Surface Disinfection to Protect against Microorganisms: Overview of Traditional Methods and Issues of Emergent Nanotechnologies
Abstract
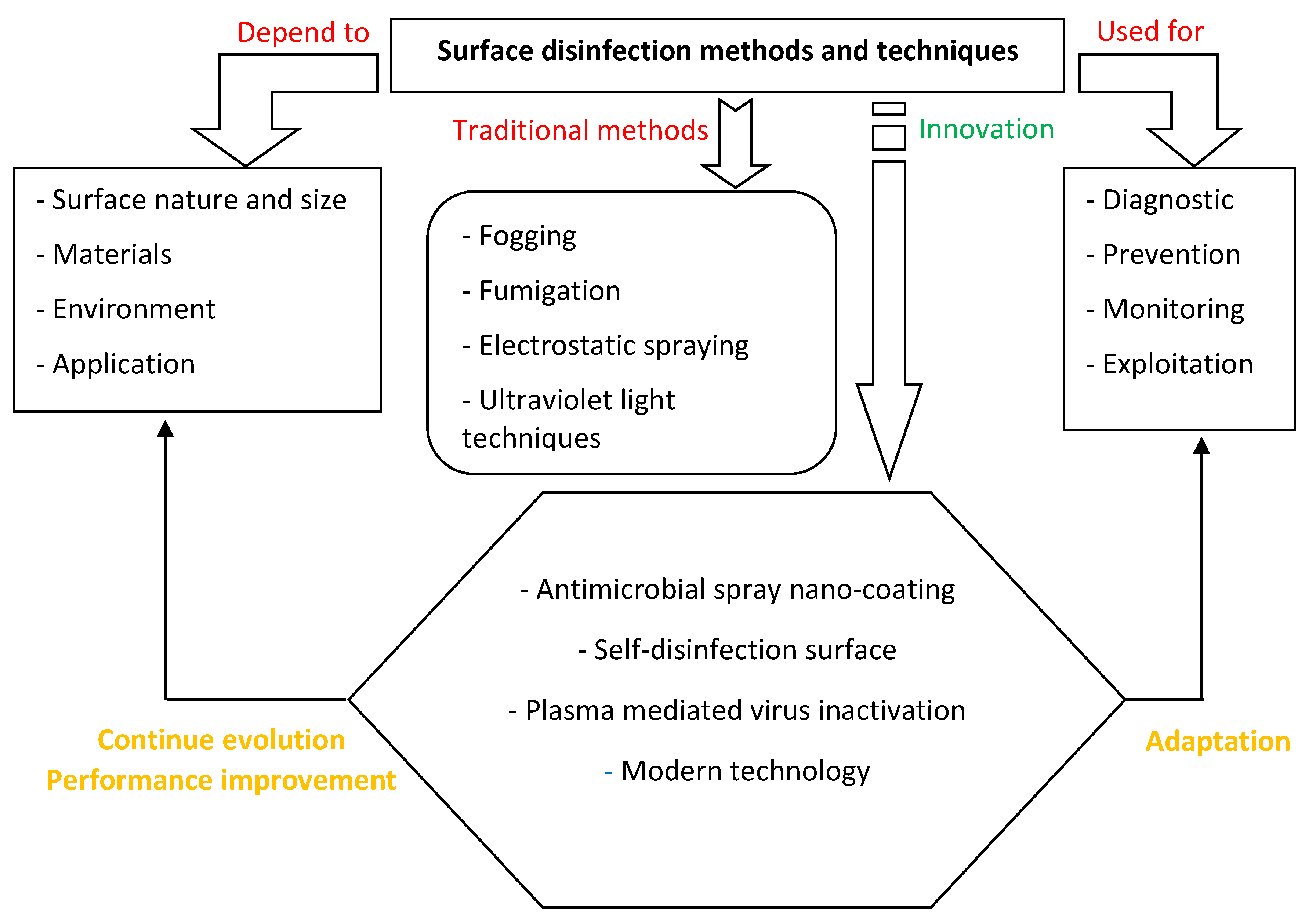
:1. Introduction
2. Traditional Methods of Surface Decontamination: Influence of Surface Size and Type
2.1. Fogging Method
2.2. Fumigation
2.3. Wide-Area or Electrostatic Spraying Techniques
2.4. Ultraviolet Light
3. Reproach and Complaint of EPA (Environmental Protection Agency)
4. Innovation in Surface Disinfection Method
4.1. Antimicrobial Spray Nanocoating
4.1.1. Principle
4.1.2. Possible Exploitation of Nanocoating to Protect against COVID-19
4.2. Self-Disinfection Surface
4.3. Plasma-Mediated Virus Inactivation
4.4. Modern Technology for Healthcare Environment and Surface
5. New Framework for Prevention, Diagnostic, and Monitoring of Surface Disinfection
6. Conclusions
- -
- Virus or bacteria disinfection is widely influenced by the type, size, and properties of surfaces which are considered as the major factor contributing to the dissipation of an epidemic.
- -
- The choice of the disinfection technique is based on Multiphysics rules. Traditional techniques such as fogging, Fumigation, wide-area or electrostatic spraying, and ultraviolet light techniques are still used to conserve equipment and surface from viruses. However, many approaches specified by the Environmental Protection Agency narrow their evolution despite the progress of sterilized solution and light technology.
- -
- An antimicrobial spray nanocoating was introduced as an emergent technology to produce efficient and inhalable nanopowder pulverization for healthy surfaces and presented as possible innovative methods to protect against COVID-19.
- -
- Relatively, self-disinfection surfaces were recently developed using chemical and physical modifications of the surface to kill or eject microorganisms. As there are several points to be more standard and well-controlled, this technique is considered a step forward to the future of the controlling protocol of disinfection.
- -
- Plasma-mediated virus inactivation was in higher microorganism inactivation potential. Given the fact that this technology is clean, effective, and human friendly, it can become a promising solution when related to modern technology.
