Spatial and Temporal Variation in Microbial Diversity and Community Structure in a Contaminated Mangrove Wetland
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description and Sampling Methods
2.2. Analytical Methods
2.2.1. Soil Pretreatment
2.2.2. Soil Chemical Analysis
2.2.3. Microbial DNA Isolation, PCR Amplification and MiSeq PE250 Sequencing
2.2.4. Illumina Read Data Processing and Analysis
2.3. Statistical Analysis
3. Results
3.1. Soil Physicochemical Characteristics
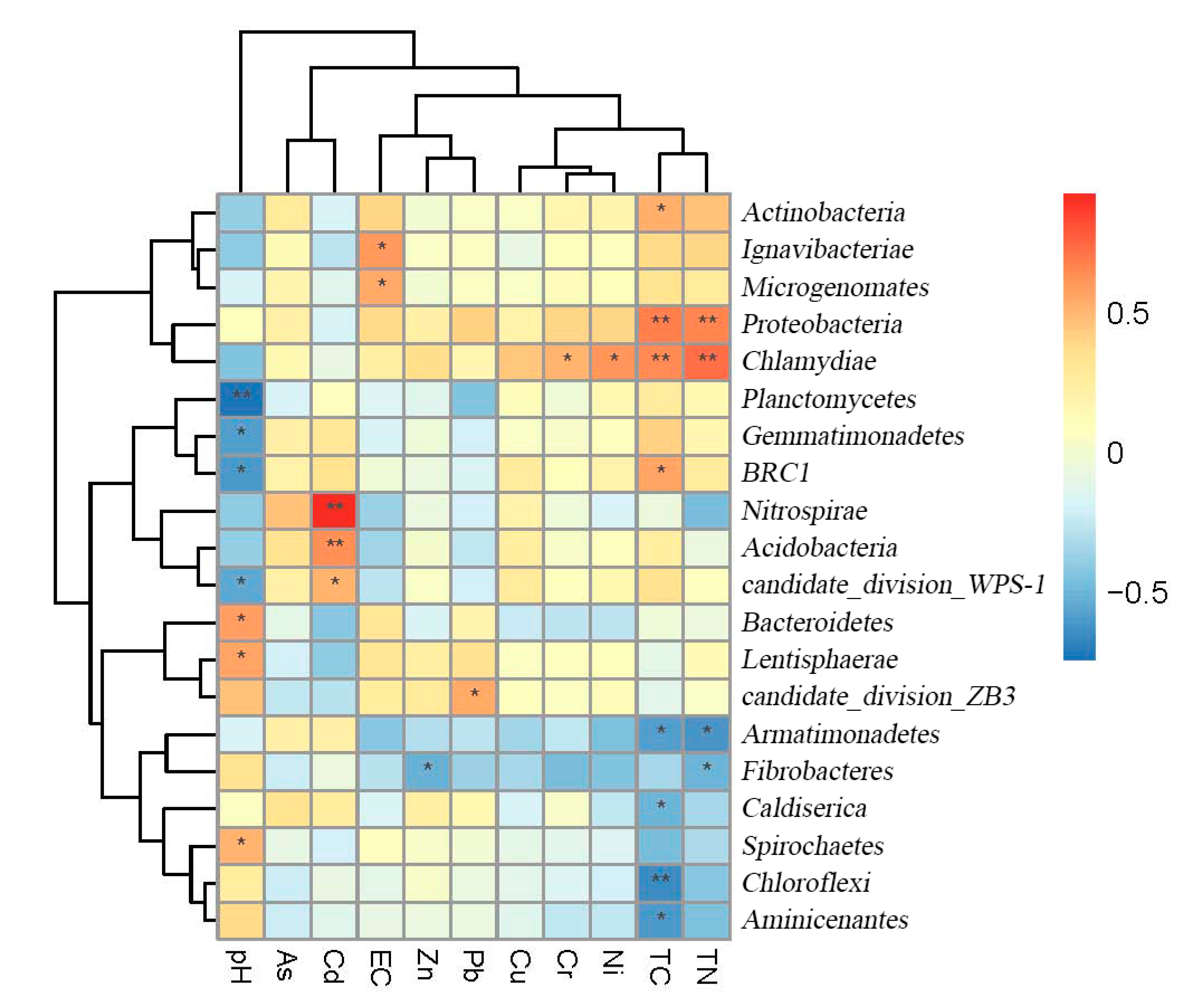
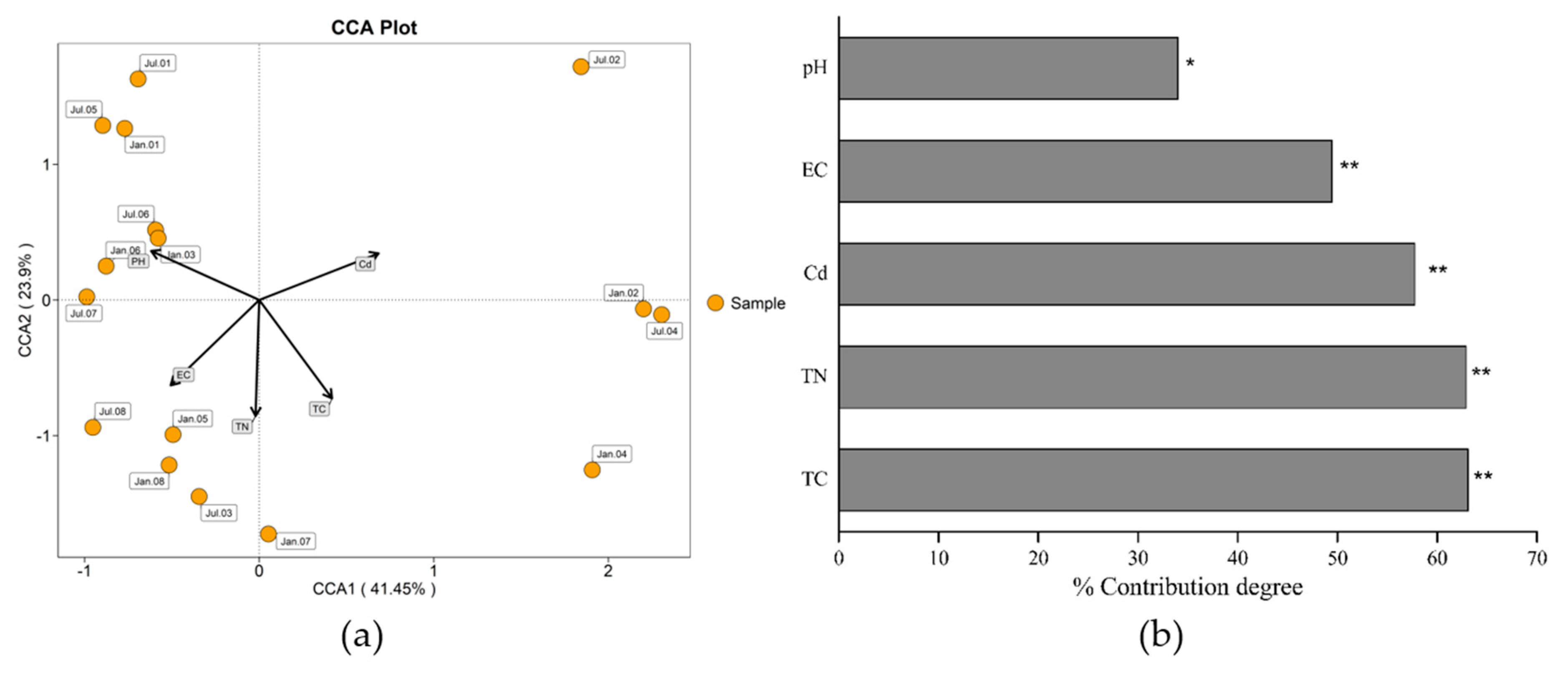
3.2. Soil Microbial Community
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Queiroz, L.d.S.; Rossi, S.; Calvet-Mir, L.; Ruiz-Mallén, I.; García-Betorz, S.; Salvà-Prat, J.; Meireles, A.J.d.A. Neglected ecosystem services: Highlighting the socio-cultural perception of mangroves in decision-making processes. Ecosyst. Serv. 2017, 26, 137–145. [Google Scholar] [CrossRef]
- Sandilyan, S.; Kathiresan, K. Mangrove conservation: A global perspective. Biodivers. Conserv. 2012, 21, 3523–3542. [Google Scholar] [CrossRef]
- Giri, C.; Ochieng, E.; Tieszen, L.L.; Zhu, Z.; Singh, A.; Loveland, T.; Masek, J.; Duke, N. Status and distribution of mangrove forests of the world using earth observation satellite data. Glob. Ecol. Biogeogr. 2011, 20, 154–159. [Google Scholar] [CrossRef]
- Li, R.; Chai, M.; Qiu, G.Y. Distribution, Fraction, and Ecological Assessment of Heavy Metals in Sediment-Plant System in Mangrove Forest, South China Sea. PLoS ONE 2016, 11, e0147308. [Google Scholar] [CrossRef] [Green Version]
- Holguin, G.; Vazquez, P.; Bashan, Y. The role of sediment microorganisms in the productivity, conservation, and rehabilitation of mangrove ecosystems: An overview. Biol. Fertil. Soils 2001, 33, 265–278. [Google Scholar] [CrossRef]
