An Experimental and Statistical Study on Rebar Corrosion Considering the Temperature Effect Using Gaussian Process Regression
Abstract
1. Introduction
2. Experimental and Statistical Methods
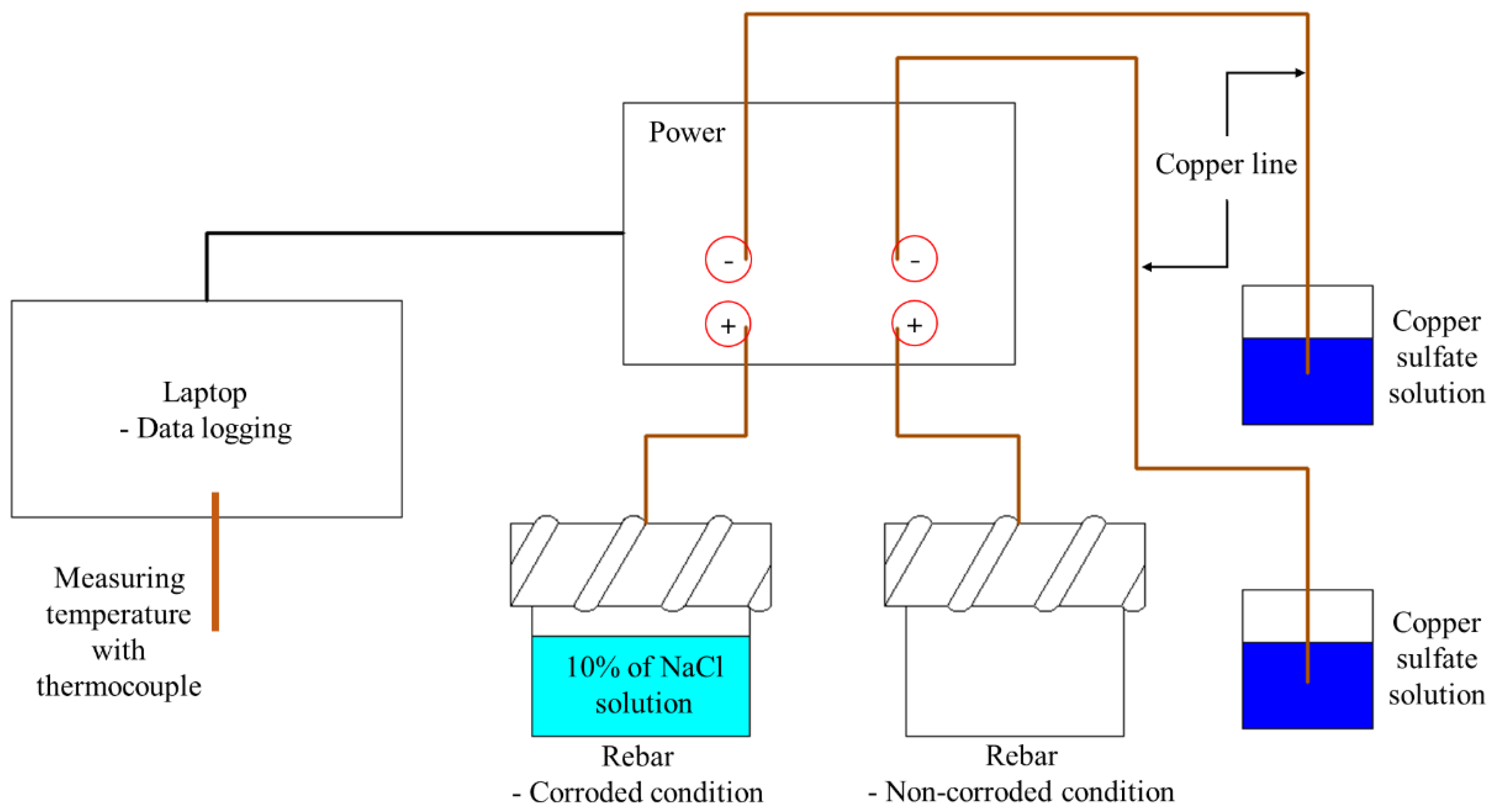
2.1. Preparing the Specimens
