Abstract
β-silicon carbide (SiC) powders were synthesized by the carbothermal reduction of methyl-modified silica aerogel/carbon mixtures. The correlations between the phase evolution and morphologies of the SiC powders and the C/SiO2 ratio were investigated. At a C/SiO2 ratio of 3, β-SiC formed at 1425 °C and single-phase SiC powders were obtained at 1525 °C. The methyl groups (-CH3) on the silica aerogel surfaces played important roles in the formation of SiC during the carbothermal reduction. SiC could be synthesized from the silica aerogel/carbon mixtures under lower temperature and C/SiO2 ratios than those needed for quartz or hydrophilic silica. The morphology of the SiC powder depended on the C/SiO2 ratio. A low C/SiO2 ratio resulted in β-SiC powder with spherical morphology, while agglomerates consisting of fine SiC particles were obtained at the C/SiO2 ratio of 3. High-purity SiC powder (99.95%) could be obtained with C/SiO2 = 0.5 and 3 at 1525 °C for 5 h.
1. Introduction
Silicon carbide (SiC) is a typical non-oxide ceramic material that forms covalent bonds in structural units. Because of its strong covalent bonds and chemical stability, SiC exhibits excellent properties at temperatures up to 1400 °C such as a high hardness, wear resistance, corrosion resistance, and strength [1,2]. Other notable features of SiC are high thermal and electrical conductivities. Based on these excellent mechanical and electronic properties, SiC has been actively studied in various fields with respect to different applications such as for gas turbine components, heat exchangers, high temperature gas filters, power devices, heat dissipation substrates, and catalyst support materials.
The SiC ceramics can be fabricated by sintering SiC powders or preceramic polymers [3,4,5] at high temperature because of the covalent nature of the Si-C bond and low self-diffusion coefficient [6]. Single crystals of SiC for power device applications can be grown from SiC powder compacts by physical vapor transport (PVT) or sublimation epitaxial growth (SEG) [7,8]. The defects (stacking faults and dislocations) in SiC single crystals, sinterability, microstructure, and properties of sintered SiC strongly depend on the purity, morphology, size, and distribution of the SiC starting powders [9,10,11,12].
Various techniques have been developed for the synthesis of high-quality SiC powders such as the carbothermal reduction of silica (SiO2) [13,14,15], direct carbonization [16,17], the thermal decomposition of preceramic polymers [18], and chemical vapor deposition (CVD) [19,20]. The synthesis of α-SiC using the carbothermal reduction of SiO2 is the most common technique, which is characterized by relatively low-cost processing (inexpensive starting materials) and can be easily scaled up [21,22]. The carbothermal reaction can be expressed as follows:
SiO2(s) + 3C(s) → SiC(s) + 2CO(g)
A C/SiO2 powder mixture with a stoichiometric C/SiO2 ratio above 3 is required for the reaction to obtain SiO2-free SiC powder [23]. Because gaseous silicon monoxide (SiO) species may form and the SiC particles grow based on the reaction of SiO and C [24], the morphology and characteristics of the SiC powder obtained using carbothermal reduction strongly depend on starting the starting material, that is, SiO2 and C, as well as on the reaction conditions.
Parmentier et al. synthesized SiC powders with high specific surface areas through the carbothermal reaction of mesoporous MCM-48 silica and pyrolytic carbon using the chemical vapor infiltration of propylene [25]. Meng et al. proposed a novel sol-gel process to obtain SiO2 xerogels containing carbon nanoparticles [26]. They fabricated nanostructured α-SiC powder at 1650 °C using the carbothermal reduction of the precursor powder mentioned above.
In our previous study [27], we developed a novel technique for the synthesis of silica aerogel powders with spherical shapes using emulsion polymerization from water glass. The synthesized silica aerogels were highly porous (~95% porosity), and their surfaces were modified to contain methyl groups (-CH3). When the surface-modified silica aerogel is employed as a starting material for the carbothermal reduction to synthesize SiC powder, the covalent bonds of Si-C and the high surface energy, owing to mesoporous nature of the silica aerogels, can accelerate the conversion of silica to SiC. In addition, the use of the surface-modified silica aerogel is expected to produce a spherical SiC powder. To our knowledge, there has been no attempt to use silica aerogels containing surface methyl groups to synthesize SiC powder.
This study focuses on the synthesis of spherical β-SiC powders through the carbothermal reduction of surface-modified SiO2 aerogels. The effects of the surface methyl groups (-CH3) and mesoporous structure of the silica aerogel on the carbothermal reduction reaction were investigated for different SiO2/C ratios. The synthesized SiC powders were characterized by X-ray diffraction (XRD) and scanning electron microscopy (SEM).
2. Materials and Methods
2.1. Preparation of Silica Aerogel
Silica aerogel and carbon black (Raven425, Birla Carbon, Seoul, Korea) were used as starting materials for the carbothermal reduction. Silica aerogel powders were prepared from water glass (silica content, 28–30 wt.%; SiO2/Na2O = 3.52:1; Young IL Chemical, Incheon, Korea) and n-hexane (95%, Samchun Pure Chemicals, Pyeongtaek, Korea) using an emulsion polymerization technique. The detailed procedure is described elsewhere [27]. Silica aerogel and carbon black were mixed in n-hexane using an ultrasonic bath. Homogeneous slurry was obtained after 10 min and dried at 100 °C in an electric oven. The C/SiO2 molar ratios were 0, 0.5, 1, 2, and 3. The SiO2/C powder mixture was poured into an alumina (Al2O3) boat, which was placed inside a tube furnace under a constant argon flow (100 sccm). The carbothermal reduction was performed at 1450–1525 °C for 1 and 5 h. The synthesized powders were further heat-treated at 800 °C for 1 h in air to remove residual carbon black.
2.2. Evaluation
To measure tap density, 0.2 g of the silica aerogel powder was placed in a 5 mL cylinder (9 mm in diameter) and tapped 1000 times using a tapping density tester (TAP-2S, Logan Instruments Co., Somerset, NJ, USA). Fourier transform infrared (FT-IR) spectroscopy (FTS-165, Bio-Rad, Hercules CA, USA) was used to confirm the surface chemical structure of the aerogels in the wave number range of 400–4000 cm−1. The powder was mixed with potassium bromide (KBr) and pressed to form a sample disk for FT-IR measurements. The phases of the obtained powders were identified using XRD (D/MAX 2200V/PC, RIGAKU Co., Ltd., Tokyo, Japan) with Ni-filtered CuKα radiation. The thermogravimetry (TG) and differential thermal analysis (DTA) (Diamond TG/DTA Lab System, Perkin Elmer) was performed in air up to 800 °C at a heating rate of 5 °C · min−1 to investigate the thermal behavior of the silica aerogel powder. The carbon, hydrogen, and oxygen contents in the starting silica aerogel and synthesized SiC powders were determined using an elemental analyzer (EA, Thermo EA1112, Thermo Fisher Scientific, Waltham, MA, USA) and an oxygen–nitrogen analyzer (EMGA-920, Horiba, Japan), respectively. The surface area, pore volume, and mean pore size of the starting silica aerogel powder were measured using Brunauer–Emmett–Teller (BET) equipment (0.01 < p/p0 < 1; ASAP 2010; Micrometrics, Norcross, GA, USA). The equipment is used to measure the amount of nitrogen that is adsorbed as the pressure changes. Before N2 adsorption, the powder sample was degassed at 200 °C. The microstructures of the obtained powder samples were examined using field-emission SEM (FESEM, S-4300, Hitachi, Japan).