- Gong, B.; Cao, H.; Peng, C.; Perculija, V.; Tong, G.; Fang, H.; Wei, X.; Ouyang, S. High-throughput sequencing and analysis of microbial communities in the mangrove swamps along the coast of Beibu Gulf in Guangxi, China. Sci. Rep. 2019, 9, 9377. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, H.F.; Cury, J.C.; do Carmo, F.L.; dos Santos, A.L.; Tiedje, J.; van Elsas, J.D.; Rosado, A.S.; Peixoto, R.S. Mangrove bacterial diversity and the impact of oil contamination revealed by pyrosequencing: Bacterial proxies for oil pollution. PLoS ONE 2011, 6, e16943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, G.F.; Li, P.F.; Zhong, J.X.; Shen, Z.J.; Chen, J.; Li, Y.T.; Isabwe, A.; Zhu, X.Y.; Ding, Q.S.; Zhang, S.; et al. Spartina alterniflora invasion alters soil bacterial communities and enhances soil N2O emissions by stimulating soil denitrification in mangrove wetland. Sci. Total Environ. 2019, 653, 231–240. [Google Scholar] [CrossRef]
- Chambers, L.G.; Guevara, R.; Boyer, J.N.; Troxler, T.G.; Davis, S.E. Effects of Salinity and Inundation on Microbial Community Structure and Function in a Mangrove Peat Soil. Wetlands 2016, 36, 361–371. [Google Scholar] [CrossRef]
- Fernandez-Cadena, J.C.; Ruiz-Fernandez, P.S.; Fernandez-Ronquillo, T.E.; Diez, B.; Trefault, N.; Andrade, S.; De la Iglesia, R. Detection of sentinel bacteria in mangrove sediments contaminated with heavy metals. Mar. Pollut. Bull. 2020, 150, 110701. [Google Scholar] [CrossRef]
- Hossain, M.; Siddique, M.R.H.; Abdullah, S.M.R.; Saha, S.; Ghosh, D.C.; Rahman, M.S.; Limon, S.H. Nutrient Dynamics Associated with Leaching and Microbial Decomposition of Four Abundant Mangrove Species Leaf Litter of the Sundarbans, Bangladesh. Wetlands 2013, 34, 439–448. [Google Scholar] [CrossRef]
- Enya, O.; Lin, C.; Qin, J. Heavy metal contamination status in soil-plant system in the Upper Mersey Estuarine Floodplain, Northwest England. Mar. Pollut. Bull. 2019, 146, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Enya, O.; Heaney, N.; Iniama, G.; Lin, C. Effects of heavy metals on organic matter decomposition in inundated soils: Microcosm experiment and field examination. Sci. Total Environ. 