2.2. Corrosion Potential
2.3. Gaussian Process Regression (GPR)
2.4. Root Mean Square Error (RMSE)
2.5. Coefficient of Determination (R2)
2.6. Temperature-Potential Index (TPI)
3. Results and Discussion
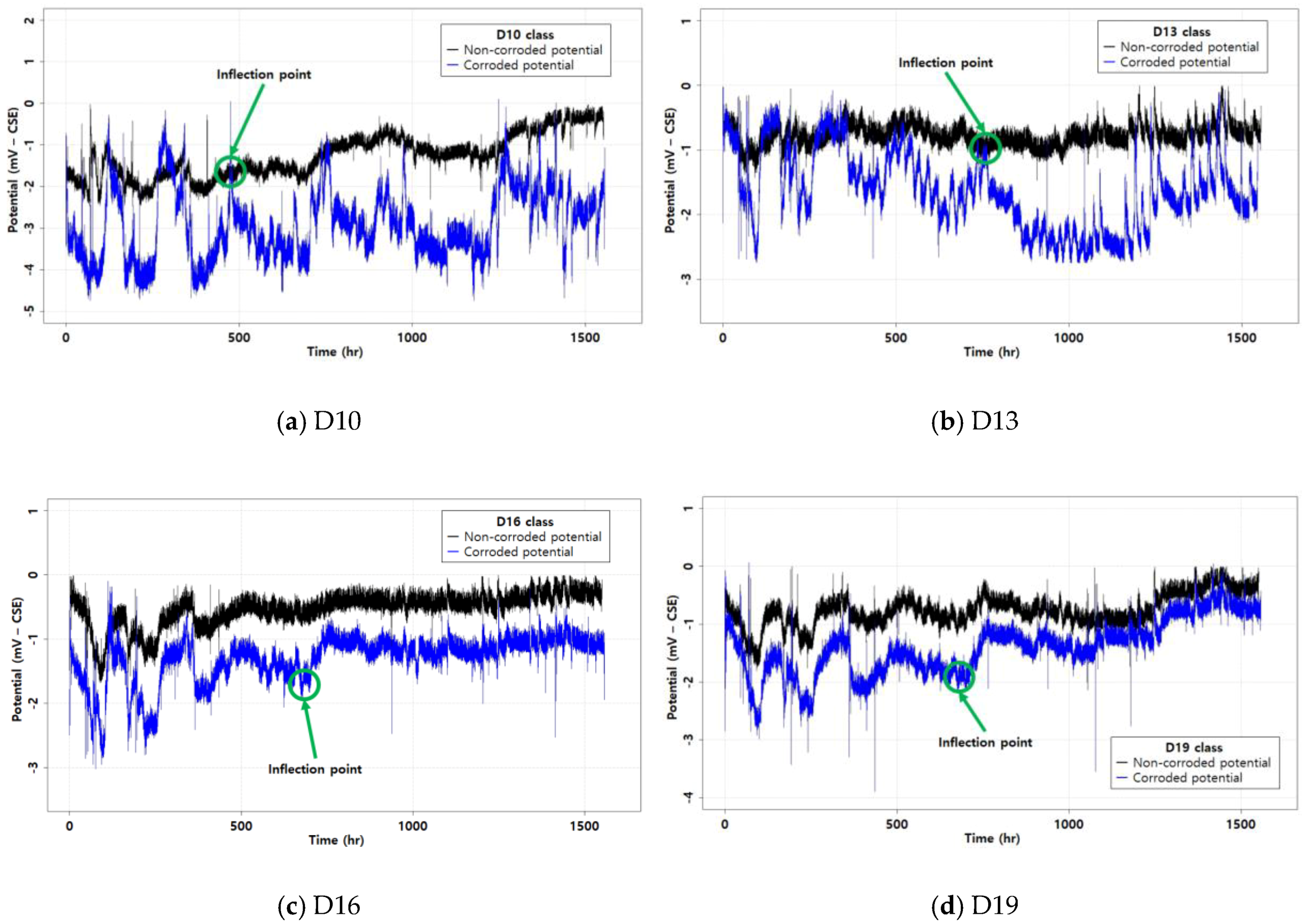
3.1. The Corrosion Potential Result