3. Results and Discussion
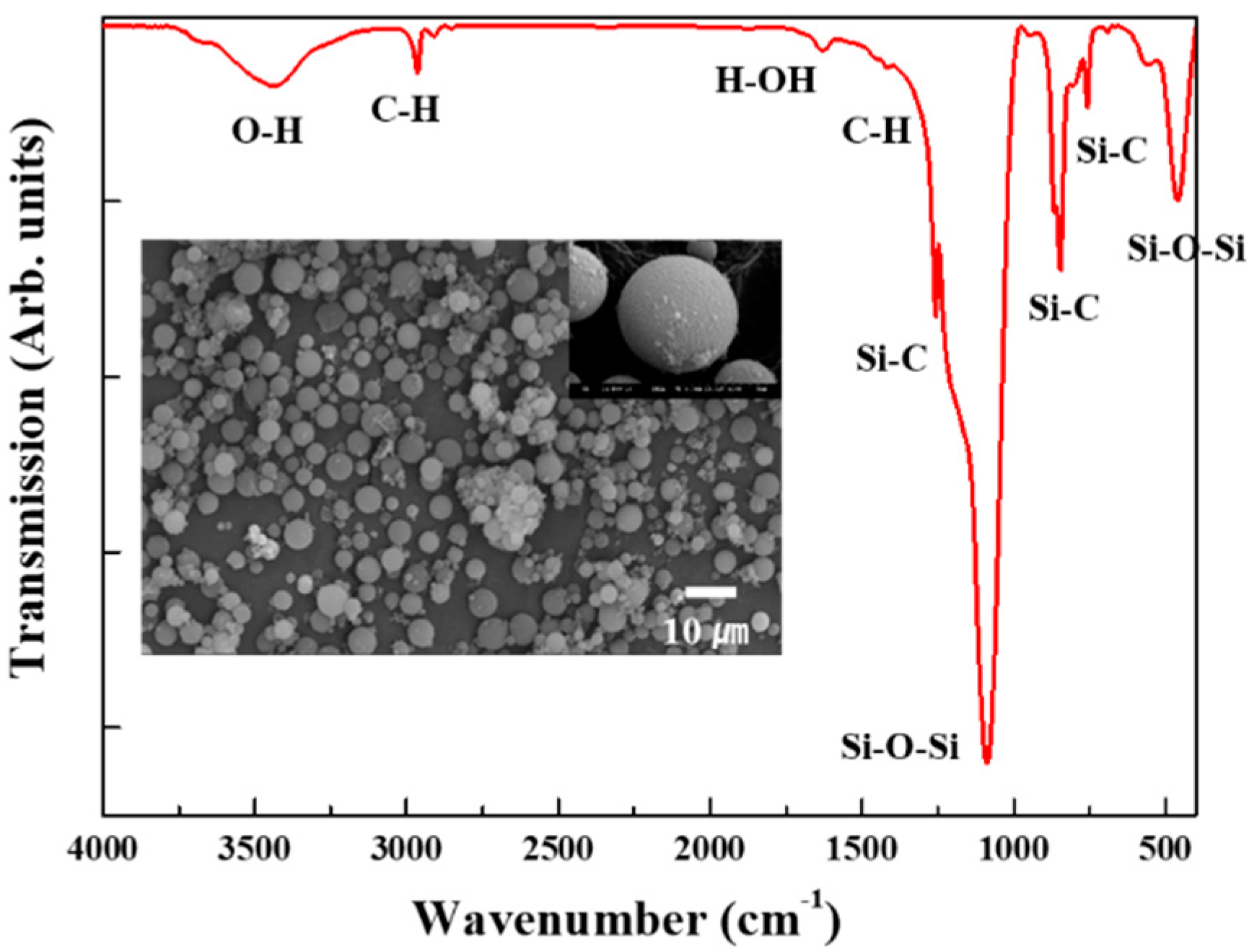
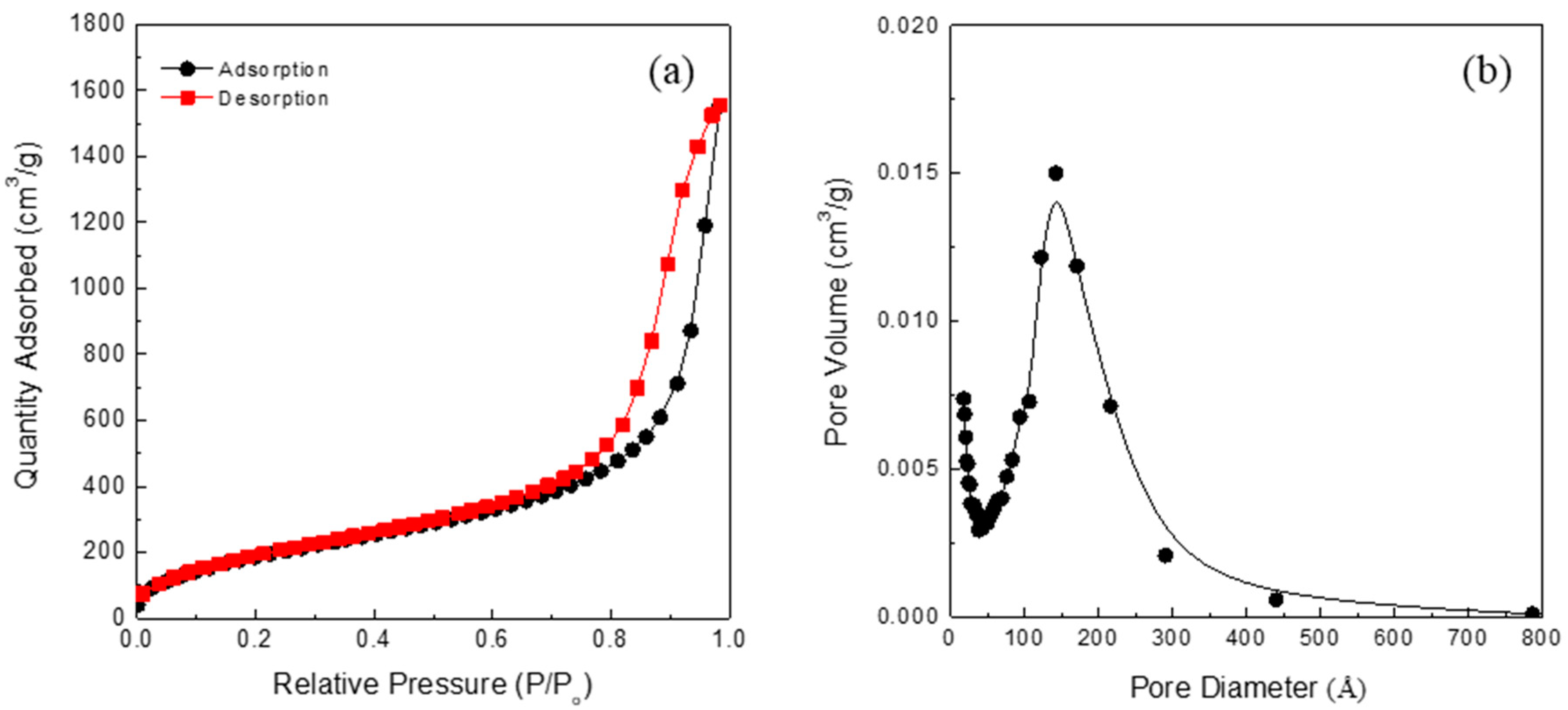
Some of the physical properties of the starting silica aerogel powder are listed in Table 1. The silica aerogel has a highly porous structure with mesopores, and its surface was modified by methyl groups (-CH3). The Fourier transform infrared spectra of the starting silica aerogel powder are shown in Figure 1. The absorption peaks near 1100, 800, and 460 cm−1 were assigned to the asymmetry, symmetry, and bending modes of Si-O-Si, respectively [28,29]. These peaks are characteristic peaks showing a typical silica aerogel network structure. By contrast, the peaks at 1260 and 850 cm−1 indicate the presence of a Si-C bond, while the peaks at 2900 and 1450 cm−1 are due to C-H stretching [30,31]. Thus, it can be inferred that the silica aerogel was modified into a hydrophobic form by the surface methyl groups (-CH3). A N2 adsorption–desorption isotherm of the starting silica aerogel powder is shown in Figure 2. N2 absorption sharply increases near the high relative pressure (Type IV adsorption–desorption isotherm), which indicates that the silica aerogel is mesoporous [32,33].

Table 1.
Some physical properties of the starting silica aerogel powder.
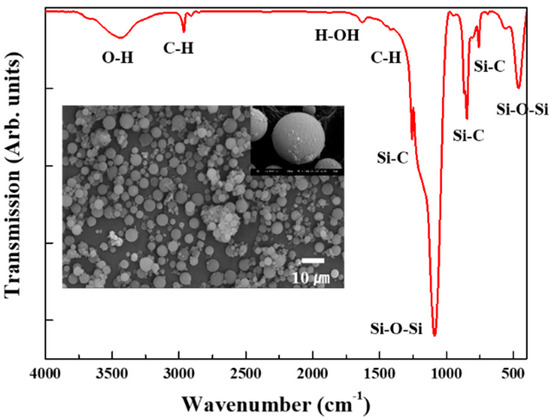
Figure 1.
FT-IR spectra of the starting silica aerogel powder. The inset is an SEM image of the starting silica aerogel powder.
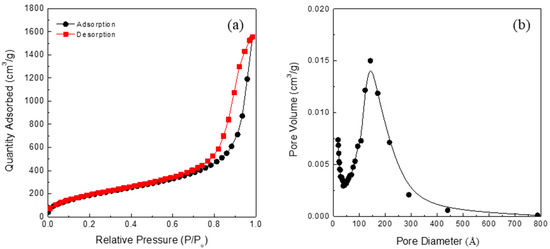
Figure 2.
N2 adsorption–desorption isotherm (a) and pore size distribution of the starting silica aerogel powder (b).
