Changes in Electroencephalography by Modulation of Interferential Current Stimulation
Abstract
1. Introduction
2. Methods
2.1. Participants
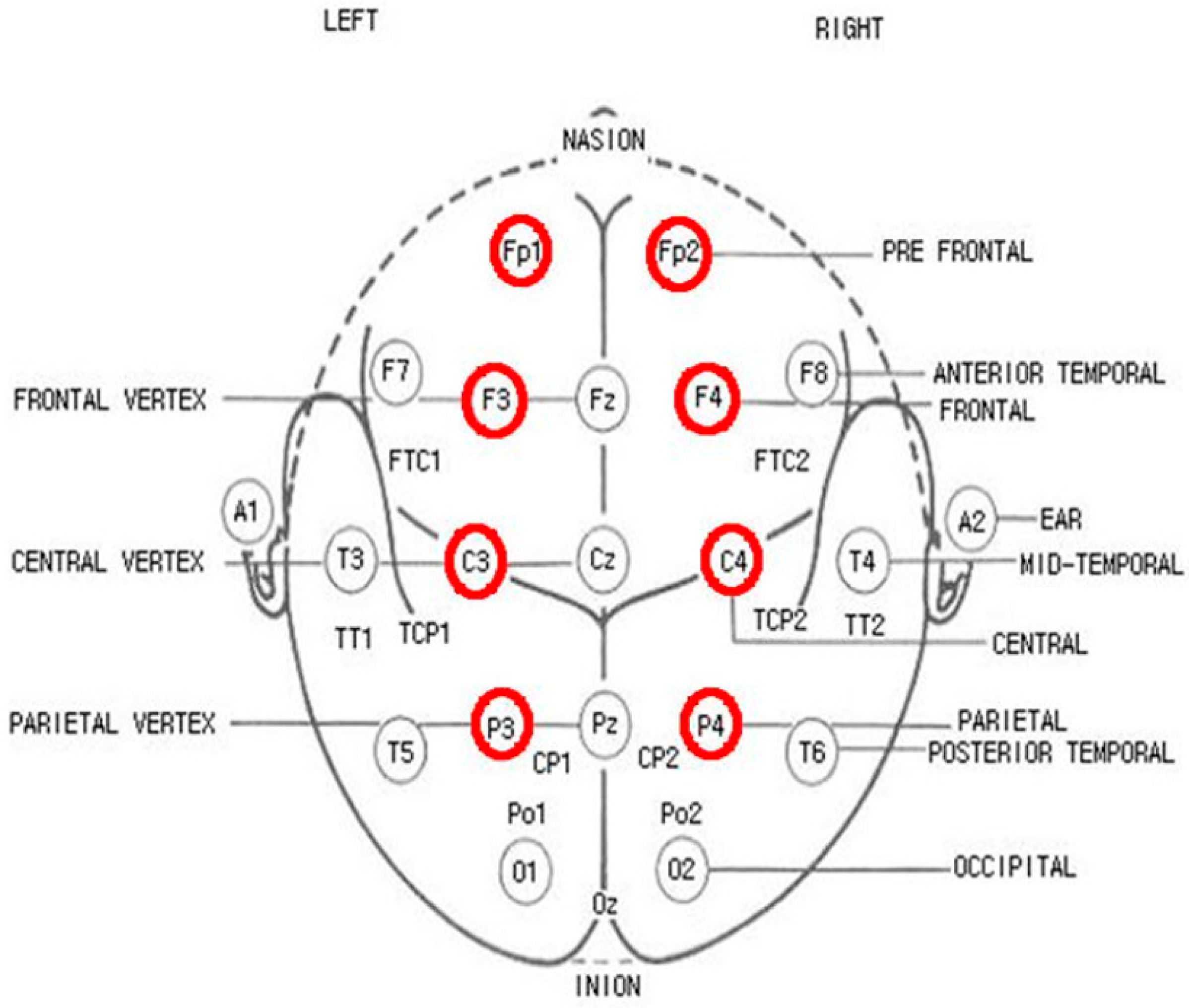
2.2. EEG Recordings
2.3. IFC Application and EEG Measurements
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dhruv, N.T.; Niemi, J.B.; Harry, J.D.; Lipsitz, L.A.; Collins, J.J. Enhancing tactile sensation in older adults with electrical noise stimulation. Neuroreport 2002, 13, 597–600. [Google Scholar] [CrossRef]
- Valeriani, M.; Tonali, P.; Le Pera, D.; Restuccia, D.; De Armas, L.; Del Vesco, C.; Miliucci, R.; Fiaschi, A.; Vigevano, F.; Arendt-Nielsen, L.; et al. Modulation of laser-evoked potentials by experimental cutaneous tonic pain. Neuroscience 2006, 140, 1301–1310. [Google Scholar] [CrossRef] [PubMed]
- Araújo, F.M.; Menezes, M.A.; De Araújo, A.M.; Sousa, T.A.D.S.; Lima, L.V.; Carvalho, E.Á.N.; DeSantana, J.M. Validation of a New Placebo Interferential Current Method: A New Placebo Method of Electrostimulation. Pain Med. 2016, 18, 86–94. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Corrêa, J.B.; Costa, L.O.; Oliveira, N.T.; Lima, W.P.; Sluka, K.A.; Liebano, R.E. Effects of the carrier frequency of interferential current on pain modulation and central hypersensitivity in people with chronic nonspecific low back pain: A randomized placebo-controlled trial. Eur. J. Pain 2016, 20, 1653–1666. [Google Scholar] [CrossRef] [PubMed]
- Kottink, A.I.; Oostendorp, L.J.; Buurke, J.H.; Nene, A.V.; Hermens, H.J.; IJzerman, M.J. The orthotic effect of functional electrical stimulation on the improvement of walking in stroke patients with a dropped foot: A systematic review. Artif. Organs 2004, 28, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, M.J.; Lynch, M.E.; Clark, A.J. Dimensions of catastrophic thinking associated with pain experience and disability in patients with neuropathic pain conditions. Pain 2005, 113, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Sokhadze, T.M.; Cannon, R.L.; Trudeau, D.L. EEG biofeedback as a treatment for substance use disorders: Review, rating of efficacy, and recommendations for further research. Appl. Psychophysiol. Biofeedback 2008, 33, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Nangini, C.; Ross, B.; Tam, F.; Graham, S.J. Magnetoencephalographic study of vibrotactile evoked transient and steady-state responses in human somatosensory cortex. Neuroimage 2006, 33, 252–262. [Google Scholar] [CrossRef]
