Beta-Catenin in Pseudoexfoliation Syndrome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tissue Extraction
2.2. The Ultrastructural Localization of β-Catenin Using AU
2.3. The Fluorescent Labelling of Cell–Cell Junctions
2.4. Statistical Analysis
3. Results
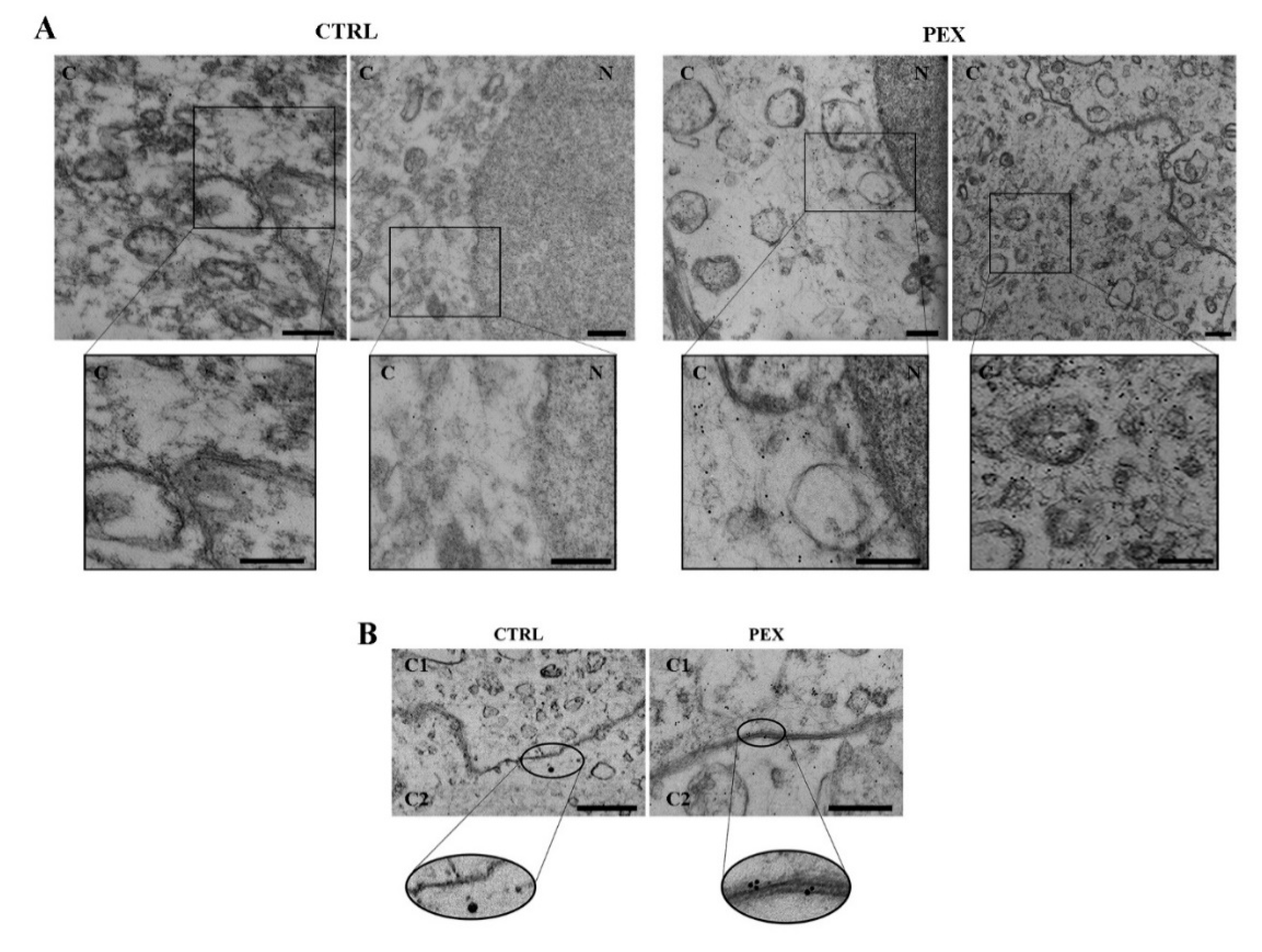
3.1. The Ultrastructural Localization of β-Catenin
3.2. The Fluorescence Staining of β-Catenin
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shapiro, L.; Weis, W.I. Structure and biochemistry of cadherins and catenins. Cold Spring Harb. Perspect. Biol. 2009, 1, a003053. [Google Scholar] [CrossRef] [PubMed]
- Clevers, H. Wnt/beta-catenin signaling in development and disease. Cell 2006, 127, 469–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamai, K.; Semenov, M.; Kato, Y.; Spokony, R.; Liu, C.; Katsuyama, Y.; Hess, F.; Saint-Jeannet, J.P.; He, X. LDL-receptor-related proteins in Wnt signal transduction. Nature 2000, 407, 530–535. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Hu, Y.; Chen, Y.; Zhou, K.K.; Zhang, B.; Gao, G.; Ma, J.X. The pathogenic role of the canonical Wnt pathway in age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2010, 51, 4371–4379. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; He, X. Wnt/beta-catenin signaling: New (and old) players and new insights. Curr. Opin. Cell Biol. 2008, 20, 119–125. [Google Scholar] [CrossRef] [Green Version]
- Fodde, R.; Brabletz, T. Wnt/beta-catenin signaling in cancer stemness and malignant behavior. Curr. Opin. Cell Biol. 2007, 19, 150–158. [Google Scholar] [CrossRef]
- Xiao, L.; Zhou, D.; Tan, R.J.; Fu, H.; Zhou, L.; Hou, F.F.; Liu, Y. Sustained Activation of Wnt/β-Catenin Signaling Drives AKI to CKD Progression. J. Am. Soc. Nephrol. 2016, 27, 1727–1740. [Google Scholar] [CrossRef] [Green Version]
- Yao, L.; Sun, Y.T.; Sun, W.; Xu, T.H.; Ren, C.; Fan, X.; Sun, L.; Liu, L.L.; Feng, J.M.; Ma, J.F.; et al. High phosphorus level leads to aortic calcification via β-catenin in chronic kidney disease. Am. J. Nephrol. 2015, 41, 28–36. [Google Scholar] [CrossRef]
- Zhou, G.; Wu, J.; Gu, C.; Wang, B.; Abel, E.D.; Cheung, A.K.; Huang, Y. Prorenin independently causes hypertension and renal and cardiac fibrosis in cyp1a1-prorenin transgenic rats. Clin. Sci. 2018, 132, 1345–1363. [Google Scholar] [CrossRef] [Green Version]
- Heiser, P.W.; Cano, D.A.; Landsman, L.; Kim, G.E.; Kench, J.G.; Klimstra, D.S.; Taketo, M.M.; Biankin, A.V.; Hebrok, M. Stabilization of beta-catenin induces pancreas tumor formation. Gastroenterology 2008, 135, 1288–1300. [Google Scholar] [CrossRef] [Green Version]
- Zeng, G.; Germinaro, M.; Micsenyi, A.; Monga, N.K.; Bell, A.; Sood, A.; Malhotra, V.; Sood, N.; Midda, V.; Monga, D.K.; et al. Aberrant Wnt/beta-catenin signaling in pancreatic adenocarcinoma. Neoplasia 2006, 8, 279–289. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Heidt, D.G.; Lee, C.J.; Yang, H.; Logsdon, C.D.; Zhang, L.; Fearon, E.R.; Ljungman, M.; Simeone, D.M. Oncogenic function of ATDC in pancreatic cancer through Wnt pathway activation and beta-catenin stabilization. Cancer Cell 2009, 15, 207–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pramanik, K.C.; Fofaria, N.M.; Gupta, P.; Ranjan, A.; Kim, S.H.; Srivastava, S.K. Inhibition of β-Catenin signaling suppresses pancreatic tumor growth by disrupting nuclear β-Catenin/TCF-1 complex: Critical role of STAT-3. Oncotarget 2015, 10, 11561–11574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddy, H.S.; Keene, C.D.; Chang, S.H.; Jian-Amadi, A.; Cimino, P.J. Immunohistochemical profiling including beta-catenin in conjunctival melanocytic lesions. Exp. Mol. Pathol. 2017, 102, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Dissanayake, W.C.; Sorrenson, B.; Shepherd, P.R. The role of adherens junction proteins in the regulation of insulin secretion. Biosci. Rep. 2018, 38, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sorrenson, B.; Cognard, E.; Lee, K.L.; Dissanayake, W.C.; Fu, Y.; Han, W.; Hughes, W.E.; Shepherd, P.R. A critical role for β-catenin in modulating levels of insulin secretion from β-cells by regulating actin cytoskeleton and insulin vesicle localization. J. Biol. Chem. 2016, 291, 25888–25900. [Google Scholar] [CrossRef] [Green Version]
- Stafiej, J.; Hałas-Wiśniewska, M.; Izdebska, M.; Gagat, M.; Grzanka, D.; Grzanka, A.; Malukiewicz, G. Immunohistochemical analysis of microsomal glutathione S-transferase 1 and clusterin expression in lens epithelial cells of patients with pseudoexfoliation syndrome. Exp. Ther. Med. 2017, 13, 1057–1063. [Google Scholar] [CrossRef]
- Izdebska, M.; Gagat, M.; Grzanka, D.; Grzanka, A. Ultrastructural localization of F-actin using phalloidin and quantum dots in HL-60 promyelocytic leukemia cell line after cell death induction by arsenic trioxide. Acta Histochem. 2013, 115, 487–495. [Google Scholar] [CrossRef]
- Schumacher, S.; Schlötzer-Schrehardt, U.; Martus, P.; Lang, W.; Naumann, G.O. Pseudoexfoliation syndrome and aneurysms of the abdominal aorta. Lancet 2001, 357, 359–360. [Google Scholar] [CrossRef]
- Dai, C.; Stolz, D.B.; Kiss, L.P.; Monga, S.P.; Holzman, L.B.; Liu, Y. Wnt/beta-catenin signaling promotes podocyte dysfunction and albuminuria. J. Am. Soc. Nephrol. 2009, 20, 1997–2008. [Google Scholar] [CrossRef] [Green Version]
- von Toerne, C.; Schmidt, C.; Adams, J.; Kiss, E.; Bedke, J.; Porubsky, S.; Gretz, N.; Lindenmeyer, M.T.; Cohen, C.D.; Gröne, H.J.; et al. Wnt pathway regulation in chronic renal allograft damage. Am. J. Transplant. 2009, 9, 2223–2239. [Google Scholar] [CrossRef]
- Lin, X.; Zha, Y.; Zeng, X.Z.; Dong, R.; Wang, Q.H.; Wang, D.T. Role of the Wnt/β-catenin signaling pathway in inducing apoptosis and renal fibrosis in 5/6-nephrectomized rats. Mol. Med. Rep. 2017, 15, 3575–3582. [Google Scholar] [CrossRef] [PubMed]
- Hartsock, A.; Nelson, W.J. Adherens and tight junctions: Structure, function and connections to the actin cytoskeleton. Biochim. Biophys. Acta 2008, 1778, 660–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vermette, D.; Hu, P.; Canarie, M.F.; Funaro, M.; Glover, J.; Pierce, R.W. Tight junction structure, function, and assessment in the critically ill: A systematic review. Intensive Care Med. Exp. 2018, 6, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McEwen, A.E.; Escobar, D.E.; Gottardi, C.J. Signaling from the adherens junction. Sub-Cell. Biochem. 2012, 60, 171–196. [Google Scholar]