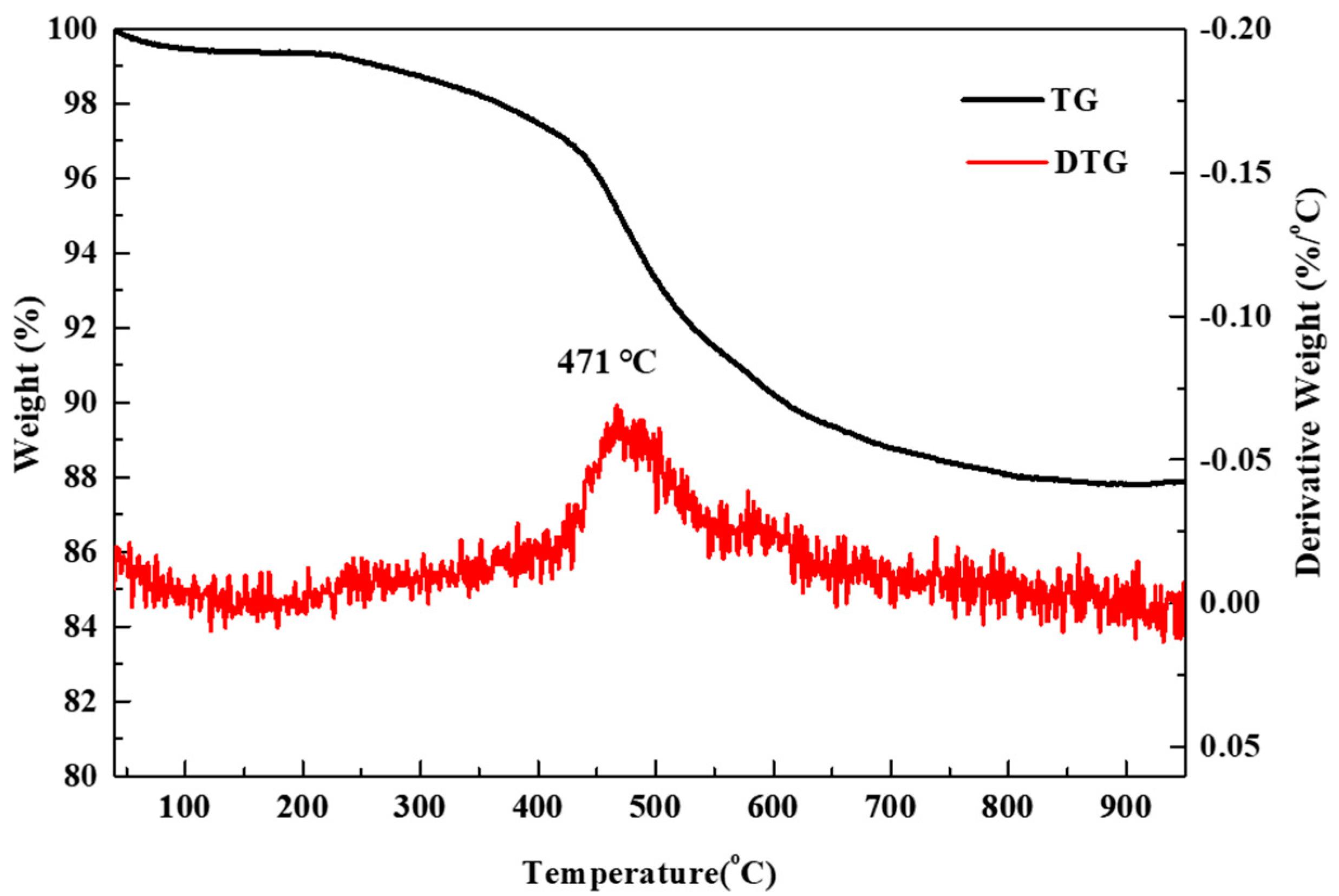
The TG was carried out to quantitatively analyze the presence and content of surface carbon in the silica aerogel powder. The TG curves are illustrated in Figure 3. Weight loss was observed in two temperature regions. The weight loss of ~0.5% observed from room temperature to 200 °C is due to the release of physically adsorbed water or the evaporation of residual solvents on the surface of the silica aerogel. On the other hand, the weight loss of 11.8% in the temperature range of 300 to 800 °C is due to the combustion of the surface methyl group (-CH3). It is assumed that the DTG peak and sharp weight loss at ~475 °C can be attributed to the oxidation of the surface methyl groups (-CH3) of hydrophobic silica aerogel particles [34]. The silica aerogel, which is chemically modified by surface methyl groups, exhibits a hydrophobic–hydrophilic transition upon heating in air, which is due to the oxidation of-CH3. The EA analysis revealed that the carbon and hydrogen contents of the silica aerogel powder were 11.78 and 3.04 wt.%, respectively. This value (11.78 wt.% of carbon) corresponds to ~0.70 mol% of SiO2. Therefore, the actual C/SiO2 ratios are 0.70, 1.30, 1.88, 3.06, and 4.23 for the nominal C/SiO2 ratios of 0, 0.5, 1, 2, and 3, respectively.
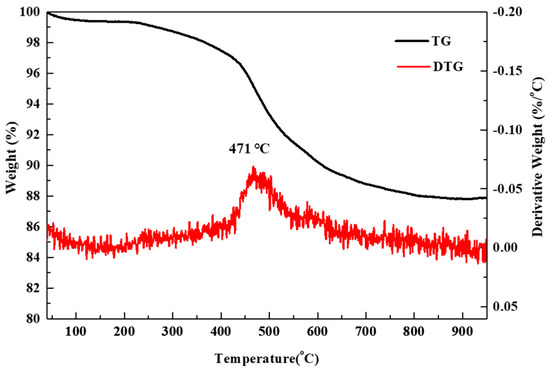
Figure 3.
TG and its derivative curves for the silica aerogel powder.
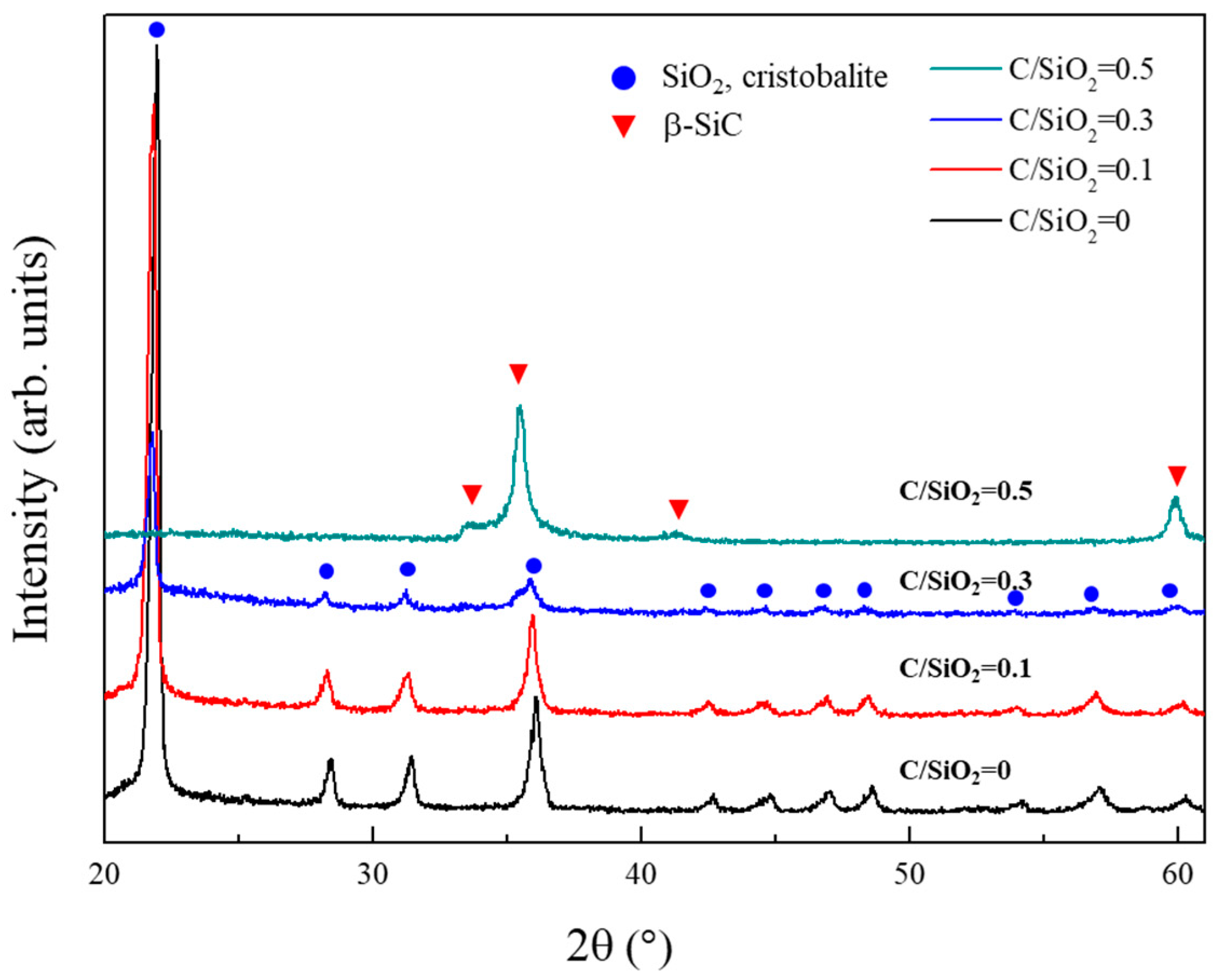
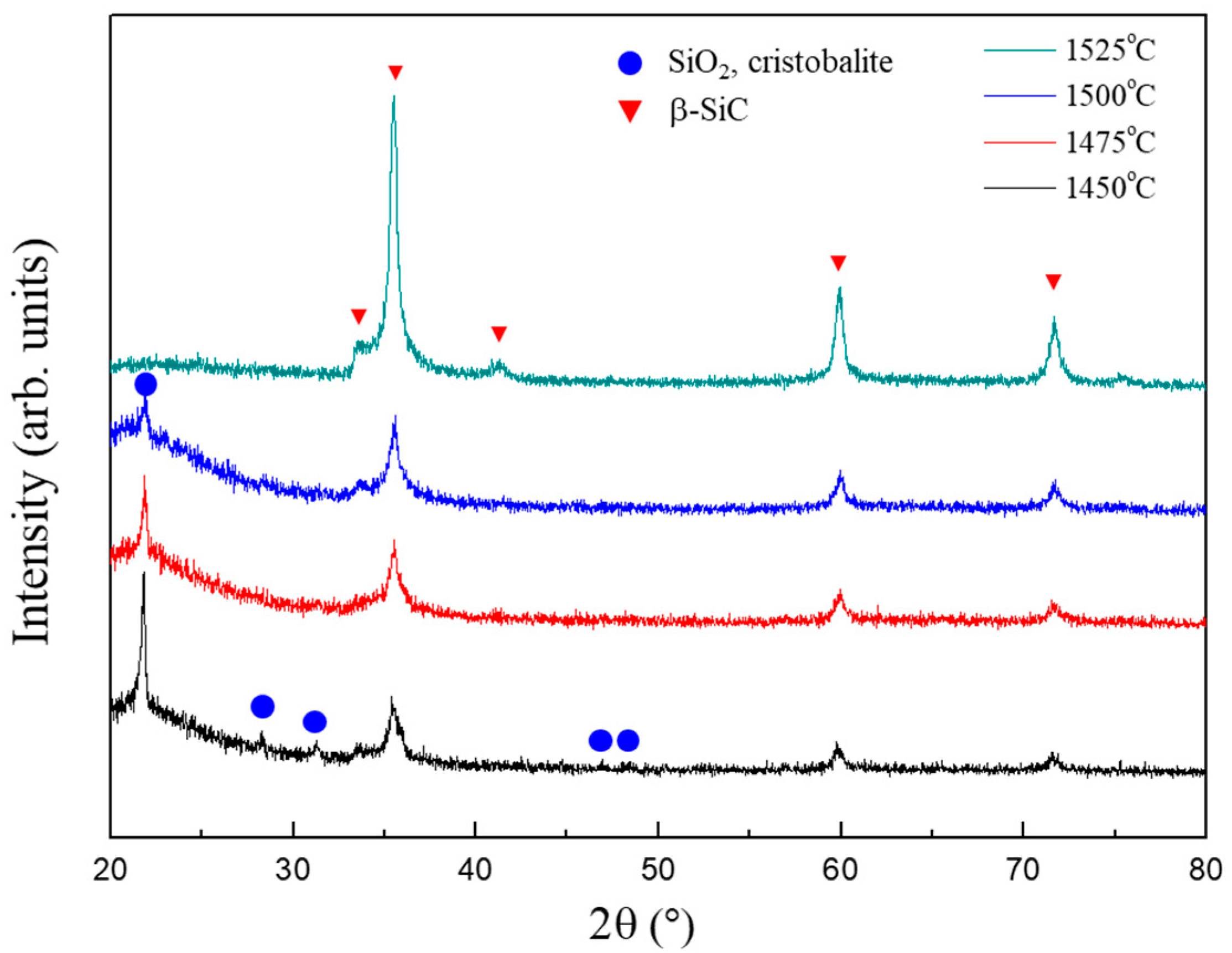
Figure 4 illustrates the XRD patterns of the powder samples synthesized from SiO2-C mixtures with various C/SiO2 ratios at 1525 °C for 1 h. The powder samples obtained at C/SiO2 ratios of 0 and 0.1 are α-cristobalite that formed from amorphous silica. This indicates that the carbothermal reduction did not proceed under these two conditions. This is reasonable because the C/SiO2 ratios of 0 and 0.1 are much lower than the stoichiometric value (3). On the other hand, in the powder sample synthesized from the SiO2-C mixture with a C/SiO2 ratio of 0.3, the α-cristobalite peak intensity was decreased and peaks corresponding to β-SiC appeared. At a C/SiO2 ratio of 0.5, all peaks due to α-cristobalite disappeared and single-phase β-SiC powder was obtained. Three peaks (35.5°, 41.5°, and 59.9°) are attributable to various β-SiC facets, and the small peak observed at 34.0° is due to stacking faults in