A Palette of Efficient and Stable Far-Red and NIR Dye Lasers
Abstract
:Featured Application
Abstract
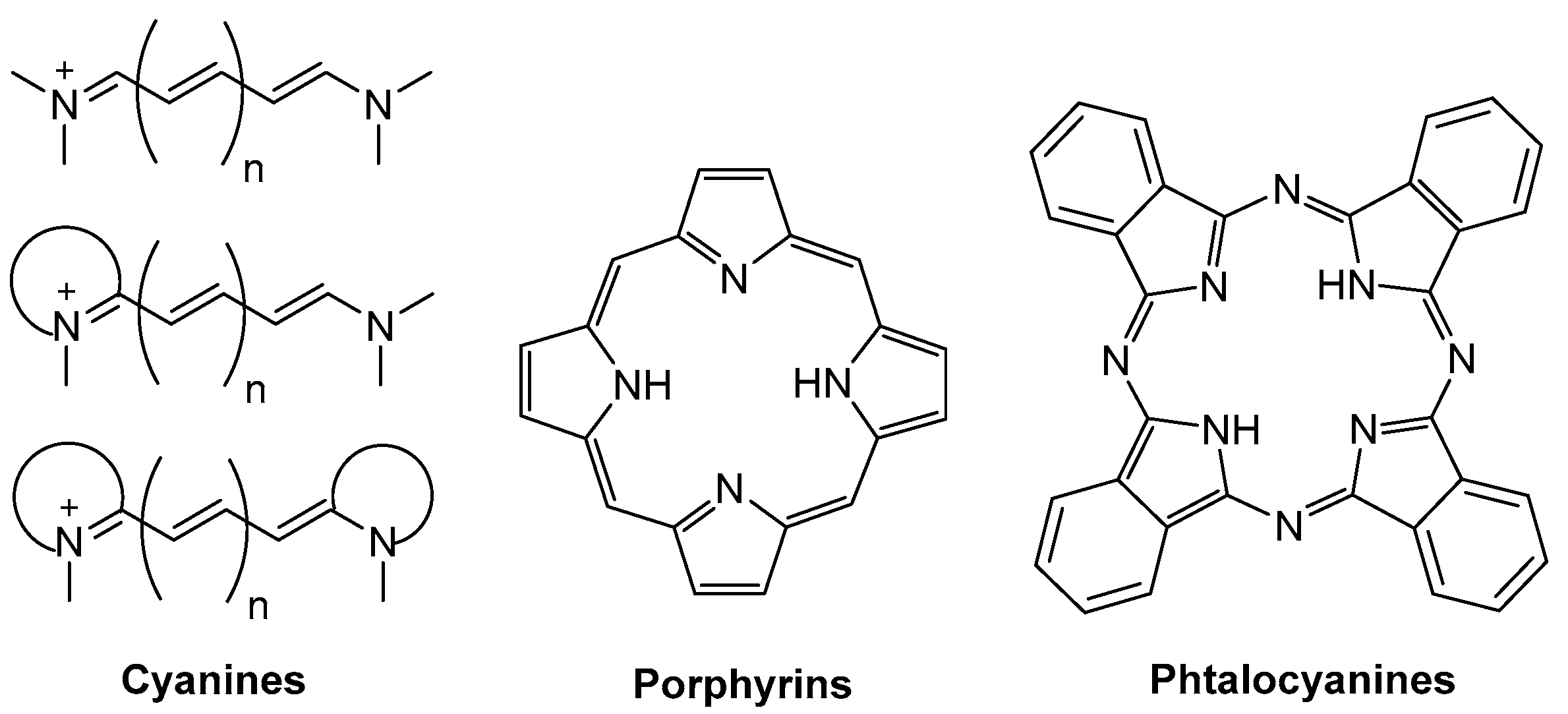
1. Introduction
- (1)
- The induction of intramolecular charge transfer (ICT) phenomena in BODIPYs via the grafting of electron donor and acceptor moieties (push–pull dyes) [30,31]. The emission from the ICT is strongly shifted to longer wavelengths. However, it is usually very weak and very sensitive to the environmental conditions.
- (2)
- The extension of the delocalized π-system through aromatic frameworks. To this aim, aromatic rings are annulated at the dipyrrin core, leading to conformationally restricted BODIPYs [32,33] or directly connected to the core via single bonds [34,35]. Moreover, according to this last procedure, two (bis-BODIPYs) [36] or several BODIPY units (oligomers) [37] can be linked through spacers allowing electronic coupling or directly fused through aromatic rings (fused bis-BODIPYs) [38,39,40]. In this way, NIR absorbers are achieved. However, the electronic rearrangement upon resonant interactions between the electronic clouds of the BODIPYs usually leads to poorly emissive compounds.