- -
- A new framework for prevention, diagnostic, and monitoring of surface disinfection was proposed.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A novelcoronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Cyranoski, D. Mystery deepens over animal source of coronavirus. Nature 2020, 579, 18–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, T.; Wu, Q.; Zhang, Z. Probable Pangolin Origin of SARS-CoV-2 Associated with the COVID-19 Outbreak. Curr. Biol. 2020, 30, 1346–1351.e2. [Google Scholar] [CrossRef] [PubMed]
- Querido, M.M.; Aguiar, L.; Neves, P.; Pereira, C.C.; Teixeira, J.P. Self-disinfecting surfaces and infection control. Colloids Surf. B Biointerfaces 2019, 178, 8–21. [Google Scholar] [CrossRef]
- Ahmadabadi, H.Y.; Yu, K.; Kizhakkedathu, J.N. Surface modification approaches for prevention of implant associated infections. Colloids Surf. B Bio-Interfaces 2020, 193, 111116. [Google Scholar] [CrossRef]
- Wang, B.; Zeng, J.; Guo, Y.; Liang, L.; Jin, Y.; Qian, S.; Miao, R.; Hu, L.; Lu, F. Reversible grafting of antibiotics onto contact lens mediated by labile chemical bonds for smart prevention and treatment of corneal bacterial infections. J. Mater. Sci. Technol. 2020, in press. [Google Scholar] [CrossRef]
- Zhou, L.; Xu, Z.; Castiglione, G.M.; Soiberman, U.S.; Eberhart, C.G.; Duh, E.J. ACE2 and TMPRSS2 are expressed on the human ocular surface, suggesting susceptibility to SARS-CoV-2 infection. Ocul. Surf. 2020, 18, 537–544. [Google Scholar] [CrossRef]
- Stein, H.; Schulz, J.; Kemper, N.; Tichy, A.; Krauss, I.; Knecht, C.; Hennig-Pauka, I. Fogging low concentrated organic acid in a fattening pig unit—Effect on animal health and microclimate. Ann. Agric. Environ. Med. 2016, 23, 581–586. [Google Scholar] [CrossRef]
- Fiorillo, L.; Cervino, G.; Matarese, M.; D’Amico, C.; Surace, G.; Paduano, V.; Fiorillo, M.T.; Moschella, A.; la Bruna, A.; Luca, G.R.; et al. COVID-19 Surface Persistence: A Recent Data Summary and Its Importance for Medical and Dental Settings. Int. J. Environ. Res. Public Health 2020, 17, 3132. [Google Scholar] [CrossRef]
- Krishnan, J.; Fey, G.; Stansfield, C.; Landry, L.; Nguy, H.; Klassen, S.; Robertson, C. Evaluation of a Dry Fogging System for Laboratory Decontamination. Appl. Biosaf. 2012, 17, 132–141. [Google Scholar] [CrossRef]
- Pereira, S.S.P.; de Oliveira, H.M.; Turrini, R.N.T.; Lacerd, R.A.A. Disinfection with sodium hypochlorite in hospital environmental surfaces in the reduction of contamination and infection prevention: A systematic review. Rev. Esc. Enferm. USP 2015, 49, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Saccucci, M.; Bruni, E.; Uccelletti, D.; Bregnocchi, A.; Sarto, M.S.; Bossù, M.; di Carlo, G.; Polimeni, A. Surface Disinfections: Present and Future. J. Nanomater. 2018, 2018, 8950143. [Google Scholar] [CrossRef]
- Bore, E.; Langsrud, S. Characterization of micro-organisms isolated from dairy industry after cleaning and fogging disinfection with alkyl amine and peracetic acid. J. Appl. Microbiol. 2005, 98, 96–105. [Google Scholar] [CrossRef]
- Park, G.W.; Boston, D.M.; Kase, J.A.; Sampson, M.N.; Sobsey, M.D. Evaluation of Liquid- and Fog-Based Application of Sterilox Hypochlorous Acid Solution for Surface Inactivation of Human Norovirus. Appl. Environ. Microbiol. 2007, 73, 4463–4468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pedreira, W.; Zeballos, J.; Angenscheidt, M. Efficacy and safety in environmental decontamination with fogging with superoxidized water XTERIDES® (SW) against Bacillus atrophaeus spores (BA) and Methicillin resistant staphylococcus epidermidis (MRSE). Int. J. Infect. Dis. 2012, 16, e382. [Google Scholar] [CrossRef] [Green Version]
- Tanner, B.D. Reduction in Infection Risk Through Treatment of Microbially Contaminated Surfaces with a Novel, Portable, Saturated Steam Vapor Disinfection System. Am. J. Infect. Control 2009, 37, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Muzslay, M.; Bruce, M.; Jeanes, A.; Moore, G.; Wilson, A.P.R. Efficacy of Two Hydrogen Peroxide Vapour Aerial Decontamination Systems for Enhanced Disinfection of Meticillin-Resistant Staphylococcus Aureus, Klebsiella Pneumoniae and Clostridium Difficile in Single Isolation Rooms. J. Hosp. Infect. 2016, 93, 70–77. [Google Scholar] [CrossRef]