β-SiC. This result is unexpected because the C/SiO2 ratio of 0.5 is also much lower than the stoichiometric ratio required for the carbothermal reduction of silica. Generally, a C/SiO2 ratio above the stoichiometric value is required for the carbothermal reduction of silica [14,23,26]. Thus, the result observed in Figure 4 strongly suggests that the surface methyl groups, which modify the silica aerogel particles, represent an efficient carbon source for the carbothermal reduction.
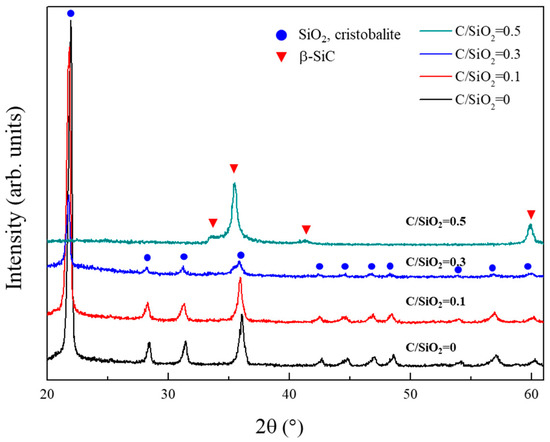
Figure 4.
XRD patterns of the powder samples synthesized under various C/SiO2 ratios at 1525 °C.
Figure 5 illustrates the XRD patterns of the powder samples synthesized from the SiO2-C mixture with a C/SiO2 ratio of 0.5 at various reduction temperatures for 1 h. At 1450 °C, the powder sample consists of β-SiC and α-cristobalite. As the reduction temperature increases, the peak intensity of α-cristobalite gradually decreases, while that of β-SiC increases. Based on Figure 5, it can be inferred that a reduction at 1525 °C is required to synthesize a single-phase β-SiC at a C/SiO2 ratio of 0.5.
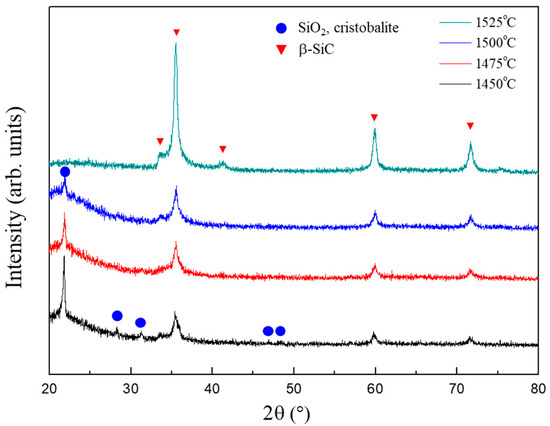
Figure 5.
XRD patterns of the powder samples synthesized from the SiO2–C mixtures with the C/SiO2 ratio of 0.5 at various reduction temperatures.
We calculated the equilibrium compositions for the carbothermal reduction reaction as a function of temperature in a previous study. The results indicated that β-SiC forms at temperatures above 1516 °C [35]. Based on the XRD analysis (Figure 5), β-SiC formed at 1450 °C, which is ~70 °C lower than the temperature obtained from the thermodynamic calculation (1516 °C). This discrepancy can be attributed to the enhanced reactivity between silica and carbon or the increased surface energy (nano-size effect) [36,37]. In this study, surface methyl groups are the carbon source, which are bonded to the silica aerogels at the atomistic level. Therefore, it can be expected that the carbothermal reaction proceeds in a highly reactive state. In addition, the high mesopore volume and high specific surface area of the silica aerogels allow the nano-size effect on the conversion of silica to SiC [25]. The thermodynamic calculation of the carbothermal reduction of silica considering the surface energy contribution indicates that the formation temperature for SiC can be reduced to 1410 °C [35].
