choi, C.; Yoo, H.S.; Oh, J.M. Preparation and heat transfer properties of nanoparticle-in-transformer oil dispersions as advanced energy-efficient coolants. Curr. Appl. Phys. 2008, 8, 710–712. [Google Scholar] [CrossRef]
- Ijam, A.; Saidur, R.; Ganesan, P.; Golsheikh, A.M. Stability, thermo-physical properties, and electrical conductivity of graphene oxide-deionized water/ethylene glycol based nanofluid. Int. J. Heat Mass Transf. 2015, 87, 92–103. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Wang, S.X.; Lin, Y.X.; Feng, M.; Wu, Q. Stability, thermal conductivity, and rheological properties of controlled reduced graphene oxide dispersed nanofluids. Appl. Therm. Eng. 2017, 119, 132–139. [Google Scholar] [CrossRef]
- Jing, F.; Yixin, L.; Pengjv, M.; Yongkun, G.; Shichao, G.; Bing, C.; Junkun, T.; Liu, Y. Supercooling and heterogeneous nucleation in acoustically levitated deionized water and graphene oxide nanofluids droplets. Exp. Therm. Fluid Sci. 2019, 103, 143–148. [Google Scholar] [CrossRef]
- Esfahani, M.R.; Languri, E.M.; Nunna, M.R. Effect of particle size and viscosity on thermal conductivity enhancement of graphene oxide nanofluid. Int. Commun. Heat Mass Transf. 2016, 76, 308–315. [Google Scholar] [CrossRef]
- Mohammad, A.N.; Roghayeh, G.; Mohammad, H.A. Experimental investigation of graphene oxide nanofluid on heat transfer enhancement of pulsating heat pipe. Int. Commun. Heat Mass 2018, 91, 90–94. [Google Scholar]
- Bahiraei, M.; Heshmatian, S. Graphene family nanofluids: A critical review and future research directions. Energy Convers. Manag. 2019, 196, 1222–1256. [Google Scholar] [CrossRef]
- Agarwal, R.; Verma, K.; Agrawal, N.K.; Singh, R. Sensitivity of thermal conductivity for Al2O3 nanofluids. Exp. Therm. Fluid Sci. 2017, 80, 19–26. [Google Scholar] [CrossRef]
- Kumar, N.; Sonawane, S.S.; Sonawane, S.H. Experimental study of thermal conductivity, heat transfer and friction factor of Al2O3 based nanofluid. Int. Commun. Heat Mass Transf. 2018, 90, 1–10. [Google Scholar] [CrossRef]