2020, 724, 138223. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Sun, Y.; Pei, N.; Zhang, Z.; Li, H.; Wang, W.; Xie, J.; Xu, X.; Luo, X.; Mai, B. Polybrominated diphenyl ethers and alternative halogenated flame retardants in mangrove plants from Futian National Nature Reserve of Shenzhen City, South China. Environ. Pollut. 2020, 260, 114087. [Google Scholar] [CrossRef]
- Feng, J.; Zhu, X.; Wu, H.; Ning, C.; Lin, G. Distribution and ecological risk assessment of heavy metals in surface sediments of a typical restored mangrove-aquaculture wetland in Shenzhen, China. Mar. Pollut. Bull. 2017, 124, 1033–1039. [Google Scholar] [CrossRef]
- Xu, S.; Lin, C.; Qiu, P.; Song, Y.; Yang, W.; Xu, G.; Feng, X.; Yang, Q.; Yang, X.; Niu, A. Tungsten- and cobalt-dominated heavy metal contamination of mangrove sediments in Shenzhen, China. Mar. Pollut. Bull. 2015, 100, 562–566. [Google Scholar] [CrossRef]
- Angola, G.; Arroyo, P.; de Miera, L.E.S. Characterization of the soil bacterial community structure and composition of natural and constructed wetlands. Sci. Total Environ. 2014, 473, 63–71. [Google Scholar]
- Li, X.; Yuan, Y.; Huang, Y.; Liu, H.W.; Bi, Z.; Yuan, Y.; Yang, P.B. A novel method of simultaneous NH4+ and NO3− removal using Fe cycling as a catalyst: Feammox coupled with NAFO. Sci Total Environ. 2018, 631, 153–157. [Google Scholar] [CrossRef]
- Niu, A.; Ma, J.; Gao, Y.; Xu, S.; Lin, C. Mangrove Soil-Borne Trace Elements in Qi’ao Island: Implications for Understanding Terrestrial Input of Trace Elements into Part of the Pearl River Estuary. Appl. Sci. 2020, 10, 2439. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Wu, Z.; Chu, B.; Zhang, N.; Cai, S.; Fang, J. Heavy metals in coastal wetland sediments of the Pearl River Estuary, China. Environ. Pollut. 2007, 149, 158–164. [Google Scholar] [CrossRef]