To confirm that the surface methyl groups of the silica aerogel were the carbon source for the carbothermal reduction of silica, the surface methyl groups were removed by calcining the silica aerogel at 600 °C for 3 h in air. The resulting hydrophilic silica aerogel powder was mixed with carbon black, and the carbothermal reduction was performed as described in Section 2. For comparison, a commercial quartz powder was purchased from Junsei (extra pure, Japan) and used for an additional carbothermal reduction experiment.
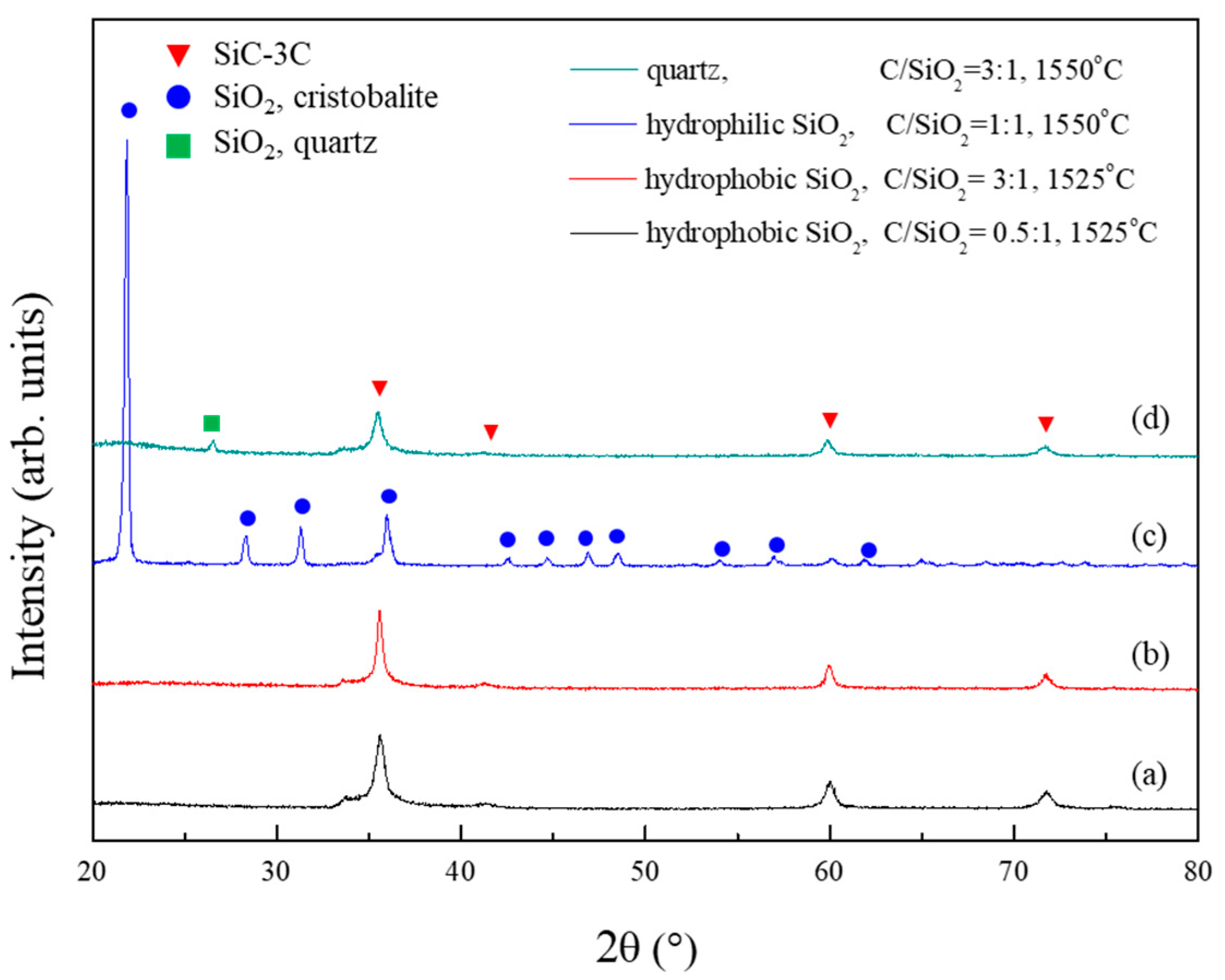
Figure 6 illustrates the XRD patterns of the powder samples synthesized from the hydrophobic silica aerogel, aerogel without surface methyl groups (hydrophilic silica aerogel), and quartz powders. The carbothermal reductions of the hydrophobic silica aerogel (Figure 6a,b) and other samples (Figure 6c,d) were performed at 1525 and 1550 °C, respectively. As described above, the powder sample synthesized from the hydrophobic silica aerogel containing surface methyl groups was single-phase β-SiC, although the C/SiO2 ratio was 0.5 and the reduction temperature was 1525 °C. By contrast, the XRD analysis indicates that the powder sample synthesized from the hydrophilic silica aerogel is α-cristobalite. Characteristic peaks corresponding to the crystalline β-SiC phase could not be observed. This phenomenon suggests that the surface methyl groups play important roles in the enhancement of the carbothermal reduction reaction of silica. With respect to quartz, α-cristobalite remained in the synthesized powder, although the reduction temperature and C/SiO2 ratio were higher than those of the hydrophobic silica aerogel.
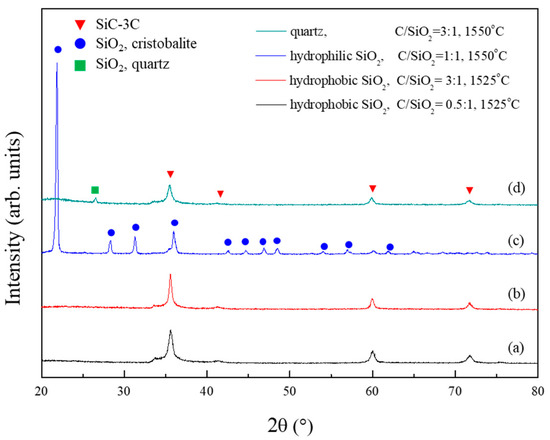
Figure 6.
XRD patterns of the powder samples synthesized from (a) the hydrophobic silica aerogel (C/SiO2 ratio of 0.5), (b) the hydrophobic silica aerogel (C/SiO2 ratio of 3), (c) the hydrophilic silica aerogel (C/SiO2 ratio of 1), and (d) the quartz (C/SiO2 ratio of 3).
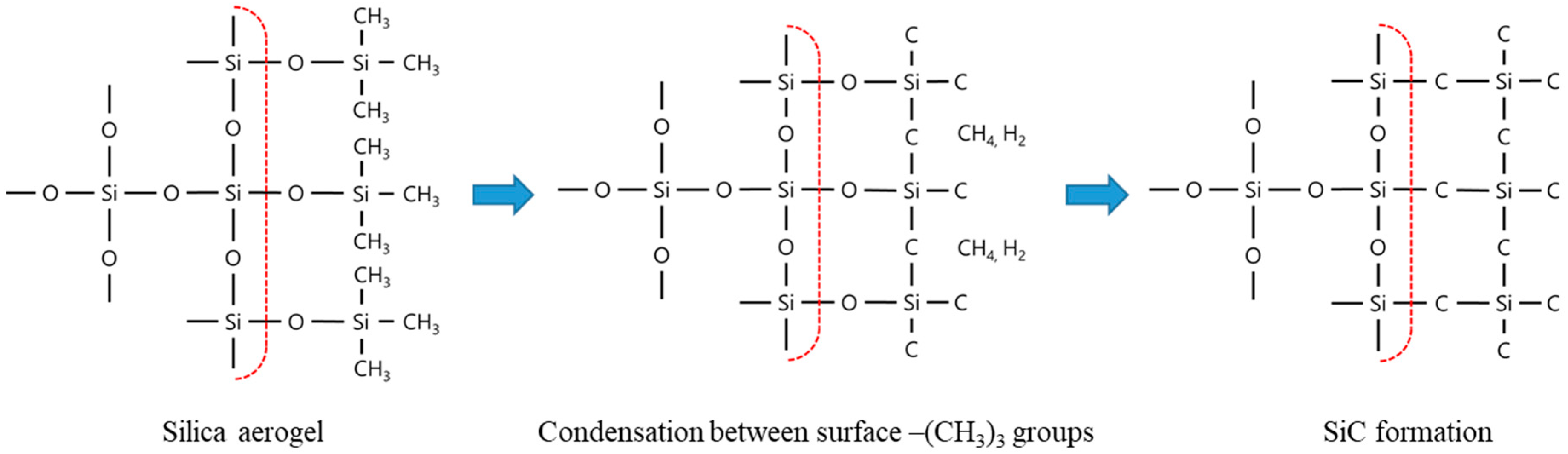
A schematic illustration of the proposed carbothermal reduction reaction mechanism for silica aerogels is shown in Figure 7. The surface of the hydrophobic silica aerogel was modified to contain methyl groups. During the carbothermal reduction of the aerogel, the C-H bonds were broken, whereas the tetrahedral environments of silicon and carbon were maintained. It can be inferred that amorphous hydrogenated SiC or hydrogenated Si-C-O (silicon oxycarbide) has a cristobalite form at temperatures between 1000 and 1400 °C [38]. During this process, condensation reactions between –Si(CH3)3 and neighboring –Si(CH3)3 groups occur, which lead to the formation of CH4 and H2 from the consumed CH3 groups [39]. Above 1400 °C, SiC particles nucleate and grow toward the inside of the silica aerogel particles via a gas-phase reaction between SiO and CO, as has typically been observed for the carbothermal reduction of silica. It appears that the surface methyl groups not only act as the carbon source but also serve as a template for the formation of the SiC particles [40,41,42]. As observed in Figure 6 and Figure 7, the SiC particles maintained the spherical morphology of the silica aerogel powder.