- Xie, Y.D.; Kocaefe, D.; Kocaefe, Y.; Cheng, J.; Liu, W. The Effect of Novel Synthetic Methods and Parameters Control on Morphology of Nano-alumina Particles. Nanoscale Res. Lett. 2016, 11, 259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmudul, H.A.; Sedong, K.; Junhyo, K.; Jungpil, N.; Sunchul, H.; Byeongkeun, C.; Hanshik, C.; Hyomin, J. Forced Convective Heat Transfer of Aqueous Al2O3 Nanofluid Through Shell and Tube Heat Exchanger. J. Nanosci. Nanotechnol. 2018, 18, 1730–1740. [Google Scholar]
- Charab, A.A.; Movahedirad, S.; Norouzbeigi, R. Thermal conductivity of Al2O3 + TiO2/water nanofluid: Model development and experimental validation. Appl. Therm. Eng. 2017, 119, 42–51. [Google Scholar] [CrossRef]
- Abdullah, M.; Malik, S.R.; Iqbal, M.H.; Sajid, M.M.; Shad, N.A.; Hussain, S.Z.; Razzaq, W.; Javed, Y. Sedimentation and Stabilization of Nano-Fluids with Dispersant. Colloid Surf. A 2018, 554, 86–92. [Google Scholar] [CrossRef]
- Said, Z.; Saidur, R.; Rahim, N.A. Energy and exergy analysis of a flat plate solar collector using different sizes of aluminium oxide based nanofluid. J. Clean. Prod. 2016, 133, 518–530. [Google Scholar] [CrossRef]
- Saleemi, M.; Vanapalli, S.; Nikkam, N.; Toprak, M.S.; Muhammed, M. Classical behavior of alumina (Al2O3) nanofluids in antifrogen N with experimental evidence. J. Nanomater. 2015, 2015, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Ezzat, A.W.; Hassan, I.M. Investigation of Alumina Nano Fluid Thermal Conductivity. Int. J. Comput. Appl. 2014, 102, 15–23. [Google Scholar] [CrossRef]
- Issa, R.J. Effect of nanoparticles size and concentration on thermal and rheological properties of Al2O3-water nanofluids. In Proceedings of the World Congress on Momentum, Prague, Czech Republic, 4–5 April 2016; pp. 1–7. [Google Scholar]
- Cui, W.; Jia, L.S.; Chen, Y.; Li, Y.; Li, J.; Mo, S. Supercooling of Water Controlled by Nanoparticles and Ultrasound. Nanoscale Res. Lett. 2018, 13, 145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Y.G.; Xi, Y.; Zhenzhong, Y.; Sasmito, A.P.; Mujumdar, A.S.; Wang, L. Experimental investigation of specific heat of aqueous graphene oxide Al2O3 hybrid nanofluid. Therm. Sci. 2019, 381. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.G.; Wang, H.C.; Sasmito, A.P.; Mujumdar, A.S. Measurement and modeling of thermal conductivity of graphene nanoplatelet water and ethylene glycol base nanofluids. Int. J. Heat Mass Transf. 2018, 123, 97–109. [Google Scholar] [CrossRef]
- Marsha, A.P.; Philip, R.C. Thermal conductivity measurements of particulate materials. J. Geophys. Res. 1997, 102, 6535–6549. [Google Scholar]
- Klarsfield, S.; Maglic, E.K. Compendium of Thermophysical Properties Measurement Methods; Springer: Berlin/Heidelberg, Germany, 1984. [Google Scholar]
- Nagamani, G.; Raghavendra, R.K. Design and development of embedded system for the measurement of thermal conductivity of liquids by transient hot wire method. Sens. Transducers J. 2011, 129, 42–56. [Google Scholar]
- Zhang, Y.; Liu, L.; Li, K.L.; Hou, D.; Wang, J. Enhancement of energy utilization using nanofluid in solar powered membrane distillation. Chemosphere 2018, 212, 554–562. [Google Scholar] [CrossRef]
- Zeng, J.; Xuan, Y.M. Enhanced solar thermal conversion and thermal conduction of MWCNTSiO2/Ag binary nanofluids. Appl. Energy 2018, 212, 809–819. [Google Scholar] [CrossRef]
- Wang, W.J.; Zhang, X.; Xin, C.Y.; Rao, Z. An experimental study on thermal management of lithium ion battery packs using an improved passive method. Appl. Therm. Eng. 2018, 134, 163–170. [Google Scholar] [CrossRef]
- Parveen, N.; Ansari, S.A.; Ansari, M.O.; Singh, A. Manganese dioxide nanorods intercalated reduced graphene oxide nanocomposite toward high performance electrochemical supercapacitive electrode materials. J. Colloid Interface Sci. 2017, 506, 613–619. [Google Scholar] [CrossRef]
- Johra, F.T.; Lee, J.-W.; Jung, W.-G. Facile and safe graphene preparation on solution based platform. J. Ind. Eng. Chem. 2014, 20, 2883–2887. [Google Scholar] [CrossRef]
- Li, Z.; Wu, K.Y.; Cao, J.; Wang, Y. Controlled synthesis of α-Al2O3 via the hydrothermal-pyrolysis method. IOP Conf. Ser. Mater. Sci. Eng. 2017, 207, 12004. [Google Scholar] [CrossRef]