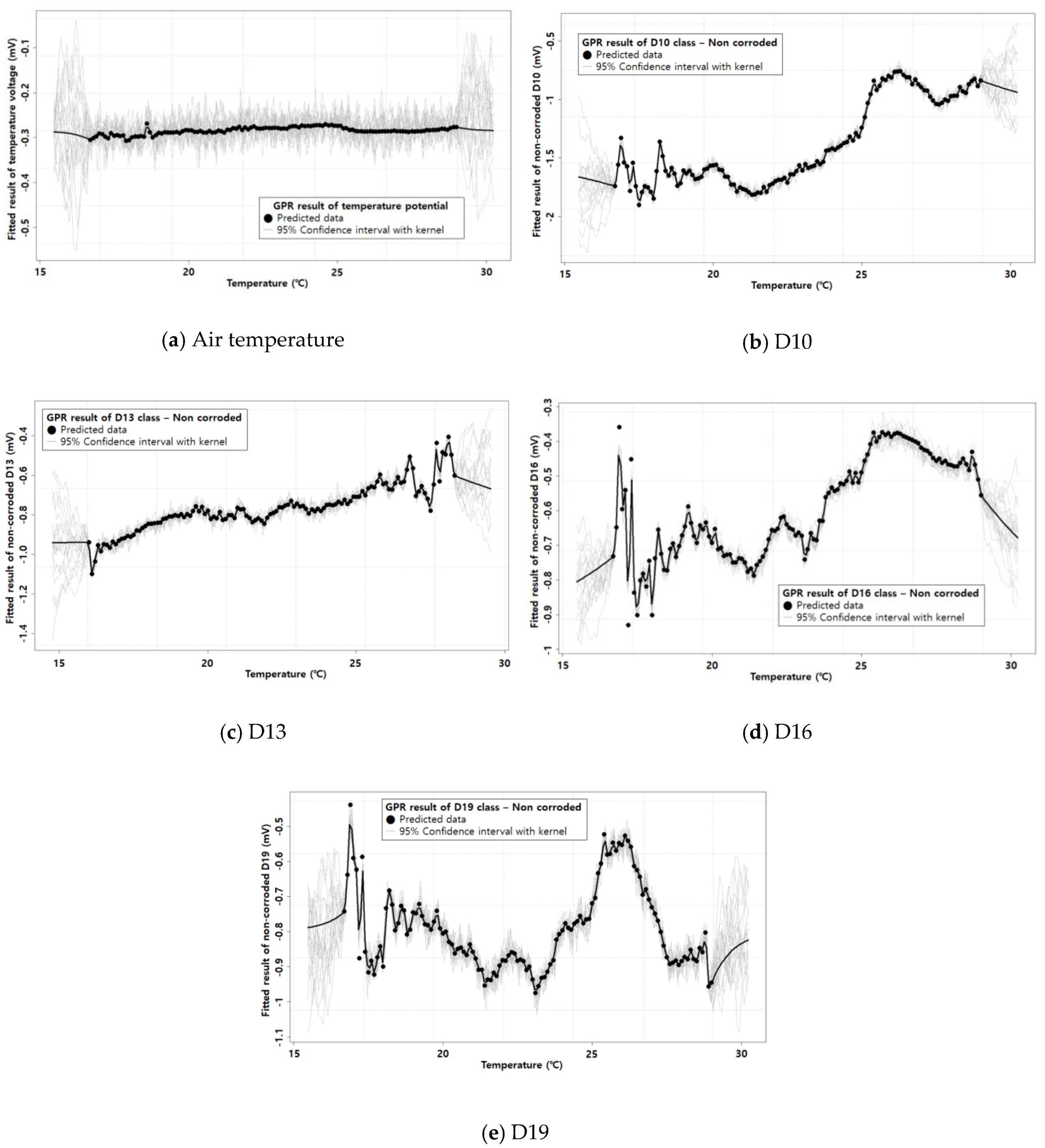
3.2. The GPR Result
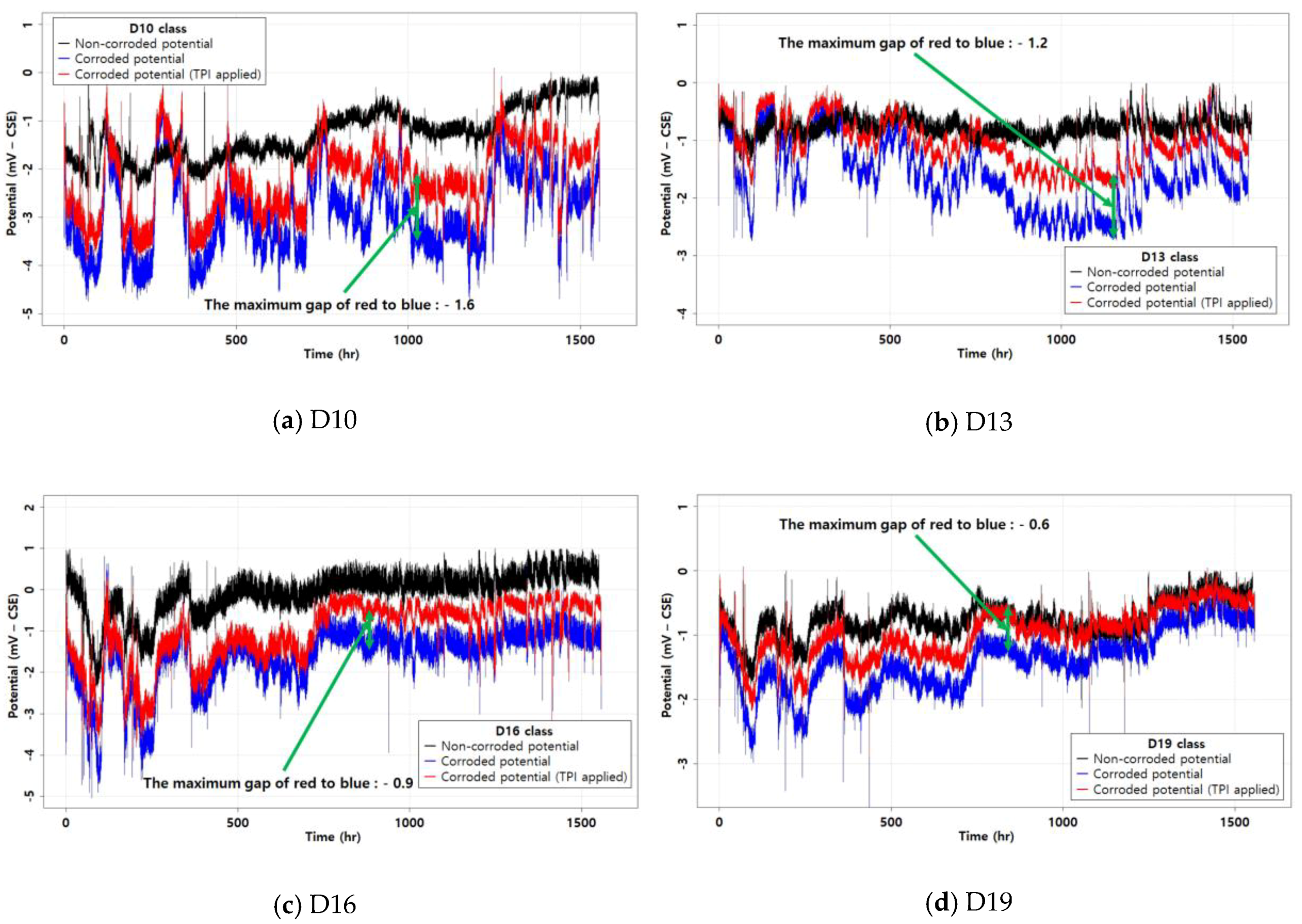
3.3. Removing the Temperature Effect
4. Conclusions
- In the uncontrolled air environment, the corrosion potential was highly sensitive to the environmental conditions. Its fluctuation was evidence of the environmental effects. In particular, because the air temperature was not controlled, the rebars showed that the temperature had an effect on the specimens. In addition, the corrosion potential behavior showed different trends by rebar diameter.