- Kaelin-Lang, A.; Luft, A.R.; Sawaki, L.; Burstein, A.H.; Sohn, Y.H.; Cohen, L.G. Modulation of human corticomotor excitability by somatosensory input. J. Physiol. 2002, 540, 623–633. [Google Scholar] [CrossRef]
- Wang, L. Brain plasticity: Dynamic changes of the cerebral activity as effect of pain modulation. Ph.D. Thesis, Aalborg University, Aalborg, Denmark, 2007. [Google Scholar]
- Niemiec, A.; Lithgow, B. Alpha-band characteristics in EEG spectrum indicate reliability of frontal brain asymmetry measures in diagnosis of depression. In Proceedings of the IEEE Engineering in Medicine and Biology 27th Annual Conference, Shanghai, China, 17–18 January 2006; pp. 7517–7520. [Google Scholar] [CrossRef]
- Dornhege, G.; Millán, J.R.; Hinterberger, T.; McFarland, D.; Müller, K.R. Toward Brain-Computer Interfacing; MIT Press: Cambridge, MA, USA, 2007; pp. 31–42. [Google Scholar]
- Eslamian, F.; Farhoudi, M.; Jahanjoo, F.; Sadeghi-Hokmabadi, E.; Darabi, P. Electrical interferential current stimulation versus electrical acupuncture in management of hemiplegic shoulder pain and disability following ischemic stroke-a randomized clinical trial. Arch. Physiother. 2020, 10, 2. [Google Scholar] [CrossRef]
- Furuta, T.; Takemura, M.; Tsujita, J.; Oku, Y. Interferential electric stimulation applied to the neck increases swallowing frequency. Dysphagia 2012, 27, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.J.; Stinstra, G.; Hendriks, M. Estimation of the electrical conductivity of human tissue. Electromagnetics 2001, 21, 545–557. [Google Scholar] [CrossRef]
- Ebrahimian, M.; Razeghi, M.; Zamani, A.; Bagheri, Z.; Rastegar, K.; Motealleh, A. Does high frequency transcutaneous electrical nerve stimulation (TENS) affect EEG gamma band activity? J. Biomed. Phys. Eng. 2018, 8, 271–280. [Google Scholar] [CrossRef]
- Yıldırım, E.; Güntekin, B.; Hanoğlu, L.; Algun, C. EEG alpha activity increased in response to transcutaneous electrical nervous stimulation in young healthy subjects but not in the healthy elderly. PeerJ 2020, 8, e8330. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef]