- Fan, Y. Dispersion stability of thermal nanofluids (review). Prog. Nat. Sci. Mater. 2017, 27, 531–542. [Google Scholar]
- Zhang, K. Preparation, Characterization and Applications of Functionality Inorganics/Organics Composite Particles. Ph.D. Thesis, Sichuan University, Chengdu, China, 2005. [Google Scholar]
- Wang, X.J.; Zhu, D.S.; Li, X.F. Effects of pH on stability and thermal conductivity of Al2O3-H2O nanonuids. J. Funct. Mater. 2007, 38, 3140–3143. [Google Scholar]
- Somasundaran, P.; Markovic, B.; Krishnakumar, S.; Yu, X. Handbook of Surface and Colloid Chemistry; CRC Press: Boca Raton, FL, USA, 1997. [Google Scholar]
- Russel, W.B.; Saville, D.A.; Schowalter, W.R. Colloidal Dispersions; Cambridge University Press: Cambridge, UK, 1989. [Google Scholar]
- Bao, C.C.; Pi, Z.B. Effect of surfactant on stability of CdSSe-H2O nanofluids. Chem. Eng. Equip. 2012, 7, 32–36. [Google Scholar]
- Al-Anssari, S.; Arif, M.; Wang, S.; Barifcani, A.; Iglauer, S. Stabilising nanofluids in saline environments. J. Colloid Interface Sci. 2017, 508, 222–229. [Google Scholar] [CrossRef]
- Iglesias, G.R.; Wächter, W.; Ahualli, S.; Glatter, O. Interactions between large colloids and surfactants. Soft Matter 2011, 7, 4619. [Google Scholar] [CrossRef]
- Tadros, T.F. Applied Surfactants: Principles and Applications; Wiley-Vch: Weinheim, Germany, 2006. [Google Scholar]
- American Society of Heating, Refrigerating and Air-Conditioning Engineers. ASHRAE Handbook Fundamentals; American Society of Heating, Refrigerating and Air-Conditioning Engineers: Peachtree Corners, GA, USA, 2001. [Google Scholar]
- Esfe, M.H.; Yan, W.-M.; Akbari, M.; Karimipour, A.; Hassani, M. Experimental study on thermal conductivity of DWCNT-ZnO/water-EG nanofluids. Int. Commun. Heat Mass Transf. 2015, 68, 248–251. [Google Scholar] [CrossRef]
- Harandi, S.S.; Karimipour, A.; Afrand, M. An experimental study on thermal conductivity of f-MWCNTs-Fe3O4/EG hybrid nanofluid: Effects of temperature and concentration. Int. Commun. Heat Mass 2016, 76, 171–177. [Google Scholar] [CrossRef] [Green Version]