- Li, R.; Li, R.; Chai, M.; Shen, X.; Xu, H.; Qiu, G. Heavy metal contamination and ecological risk in Futian mangrove forest sediment in Shenzhen Bay, South China. Mar. Pollut. Bull. 2015, 101, 448–456. [Google Scholar] [CrossRef]
- Romigh, M.M.; Davis, S.E.; Rivera-Monroy, V.H.; Twilley, R.R. Flux of organic carbon in a riverine mangrove wetland in the Florida Coastal Everglades. Hydrobiologia 2006, 569, 505–516. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Nie, S.; Liang, J.; Zeng, G.; Wu, H.; Hua, S.; Liu, J.; Yuan, Y.; Xiao, H.; Deng, L.; et al. Effects of heavy metals and soil physicochemical properties on wetland soil microbial biomass and bacterial community structure. Sci. Total Environ. 2016, 557, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-P.; Chen, H.-Y.; Sun, J.; Zhang, X.; He, C.-Q.; Liu, X.-Y.; Huang, X.; Yang, M.; Wang, F.-S.; Väänänen, K. Shifts in the structure and function of the microbial community in response to metal pollution of fresh water sediments in Finland. J. Soils Sediments 2017, 18, 3324–3333. [Google Scholar] [CrossRef]
- Sundaramanickam, A.; Shanmugam, N.; Cholan, S.; Kumaresan, S.; Madeswaran, P.; Balasubramanian, T. Spatial variability of heavy metals in estuarine, mangrove and coastal ecosystems along Parangipettai, Southeast coast of India. Environ. Pollut. 2016, 218, 186–195. [Google Scholar] [CrossRef]
- Hayatsu, M.; Tago, K.; Saito, M. Various players in the nitrogen cycle: Diversity and functions of the microorganisms involved in nitrification and denitrification. Soil Sci. Plant Nutr. 2008, 54, 33–45. [Google Scholar] [CrossRef]
- Zhou, Z.; Meng, H.; Liu, Y.; Gu, J.D.; Li, M. Stratified Bacterial and Archaeal Community in Mangrove and Intertidal Wetland Mudflats Revealed by High Throughput 16S rRNA Gene Sequencing. Front. Microbiol. 2017, 8, 2148. [Google Scholar] [CrossRef] [PubMed]
- Behera, P.; Mohapatra, M.; Kim, J.Y.; Adhya, T.K.; Pattnaik, A.K.; Rastogi, G. Spatial and temporal heterogeneity in the structure and function of sediment bacterial communities of a tropical mangrove forest. Environ. Sci. Pollut. Res. Int. 2019, 26, 3893–3908. [Google Scholar] [CrossRef]