- Niedermeyer, E.; da Silva, F.H.L. Electroencephalography: Basic Principles, Clinical Applications, and Related Fields, 5th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2005. [Google Scholar]
- Hirsch, L.J.; Brenner, R.P. Atlas of EEG in Critical Care; John Wiley and Sons: Hoboken, NJ, USA, 2010; Volume 30, pp. 187–216. [Google Scholar]
- Nunez, P.L.; Srinivasan, R. Electric Fields of the Brain: The Meurophysics of EEG, 2nd ed.; Oxford University Press: New York, NY, USA, 2005; pp. 154–169. [Google Scholar]
- Wang, H.; Lei, X.; Zhan, Z.; Yao, L.; Wu, X. A new fMRI informed mixed-norm constrained algorithm for EEG source localization. IEEE Access 2018, 6, 8258–8269. [Google Scholar] [CrossRef]
- Babiloni, C.; Ferri, R.; Moretti, D.V.; Strambi, A.; Binetti, G.; Dal Forno, G.; Ferreri, F.; Lanuzza, B.; Bonato, C.; Nobili, F.; et al. Abnormal fronto-parietal coupling of brain rhythms in mild Alzheimer’s disease: A multicentric EEG study. Eur. J. Neurosci. 2004, 19, 2583–2590. [Google Scholar] [CrossRef]
- Murugappan, M.; Ramachandran, N.; Sazali, Y. Combining spatial filtering and wavelet transform for classifying human emotions using EEG signals. J. Med. Biol. Eng. 2010, 31, 45–51. [Google Scholar] [CrossRef]
- Fisch, B. Fisch and Spehlmann’s EEG Primer: Basic Principles of Digital and Analog EEG, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Selkowitz, D.M. Improvement in isometric strength of the quadriceps femoris muscle after training with electrical stimulation. Phys. Ther. 1985, 65, 186–196. [Google Scholar] [CrossRef]
- Schoffelen, J.M.; Oostenveld, R.; Fries, P. Neuronal coherence as a mechanism of effective corticospinal interaction. Science 2005, 308, 111–113. [Google Scholar] [CrossRef] [PubMed]
- Sanei, S.; Chambers, J.A. EEG Signal Processing, 1st ed.; John Wiley & Sons: Hoboken, NJ, USA, 2007; pp. 10–13. [Google Scholar]
- Jin, H.K.; Cho, S.H. Comparison of relative α-power spectral electroencephalogram activity analysis according to electrical stimulation levels in normal adults. J. Psychiatry 2015, 18, 5. [Google Scholar] [CrossRef]
- Jiang, X.; Bian, G.B.; Tian, Z. Removal of artifacts from EEG signals: A review. Sensors 2019, 19, 987. [Google Scholar] [CrossRef] [PubMed]