- It was confirmed that temperature is an important factor for measuring the corrosion potential. This evidence was the behavior of corrosion potential with fluctuation. From these results, temperature has to be considered when measuring the corrosion potential.
- The GPR with a kernel performed close to the ML with a high R2 value. This confirmed the usability of GPR in corrosion analysis, especially in removing the temperature effect. The derived GPR model was able to produce a reliable R2 and RMSE. Therefore, the GPR method can be appropriate for corrosion analysis considering the temperature effect.
Author Contributions
Funding
Conflicts of Interest
References
- Faroz, S.A.; Pujari, N.N.; Ghosh, S. Reliability of a corroded RC beam based on Bayesian updating of the corrosion model. Eng. Struct. 2016, 126, 457–468. [Google Scholar] [CrossRef]
- Chen, D.; Mahadevan, S. Chloride-induced reinforcement corrosion and concrete cracking simulation. Cem. Concr. Compos. 2008, 30, 227–238. [Google Scholar] [CrossRef]
- Verma, S.K.; Bhadauria, S.S.; Akhtar, S. Monitoring corrosion of steel bars in reinforced concrete structures. Sci. World J. 2014, 2014, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Tan, X.; Zhang, Q.; Meng, W.; Chen, G.; Bao, Y. Monitoring corrosion of steel bars in reinforced concrete based on helix strains measured from a distributed fiber optic sensor. Eng. Struct. 2020, 204, 110039. [Google Scholar] [CrossRef]
- Goyal, A.; Pouya, H.S.; Ganjian, E.; Olubanwo, A.O.; Khorami, M. Predicting the corrosion rate of steel in cathodically protected concrete using potential shift. Constr. Build. Mater. 2019, 194, 344–349. [Google Scholar] [CrossRef]
- Joh, S.-H.; Lim, Y.-C.; Ismail, M.; Lee, H.-S. Fundamental Study on Developing Embedded Mini-Sensor for Nondestructive Diagnosis Corrosion of Rebar. J. Korea Inst. Struct. Maint. Insp. 2010, 14, 179–187. [Google Scholar]
- Liu, L.; Zheng, D.; Zhou, J.; Yang, J.; Zhang, H. Parameters That Influence Corrosion Detection in Reinforced Concrete Based on Eddy Current Thermography. Adv. Civ. Eng. 2020, 2020, 1–9. [Google Scholar] [CrossRef]
- Wang, W.; Lu, C. Time-varying law of rebar corrosion rate in fly ash concrete. J. Hazard. Mater. 2018, 360, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yu, J.; Li, G.; Zhang, M.; Leung, C.K. Corrosion behavior of steel rebar embedded in hybrid CNTs-OH/polyvinyl alcohol modified concrete under accelerated chloride attack. Cem. Concr. Compos. 2019, 100, 120–129. [Google Scholar] [CrossRef]
- Wasim, M.; Ngo, T.D.; Abid, M. Investigation of long-term corrosion resistance of reinforced concrete structures constructed with various types of concretes in marine and various climate environments. Constr. Build. Mater. 2020, 237, 117701. [Google Scholar] [CrossRef]
- Wu, Z.; Yu, H.; Ma, H.; Zhang, J.; Da, B.; Zhu, H. Rebar corrosion in coral aggregate concrete: Determination of chloride threshold by LPR. Corros. Sci. 2020, 163, 108238. [Google Scholar] [CrossRef]
- Xu, Q.; Ji, T.; Yang, Z.; Ye, Y. Steel rebar corrosion in artificial reef concrete with sulphoaluminate cement, sea water and marine sand. Constr. Build. Mater. 2019, 227, 116685. [Google Scholar] [CrossRef]
- Zou, X.; Schmitt, T.; Perloff, D.; Wu, N.; Yu, T.-Y.; Wang, X. Nondestructive corrosion detection using fiber optic photoacoustic ultrasound generator. Measurement 2015, 62, 74–80. [Google Scholar] [CrossRef]