- Guo, H.; Nasir, M.; Lv, J.; Dai, Y.; Gao, J. Understanding the variation of microbial community in heavy metals contaminated soil using high throughput sequencing. Ecotoxicol. Environ. Saf. 2017, 144, 300–306. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, B.X.; Ren, H.; Zhang, J. Composition and functional diversity of microbial community across a mangrove-inhabited mudflat as revealed by 16S rDNA gene sequences. Sci. Total Environ. 2018, 633, 518–528. [Google Scholar] [CrossRef]
- Zhu, P.; Wang, Y.; Shi, T.; Zhang, X.; Huang, G.; Gong, J. Intertidal zonation affects diversity and functional potentials of bacteria in surface sediments: A case study of the Golden Bay mangrove, China. Appl. Soil Ecol. 2018, 130, 159–168. [Google Scholar] [CrossRef]
- Yin, Y.; Yan, Z. Variations of soil bacterial diversity and metabolic function with tidal flat elevation gradient in an artificial mangrove wetland. Sci Total Environ. 2020, 718, 137385. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Huang, H.; Bao, S.; Tong, Y. Microbial community structure of soils in Bamenwan mangrove wetland. Sci. Rep. 2019, 9, 8406. [Google Scholar] [CrossRef] [Green Version]
- Mendes, L.W.; Tsai, S.M. Distinct taxonomic and functional composition of soil microbiomes along the gradient forest-restinga-mangrove in southeastern Brazil. Antonie Van Leeuwenhoek 2018, 111, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, A.; Sengupta, S.; Bhattacharyya, M. Microbial Diversity and Community Analysis of the Sundarbans Mangrove, a World Heritage Site. In Microbial Diversity in the Genomic Era; Elsevier: Amsterdam, The Netherlands, 2019; pp. 65–76. [Google Scholar] [CrossRef]
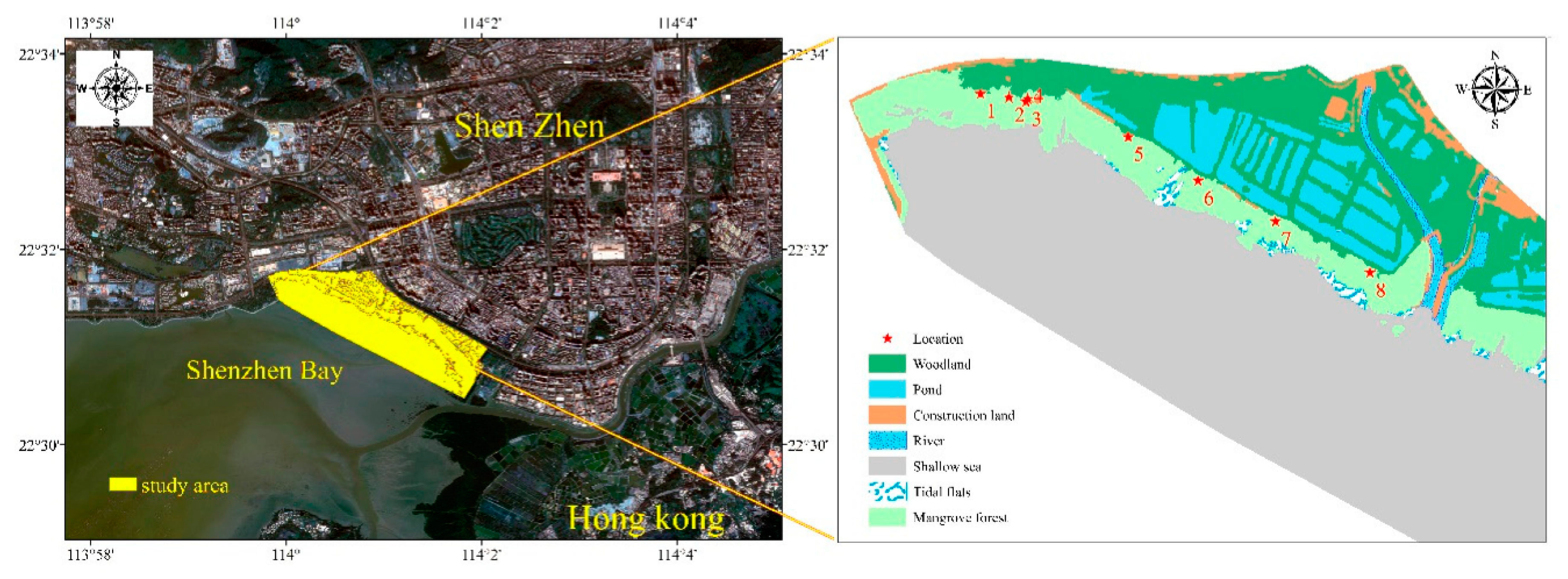
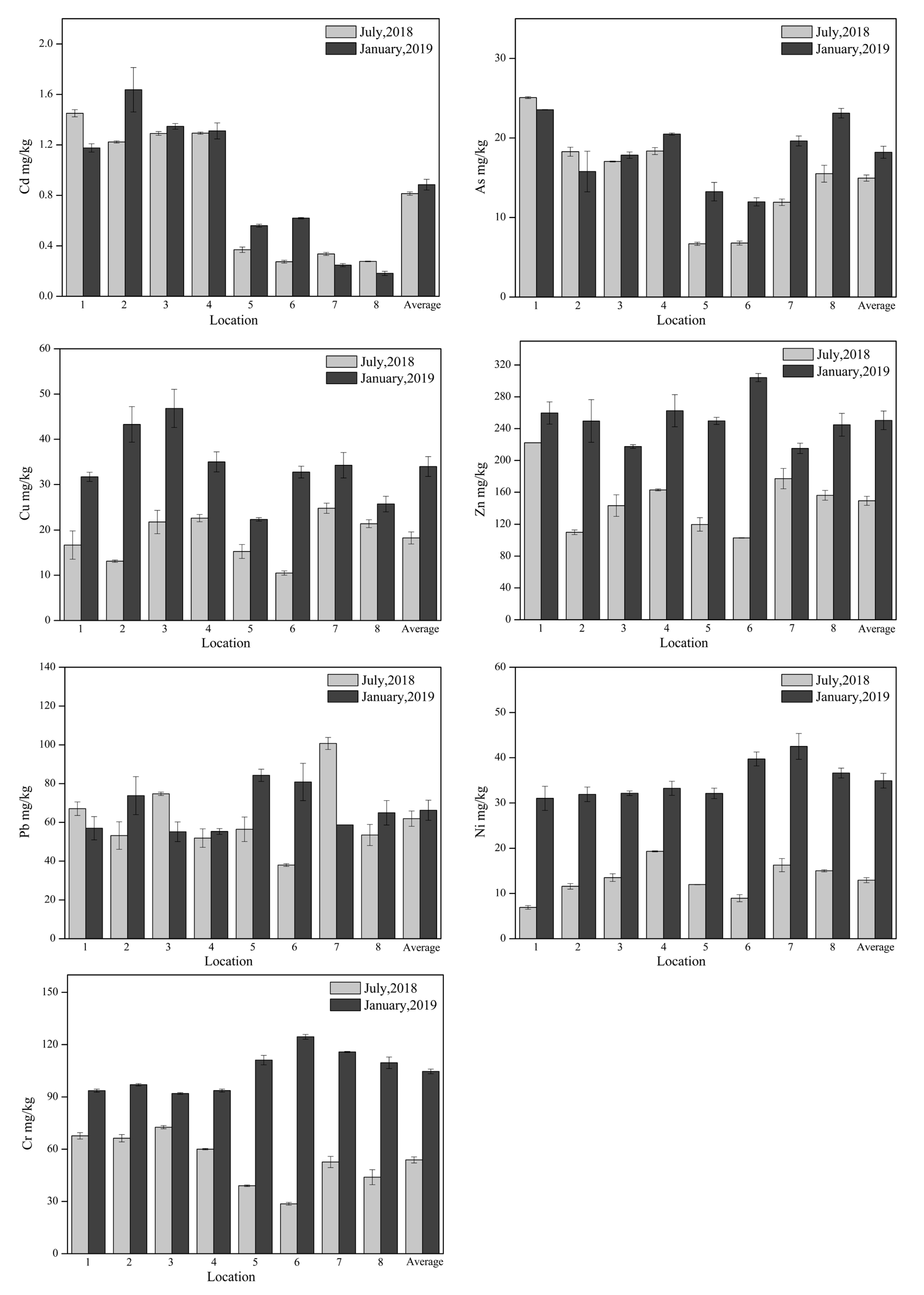
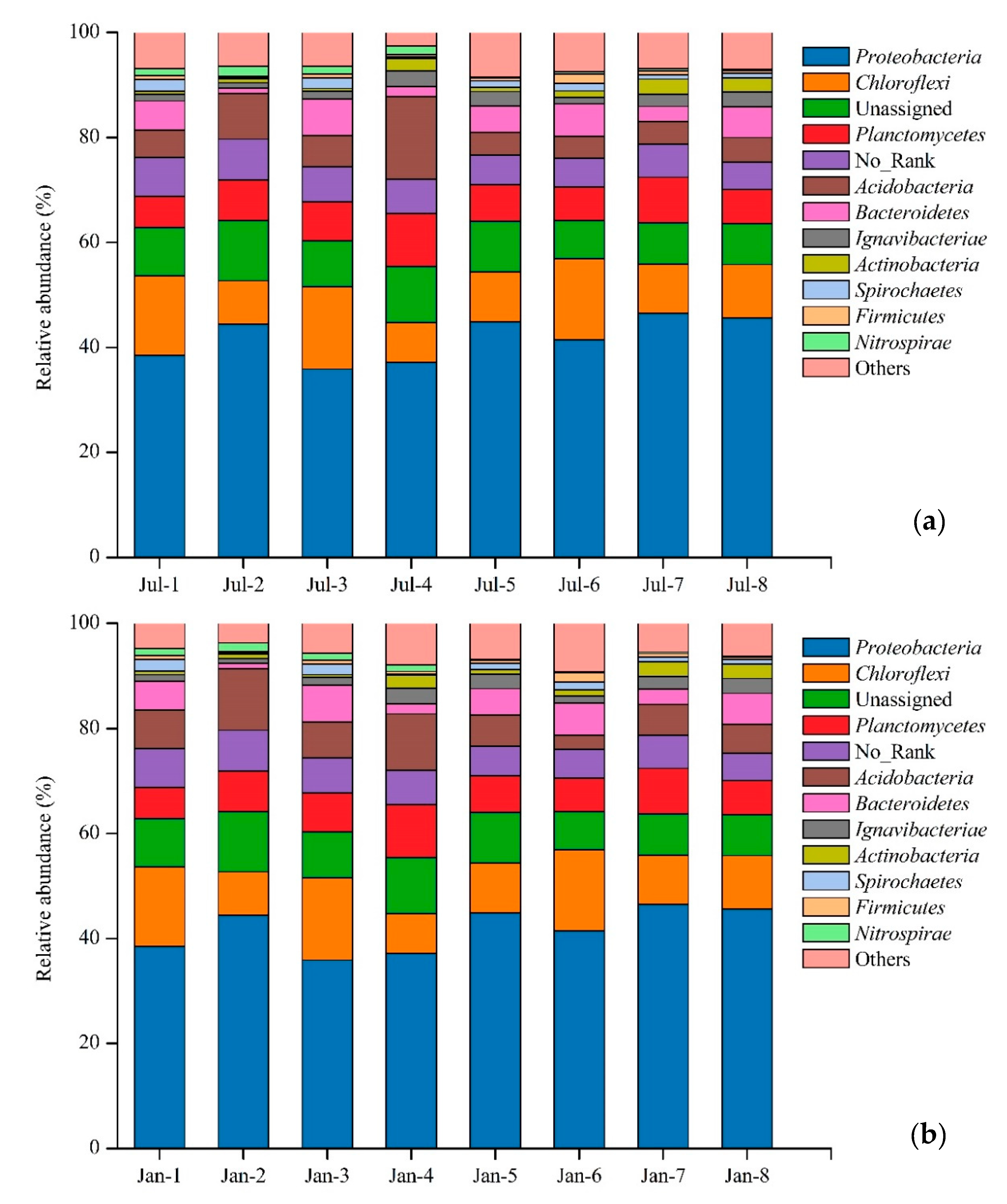
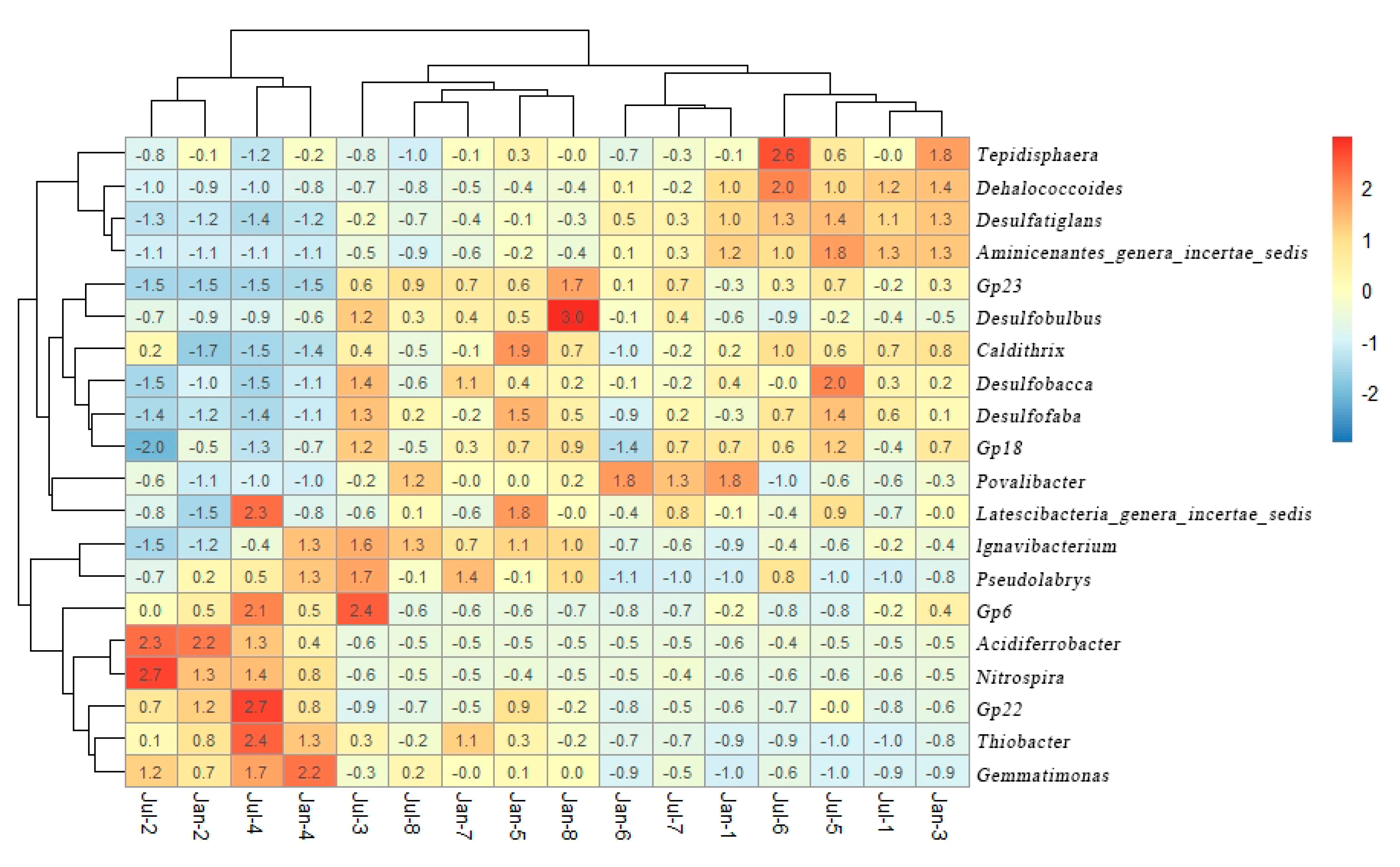