- Grabiańska, E.; Leśniewicz, J.; Pieszyński, I.; Kostka, J. Comparison of the analgesic effect of interferential current (IFC) and TENS in patients with low back pain. Wiad. Lek. 2015, 68, 13–19. (In Polish) [Google Scholar] [PubMed]
- Cincotti, F.; Kauhanen, L.; Aloise, F.; Palomäki, T.; Caporusso, N.; Jylänki, P.; Mattia, D.; Babiloni, F.; Vanacker, G.; Nuttin, M.; et al. Vibrotactile feedback for brain-computer interface operation. Comput. Intell. Neurosci. 2007, 2007, 48937. [Google Scholar] [CrossRef][Green Version]
- Cho, S.H. Frequency and intensity of electrical stimulation of human sympathetic ganglia affect heart rate variability and pain threshold. Appl. Sci. 2019, 9, 4490. [Google Scholar] [CrossRef]
- Ozcan, J.; Ward, A.R.; Robertson, V.J. A comparison of true and premodulated interferential currents. Arch. Phys. Med. Rehabil. 2004, 85, 409–415. [Google Scholar] [CrossRef]
- Norman, G.R.; Streiner, D.L. Biostatistics: The Bare Essentials, 2nd ed.; B.C. Decker Inc.: London, UK, 2000. [Google Scholar]
- Bakeman, R. Recommended effect size statistics for repeated measures designs. Behav. Res. Methods 2005, 37, 379–384. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Erlbaum: Hillsdale, NJ, USA, 1988; pp. 413–414. [Google Scholar]
- Field, A. Discovering Statistics Using IBM SPSS Statistics, 4th ed.; Sage Publications Ltd.: London, UK, 2013; p. 267. [Google Scholar]
- Robertson, V.; Ward, A.; Low, J.; Reed, A. Electrotherapy Explained: Principles and Practice, 4th ed.; Elsevier Butterworth-Heinemann: Philadelphia, PA, USA, 2006. [Google Scholar]
- Ge, H.Y.; Fernández-de-las-Peñas, C.; Arendt-Nielsen, L. Sympathetic facilitation of hyperalgesia evoked from myofascial tender and trigger points in patients with unilateral shoulder pain. Clin. Neurophysiol. 2006, 117, 1545–1550. [Google Scholar] [CrossRef]
- Watson, N.F.; Buchwald, D.; Goldberg, J.; Noonan, C.; Ellenbogen, R.G. Neurologic signs and symptoms in fibromyalgia. Arthritis Rheum. 2009, 60, 2839–2844. [Google Scholar] [CrossRef]
- Sadock, B.J.; Sadock, V.A. Kaplan and Sadock’s Synopsis of Psychiatry: Behavioral Sciences/Clinical Psychiatry, 10th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2007. [Google Scholar]