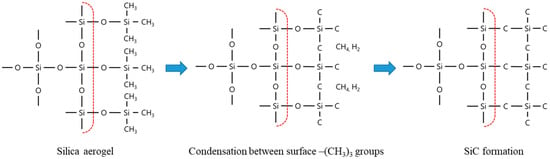
Figure 7.
Surface structure of the hydrophobic silica aerogel used in this study.
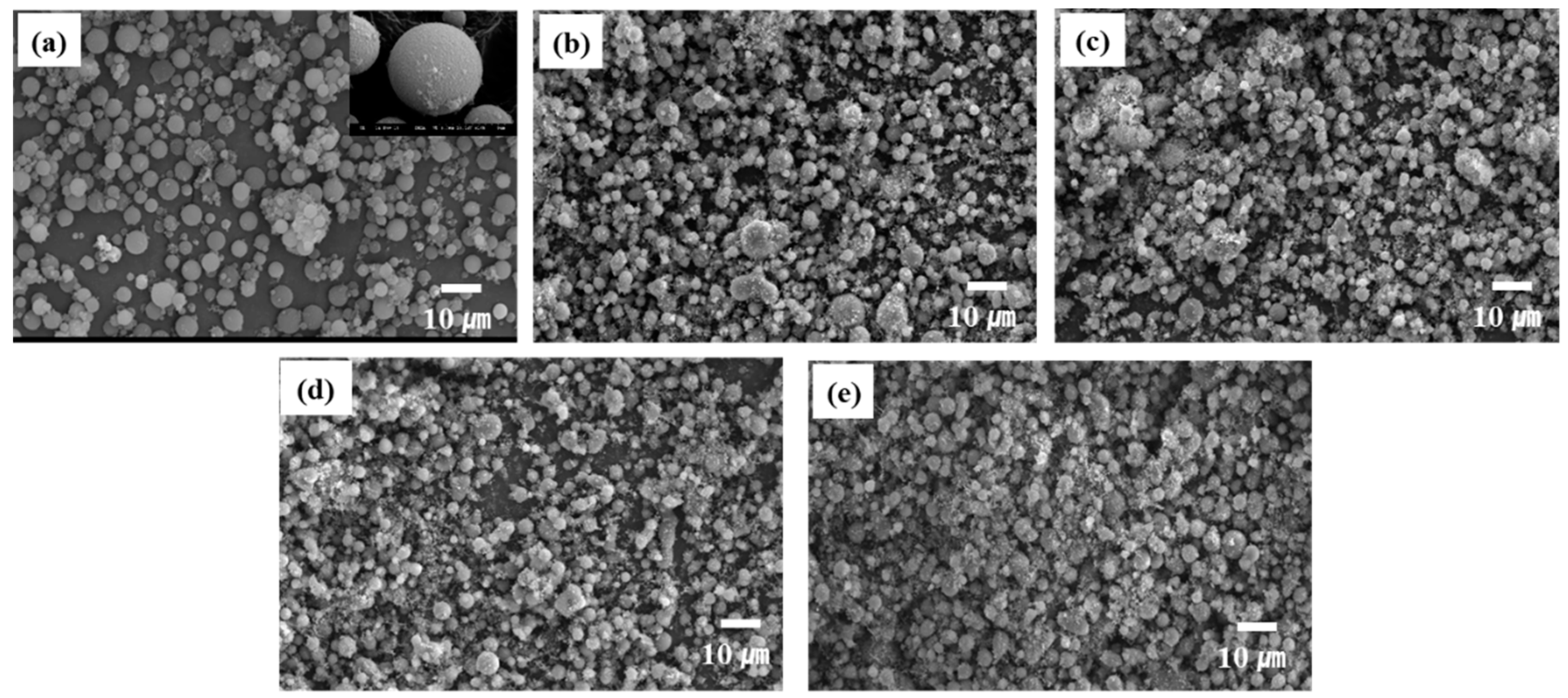
Figure 8 shows the SEM images of the silica aerogel and β-SiC powders synthesized from the SiO2-C mixtures at various reduction temperatures. The C/SiO2 ratio was 0.5. Based on Figure 8a, the starting silica aerogel had spherical morphology and significant aggregation cannot be observed. The particle size was estimated to be 5 to 10 μm. An interesting feature can be observed in Figure 8b–e, that is, the synthesized β-SiC powders exhibit spherical morphology, regardless of the reduction temperature.
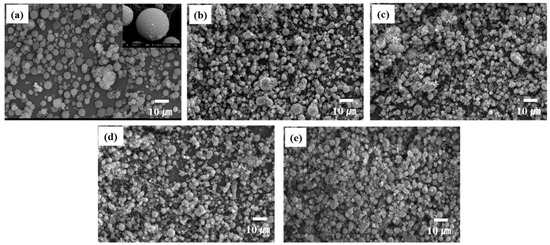
Figure 8.
SEM images of (a) the starting spherical silica aerogel and b-SiC powders synthesized from the SiO2–C mixtures at (b) 1450 °C, (c) 1475 °C, (d) 1500 °C, and (e) 1525 °C for 3 h. The C/SiO2 ratio was 0.5.
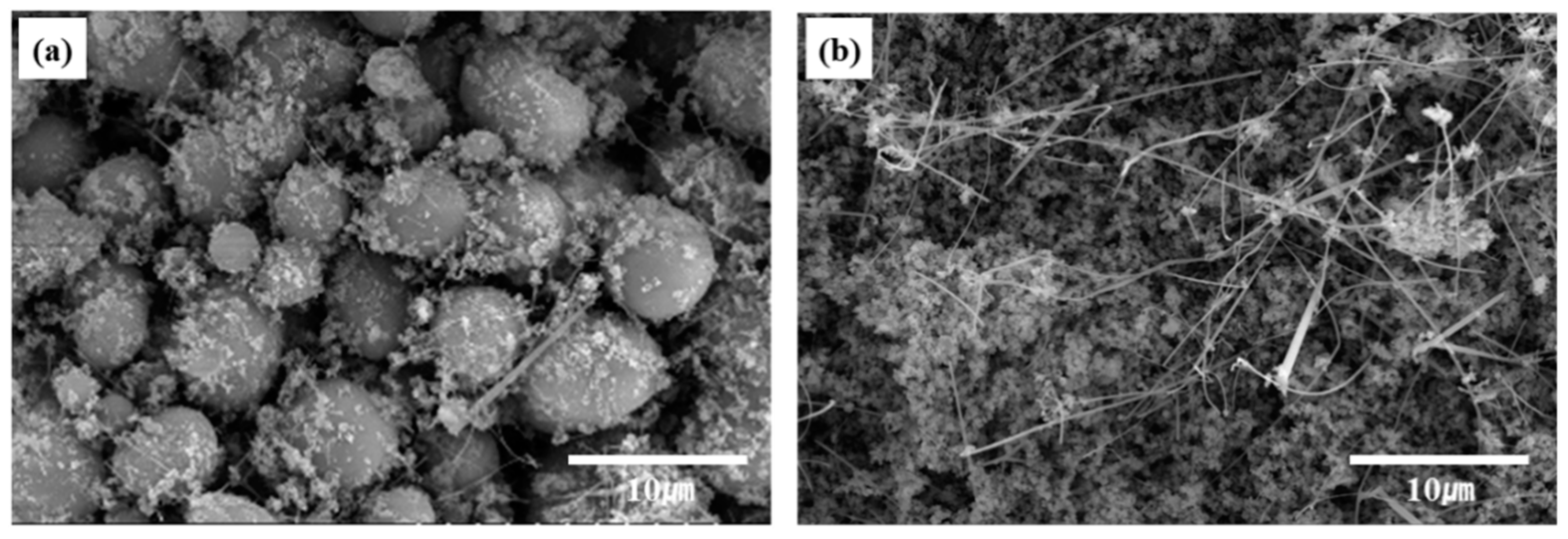
The particle size and morphology of the SiC powder samples obtained at different C/SiO2 ratios were analyzed using SEM. Figure 9 shows the SEM images of the β-SiC powders synthesized from SiO2-C mixtures with C/SiO2 ratios of 0.5 and 3. The carbothermal reduction was performed at 1525 °C for 1 h. The comparison of Figure 9a,b shows that the morphology of the SiC particles was completely different. At a C/SiO2 ratio of 0.5, the SiC particles were spherical. By contrast, a high C/SiO2 ratio of 3 yielded an agglomerated structure consisting of fine SiC particles. In addition, nanofiber-like SiC was observed between the fine SiC particles. The BET specific surface areas of the SiC powders prepared using C/SiO2 ratios of 1 and 3 were estimated to be 6.4 and 12.7 m2/g, respectively. This shows that the specific surface areas increase with the C/SiO2 ratio. These results suggest that the C/SiO2 ratio affects the nucleation and growth mechanisms during the synthesis of SiC powder.