| Sampling Time | Location | pH | EC | TC | TN |
|---|---|---|---|---|---|
| July, 2018 | 1 | 6.85 ± 0.21 Bb | 7.83 ± 0.38 Ca | 13.27 ± 0.69 Cb | 0.95 ± 0.01 Cb |
| 2 | 6.77 ± 0.06 Bb | 3.45 ± 0.29 Db | 52.18 ± 0.60 Bb | 1.70 ± 0.10 Bb | |
| 3 | 6.44 ± 0.24 Db | 15.63 ± 0.09 Ba | 66.00 ± 2.50 Ab | 2.24 ± 0.09 Ab | |
| 4 | 6.76 ± 0.08 Ba | 5.97 ± 0.77 Cb | 69.20 ± 8.77 Aa | 2.74 ± 0.22 Ab | |
| 5 | 7.09 ± 0.12 Aa | 7.23 ± 0.48 Cb | 25.68 ± 3.93 Cb | 1.69 ± 0.16 Bb | |
| 6 | 6.63 ± 0.09 Cb | 7.70 ± 0.06 Cb | 26.62 ± 2.32 Cb | 1.64 ± 0.14 Bb | |
| 7 | 7.03 ± 0.02 Aa | 13.57 ± 1.25 Aa | 62.02 ± 4.07 Ab | 2.57 ± 0.10 Ab | |
| 8 | 7.13 ± 0.03 Ba | 19.10 ± 2.23 Aa | 44.69 ± 5.67 Bb | 2.13 ± 0.26 Ab | |
| Average | 6.83 ± 0.08 a | 9.56 ± 1.90 b | 50.53 ± 9.26 b | 2.13 ± 0.28 b | |
| January, 2019 | 1 | 7.03 ± 0.06 Aa | 8.45 ± 0.01 Ca | 28.65 ± 3.66 Da | 1.79 ± 0.32 Ca |
| 2 | 6.86 ± 0.21 Cb | 9.78 ± 0.00 Ba | 70.18 ± 2.47 Ba | 2.78 ± 0.06 Ca | |
| 3 | 6.93 ± 0.36 Ba | 16.41 ± 0.29 Aa | 93.91 ± 2.04 Aa | 3.14 ± 0.24 Ba | |
| 4 | 6.09 ± 0.27 Db | 10.06 ± 1.90 Ba | 76.94 ± 4.28 Ba | 2.13 ± 0.28 Ca | |
| 5 | 6.97 ± 0.02 Bb | 18.42 ± 1.26 Aa | 75.52 ± 4.96 Ba | 4.18 ± 0.07 Ba | |
| 6 | 6.95 ± 0.06 Ba | 12.17 ± 1.22 Ba | 49.80 ± 2.94 Ca | 3.96 ± 0.39 Ba | |
| 7 | 6.81 ± 0.15 Cb | 11.50 ± 0.15 Bb | 102.93 ± 9.81 Aa | 5.29 ± 0.48 Aa | |
| 8 | 6.84 ± 0.09 Cb | 9.34 ± 0.72 Bb | 65.15 ± 5.04 Ba | 4.09 ± 0.36 Ba | |
| Average | 6.81 ± 0.01 a | 12.16 ± 1.23 a | 70.38 ± 8.31 a | 3.55 ± 0.37 a |
| Time | Location | OTUs | ACE | Chao | Evenness | Shannon | Coverage |
|---|---|---|---|---|---|---|---|
| July, 2018 | 1 | 162,460 | 11,278 | 11,271 | 0.83 | 7.14 | 0.98 |
| 2 | 154,322 | 11,990 | 11,818 | 0.79 | 7.22 | 0.98 | |
| 3 | 126,439 | 10,438 | 10,256 | 0.81 | 7.30 | 0.98 | |
| 4 | 147,421 | 8,326 | 8,255 | 0.77 | 6.79 | 0.99 | |
| 5 | 155,661 | 14,206 | 14,086 | 0.80 | 7.42 | 0.98 | |
| 6 | 162,041 | 13,192 | 13,213 | 0.81 | 7.49 | 0.98 | |