- Afrand, M. Experimental study on thermal conductivity of ethylene glycol containing hybrid nano-additives and development of a new correlation. Appl. Therm. Eng. 2017, 110, 1111–1119. [Google Scholar] [CrossRef]
- Hamid, K.A.; Azmi, W.H.; Nabil, M.F.; Mamat, R. Improved thermal conductivity of TiO2-SiO2 hybrid nanofluid in ethylene glycol and water mixture. IOP Conf. Ser. Mater. Sci. Eng. 2017, 257, 12067. [Google Scholar] [CrossRef]
- Esfahani, N.N.; Toghraie, D.; Afrand, M. A new correlation for predicting the thermal conductivity of ZnO-Ag (50%–50%)/water hybrid nanofluid: An experimental study. Powder Technol. 2018, 323, 367–373. [Google Scholar] [CrossRef]
- Maxwell, J.C. A TREATISE on Electricity and Magnetism; Clarendon Press (Oxford): Cambridge, UK, 1873; Volume 1. [Google Scholar]
- Takabi, B.; Salehi, S. Augmentation of the Heat Transfer Performance of a Sinusoidal Corrugated Enclosure by Employing Hybrid Nanofluid. Adv. Mech. Eng. 2014, 6, 147059. [Google Scholar] [CrossRef]
- Khan, M.F.S.; Alexander, A.B. Thermal properties of graphene and multilayer graphene: Applications in thermal interface materials. Solid State Commun. 2012, 152, 1331–1340. [Google Scholar]
- Im, H.; Kim, J. Thermal conductivity of a graphene oxide-carbon nanotube hybrid/epoxy composite. Carbon 2012, 50, 5429–5440. [Google Scholar] [CrossRef]
- Wang, X.-Q.; Mujumdar, A.S. Heat transfer characteristics of nanofluids: A review. Int. J. Therm. Sci. 2007, 46, 1–19. [Google Scholar] [CrossRef]
- Nan, C.-W. Physics of inhomogeneous inorganic materials. Prog. Mater. Sci. 1993, 37, 1–116. [Google Scholar] [CrossRef]
- Xuan, Y.M. An overview on nanofluids and applications. Sci. Sin. Technol. 2014, 44, 269–279. [Google Scholar] [CrossRef]
- Hamilton, R.L.; Crosser, O. Thermal Conductivity of Heterogeneous Two-Component Systems. Ind. Eng. Chem. Fundam. 1962, 1, 187–191. [Google Scholar] [CrossRef]
- Chu, K.; Li, W.-S.; Tang, F.-L. Flatness-Dependent thermal conductivity of graphene-based composites. Phys. Lett. A 2013, 377, 910–914. [Google Scholar] [CrossRef]