- Jo, G.J. A Study on the Corrosion and Cathodic Protection Monitoring for the Reinforced Concrete Structures with Multi-Functional Sensor. Master’s Thesis, Korea Maritime & Ocean University, Busan, Korea, 2013. [Google Scholar]
- Du, C.; Owusu Twumasi, J.; Tang, Q.; Guo, X.; Zhou, J.; Yu, T.; Wang, X. All-optical photoacoustic sensors for steel rebar corrosion monitoring. Sensors 2018, 18, 1353. [Google Scholar] [CrossRef] [PubMed]
- Leelalerkiet, V.; Kyung, J.-W.; Ohtsu, M.; Yokota, M. Analysis of half-cell potential measurement for corrosion of reinforced concrete. Constr. Build. Mater. 2004, 18, 155–162. [Google Scholar] [CrossRef]
- Roux, S.; Bur, N.; Ferrari, G.; Tribollet, B.; Feugeas, F. Influence of a biopolymer admixture on corrosion behaviour of steel rebars in concrete. Mater. Corros. 2010, 61, 1026–1033. [Google Scholar] [CrossRef]
- Pour-Ghaz, M.; Isgor, O.B.; Ghods, P. The effect of temperature on the corrosion of steel in concrete. Part 1: Simulated polarization resistance tests and model development. Corros. Sci. 2009, 51, 415–425. [Google Scholar] [CrossRef]
- Deus, J.; Freire, L.; Montemor, M.; Nóvoa, X. The corrosion potential of stainless steel rebars in concrete: Temperature effect. Corros. Sci. 2012, 65, 556–560. [Google Scholar] [CrossRef]
- Chen, L.; Su, R.K.L. Effect of high rebar temperature during casting on corrosion in carbonated concrete. Constr. Build. Mater. 2020, 249, 118718. [Google Scholar] [CrossRef]
- López, W.; González, J.; Andrade, C. Influence of temperature on the service life of rebars. Cem. Concr. Res. 1993, 23, 1130–1140. [Google Scholar] [CrossRef]
- Zhang, S.; Zhou, W.; Qin, H. Inverse Gaussian process-based corrosion growth model for energy pipelines considering the sizing error in inspection data. Corros. Sci. 2013, 73, 309–320. [Google Scholar] [CrossRef]
- Liu, Y.; Song, Y.; Keller, J.; Bond, P.; Jiang, G. Prediction of concrete corrosion in sewers with hybrid Gaussian processes regression model. RSC Adv. 2017, 7, 30894–30903. [Google Scholar] [CrossRef]
- Muthulingam, S. Space-time Gaussian models for evaluating corrosion-induced damages in reinforcing bars. Sādhanā 2019, 44, 13. [Google Scholar] [CrossRef]
- Yodsudjai, W.; Pattarakittam, T. Factors influencing half-cell potential measurement and its relationship with corrosion level. Measurement 2017, 104, 159–168. [Google Scholar] [CrossRef]
- Pérez-Cruz, F.; Van Vaerenbergh, S.; Murillo-Fuentes, J.J.; Lázaro-Gredilla, M.; Santamaria, I. Gaussian processes for nonlinear signal processing: An overview of recent advances. IEEE Signal. Process. Mag. 2013, 30, 40–50. [Google Scholar] [CrossRef]
- Shi, J.; Wang, B.; Murray-Smith, R.; Titterington, D. Gaussian process functional regression modeling for batch data. Biometrics 2007, 63, 714–723. [Google Scholar] [CrossRef]
- Vasudevan, S.; Ramos, F.; Nettleton, E.; Durrant-Whyte, H. Gaussian process modeling of large-scale terrain. J. Field Robot. 2009, 26, 812–840. [Google Scholar] [CrossRef]
- Geng, D.; Zhang, H.; Wu, H. Short-Term Wind Speed Prediction Based on Principal Component Analysis and LSTM. Appl. Sci. 2020, 10, 4416. [Google Scholar] [CrossRef]
- Song, Y.; Lee, H.; Kim, J.; Park, J.; Park, M. Developing Drought Damage Prediction Equation Considering Economic Indicators and Basic Indicators of Asian Countries. J. Korean Soc. Hazard. Mitig. 2019, 19, 451–461. [Google Scholar] [CrossRef]