- Montazeri, N.; Manuel, C.; Moorman, E.; Khatiwada, J.R.; Williams, L.L.; Jaykus, L.A. Virucidal Activity of Fogged Chlorine Dioxide- and Hydrogen Peroxide-Based Disinfectants against Human Norovirus and Its Surrogate, Feline Calicivirus, on Hard-to-Reach Surfaces. Front. Microbiol. 2017, 8, 1031. [Google Scholar] [CrossRef]
- Mohagheghzadeh, A.; Faridi, P.; Shams-Ardakani, M.; Ghasemi, Y. Medicinal smokes. J. Ethnopharmacol. 2006, 108, 161–184. [Google Scholar] [CrossRef]
- Vishnuprasad, C.N.; Pradeep, N.S.; Cho, Y.W.; Gangadharan, G.G.; Han, S.S. Fumigation in Ayurveda: Potential strategy for drug discovery and drug delivery. J. Ethnopharmacol. 2013, 149, 409–415. [Google Scholar] [CrossRef]
- Zhang, L.; Tian, X.; Ma, Y.; Jin, Y.H.; Meng, F.J. Efficacy of combining traditional Chinese medicine fumigation with Western medicine for diabetic peripheral neuropathy: A systematic review and meta-analysis. Int. J. Nurs. Sci. 2015, 2, 295–303. [Google Scholar] [CrossRef] [Green Version]
- Rajendran, S.; Sriranjini, V. Plant products as fumigants for stored-product insect control. J. Stored. Prod. Res. 2008, 44, 126–135. [Google Scholar] [CrossRef]
- Bhatwalkar, S.B.; Shukla, P.; Srivastava, R.K.; Mondal, R.; Anupam, R. Validation of environmental disinfection efficiency of traditional Ayurvedic fumigation practices. J. Ayurveda Integr. Med. 2009, 10, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Nautiyal, C.S.; Chauhan, P.S.; Nene, Y.L. Medicinal smoke reduces airborne bacteria. J. Ethnopharmacol. 2007, 114, 446–451. [Google Scholar] [CrossRef]
- Bisht, L.S.; Brindavanam, N.B.; Kimothic, P. Comparative study of herbal agents used for fumigtation in relations to formulation. Anc. Sci. Life 1988, 8, 125–132. [Google Scholar]
- Bartlett, D.F.; Goldhagen, P.E.; Phillips, E.A. Experimental Test of Coulomb’s Law. Available online: https://www.princeton.edu/~romalis/PHYS312/Coulomb%20Ref/BartlettCoulomb (accessed on 12 October 2016).
- Tang, K.; Smith, R.D. Physical/chemical separations in the break-up of highly charged droplets from electrosprays. J. Am. Soc. Mass Spectrom. 2001, 12, 343–347. [Google Scholar] [CrossRef] [Green Version]
- Robertson, J.T. Electrostatic Technology for Surface Disinfection in Healthcare Facilities. Infection Control. Tips. Available online: https://emist.com/electrostatic-technology-for-surface-disinfection/ (accessed on 14 October 2016).
- Sasaki, R.S.; Teixeira, M.M.; Fernandes, H.C.; Monteiro, P.M.d.; Rodrigues, D.E.; de Alvarenga, C.B. Parameters of electrostatic spraying and its influence on the application efficiency. Rev. Ceres Viçosa 2013, 60, 474–479. [Google Scholar] [CrossRef]
- Cadnum, J.L.; Jencson, A.L.; Livingston, S.H.; Li, D.; Redmond, S.N.; Pearlmutter, B.; Wilson, B.M.; Donskey, C.J. Evaluation of an electrostatic spray disinfectant technology for rapid decontamination of portable equipment and large open areas in the era of SARS-CoV-2. Am. J. Infect. Control 2020, 48, 951–954. [Google Scholar] [CrossRef]
- COVID-19: Interim Guidance; World Health Organization. Available online: https://apps.who.int/iris/bitstream/handle/10665/332096/WHO-2019-nCoV-Disinfection-2020.1-eng.pdf?sequence=1&isAllowed=y (accessed on 15 May 2020).
- Centers for Disease Control and Prevention (U.S.); Public Health Service (U.S.); National Institutes of Health (Eds.) Biosafety in Microbiological and Biomedical Laboratories, 5th ed.; HHS Publication: Washington, DC, USA, 1999.
- Boyce, J.M. Modern technologies for improving cleaning and disinfection of environmental surfaces in hospitals. Antimicrob. Resist. Infect. Control 2016, 5, 10. [Google Scholar] [CrossRef] [Green Version]
- Available online: https://19january2017snapshot.epa.gov/sites/production/files/2015-09/documents/fogger-mister-final-signed-letter.pdf (accessed on 27 August 2020).