- Walker, J.E. Recent advances in quantitative EEG as an aid to diagnosis and as a guide to neurofeedback training for cortical hypofunctions, hyperfunctions, disconnections, and hyperconnections: Improving efficacy in complicated neurological and psychological disorders. Appl. Psychophysiol. Biofeedback 2010, 35, 25–27. [Google Scholar] [CrossRef] [PubMed]
- Ridding, M.C.; McKay, D.R.; Thompson, P.D.; Miles, T.S. Changes in corticomotor representations induced by prolonged peripheral nerve stimulation in humans. Clin. Neurophysiol. 2001, 112, 1461–1469. [Google Scholar] [CrossRef]
- Thornton, K.E.; Carmody, D.P. Electroencephalogram biofeedback for reading disability and traumatic brain injury. Child Adolesc. Psychiatr. Clin. N. Am. 2005, 14, 137–162. [Google Scholar] [CrossRef] [PubMed]
- Gazzaniga, M.S.; Ivry, R.B.; Mangun, G.R. Cognitive Neuroscience—The Biology of the Mind, 2nd ed.; Norton & Company. Ltd.: New York, NY, USA, 2002; pp. 301–350. [Google Scholar]
- Klimesch, W. Memory processes described as brain oscillations in the EEG-alpha and theta bands. Psycoloquy 1995, 11, 134–143. [Google Scholar]
- Klimesch, W. EEG alpha and theta oscillations reflect cognitive and memory performance: A review and analysis. Brain Res. Brain Res. Rev. 1999, 29, 169–195. [Google Scholar] [CrossRef]
- Vernon, D.; Egner, T.; Cooper, N.; Compton, T.; Neilands, C.; Sheri, A.; Gruzelier, J. The effect of training distinct neurofeedback protocols on aspects of cognitive performance. Int. J. Psychophysiol. 2003, 47, 75–85. [Google Scholar] [CrossRef]
- Seminowicz, D.A.; Davis, K.D. Cortical responses to pain in healthy individuals depends on pain catastrophizing. Pain 2006, 120, 297–306. [Google Scholar] [CrossRef]
- Cheing, G.L.; Chan, W.W. Influence of choice of electrical stimulation site on peripheral neurophysiological and hypoalgesic effects. J. Rehabil. Med. 2009, 41, 412–417. [Google Scholar] [CrossRef]
- Bolanowski, S.J.; Verrillo, R.T.; McGlone, F. Passive, active and intra-active (self) touch. Somatosens. Mot. Res. 1999, 16, 304–311. [Google Scholar] [CrossRef]

| Characteristics | HF–LI (N = 15) | LF–HI (N = 15) | HF–HI (N = 15) | F | P |
|---|---|---|---|---|---|
| Sex (male/female) | 7/8 | 8/7 | 8/7 | ||
| Age (years) | 22.64 ± 1.41 | 22.45 ± 1.44 | 22.20 ± 1.63 | 0.527 | 0.566 |
| Height (cm) | 170.27 ± 7.46 | 167.09 ± 10.50 | 166.64 ± 11.08 | 0.615 | 0.753 |
| Weight (kg) | 63.55 ± 4.55 | 61.82 ± 4.08 | 64.09 ± 6.93 | 0.212 | 0.210 |