- Cadigan, K.M.; Peifer, M. Wnt signaling from development to disease: Insights from model systems. Cold Spring Harb. Perspect. Biol. 2009, 1, a002881. [Google Scholar] [CrossRef] [Green Version]
- Kam, Y.; Quaranta, V. Cadherin-Bound β-Catenin Feeds into the Wnt Pathway upon Adherens Junctions Dissociation: Evidence for an Intersection between β-Catenin Pools. PLoS ONE 2009, 4, e4580. [Google Scholar] [CrossRef] [PubMed]
- Valenta, T.; Hausmann, G.; Basler, K. The many faces and functions of β-catenin. EMBO J. 2012, 31, 2714–2736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasioukhin, V. Adherens junctions and cancer. Sub-Cell. Biochem. 2012, 60, 379–414. [Google Scholar]
- Yang, S.; Liu, Y.; Li, M.Y.; Ng, C.S.H.; Yang, S.L.; Wang, S.; Zou, C.; Dong, Y.; Du, J.; Long, X.; et al. FOXP3 promotes tumor growth and metastasis by activating Wnt/β-catenin signaling pathway and EMT in non-small cell lung cancer. Mol. Cancer 2017, 16, 124. [Google Scholar] [CrossRef]
- Sedgwick, A.E.; D’Souza-Schorey, C. Wnt Signaling in Cell Motility and Invasion: Drawing Parallels between Development and Cancer. Cancers 2016, 8, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antosova, B.; Smolikova, J.; Borkovcova, R.; Strnad, H.; Lachova, J.; Machon, O.; Kozmik, Z. Ectopic activation of Wnt/β-catenin signaling in lens fiber cells results in cataract formation and aberrant fiber cell differentiation. PLoS ONE 2013, 8, e78279. [Google Scholar] [CrossRef] [Green Version]
- Chong, C.C.; Stump, R.J.; Lovicu, F.J.; McAvoy, J.W. TGFbeta promotes Wnt expression during cataract development. Exp. Eye Res. 2009, 88, 307–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bao, X.L.; Song, H.; Chen, Z.; Tang, X. Wnt3a promotes epithelial-mesenchymal transition, migration, and proliferation of lens epithelial cells. Mol. Vis. 2012, 18, 1890–1983. [Google Scholar] [PubMed]
- Zhang, Y.; Jeffrey, J.; Dong, F.; Zhang, J.; Kao, W.W.; Liu, C.Y.; Yuan, Y. Repressed Wnt Signaling Accelerates the Aging Process in Mouse Eyes. J. Ophthalmol. 2019, 2019, 7604396. [Google Scholar] [CrossRef] [Green Version]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stafiej, J.; Hałas-Wiśniewska, M.; Izdebska, M.; Gagat, M.; Grzanka, A.; Malukiewicz, G. Beta-Catenin in Pseudoexfoliation Syndrome. Appl. Sci. 2020, 10, 6199. https://doi.org/10.3390/app10186199
Stafiej J, Hałas-Wiśniewska M, Izdebska M, Gagat M, Grzanka A, Malukiewicz G. Beta-Catenin in Pseudoexfoliation Syndrome. Applied Sciences. 2020; 10(18):6199. https://doi.org/10.3390/app10186199
Chicago/Turabian StyleStafiej, Joanna, Marta Hałas-Wiśniewska, Magdalena Izdebska, Maciej Gagat, Alina Grzanka, and Grażyna Malukiewicz. 2020. "Beta-Catenin in Pseudoexfoliation Syndrome" Applied Sciences 10, no. 18: 6199. https://doi.org/10.3390/app10186199