- Starr, T.N.; Greaney, A.J.; Hilton, S.K.; Crawford, K.H.; Navarro, M.J.; Bowen, J.E.; Tortorici, M.A.; Walls, A.C.; Veesler, D.; Bloom, J.D. Deep mutational scanning of SARS-CoV-2 receptor binding domain reveals constraints on folding and ACE2 binding. CellPress 2020, 182, 1–16. [Google Scholar] [CrossRef]
- Heng, D.; Lee, S.H.; Ng, W.K.; Tan, R.B. The nano spray dryer B-90. Expert Opin. Drug Deliv. 2011, 8, 965–972. [Google Scholar] [CrossRef]
- Nandiyanto, A.B.D.; Okuyama, K. Progress in developing spray-drying methods for the production of controlled morphology particles: From the nanometer to submicrometer size ranges. Adv. Powder Technol. 2011, 22, 1–19. [Google Scholar] [CrossRef]
- Kawabata, Y.; Wada, K.; Nakatani, M.; Yamada, S.; Onoue, S. Formulation design for poorly water-soluble drugs based on biopharmaceutics classification system: Basic approaches and practical applications. Int. J. Pharm. 2011, 420, 1–10. [Google Scholar] [CrossRef]
- Huang, Y.; Dai, W.G. Fundamental aspects of solid dispersion technology for poorly soluble drugs. Acta Pharm. Sin. 2014, 4, 18–25. [Google Scholar] [CrossRef] [Green Version]
- Brough, C.; Williams, R.O., III. Amorphous solid dispersions, and nano-crystal technologies for poorly watersoluble drug delivery. Int. J. Pharm. 2013, 453, 157–166. [Google Scholar] [CrossRef]
- Lam, J.; Vaughan, S.; Parkins, M.D. Tobramycin Inhalation Powder (TIP): An Efficient Treatment Strategy for the Management of Chronic Pseudomonas Aeruginosa Infection in Cystic Fibrosis. Clin. Med. Insights. Circ. Respir. Pulm. Med. 2013, 7, 61–77. [Google Scholar] [CrossRef] [PubMed]
- Konstan, M.W.; Geller, D.E.; Minić, P.; Brockhaus, F.; Zhang, J.; Angyalosi, G. Tobramycin inhalation powder for P. aeruginosa infection in cystic fibrosis: The EVOLVE trial. Pediatr. Pulmonol. 2011, 46, 230–238. [Google Scholar] [CrossRef] [Green Version]
- Anderson, S.D.; Daviskas, E.; Brannan, J.D.; Chan, H.K. Repurposing excipients as active inhalation agents: The mannitol story. Adv. Drug Deliv. Rev. 2018, 133, 45–56. [Google Scholar] [CrossRef]
- Guntur, V.P.; Dhand, R. Inhaled Insulin: Extending the Horizons of Inhalation therapy. Respir. Care 2007, 52, 911–922. [Google Scholar]
- Arpagaus, C. Pharmaceutical Particle Engineering via Nano Spray Drying-Process Parameters and Application Examples on the Laboratory-scale. Arpagaus. Int. J. Med. Nano Res. 2018, 5, 26. [Google Scholar] [CrossRef]
- Li, X.; Anton, N.; Arpagaus, C.; Belleteix, F.; Vandamme, T.F. Nanoparticles by spray drying using innovative new technology: The Büchi nano spray dryer B-90. J. Control. Release 2010, 147, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Schafroth, N.; Arpagaus, C.; Jadhav, U.Y.; Makne, S.; Douroumis, D. Nano and microparticle engineering of water insoluble drugs using a novel spray-drying process. Colloids Surf. B Biointerfaces 2012, 90, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Arpagaus, C.; John, P.; Collenberg, A.; Rütti, D. Nanocapsules formation by nano spray drying. In Nanoencapsulation Technologies for the Food and Nutraceutical Industries; Seid, M.J., Ed.; Elsevier Science: Amsterdam, The Netherland, 2017; pp. 346–401. ISBN 978-0-12-809436-5. [Google Scholar]
- Schmid, K.; Arpagaus, C.; Friess, W. Evaluation of the Nano Spray Dryer B-90 for pharmaceutical applications. Pharm. Dev. Technol. 2011, 16, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Arpagaus, C.; Schafroth, N. Laboratory scale spray drying of biodegradable polymers. In Conference, Respiratory Drug Delivery Europe; RDD Europe 2009; RDD: Lisbon, Portugal, 2009; ISBN 1933722312. [Google Scholar]
- Haggag, Y.A.; Faheem, A.M. Evaluation of nanospray drying as a method for drying and formulation of therapeutic peptides and proteins. Front. Pharmacol. 2015, 6. [Google Scholar] [CrossRef] [Green Version]
- Arpagaus, C.; Collenberg, A.; Rütti, D.; Assadpour, E.; Jafari, S.M. Nano spray drying for encapsulation of pharmaceuticals. Int. J. Pharm. 2018, 546, 194–214. [Google Scholar] [CrossRef] [PubMed]
- Aquino, R.P.; Stigliani, M.; Del Gaudio, P.; Mencherini, T.; Sansone, F.; Russo, P. Nanospray drying as a novel technique for the manufacturing of inhalable NSAID powders. Hindawi Publ. Corp. Sci. World J. 2014, 2014, 838410. [Google Scholar]
- Arpagaus, C. Nano Spray Drying of Pharmaceuticals. In IDS’2018—21st International Drying Symposium; Editorial Universitat Politècnica de València: Valencia, Spain, 2018; pp. 1–8. [Google Scholar]
- Fang, Z.; Bhandari, B. Encapsulation techniques for food ingredient systems. In Food Materials Science and Engineering; Bhandari, B., Roos, Y.H., Eds.; Blackwell Publishing Ltd.: Oxford, UK, 2012; Chapter 12; pp. 320–348. [Google Scholar]
- Couvreur, P.; Dubernet, C.; Puisieux, F. Controlled drug delivery with nanoparticles—Current possibilities and future trends. Eur. J. Pharm. Biopharm. 2005, 41, 2–13. [Google Scholar]
- Tantawy, A.S.; Salama, Y.A.M.; Abdel–Mawgoud, A.M.R.; Ghoname, A.A. Comparison of chelated calcium with nano-calcium on alleviation of salinity negative effects on tomato plants. Middle East J. Agric. Res. 2014, 3, 912–916. [Google Scholar]
- Ranjbar, S.; Rahemi, M.; Ramezanian, A. Comparison of nano-calcium and calcium chloride spray on postharvest quality and cell wall enzymes activity in apple cv. Red Delicious. Sci. Hortic. 2008, 240, 57–64. [Google Scholar] [CrossRef]
- Hang, X.; Peng, H.; Song, H.; Qi, Z.; Miao, X.; Xu, W. Antiviral activity of cuprous oxide nanoparticles against Hepatitis C Virus in vitro. J. Virol. Methods 2015, 222, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Salah, M.A. Coronavirus: Can Nano-Coatings and Sprays Help? Available online: https://kashmirlife.net/coronavirus-can-nano-coatings-and-sprays-help-227570/ (accessed on 30 March 2020).