| 7 | 157,612 | 17,301 | 17,203 | 0.83 | 7.81 | 0.97 | |
| 8 | 166,432 | 15,267 | 15,267 | 0.83 | 7.75 | 0.98 | |
| Average | 154,049 | 12,750 | 12,671 | 0.81 | 7.37 | 0.98 | |
| Jan, 2019 | 1 | 158,324 | 13,523 | 13,429 | 0.80 | 7.36 | 0.98 |
| 2 | 162,643 | 7,975 | 8,070 | 0.74 | 6.45 | 0.99 | |
| 3 | 163,763 | 17,650 | 17,587 | 0.82 | 7.82 | 0.97 | |
| 4 | 153,204 | 9,287 | 9,310 | 0.80 | 7.10 | 0.99 | |
| 5 | 161,733 | 12,548 | 12,458 | 0.80 | 7.36 | 0.98 | |
| 6 | 163,450 | 15,966 | 15,689 | 0.82 | 7.73 | 0.98 | |
| 7 | 145,143 | 11,144 | 11,018 | 0.81 | 7.31 | 0.98 | |
| 8 | 165,804 | 14,671 | 14,546 | 0.81 | 7.55 | 0.98 | |
| Average | 159,258 | 12,864 | 12,763 | 0.80 | 7.34 | 0.98 |
| OTUs | ACE | Chao1 | Evenness | Shannon | Coverage | |
|---|---|---|---|---|---|---|
| TC | −0.28 | −0.12 | −0.13 | −0.21 | −0.07 | 0.01 |
| TN | 0.05 | 0.10 | 0.10 | 0.05 | 0.19 | 0.02 |
| pH | 0.50 * | 0.57 * | 0.57 * | 0.17 | 0.36 | −0.48 |
| EC | 0.04 | 0.37 | 0.38 | 0.41 | 0.46 | −0.36 |
| Cr | 0.05 | −0.07 | −0.08 | −0.13 | −0.08 | 0.11 |
| Ni | 0.14 | 0.03 | 0.03 | −0.16 | 0.01 | 0.12 |
| Cu | 0.11 | 0.02 | 0.03 | −0.23 | −0.09 | 0.04 |
| Zn | 0.32 | 0.01 | 0.01 | −0.04 | −0.06 | 0.15 |
| As | −0.08 | −0.29 | −0.29 | 0.00 | −0.26 | 0.18 |
| Cd | −0.23 | −0.53 * | −0.53 * | −0.47 | −0.62 * | 0.38 |
| Pb | −0.03 | 0.21 | 0.20 | 0.13 | 0.14 | −0.29 |
| Study Area | Diversity | Community Structure |
|---|---|---|
| Shannon | Five Most Abundant Bacteria (%) at the Phylum Level | |
| Beibu Gulf, China [6] | 9.44–10.46 | Proteobacteria (52.3%), Bacteroidetes (7.73%), Chloroflexi (6.09%), Actinobacteria (5.02%), Parvarchaeota (4.10%) |
| Mai Po Wetland, China [27] | 6.94–10.27 | Proteobacteria (45.6%), Chloroflexi (14.7%), Bacteroidetes (12.0%), Cyanobacteria (7.6%), Planctomycetes (4.5%) |
| Zhangjiang River, China [8] | 5.16–5.23 | Proteobacteria, Chloroflexi, Actinobacteria, Bacteroidetes, Planctomycetes |