| Variable | Group | Pre-Test | Post-Test | Post-30 | F (P) | Group × Time η2 | ||
|---|---|---|---|---|---|---|---|---|
| Group | Time | Group × Time | ||||||
| Fp1 | HF–LI | 0.17 ± 0.07 | 0.19 ± 0.05 | 0.18 ± 0.09 | 1.834 (0.178) | 0.521 (0.476) | 0.114 (0.892) | 0.008 |
| LF–HI | 0.18 ± 0.08 | 0.18 ± 0.07 | 0.19 ± 0.08 | |||||
| HF–HI | 0.23 ± 0.10 | 0.25 ± 0.08 | 0.25 ± 0.08 | |||||
| Fp2 | HF–LI | 0.16 ± 0.06 | 0.18 ± 0.04 | 0.17 ± 0.07 | 2.098 (0.141) | 0.894 (0.352) | 0.010 (0.990) | 0.001 |
| LF–HI | 0.18 ± 0.07 | 0.18 ± 0.07 | 0.18 ± 0.08 | |||||
| HF–HI | 0.21 ± 0.11 | 0.24 ± 0.08 | 0.24 ± 0.10 | |||||
| F3 | HF–LI | 0.18 ± 0.05 | 0.19 ± 0.06 | 0.17 ± 0.05 | 2.279 (0.120) | 0.054 (0.818) | 0.307 (0.738) | 0.021 |
| LF–HI | 0.18 ± 0.07 | 0.18 ± 0.07 | 0.18 ± 0.07 | |||||
| HF–HI | 0.24 ± 0.10 | 0.26 ± 0.09 | 0.25 ± 0.10 | |||||
| F4 | HF–LI | 0.18 ± 0.05 | 0.18 ± 0.05 | 0.17 ± 0.06 | 1.882 (0.170) | 0.342 (0.563) | 0.317 (0.731) | 0.021 |
| LF–HI | 0.19 ± 0.08 | 0.18 ± 0.07 | 0.18 ± 0.08 | |||||
| HF–HI | 0.25 ± 0.10 | 0.26 ± 0.10 | 0.25 ± 0.11 | |||||
| C3 | HF–LI | 0.17 ± 0.04 | 0.17 ± 0.05 | 0.17 ± 0.05 | 3.507 (0.043 *) | 0.537 (0.470) | 0.004 (0.996) | 0.000 |
| LF–HI | 0.17 ± 0.07 | 0.17 ± 0.06 | 0.17 ± 0.07 | |||||
| HF–HI | 0.23 ± 0.09 | 0.26 ± 0.08 | 0.25 ± 0.10 | |||||
| C4 | HF–LI | 0.17 ± 0.05 | 0.16 ± 0.06 | 0.15 ± 0.05 | 3.275 (0.052) | 0.206 (0.653) | 0.334 (0.718) | 0.023 |
| LF–HI | 0.17 ± 0.08 | 0.17 ± 0.06 | 0.17 ± 0.07 | |||||
| HF–HI | 0.24 ± 0.09 | 0.26 ± 0.09 | 0.25 ± 0.10 | |||||
| P3 | HF–LI | 0.16 ± 0.05 | 0.16 ± 0.05 | 0.16 ± 0.06 | 4.379 (0.022) | 0.712 (0.406) | 0.578 (0.567) | 0.038 |
| LF–HI | 0.17 ± 0.08 | 0.15 ± 0.05 | 0.16 ± 0.08 | |||||
| HF–HI | 0.20 ± 0.10 | 0.25 ± 0.08 | 0.23 ± 0.10 | |||||
| P4 | HF–LI | 0.17 ± 0.05 | 0.17 ± 0.07 | 0.16 ± 0.06 | 3.453 (0.045 *) | 0.173 (0.680) | 0.079 (0.924) | 0.005 |
| LF–HI | 0.16 ± 0.08 | 0.16 ± 0.06 | 0.16 ± 0.08 | |||||
| HF–HI | 0.22 ± 0.09 | 0.26 ± 0.09 | 0.25 ± 0.11 | |||||
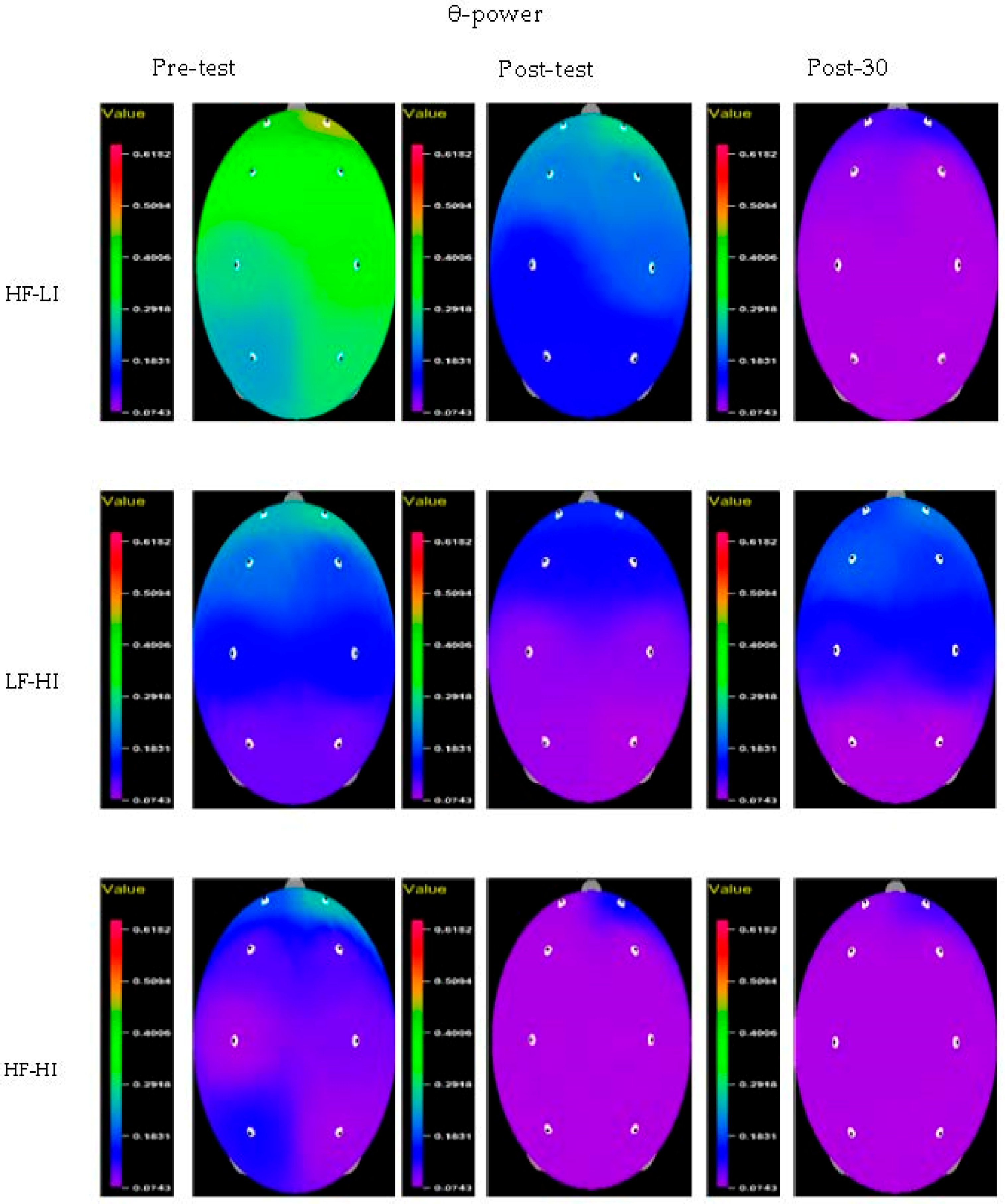
| Variable | Group | Pre-Test | Post-Test | Post-30 | F (P) | Group × Time η2 | ||
|---|---|---|---|---|---|---|---|---|
| Group | Time | Group × Time | ||||||
| Fp1 | HF–LI | 0.11 ± 0.12 | 0.09 ± 0.06 | 0.08 ± 0.07 | 1.916 (0.165) | 0.292 (0.593) | 0.453 (0.640) | 0.030 |