- Sun, Z.; Ostrikov, K. Future antiviral surfaces: Lessons from COVID-19 pandemic. Sustain. Mater. Technol. 2020, 25, e00203. [Google Scholar] [CrossRef]
- Miranda-Linares, V.; Quintanar-Guerrero, D.; Del Real, A.; Zambrano-Zaragoza, M.L. Spray-drying method for the encapsulation of a functionalized ingredient in alginate-pectin nano- and microparticles loaded with distinct natural actives: Stability and antioxidant effect. Food Hydrocoll. 2020, 101, 105560. [Google Scholar] [CrossRef]
- Ghasemi, S.; Jafari, S.M.; Assadpour, E.; Khomeiri, M. Production of pectin-whey protein nano-complexes as carriers of orange peel oil. Carbohydr. Polym. 2017, 177, 369–377. [Google Scholar] [CrossRef]
- Anna, G.; Elizaveta, K.; Elena, Y.; Galina, L.; Natalia, K.; Denis, K. Stability study of ZnO nanoparticles in aqueous solutions of carboxylate anions. J. Nanopart. Res. 2015, 17, 123. [Google Scholar]
- Lee, S.H.; Heng, D.; Ng, W.K.; Chan, H.K.; Tan, R.B. Nano spray drying: A novel method for preparing protein nanoparticles for protein therapy. Int. J. Pharm. 2011, 403, 192–200. [Google Scholar] [CrossRef]
- Beck-Broichsitter, M.; Schweiger, C.; Schmehl, T.; Gessler, T.; Seeger, W.; Kissel, T. Characterization of novel spray-dried polymeric particles for controlled pulmonary drug delivery. J. Control. Release 2012, 158, 329–335. [Google Scholar] [CrossRef]
- Gonzattia, M.B.; Sousaa, M.E.P.; Tunissib, A.S.; Mortaraa, R.A.; de Oliveirab, A.M.; Cerizeb, N.N.P.; Kellera, A.D. Nano spray dryer for vectorizing α-galactosylceramide in polymeric nanoparticles: A single step process to enhance invariant Natural Killer T lymphocyte responses. Int. J. Pharm. 2019, 565, 123–132. [Google Scholar] [CrossRef]
- Arpagaus, C. Nano Spray Dryer B-90: Literature review and applications. Inf. Bull. 2011, 63, 2011. [Google Scholar]
- Arpagaus, C.; Schafroth, N.; Meuri, M. Laboratory scale spray drying of lactose: A review. Inf. Bull. 2010, 57, 1–12. [Google Scholar]
- Büchi Labortechnik, A.G. Nano Spray Dryer B-90. Tech. Data Sheet 2013, en 1311. Available online: http://static2.buchi.com/sites/default/files/technical-data-pdf/B-90_Data_Sheet_en_A_0.pdf (accessed on 2 June 2020).
- Giannossa, L.C.; Longano, D.; Cioffi, N.; Nitti, M.A.; Paladini, F.; Pollini, M.; Rai, M.; Sannino, A.; Valentini, A.; Cioffi, N. Metal nanoantimicrobials for textile applications. Nanotechnol. Rev. 2013, 2, 307–331. [Google Scholar] [CrossRef]
- Zendehdel, R.; Goli, F.; Hajibabaei, M. Comparing the microbial inhibition of nanofibres with multi-metal ion exchanged nano-zeolite Y in air sampling. J. Appl. Microbiol. 2020, 128, 202–208. [Google Scholar] [CrossRef] [Green Version]
- De Jong, B.; Meeder, A.M.; Koekkoek, K.W.A.C.; Schouten, M.A.; Westers, P.; Van Zanten, A.R.H. Pre-post evaluation of effects of a titanium dioxide coating on environmental contamination of an intensive care unit: The TITANIC study. J. Hosp. Infect. 2018, 99, 256–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenberg, M.; Ilić, K.; Juganson, K.; Ivask, A.; Ahonen, M.; Vrček, I.V.; Kahru, A. Potential ecotoxicological effects of antimicrobial surface coatings: A literature survey backed up by analysis of market reports. PeerJ 2019, 7, e6315. [Google Scholar] [CrossRef] [PubMed]
- Biller, K. Focus on Powder Coatings; Elsevier: Amsterdam, The Netherlands, 2020; Volume 2020. [Google Scholar]
- Ghosh, S.K. Anti-Viral Surface Coating to Prevent Spread of Novel Coronavirus (COVID-19) Through Touch. Coatings World. Available online: https://www.coatingsworld.com/content-microsite/cw_covid-19/2020-04-15/anti-viral-surface-coating-to-prevent-spread-of-novel-coronavirus-covid-19-through-touch (accessed on 15 April 2020).
- Li, Y.; Leung, W.K.; Yeung, K.L.; Lau, P.S.; Kwan, J.K. A Multilevel Antimicrobial Coating Based on Polymer-Encapsulated ClO2. Langmuir 2009, 25, 13472–13480. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.; Cristancho, D.E.; Rachford, A.A.; Reder, A.L.; Williamson, A.; Grzesiak, A.L. Controlled Release of Antimicrobial ClO2 Gas from a Two-Layer Polymeric Film System. J. Agric. Food Chem. 2016, 64, 8647–8652. [Google Scholar] [CrossRef]
- Pulit-Prociak, J.; Banach, M. Silver nanoparticles—A material of the future…? Open Chem. 2016, 14, 76. [Google Scholar] [CrossRef]
- Deshmukh, S.P.; Patil, S.M.; Mullani, S.B.; Delekar, S.D. Silver nanoparticles as an effective disinfectant: A review. Mater. Sci. Eng. C 2019, 97, 954–965. [Google Scholar] [CrossRef]
- EPA-Approved Antimicrobial Surface Coating Represents Breakthrough in the Control and Spread of Infectious Diseases. Press Release. 19 March 2020. Available online: https://www.prnewswire.com/news-releases/epa-approved-antimicrobial-surface-coating-represents-breakthrough-in-the-control-and-spread-of-infectious-diseases-301027074.html (accessed on 22 June 2020).