| Daya Bay, China [30] | 7.90–9.90 | Proteobacteria (55%), Bacteroidetes (8.1%), Actinobacteria (5.1%), Acidobacteria (5.1%), Chloroflexi (4.6%) |
| Golden Bay, China [31] | 8.0–9.0 | Proteobacteria (50%), Cyanobacteria (11.5%), Bacteroidetes (11.4%), Actinobacteria (6.8%), Chloroflexi (5.4%) |
| Xiamen, China [32] | 5.39–9.28 | Proteobacteria, Fusobacteria, Epsilonbacteraeota, Chlorofloxi, Bacteroides |
| Hainan, China [33] | 5.10–5.85 | Proteobacteria, Actinobacteria, Chloroflexi, Acidobacteria, Firmicutes |
| Cananeia Estuary, Brazil [34] | 6.10–6.30 | Proteobacteria (60%), Firmicutes (8.1%), Acidobacteria (6.9%) Actinobacteria (6.2%), |
| Bengal Bay, India and Bangladesh [35] | NA 1 | Proteobacteria, Flexibacteria, Actinobacteria, Acidobacteria, Chloroflexi, |
| Guayaquil Gulf, Brazil [10] | NA | Proteobacteria (33.8%), Firmicutes (25.14%), Bacteroidetes (17.59%), Chloroflexi (5.57%), Planctomycetes (1.63% ) |
| Bhitarkanika, India [28] | NA | Proteobacteria (47.0%), Actinobacteria (9.8%), Bacteroidetes (7.6%), Acidobacteria (4.7%), Firmicutes (2.9%) |
| The study | 6.45–7.81 | Proteobacteria (41.81%), Chloroflexi (11.38%), Planctomycetes (7.47%), Acidobacteria (6.86%), Bacteroidetes (4.41%) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, J.; Zhou, T.; Xu, C.; Shen, D.; Xu, S.; Lin, C. Spatial and Temporal Variation in Microbial Diversity and Community Structure in a Contaminated Mangrove Wetland. Appl. Sci. 2020, 10, 5850. https://doi.org/10.3390/app10175850
Ma J, Zhou T, Xu C, Shen D, Xu S, Lin C. Spatial and Temporal Variation in Microbial Diversity and Community Structure in a Contaminated Mangrove Wetland. Applied Sciences. 2020; 10(17):5850. https://doi.org/10.3390/app10175850
Chicago/Turabian StyleMa, Jiaojiao, Ting Zhou, Chunyu Xu, Dawen Shen, Songjun Xu, and Chuxia Lin. 2020. "Spatial and Temporal Variation in Microbial Diversity and Community Structure in a Contaminated Mangrove Wetland" Applied Sciences 10, no. 17: 5850. https://doi.org/10.3390/app10175850