- (3)
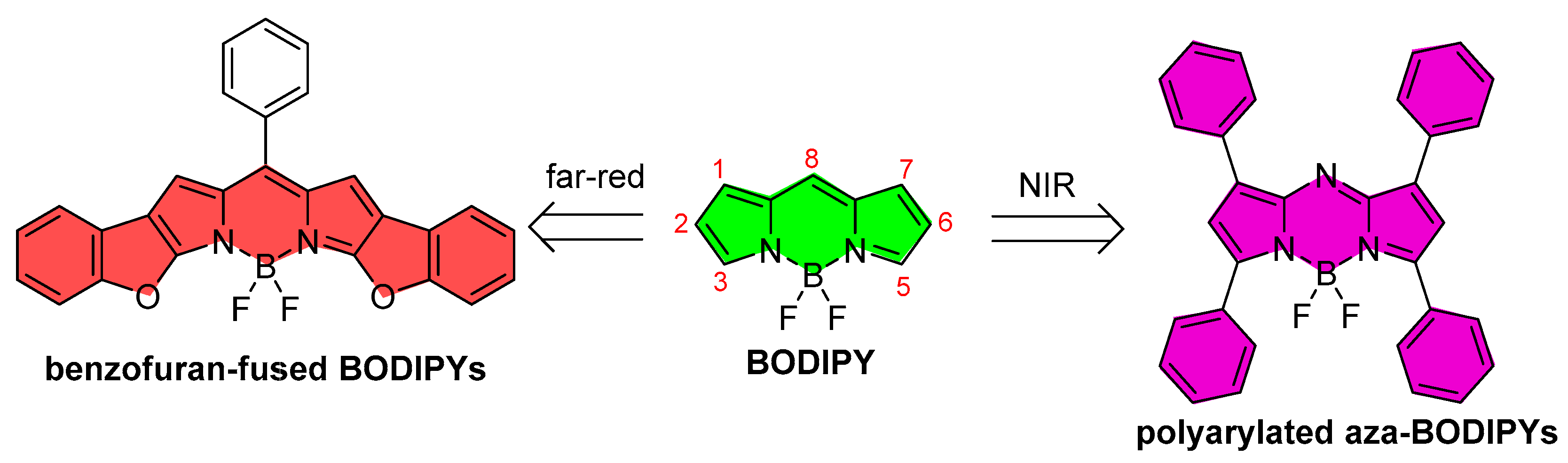
- The replacement of the meso carbon by an aza group (aza-BODIPY) [41,42,43]. Such simple modification induces pronounced bathocromic shifts [44], which can be enlarged spanning the delocalize π-system, as in the preceding point. Indeed, the aza-BODIPY dye family stands out as a promising alternative to design brighter, more stable, and compact NIR emitters than the classic phtalocyanines.
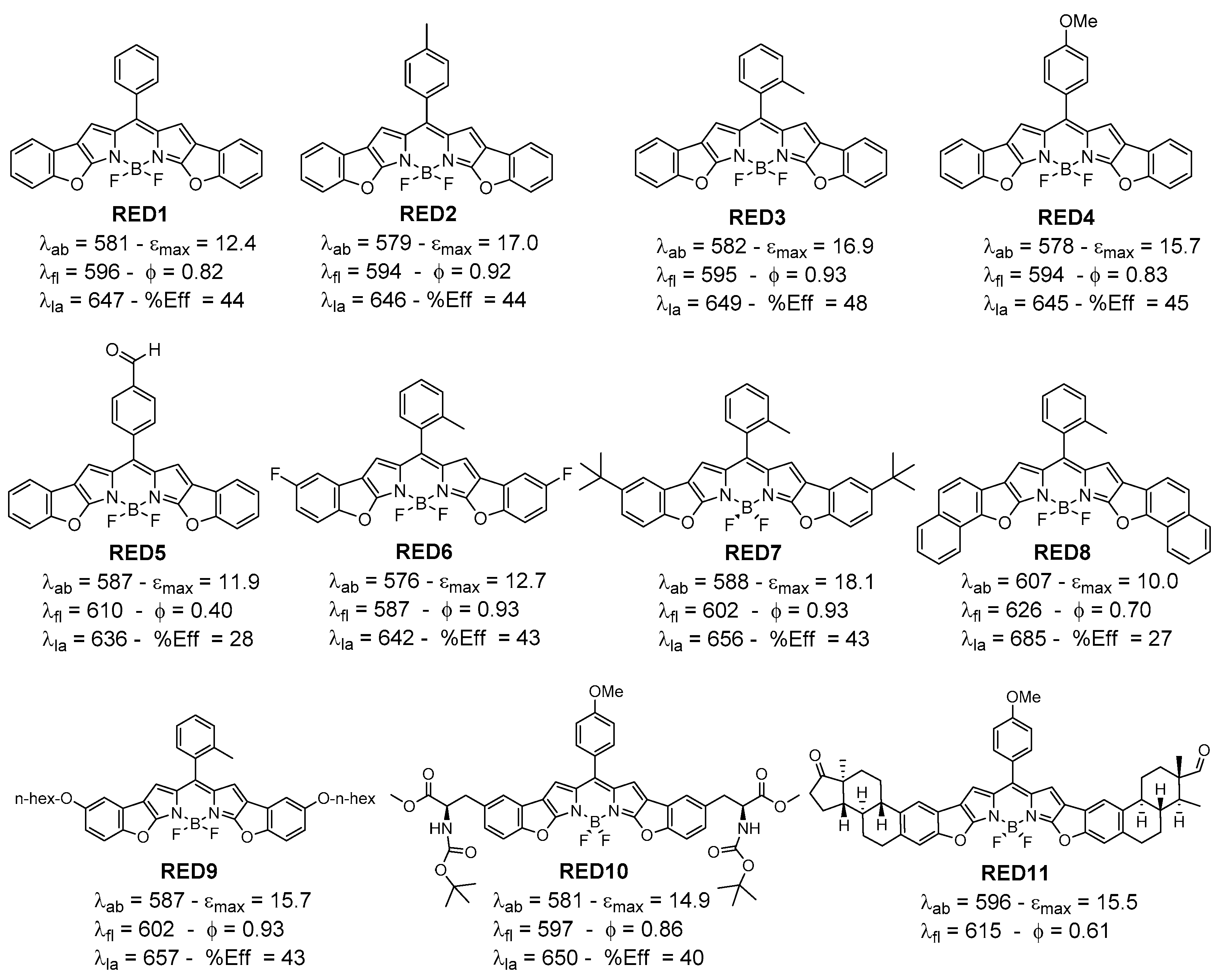
2. Red-Emitting Benzofuran-Fused BODIPYs
3. NIR-Emitting Polyarylated Aza-BODIPYs
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zampetti, A.; Minotto, A.; Cacialli, F. Near-Infrared (NIR) Organic Light-Emitting Diodes (OLEDs): Challenges and Opportunities. Adv. Funct. Mater. 2019, 29, 1807623. [Google Scholar] [CrossRef]
- Zhao, Q.; Sun, J.Z. Red and near infrared emission materials with AIE characteristics. J. Mater. Chem. C 2016, 4, 10588–10609. [Google Scholar] [CrossRef]
- Qu, Z.; Shen, J.; Li, Q.; Xu, F.; Wang, F.; Zhang, X.; Fan, C. Near-IR emissive rare-earth nanoparticles for guided surgery. Theranostics 2020, 10, 2631–2644. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-B.; Liu, H.-W.; Fu, T.; Wang, R.; Zhang, X.