- Talebian, S.; Wallace, G.G.; Schroeder, A.; Stellacci, F.; Conde, J. Nanotechnology-based disinfectants and sensors for SARS-CoV-2. Nat. Nanotechnol. 2020, 15, 618–621. [Google Scholar] [CrossRef]
- Mario, D.; Sovereto, D.; Illuzzi, G.; Laneve, E.; Raddato, B.; Arena, C.; Caponio, V.C.A.; Caloro, G.A.; Zhurakivska, K.; Troiano, G.; et al. Management of Instrument Sterilization Workflow in Endodontics: A Systematic Review and Meta-Analysis. Int. J. Dent. 2019, 2020, 5824369. [Google Scholar]
- Centers for Disease Control and Prevention. An Introduction to Applied Epidemiology and Biostatistics. Lesson 3: Measures of Risk. Section 2: Morbidity Frequency Measures. In Principles of Epidemiology in Public Health Practice, 3rd ed. Available online: https://www.cdc.gov/csels/dsepd/ss1978/index.html (accessed on 7 July 2020).
- Brühwasser, C.; Heinrich, H.; Lass-Flörl, C.; Mayr, A. Self-disinfecting surfaces and activity against Staphyloccocus aureus ATCC 6538 under real-life conditions. J. Hosp. Infect. 2017, 97, 196–199. [Google Scholar] [CrossRef]
- George, L.; Müller, A.; Röder, B.; Santala, V.; Efimov, A. Photodynamic self-disinfecting surface using pyridinium phthalocyanine. Dye. Pigment. 2017, 147, 334–342. [Google Scholar] [CrossRef]
- Weber, D.J.; Rutala, W.A. Self-Disinfecting Surfaces, Infect. Control Hosp. Epidemiol. 2012, 33, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Badrossamay, M.; Sun, G. Enhancing hygiene/antimicrobial properties of polyolefins. In Polyolefin Fibres; Woodhead Publishing: Cambridge, UK, 2017; pp. 265–284. [Google Scholar]
- Byrne, J.A.; Dunlop, P.S.M.; Hamilton, J.W.J.; Fernández-Ibáñez, P.; Polo-López, I.; Sharma, P.K.; Vennard, A.S.M. A Review of Heterogeneous Photocatalysis for Water and Surface Disinfection. Molecules 2015, 20, 5574–5615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zoccolillo, M.L.; Rogers, S.C.; Mang, T.S. Antimicrobial photodynamic therapy of S. mutansbiofilms attached to relevant dental materials. Lasers Surg. Med. 2016, 48, 995–1005. [Google Scholar] [CrossRef] [PubMed]
- Adán, C.; Marugán, J.; Mesones, S.; Casado, C.; van Grieken, R. Bacterial inactivation and degradation of organic molecules by titanium dioxide supported on porous stainless-steel photocatalytic membranes. Chem. Eng. J. 2017, 318, 29–38. [Google Scholar] [CrossRef]
- Choi, H.; Castillo, B.; Seminario-Vidal, L. Silver absorption in patients with Stevens-Johnson syndrome and toxic epidermal necrolysis treated with silver-impregnated dressings. A case series. Int. Wound J. 2018, 15, 1049–1051. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Liu, S. Antibacterial surface design—Contact kill. Prog. Surf. Sci. 2016, 91, 136–153. [Google Scholar] [CrossRef]
- Gjorgievska, E.S.; Nicholson, J.W.; Coleman, N.J.; Booth, S.; Dimkov, A.; Hurt, A. Component Release and Mechanical Properties of Endodontic Sealers following Incorporation of Antimicrobial Agents. Biomed. Res. Int. 2017, 2017, 2129807. [Google Scholar] [CrossRef] [PubMed]
- Brooks, B.D.; Brooks, A.E.; Grainger, D.W. Antimicrobial Medical Devices in Preclinical Development and Clinical Use. In Biomaterials Associated Infection; Moriarty, T., Zaat, S., Busscher, H., Eds.; Springer: New York, NY, USA, 2013. [Google Scholar]
- Weng, D.; Qi, H.; Wu, T.T.; Yan, M.; Sun, R.; Lu, Y. Visible light powered self-disinfecting coatings for influenza viruses. Nanoscale 2012, 4, 2870. [Google Scholar] [CrossRef] [PubMed]
- Kapridaki, C.; Pinho, L.; Mosquera, M.J.; Maravelaki-Kalaitzaki, P. Producing photoactive, transparent, and hydrophobic SiO2-crystalline TiO2 nanocomposites at ambient conditions with application as self-cleaning coatings. Appl. Catal. B Environ. 2014, 156–157, 416–427. [Google Scholar] [CrossRef]
- Kiwi, J.; Rtimi, S.; Sanjines, R.; Pulgarin, C. TiO2 and TiO2-Doped Films Able to Kill Bacteria by Contact: New Evidence for the Dynamics of Bacterial Inactivation in the Dark and under Light Irradiation. Int. J. Photoenergy 2014, 2014, 785037. [Google Scholar] [CrossRef] [Green Version]
- Krumdieck, S.P.; Boichot, R.; Gorthy, R.; Land, J.G.; Lay, S.; Gardecka, A.J.; Polson, M.I.; Wasa, A.; Aitken, J.E.; Heinemann, J.A.; et al. Nanostructured TiO2 anataserutile-carbon solid coating with visible light antimicrobial activity. Sci. Rep. 2019, 9, 1883. [Google Scholar] [CrossRef] [PubMed]
- Elfakhri, S.O. Antibacterial Activity of Novel Self-Disinfecting Surface Coatings. Ph.D. Thesis, The University of Salford, Salford, UK, 2014; 289p. [Google Scholar]
- Mayr, A.; Orth-Holler, D.; Heinrich, H.; Hinterberger, G.; Wille, I.; Naschberger, V.; Lass-Florl, C.; Binder, U. Galleria mellonella as a Model to Study the Effect of Antimicrobial Surfaces on Contamination by Staphylococcus aureus. Arch. Clin. Biomed. Res. 2019, 3, 315–325. [Google Scholar] [CrossRef] [Green Version]
- Weber, D.J.; Rutala, W.A.; Sickbert-Bennett, E.E.; Kanamori, H.; Anderson, D.; CDC Prevention Epicenters Program. Continuous room decontamination technologies. Am. J. Infect. Control 2019, 47, A72–A78. [Google Scholar] [CrossRef] [PubMed]