- Ferrara, L.; Krelani, V.; Carsana, M. A “fracture testing” based approach to assess crack healing of concrete with and without crystalline admixtures. Constr. Build. Mater. 2014, 68, 535–551. [Google Scholar] [CrossRef]
- Oyebisi, S.; Igba, T.; Raheem, A.; Olutoge, F. Predicting the splitting tensile strength of concrete incorporating anacardium occidentale nut shell ash using reactivity index concepts and mix design proportions. Case Stud. Constr. Mater. 2020, 19, e00393. [Google Scholar] [CrossRef]
- Hussain, R.R. Underwater half-cell corrosion potential bench mark measurements of corroding steel in concrete influenced by a variety of material science and environmental engineering variables. Measurement 2011, 44, 274–280. [Google Scholar] [CrossRef]
- Cho, S.-H.; Han, N.; Chung, L. A Study on nondestructive method to measure the corrosion level of reinforcing bar. J. Archit. Inst. Korea 2002, 18, 19–26. [Google Scholar]
- Hackerman, N.; Snavely, E. Corrosion Basics: An Introduction; National Association of Corrosion Engineers: Huston, TX, USA, 1984. [Google Scholar]
- Caines, S.; Khan, F.; Zhang, Y.; Shirokoff, J. Simplified electrochemical potential noise method to predict corrosion and corrosion rate. J. Loss Prev. Process. Ind. 2017, 47, 72–84. [Google Scholar] [CrossRef]
- Masmoudi, R.; Zaidi, A.; Gérard, P. Transverse thermal expansion of FRP bars embedded in concrete. J. Compos. Constr. 2005, 9, 377–387. [Google Scholar] [CrossRef]
- Darab, C.; Turcu, A.; Beleiu, H.G.; Pavel, S.; Birou, I.; Micu, D.D.; Ungureanu, S.; Cirstea, S.D. Hybrid Load Forecasting Using Gaussian Process Regression and Novel Residual Prediction. Appl. Sci. 2020, 10, 4588. [Google Scholar] [CrossRef]
- Qi, A.; Kang, W.; Zhang, G.; Lei, H. Coal seam thickness prediction based on transition probability of structural elements. Appl. Sci. 2019, 9, 1144. [Google Scholar] [CrossRef]


| Fe | C | Si | Mn | Cu | Ni | Cr |
|---|---|---|---|---|---|---|
| 98.22 | 0.31 | 0.29 | 1.09 | 0.02 | 0.04 | 0.03 |
| Case | Coefficients of Determination (R2) | Root Mean Square Error (RMSE) | |
|---|---|---|---|
| Air temperature | −5.385 | 0.99 | 0.00366 |
| D10 | −4.415 | 0.97 | 0.0570 |
| D13 | −5.447 | 0.97 | 0.0657 |
| D16 | −5.048 | 0.95 | 0.0394 |
| D19 | −4.639 | 0.96 | 0.0502 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woo, B.H.; Jeon, I.K.; Kim, S.S.; Lee, J.B.; Ryou, J.-S. An Experimental and Statistical Study on Rebar Corrosion Considering the Temperature Effect Using Gaussian Process Regression. Appl. Sci. 2020, 10, 5937. https://doi.org/10.3390/app10175937
Woo BH, Jeon IK, Kim SS, Lee JB, Ryou J-S. An Experimental and Statistical Study on Rebar Corrosion Considering the Temperature Effect Using Gaussian Process Regression. Applied Sciences. 2020; 10(17):5937. https://doi.org/10.3390/app10175937
Chicago/Turabian StyleWoo, Byeong Hun, In Kyu Jeon, Seong Soo Kim, Jeong Bae Lee, and Jae-Suk Ryou. 2020. "An Experimental and Statistical Study on Rebar Corrosion Considering the Temperature Effect Using Gaussian Process Regression" Applied Sciences 10, no. 17: 5937. https://doi.org/10.3390/app10175937
APA StyleWoo, B. H., Jeon, I. K., Kim, S. S., Lee, J. B., & Ryou, J.-S. (2020). An Experimental and Statistical Study on Rebar Corrosion Considering the Temperature Effect Using Gaussian Process Regression. Applied Sciences, 10(17), 5937. https://doi.org/10.3390/app10175937