- Xuan, Y.M.; Li, Q.; Hu, W. Aggregation structure and thermal conductivity of nanofluids. AIChE J. 2003, 49, 1038–1043. [Google Scholar] [CrossRef]
- Shukla, K.N.; Koller, T.M.; Rausch, M.H.; Fröba, A.P. Effective thermal conductivity of nanofluids—A new model taking into consideration Brownian motion. Int. J. Heat Mass Transf. 2016, 99, 532–540. [Google Scholar] [CrossRef]
- Waite, W.F.; Gilbert, L.Y.; Winters, W.J.; Mason, D.H. Estimating thermal diffusivity and specific heat from needle probe thermal conductivity data. Rev. Sci. Instrum. 2006, 77, 044904. [Google Scholar] [CrossRef]

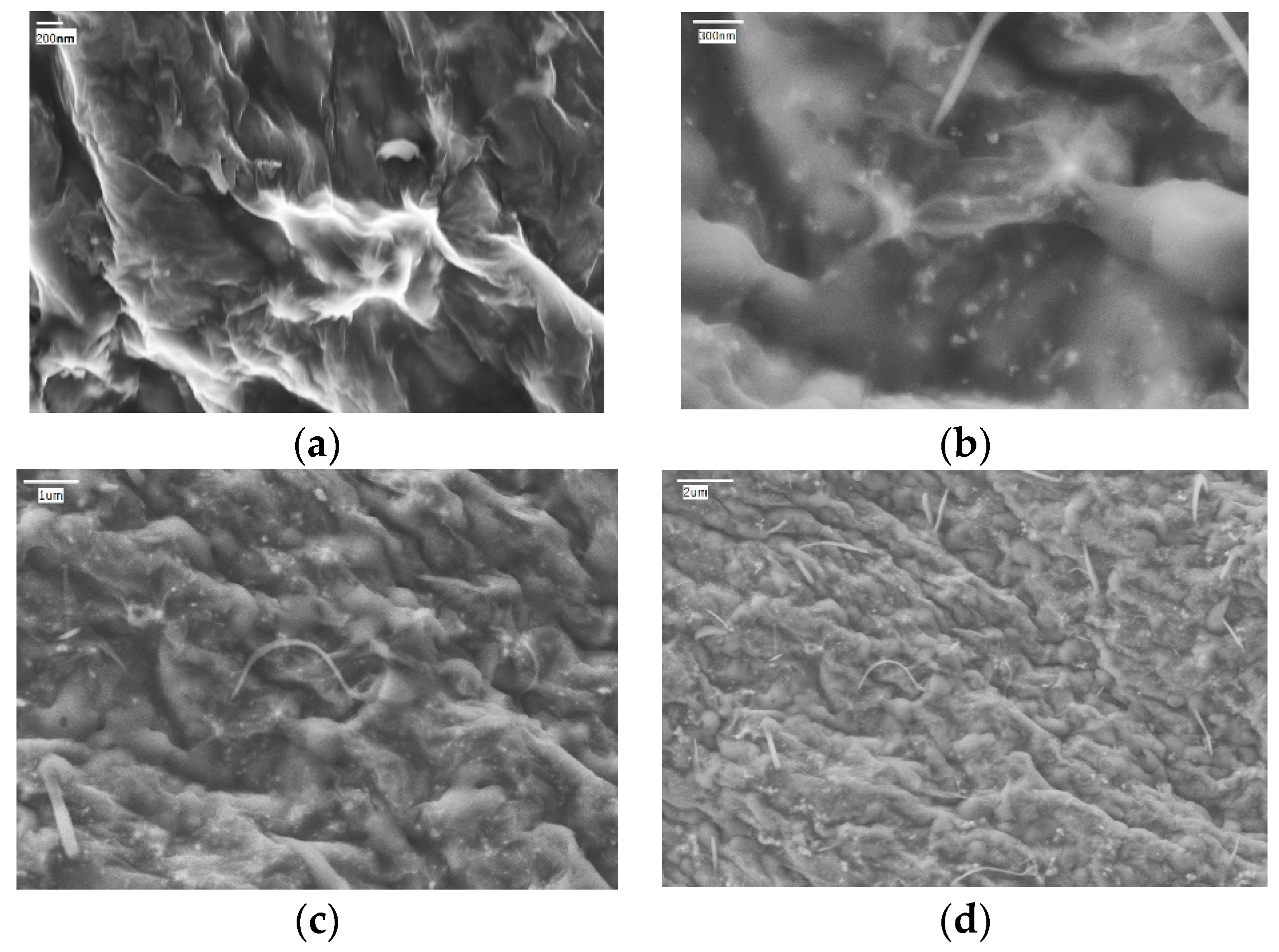
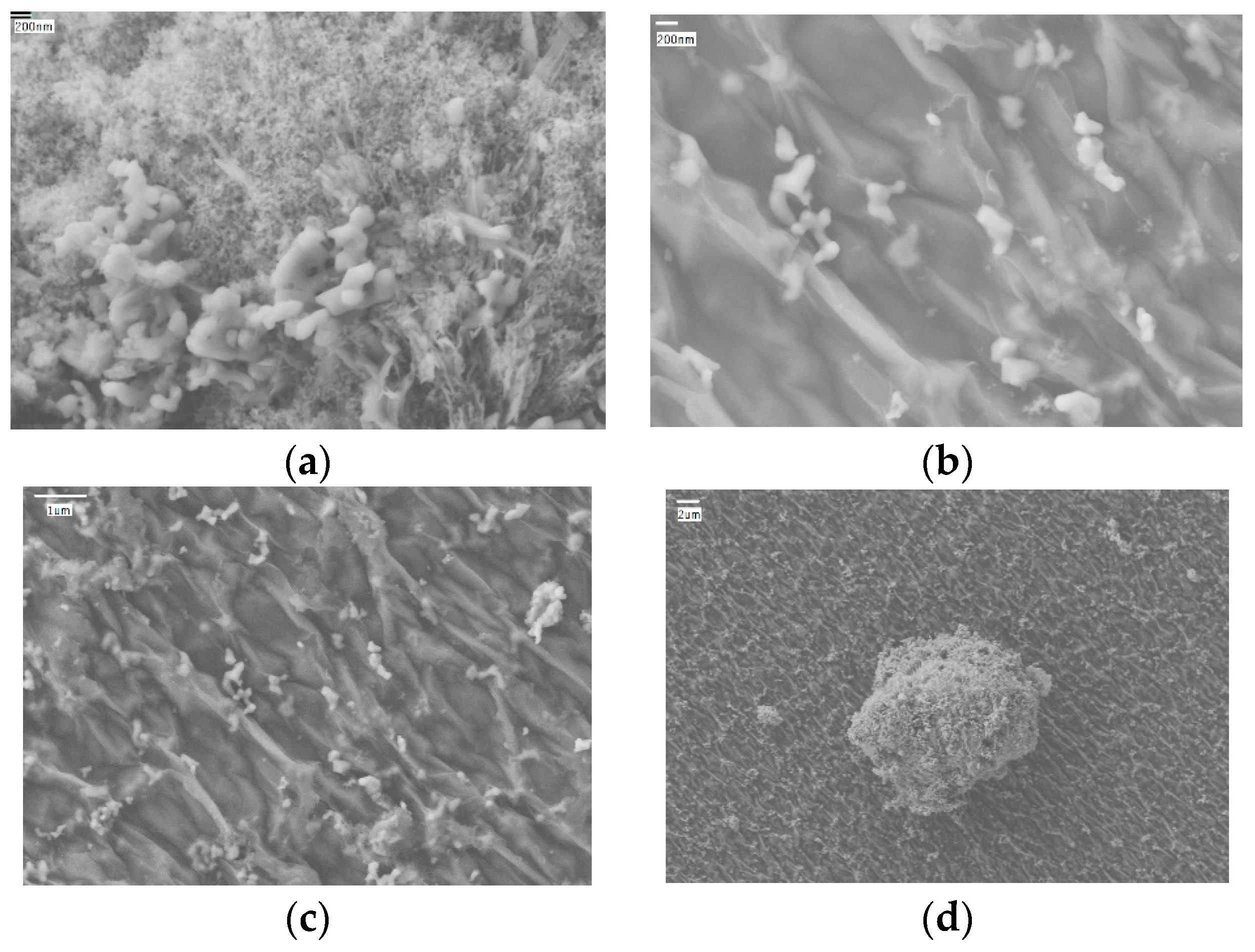






| Nanomaterials | Density, kg/m3 | Thermal Conductivity, W/(m·K) | Specific Heat, J/(kg·K) |
|---|---|---|---|
| α-Al2O3 | 3987 | 40 [46] | 773 [53] |
| GO nanosheet | 1800 [82] | 3000 [81] | 790 [53] |
| a | b | c | d | e | g | R2 |
|---|---|---|---|---|---|---|
| 0.5705 | 9.041 | 0.001868 | 1738 | −0.09111 | −7.805 × 10−6 | 0.9968 |
| η | Rk/m2Kw−1 | L/m | t/m | R2 |
|---|---|---|---|---|
| 0.3928 | 7.494 × 10−9 | 1.974 × 10−5 | 9.000 × 10−9 | 0.8658 |
| 0.3192 | 4.817 × 10−9 | 3.296 × 10−5 | 6.244 × 10−9 | 0.8658 |
| 0.3211 | 3.113 × 10−9 | 2.167 × 10−5 | 6.312 × 10−9 | 0.8658 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; An, J.; Xi, Y.; Yang, Z.; Liu, J.; Mujumdar, A.S.; Wang, L.; Sasmito, A.P. Thermal Conductivity and Stability of Novel Aqueous Graphene Oxide–Al2O3 Hybrid Nanofluids for Cold Energy Storage. Appl. Sci. 2020, 10, 5768. https://doi.org/10.3390/app10175768
Gao Y, An J, Xi Y, Yang Z, Liu J, Mujumdar AS, Wang L, Sasmito AP. Thermal Conductivity and Stability of Novel Aqueous Graphene Oxide–Al2O3 Hybrid Nanofluids for Cold Energy Storage. Applied Sciences. 2020; 10(17):5768. https://doi.org/10.3390/app10175768
Chicago/Turabian StyleGao, Yuguo, Jiancai An, Yangyang Xi, Zhenzhong Yang, Junjun Liu, Arun S. Mujumdar, Lijun Wang, and Agus P. Sasmito. 2020. "Thermal Conductivity and Stability of Novel Aqueous Graphene Oxide–Al2O3 Hybrid Nanofluids for Cold Energy Storage" Applied Sciences 10, no. 17: 5768. https://doi.org/10.3390/app10175768