- Tamimi, A.H.; Carlino, S.; Gerba, C.P. Long-term efficacy of a self-disinfecting coating in an intensive care unit. Am. J. Infect. Control 2014, 42, 1178–1181. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.J.; Rutala, W.A. Self-disinfecting surfaces: Review of current methodologies, and future prospects. Am. J. Infect. Control 2013, 41, S31–S35. [Google Scholar] [CrossRef] [PubMed]
- Santo, C.E.; Quaranta, D.; Grass, G. Antimicrobial Metallic Copper Surfaces Kill Staphylococcus haemolyticus Via Membrane Damage. Microbiologyopen 2012, 1, 46–52. [Google Scholar] [CrossRef]
- Waman, P.; Swapnil, S.; Krishna, K.; Rajshree, C.; Chetan, K.; Naresh, B.; Udit, P.; Ram, P.; Ghanshyam, L.B. Future prospects of plasma treatment technology for disinfection. In Engineering Technology of the 21st Century, 1st ed.; Roy, A.K., Ed.; New India Publishing Agency: New Delhi, India, 2015; Chapter 10. [Google Scholar]
- Sakudo, A.; Yagyu, Y.; Onodera, T. Disinfection and Sterilization Using Plasma Technology: Fundamentals and Future Perspectives for Biological Applications. Int. J. Mol. Sci. 2019, 20, 5216. [Google Scholar] [CrossRef] [Green Version]
- Dai, X.; Bazaka, K.; Richard, D.J.; Thompson, E.R.W.; Ostrikov, K.K. The Emerging Role of Gas Plasma in Oncotherapy. Trends Biotechnol. 2018, 36, 1183–1198. [Google Scholar] [CrossRef] [PubMed]
- Aboubakr, H.A.; Parra, F.S.; Collins, J.; Bruggeman, P.; Goyal, S.M. Ìn situ inactivation of human norovirus GII.4 by cold plasma: Ethidium monoazide (EMA)-coupled RT-qPCR underestimates virus reduction and fecal material suppresses inactivation. Food Microbiol. 2020, 85, 103307. [Google Scholar] [CrossRef] [PubMed]
- Machala, Z.; Tarabová, B.; Sersenová, D.; Janda, M.; Hensel, K. Chemical and antibacterial effects of plasma activated water: Correlation with gaseous and aqueous reactive oxygen and nitrogen species, plasma sources and air flow conditions. J. Phys. D Appl. Phys. 2018, 52, 034002. [Google Scholar] [CrossRef]
- Ben Belgacem, Z.; Carré, G.; Charpentier, E.; Le-Bras, F.; Maho, T.; Robert, E.; Pouvesle, J.M.; Polidor, F.; Gangloff, S.C.; Boudifa, M.; et al. Innovative non-thermal plasma disinfection process inside sealed bags: Assessment of bactericidal and sporicidal effectiveness in regard to current sterilization norms. PLoS ONE 2017, 12, e0180183. [Google Scholar] [CrossRef] [Green Version]
- Ehlbeck, J.; Schnabel, U.; Polak, M.; Winter, J.; Von Woedtke, T.; Brandenburg, R.; Von dem Hagen, T.; Weltmann, K.D. Low temperature atmospheric pressure plasma sources for microbial decontamination. J. Phys. D Appl. Phys. 2011, 44, 013002. [Google Scholar] [CrossRef] [Green Version]
- Van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and surface stability of SARS cov-2 as compared with SARS cov-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef]
- Filipić, A.; Gutierrez-Aguirre, I.; Primc, G.; Mozetič, M.; Dobnik, D. Cold Plasma, a New Hope in the Field of Virus Inactivation. Trends Biotechnol. 2020. [Google Scholar] [CrossRef]
- Rideout, K. Considering risks to healthcare workers from glutaraldehyde alternatives in high-level disinfection. J. Hosp. Infect. 2005, 59, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Quinn, M.M.; Henneberger, P.K.; Braun, B.; Delclos, G.L.; Fagan, K.; Huang, V.; Knaack, J.L.; Kusek, L.; Lee, S.J.; Le Moual, N.; et al. Cleaning and disinfecting environmental surfaces in health care: Toward an integrated framework for infection and occupational illness prevention. Am. J. Infect. Control 2015, 43, 424–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, X.; Wang, M.; Qin, C.; Tan, L.; Ran, L.; Chen, D.; Zhang, H.; Shang, K.; Xia, C.; Wang, S.; et al. Coronavirus Disease 2019 (COVID-2019) Infection Among Health Care Workers and Implications for Prevention Measures in a Tertiary Hospital in Wuhan, China. JAMA Netw. Open 2020, 3, e209666. [Google Scholar] [CrossRef] [PubMed]
- Vimercati, L.; Dell’Erba, A.; Migliore, G.; De Maria, L.; Caputi, A.; Quarato, M.; Stefanizzi, P.; Cavone, D.; Ferorelli, D.; Sponselli, S.; et al. Prevention and protection measures of healthcare workers exposed to SARS-CoV-2 in a university hospital in Bari, Apulia, Southern Italy. J. Hosp. Infect. 2020, 105, 454–458. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.J.; Rutala, W.A.; Anderson, D.J.; Chen, L.F.; Sickbert-Bennett, E.E.; Boyce, J.M. Effectiveness of ultraviolet devices and hydrogen peroxide systems for terminal room decontamination: Focus on clinical trials. Am. J. Infect. Control 2016, 44, e77–e84. [Google Scholar] [CrossRef] [PubMed]




| Ref | Disinfection Application | Disinfectant | Actions and Remarks |
|---|---|---|---|
| [8] | Pigs’ health, environmental, and hygiene parameters | Fogging a low concentrated tartaric acid solution | The ammonia concentrations are not influenced by the fogging procedure. The limitation on the tartaric acid concentration which should be a maximum of 0.1% because of the negative effect on the respiratory mucosa. |