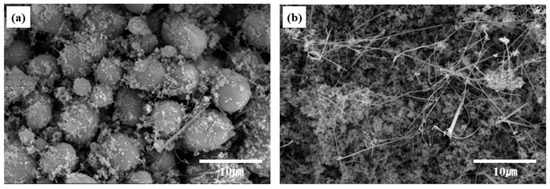
Figure 9.
SEM images of the SiC powders synthesized from SiO2–C mixtures with C/SiO2 ratios of (a) 0.5 and (b) 3 at 1525 °C for 1 h. The powder samples were heat-treated in air at 1000 °C for 1 h after reduction.
To elucidate the effect of the porous structure of the SiC on oxidation, the oxygen content in the SiC powder samples was determined using an oxygen–nitrogen analyzer. The oxygen contents in the SiC powder prepared at 1525 °C for 5 h with C/SiO2 ratios of 0.5 and 3 were 0.36 and 0.05 wt.% for the samples after the heat treatment in air, respectively. Almost-pure SiC powders were obtained when the C/SiO2 ratio was 3.
The overall carbothermal reduction reaction for silica, which is a solid (SiO2)–solid (C) reaction, is shown in Equation (1). At low temperature, the carbothermal reduction reaction can be written in as follows [43]:
SiO2(s) + C(s) → SiO(g) + CO(g)
SiO(g) + 2C(s) → SiC(s) + CO(g)
At a high temperature and high PSiO/PCO ratio, whisker- or nanofiber-like SiC can be grown on the SiC nuclei based on Equation (4), which is similar to the CVD of SiC [35,44]. The SiC particles obtained through Equation (3) serve as nucleation sites for Equation (4).
SiO(g) + 3CO(g) → SiC(s) + 2CO2(g)
The morphologies of the SiC particles differ, as shown in Figure 9a,b, which indicates that the formation mechanisms for the SiC particles differ. At a C/SiO2 ratio of 0.5, the morphologies of the synthesized SiC particles are similar to that of the starting silica aerogel, suggesting that the SiC formation mechanism relies on Equation (1), that is, the solid–solid reaction. The solid SiO2 particles directly react with carbon from the surface methyl groups, whereby spherical SiC powder was observed at a C/SiO2 ratio of 0.5.
The molar ratio of carbon in the SiO2-C mixture is of great importance because the CO gas that is produced by Equations (1) or (2) was sufficiently supplied. The high C/SiO2 ratio (3) leads to an increase in the partial pressure of gaseous species, such as SiO(g) and CO(g); consequently, SiC particles form and grow, as illustrated in Equations (3) and (4). The nanofiber-like SiC observed in Figure 9b might be due to the reaction expressed in Equation (4).
4. Conclusions
β-SiC powders were synthesized by the carbothermal reduction of methyl-bearing surface-modified silica aerogel/carbon mixtures. Based on the XRD analysis, single-phase β-SiC was synthesized at 1525 °C from the silica aerogel/carbon mixture with a C/SiO2 ratio of 3. The surface methyl groups that are covalently bonded to the silica aerogel serve as a carbon source for the carbothermal reduction reaction; therefore, the formation temperature for SiC is lower. The direct bond between silicon and carbon atoms in the surface methyl group results in the enhanced kinetics of the carbothermal reduction reaction. The results show that the C/SiO2 ratio plays an important role in both the reduction of the formation temperature for SiC and the control of the morphology of the synthesized SiC particles. At a C/SiO2 ratio of 0.5, SiC maintains the original spherical morphology of the silica aerogels. By contrast, agglomerates with fine SiC particles were synthesized from the SiO2-C mixture at a C/SiO2 ratio of 3. This phenomenon can be attributed to different formation mechanisms for SiC, which depend on the silica–carbon ratio of the starting silica/carbon mixture. Almost pure SiC powder (99.95%) was obtained when the silica aerogel/carbon mixture at a C/SiO2 ratio of 3 was reduced at 1525 °C for 5 h.
Author Contributions
Conceptualization, K.-J.L. and H.H.; methodology, Y.K., Y.H.K. and S.W.B.; analysis, K.-J.L., Y.K. and Y.H.K.; writing—original draft preparation, K.-J.L. writing—review and editing, H.H.; supervision, H.H.; project administration, H.H. and S.W.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by LG Chem. This research was supported by the Ministry of Trade, Industry & Energy (MOTIE), Korea Institute for Advancement of Technology (KIAT), through the Encouragement Program for The Industries of Economic Cooperation Region (P0002149).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shcherban, N.D. Review on synthesis, structure, physical and chemical properties and functional characteristics of porous silicon carbide. J. Ind. Eng. Chem. 2017, 50, 15–28. [Google Scholar] [CrossRef]
- Kim, K.; Hahn, Y.; Lee, S.; Choi, K.; Lee, J.-H. Mechanical Properties of Cf/SiC Composite Using a Combined Process of Chemical Vapor Infiltration and Precursor Infiltration Pyrolysis. J. Korean Ceram. Soc. 2018, 55, 392–399. [Google Scholar] [CrossRef]
- Seo, Y.; Kim, Y.; Nishimura, T.; Seo, W.S. High-temperature strength of a thermally conductive silicon carbide ceramic sintered with yttria and scandia. J. Eur. Ceram. Soc. 2016, 36, 3755–3760. [Google Scholar] [CrossRef]
- Chen, H.; Wang, X.; Xue, F.; Huang, Y.; Zhou, K.; Zhang, D. 3D printing of SiC ceramic, Direct ink writing with a solution of preceramic polymers. J. Eur. Ceram. Soc. 2018, 38, 5294–5300. [Google Scholar] [CrossRef]
- Bernardo, E.; Fiocco, L.; Parcianello, G.; Storti, E.; Colombo, P. Advanced ceramics from preceramic polymers modified at the nano-scale: A review. Materials 2014, 7, 1927–1956. [Google Scholar] [CrossRef]
- Prochazka, S.; Scanlan, R.M. Effect of boron and carbon on sintering of SiC. J. Am. Ceram. Soc. 1975, 58, 72. [Google Scholar] [CrossRef]
- Syväjärvi, M.; Ma, Q.; Jokubavicius, V.; Galeckas, A.; Sun, J.; Liu, X.; Jansson, M.; Wellmann, P.; Linnarsson, M.; Runde, P.; et al. Cubic silicon carbide as a potential photovoltaic material. Sol. Energy Mater. Sol. Cells 2016, 145, 104–108. [Google Scholar] [CrossRef]
- Kim, J.G.; Jeong, J.H.; Kim, Y.; Makarov, Y.; Choi, D.J. Evaluation of the change in properties caused by axial and radial temperature gradients in silicon carbide crystal growth using the physical vapor transport method. Acta Mater. 2014, 77, 54–59. [Google Scholar] [CrossRef]
- Sciti, D.; Bellosi, A. Effects of additives on densification, microstructure and properties of liquid-phase sintered silicon carbide. J. Mater. Sci. 2000, 35, 3849–3855. [Google Scholar] [CrossRef]
- Wang, X.; Cai, D.; Zhang, H. Increase of SiC sublimation growth rate by optimizing of powder packaging. J. Cryst. Growth 2007, 305, 122–132. [Google Scholar] [CrossRef]
- Hayun, S.; Paris, V.; Mitrani, R.; Kalabukhov, S.; Dariel, M.P.; Zaretsky, E.; Frage, N. Microstructure and mechanical properties of silicon carbide processed by Spark Plasma Sintering (SPS). Ceram. Int. 2012, 38, 6335–6340. [Google Scholar] [CrossRef]
- Kim, J.G.; Jung, E.J.; Kim, Y.; Makarov, Y.; Choi, D.J. Quality improvement of single crystal 4H SiC grown with a purified β-SiC powder source. Ceram. Int. 2014, 40, 3953–3959. [Google Scholar] [CrossRef]
- Omidi, Z.; Ghasemi, A.; Bakhshi, S.R. Synthesis and characterization of SiC ultrafine particles by means of sol-gel and carbothermal reduction methods. Ceram. Int. 2015, 41, 5779–5784. [Google Scholar] [CrossRef]