| LF–HI | 0.09 ± 0.10 | 0.07 ± 0.02 | 0.08 ± 0.04 | |||||
| HF–HI | 0.12 ± 0.08 | 0.12 ± 0.05 | 0.10 ± 0.05 | |||||
| Fp2 | HF–LI | 0.09 ± 0.10 | 0.07 ± 0.04 | 0.06 ± 0.04 | 1.528 (0.234) | 0.456 (0.505) | 0.145 (0.866) | 0.010 |
| LF–HI | 0.08 ± 0.09 | 0.07 ± 0.03 | 0.06 ± 0.04 | |||||
| HF–HI | 0.08 ± 0.05 | 0.09 ± 0.04 | 0.08 ± 0.03 | |||||
| F3 | HF–LI | 0.08 ± 0.08 | 0.07 ± 0.05 | 0.05 ± 0.04 | 0.708 (0.501) | 0.000 (0.989) | 0.139 (0.871) | 0.009 |
| LF–HI | 0.07 ± 0.07 | 0.05 ± 0.04 | 0.05 ± 0.03 | |||||
| HF–HI | 0.10 ± 0.09 | 0.08 ± 0.04 | 0.07 ± 0.05 | |||||
| F4 | HF–LI | 0.09 ± 0.09 | 0.08 ± 0.05 | 0.07 ± 0.06 | 0.485 (0.621) | 0.046 (0.832) | 0.766 (0.474) | 0.050 |
| LF–HI | 0.07 ± 0.07 | 0.06 ± 0.03 | 0.07 ± 0.06 | |||||
| HF–HI | 0.11 ± 0.09 | 0.09 ± 0.05 | 0.09 ± 0.05 | |||||
| C3 | HF–LI | 0.09 ± 0.08 | 0.10 ± 0.11 | 0.05 ± 0.03 | 1.298 (0.288) | 0.025 (0.876) | 1.698 (0.201) | 0.105 |
| LF–HI | 0.06 ± 0.07 | 0.05 ± 0.02 | 0.06 ± 0.03 | |||||
| HF–HI | 0.10 ± 0.10 | 0.09 ± 0.04 | 0.09 ± 0.05 | |||||
| C4 | HF–LI | 0.08 ± 0.07 | 0.06 ± 0.05 | 0.04 ± 0.02 | 2.686 (0.085) | 0.164 (0.689) | 0.633 (0.538) | 0.042 |
| LF–HI | 0.06 ± 0.05 | 0.05 ± 0.02 | 0.05 ± 0.04 | |||||
| HF–HI | 0.10 ± 0.08 | 0.08 ± 0.05 | 0.08 ± 0.04 | |||||
| P3 | HF–LI | 0.07 ± 0.06 | 0.06 ± 0.05 | 0.04 ± 0.03 | 3.670 (0.038 *) | 0.027 (0.870) | 1.172 (0.324) | 0.075 |
| LF–HI | 0.06 ± 0.06 | 0.03 ± 0.02 | 0.05 ± 0.04 | |||||
| HF–HI | 0.08 ± 0.08 | 0.07 ± 0.03 | 0.07 ± 0.03 | |||||
| P4 | HF–LI | 0.07 ± 0.06 | 0.08 ± 0.10 | 0.04 ± 0.03 | 1.130 (0.337) | 0.301 (0.588) | 1.065 (0.358) | 0.068 |
| LF–HI | 0.05 ± 0.05 | 0.03 ± 0.02 | 0.05 ± 0.04 | |||||
| HF–HI | 0.07 ± 0.06 | 0.07 ± 0.03 | 0.06 ± 0.03 | |||||
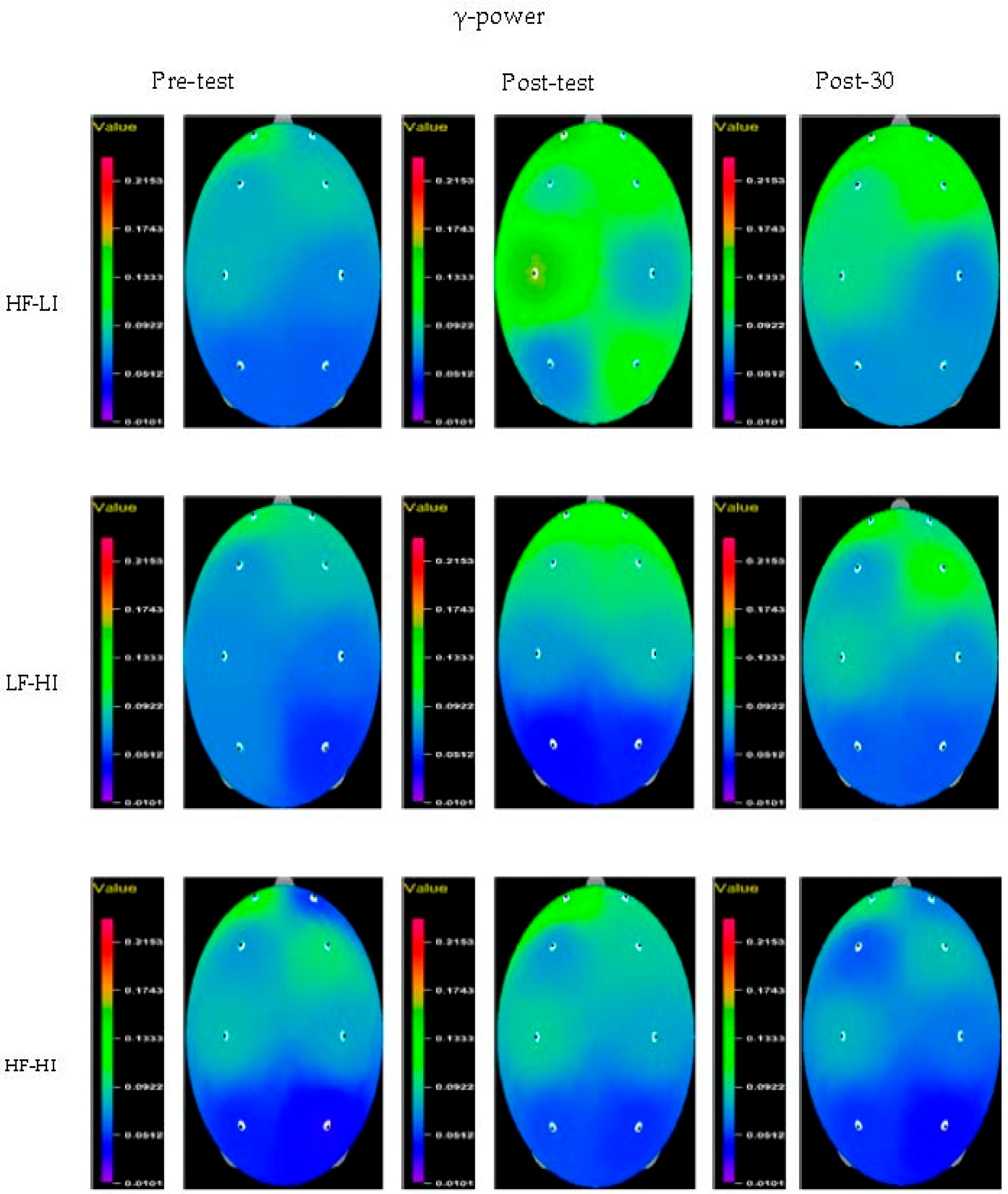
| Variable | Group | Pre-Test | Post-Test | Post-30 | F (P) | Group × Time η2 | ||
|---|---|---|---|---|---|---|---|---|
| Group | Time | Group × Time | ||||||
| Fp1 | HF–LI | 0.40 ± 0.24 | 0.39 ± 0.15 | 0.33 ± 0.11 | 0.835 (0.444) | 0.118 (0.734) | 2.212 (0.128) | 0.132 |
| LF–HI | 0.40 ± 0.21 | 0.32 ± 0.07 | 0.36 ± 0.06 | |||||
| HF–HI | 0.33 ± 0.15 | 0.31 ± 0.08 | 0.31 ± 0.08 | |||||
| Fp2 | HF–LI | 0.41 ± 0.23 | 0.41 ± 0.16 | 0.34 ± 0.12 | 0.628 (0.541) | 0.508 (0.482) | 3.351 (0.049 *) | 0.188 |