| [9] | Nosocomial and healthcare-associated infections | Fogging using garlic peel, turmeric powder, Carom seeds, and Loban | Fumigation using these plants reduces airborne bacteria. This traditional fumigation could replace the harmful and toxic chemical fumigation for healthcare environmental disinfection. |
| [10] | Electronic devices and Stainless steel, laboratory spaces, and walk-in coolers | Fogging dry fogging system (DFS) using liquid peracetic acid (PAA) | The dry fogging system is an effective disinfection technology compared to formaldehyde, vaporous hydrogen peroxide, or gaseous chlorine dioxide. |
| [11] | Environmental surfaces | Sodium hypochlorite | The sodium hypochlorite is an influential disinfectant. However, the problem of direct contact is noticed to reduce healthcare-associated infection (HAIs) remains. |
| [12] | Hospital environmental, such as the surfaces of biomedical devices | Layers of graphene-based nanomaterial | Layers of graphene-based nanomaterial have several advantages. It is used to stop infections and bacteria. Unlike the known antibiotics and detergents, the nanoparticles are stable for long periods and are intoxicants. |
| [13] | Stainless steel in the food industry | Fogging disinfection with alkyl amine/peracetic acid | Due to the surface attachment and resistance, after cleaning and fogging with alkyl amine or peracetic acid, microorganisms are detected. |
| [14] | Noroviruses (NVs) in the environment | Fogging Sterilox hypochlorous acid solution (HAS) | To decontaminate large spaces, the Sterilox hypochlorous acid solution was used as a fog. The use of these fogs is more active in the disinfection of large areas. The performance of this solution is on surfaces, contaminated with noroviruses disinfection. |
| [15] | Environmental surfaces personnel, terminal, and emergency | Fogging superoxidized water (SW) | The fogging system using the superoxidized water is not an expensive solution and it can be applied with the presence of persons. |
| [18] | Human norovirus (NoV) | Fogging using the hydrogen peroxide solution and the chlorine dioxide | The fogging system of the hydrogen peroxide promising virucidal activity, however, fogged chlorine dioxide-surfactant-based product is uniformly delivered and effectively used for the application of closed areas NoV disinfection, allowing the disinfection using the saturation of the environment air which leads to sterilize all surfaces. |
| [28] | Hospital environmental surfaces | Electrostatic spraying Electrostatic disinfection | The disinfection methods including wipes, spray, fogging, and UV lighting may be expensive for daily use, but the electrostatic disinfection systems present a reliable with acceptable costs in the environmental surface disinfection. |
| [29] | Coronavirus (COVID-19) | Ultraviolet (UV) | The UV technology is efficacy, but it has a bad effect on the human body in the case of repetitive exposure |
| [30] | Biological safety cabinets (BSC) | Ultraviolet (UV) | The radiation output of the UV should not be less than 40 mW/cm2 at a wavelength of 254 nm to kill microorganisms Ultraviolet (UV) lamps are not recommended in BSCs because it should be stopped when there is someone in the room to protect eyes and skin human from the exposure to the UV light. |
| Production Company | Product | Disease | Materials | Company | FDA Approval |
|---|---|---|---|---|---|
| Prograf | Tacrolimus | Immunosuppressant (prevents orga rejection) | HPMC | Astellas Pharma | 1994 |
| Exhubera | Insulin | Diabetes | Mannitol, glycine, sodium citrate | Pfizer/ Nektar | 2006 |
| Intelence | Etravirine | HIV medicine | HPMC | Janssen | 2008 |
| Zortress | Everolimus | Immunosuppressant (prevents orga rejection) | HPMC | Novartis | 2010 |
| Aridol/Osmohale, Bronchitol | - | Asthma/Cystic fibrosis | Mannitol | Pharmaxis | 2010 |
| TOBI Podhaler | Tobramycin | Inhalation therapy | DSPC, calcium chloride, sulfuric acid | Novartis | 2013 |
| Raplixa | - | Bleeding control during surgery | Fibrinogen/ Thrombin | Nova Laboratories | 2016 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kchaou, M.; Abuhasel, K.; Khadr, M.; Hosni, F.; Alquraish, M. Surface Disinfection to Protect against Microorganisms: Overview of Traditional Methods and Issues of Emergent Nanotechnologies. Appl. Sci. 2020, 10, 6040. https://doi.org/10.3390/app10176040
Kchaou M, Abuhasel K, Khadr M, Hosni F, Alquraish M. Surface Disinfection to Protect against Microorganisms: Overview of Traditional Methods and Issues of Emergent Nanotechnologies. Applied Sciences. 2020; 10(17):6040. https://doi.org/10.3390/app10176040
Chicago/Turabian StyleKchaou, Mohamed, Khaled Abuhasel, Mosaad Khadr, Faouzi Hosni, and Mohammed Alquraish. 2020. "Surface Disinfection to Protect against Microorganisms: Overview of Traditional Methods and Issues of Emergent Nanotechnologies" Applied Sciences 10, no. 17: 6040. https://doi.org/10.3390/app10176040
APA StyleKchaou, M., Abuhasel, K., Khadr, M., Hosni, F., & Alquraish, M. (2020). Surface Disinfection to Protect against Microorganisms: Overview of Traditional Methods and Issues of Emergent Nanotechnologies. Applied Sciences, 10(17), 6040. https://doi.org/10.3390/app10176040