- Barbouche, M.; Zaghouani, R.B.; Benammar, N.E.; Khirouni, K.; Ezzaouia, H. Synthesis and characterization of 3C-SiC by rapid silica carbothermal reduction. Int. J. Adv. Manuf. Technol. 2017, 91, 1339–1345. [Google Scholar] [CrossRef]
- Kim, J.; Choi, S.; Kim, J.; Choi, S.C. Characteristics of silicon carbide nanowires synthesized on porous body by carbothermal reduction. J. Korean Ceram. Soc. 2018, 55, 285–289. [Google Scholar] [CrossRef]
- Kwon, W.T.; Kim, S.R.; Kim, Y.; Lee, Y.J.; Won, J.Y.; Park, W.K.; Oh, S.C. Effect of temperature and carbon contents on the synthesis of β-SiC from silicon sludge by direct carbonization method. Mater Sci. Forum. 2012, 724, 45–48. [Google Scholar] [CrossRef]
- Yang, Y.; Lin, Z.; Li, J. Synthesis of SiC by silicon and carbon combustion in air. J. Eur. Ceram. Soc. 2009, 29, 175–180. [Google Scholar] [CrossRef]
- Colombo, P.; Mera, G.; Riedel, R.; Soraru, G.D. Polymer-derived ceramics: 40 years of research and innovation in advanced ceramics. J. Am. Ceram. Soc. 2010, 93, 1805–1837. [Google Scholar] [CrossRef]
- Andrievski, R.A. Synthesis, structure and properties of nanosized silicon carbide. Rev. Adv. Mater Sci. 2009, 22, 1–20. [Google Scholar]
- Yazdanfar, M.; Pedersen, H.; Sukkaew, P.; Ivanov, I.G.; Danielsson, Ö.; Kordina, O.; Janzén, E. On the use of methane as a carbon precursor in Chemical Vapor Deposition of silicon carbide. J. Cryst. Growth 2014, 390, 24–29. [Google Scholar] [CrossRef]
- Galvagno, S.; Portofino, S.; Casciaro, G.; Casu, S.; d’Aquino, L.; Martino, M.; Russo, A.; Bezzi, G. Synthesis of beta silicon carbide powders from biomass gasification residue. J. Mater. Sci. 2007, 42, 6878–6886. [Google Scholar] [CrossRef]
- Kevorkijan, V.M.; Komac, M.; Kolar, D. Low-temperature synthesis of sinterable SiC powders by carbothermic reduction of colloidal SiO2. J. Mater. Sci. 1992, 27, 2705–2712. [Google Scholar] [CrossRef]
- Bağci, C.; Arik, H. Synthesis of SiC Powders by carbothermal reduction of enriched brown sepiolite with carbon black. J. Mater. Eng. Perform. 2013, 22, 958–963. [Google Scholar] [CrossRef]
- Shimoo, T. Carbon removal and oxidation of SiC powder synthesized by carbothermic reduction of silica. J. Ceram Soc. Jpn. 1991, 99, 768–773. [Google Scholar] [CrossRef][Green Version]
- Parmentier, J.; Patarin, J.; Dentzer, J.; Vix-Guterl, C. Formation of SiC via carbothermal reduction of a carbon-containing mesoporous MCM-48 silica phase: A new route to produce high surface area SiC. Ceram. Int. 2002, 28, 1–7. [Google Scholar] [CrossRef]
- Meng, G.W.; Cui, Z.; Zhang, L.D.; Phillipp, F. Growth and characterization of nanostructured β-SiC via carbothermal reduction of SiO2 xerogels containing carbon nanoparticles. J. Cryst. Growth 2000, 209, 801–806. [Google Scholar] [CrossRef]
- Lee, K.; Kim, Y.H.; Lee, J.K.; Hwang, H.J. Fast synthesis of spherical silica aerogel powders by emulsion polymerization from water glass. ChemistrySelect 2018, 3, 1257–1261. [Google Scholar] [CrossRef]
- Sarawade, P.B.; Kim, J.K.; Hilonga, A.; Quang, D.V.; Jeon, S.J.; Kim, H.T. Synthesis of sodium silicate-based hydrophilic silica aerogel beads with superior properties: Effect of heat-treatment. J. Non-Cryst. Solids 2011, 357, 2156–2162. [Google Scholar] [CrossRef]
- Bhagat, S.D.; Kim, Y.H.; Suh, K.H.; Ahn, Y.S.; Yeo, J.G.; Han, J.H. Superhydrophobic silica aerogel powders with simultaneous surface modification, solvent exchange and sodium ion removal from hydrogels. Microporous Mesoporous Mater. 2008, 112, 504–509. [Google Scholar] [CrossRef]
- Rao, A.P.; Rao, A.V.; Pajonk, G.M. Hydrophobic and physical properties of the two step processed ambient pressure dried silica aerogels with various exchanging solvents. J. Sol-Gel Sci. Technol. 2005, 36, 285–292. [Google Scholar] [CrossRef]
- He, S.; Huang, Y.; Ghen, G.; Feng, M.; Dai, H.; Yuan, B.; Chen, X. Effect of heat treatment on hydrophobic silica aerogel. J. Hazard. Mater. 2019, 362, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.D.; Cheng, X.; Zheng, Y.M. Facile co-precursor sol-gel synthesis of a novel amine-modified silica aerogel for high efficiency carbon dioxide capture. J. Colloid Interface Sci. 2018, 530, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Stojanovic, A.; Zhao, S.; Angelica, E.; Malfait, W.J.; Koebel, M.M. Three routes to superinsulating silica aerogel powder. J. Sol-Gel Sci. Technol. 2019, 90, 57–66. [Google Scholar] [CrossRef]
- Bhagat, S.D.; Oh, C.; Kim, Y.; Ahn, Y.S.; Yeo, J.G. Methyltrimethoxysilane based monolithic silica aerogels via ambient pressure drying. Microporous Mesoporous Mater. 2007, 100, 350–355. [Google Scholar] [CrossRef]
- Jeong, S.; Seo, W.; Jung, I.; Lee, K.J.; Hwang, H.J. Thermodynamic analysis of the synthesis of silicon carbide nanofibers from exfoliated graphite and amorphous silica. CrystEngComm 2014, 16, 2348–2351. [Google Scholar] [CrossRef]
- Choi, H.; Lee, J. Continuous synthesis of silicon carbide whiskers. J. Mater. Sci. 1995, 30, 1982–1986. [Google Scholar] [CrossRef]
- Eichhammer, Y.; Roeck, J.; Moelans, N.; Iacopi, F.; Blanpain, B.; Heyns, M. Calculation of the Au-Ge phase diagram for nanoparticles. Arch. Metall. Mater. 2008, 53, 1133–1139. [Google Scholar]
- Bouillon, E.; Langlais, F.; Pailler, R.; Naslain, R.; Cruege, F.; Huong, P.V.; Sarthou, J.C.; Delpuech, A.; Laffon, C.; Lagarde, P.; et al. Conversion mechanisms of a polycarbosilane precursor into an SiC-based ceramic material. J. Mater. Sci. 1991, 26, 1333–1345. [Google Scholar] [CrossRef]
- Soraru, G.D.; Babonneau, F.; Mackenzie, J.D. Structural evolutions from polycarbosilane to SiC ceramic. J. Mater. Sci. 1990, 25, 3886–3893. [Google Scholar] [CrossRef]
- Xie, W.; Möbus, G.; Zhang, S. Molten salt synthesis of silicon carbide nanorods using carbon nanotubes as templates. J. Mater. Chem. 2011, 21, 18325–18330. [Google Scholar] [CrossRef]
- Wu, Y.J.; Qin, W.; Yang, Z.X.; Wu, J.S.; Zhang, Y.F. Preparation of high-quality ß-SiC nanowhiskers by using carbon fibres as carbon source. J. Mater. Sci. 2004, 39, 5563–5565. [Google Scholar] [CrossRef]
- Tony, V.; Voon, C.H.; Lee, C.C.; Lim, B.Y.; Arshad, M.M.; Gopinath, S.C.; Foo, K.L.; Ruslinda, A.R.; Hashim, U.; Nashaain, M.N. Novel synthesis of silicon carbide nanotubes by microwave heating of blended silicon dioxide and multi-walled carbon nanotubes: The effect of the heating temperature. Ceram. Int. 2016, 42, 17642–17649. [Google Scholar] [CrossRef]
- Van Dijen, F.K.; Metselaar, R. The chemistry of the carbothermal synthesis of β-SiC: Reaction mechanism, reaction rate and grain growth. J. Eur. Ceram. Soc. 1991, 7, 177–184. [Google Scholar] [CrossRef]
- Moshtaghioun, B.M.; Poyato, R.; Cumbrera, F.L.; de Bernardi-Martin, S.; Monshi, A.; Abbasi, M.H.; Karimzadeh, F.; Dominguez-Rodriguez, A. Rapid carbothermic synthesis of silicon carbide nano powders by using microwave heating. J. Eur. Ceram. Soc. 2012, 32, 1787–1794. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).