| LF–HI | 0.41 ± 0.21 | 0.32 ± 0.07 | 0.37 ± 0.08 | |||||
| HF–HI | 0.39 ± 0.19 | 0.34 ± 0.11 | 0.33 ± 0.09 | |||||
| F3 | HF–LI | 0.35 ± 0.19 | 0.38 ± 0.17 | 0.31 ± 0.10 | 0.232 (0.794) | 0.594 (0.447) | 3.435 (0.046 *) | 0.192 |
| LF–HI | 0.37 ± 0.21 | 0.30 ± 0.09 | 0.37 ± 0.09 | |||||
| HF–HI | 0.28 ± 0.15 | 0.30 ± 0.09 | 0.30 ± 0.10 | |||||
| F4 | HF–LI | 0.35 ± 0.19 | 0.38 ± 0.16 | 0.30 ± 0.12 | 0.374 (0.691) | 0.023 (0.880) | 3.803 (0.034 *) | 0.208 |
| LF–HI | 0.36 ± 0.21 | 0.30 ± 0.10 | 0.36 ± 0.11 | |||||
| HF–HI | 0.28 ± 0.14 | 0.28 ± 0.10 | 0.29 ± 0.09 | |||||
| C3 | HF–LI | 0.32 ± 0.20 | 0.34 ± 0.19 | 0.27 ± 0.09 | 0.035 (0.965) | 1.111 (0.301) | 3.872 (0.032 *) | 0.211 |
| LF–HI | 0.34 ± 0.22 | 0.27 ± 0.10 | 0.33 ± 0.11 | |||||
| HF–HI | 0.26 ± 0.15 | 0.28 ± 0.08 | 0.28 ± 0.09 | |||||
| C4 | HF–LI | 0.33 ± 0.20 | 0.37 ± 0.18 | 0.30 ± 0.11 | 0.371 (0.693) | 0.939 (0.341) | 4.851 (0.015 *) | 0.251 |
| LF–HI | 0.35 ± 0.23 | 0.27 ± 0.11 | 0.34 ± 0.12 | |||||
| HF–HI | 0.27 ± 0.15 | 0.27 ± 0.09 | 0.29 ± 0.09 | |||||
| P3 | HF–LI | 0.30 ± 0.21 | 0.34 ± 0.18 | 0.25 ± 0.10 | 0.150 (0.861) | 0.000 (0.994) | 2.863 (0.073) | 0.165 |
| LF–HI | 0.32 ± 0.24 | 0.27 ± 0.15 | 0.30 ± 0.11 | |||||
| HF–HI | 0.30 ± 0.17 | 0.26 ± 0.08 | 0.28 ± 0.08 | |||||
| P4 | HF–LI | 0.32 ± 0.20 | 0.34 ± 0.19 | 0.25 ± 0.10 | 0.199 (0.820) | 0.590 (0.449) | 4.301 (0.023 *) | 0.229 |
| LF–HI | 0.32 ± 0.24 | 0.24 ± 0.11 | 0.30 ± 0.12 | |||||
| HF–HI | 0.26 ± 0.15 | 0.26 ± 0.08 | 0.28 ± 0.08 | |||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, S.-H.; Kim, S.-C. Changes in Electroencephalography by Modulation of Interferential Current Stimulation. Appl. Sci. 2020, 10, 6028. https://doi.org/10.3390/app10176028
Cho S-H, Kim S-C. Changes in Electroencephalography by Modulation of Interferential Current Stimulation. Applied Sciences. 2020; 10(17):6028. https://doi.org/10.3390/app10176028
Chicago/Turabian StyleCho, Sung-Hyoun, and Seon-Chil Kim. 2020. "Changes in Electroencephalography by Modulation of Interferential Current Stimulation" Applied Sciences 10, no. 17: 6028. https://doi.org/10.3390/app10176028
APA StyleCho, S.-H., & Kim, S.-C. (2020). Changes in Electroencephalography by Modulation of Interferential Current Stimulation. Applied Sciences, 10(17), 6028. https://doi.org/10.3390/app10176028