-B.; Tan, W. Recent Progress in Small-Molecule Near-IR Probes for Bioimaging. Trends Chem. 2019, 1, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Wunsch, A.; Matuschka, K. A Controlled Trial to Determine the Efficacy of Red and Near-Infrared Light Treatment in Patient Satisfaction, Reduction of Dine Lines, Wrinkles, Skin Roughness, and Intradermal Collagen Density Increase. Photomed. Laser Surg. 2014, 32, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Pansare, V.; Hejazi, S.; Faenza, W.; Prud’Home, R.K. Review of Long-Wavelength Optical and NIR Imaging Materials: Contrast Agents, Fluorophores and Multifunctional Nano Carriers. Chem. Mater. 2012, 24, 812–827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bünzli, J.-C.G.; Eliseeva, S.V. Lanthanide NIR luminescence for telecommunications, bioanalyses and solar energy conversion. J. Rare Earths 2010, 28, 824–842. [Google Scholar] [CrossRef]
- Kaur, H.; Sundriyal, S.; Oachauri, V.; Ingebrandt, S.; Kim, K.-H.; Sharma, A.L.; Deep, A. Luminescent metal-organic frameworks and their composites: Potential future materials for organic light emitting displays. Coord. Chem. Rev. 2019, 41, 213077. [Google Scholar] [CrossRef]
- Dou, L.; Liu, Y.; Hong, Z.; Li, G.; Yang, Y. Low-Bandgap Near-IR Conjugated Polymers/Molecules for Organic Electronics. Chem. Rev. 2015, 115, 12633–12665. [Google Scholar] [CrossRef]
- Kim, K.H.; Singha, S.; Jun, Y.W.; Reo, Y.J.; Kim, H.R.; Ryu, H.G.; Bhunia, S.; Ahn, K.H. Far-red/near-infrared emitting, two-photon absorbing, and bio-stable amino-Si-pyronin dyes. Chem. Sci. 2019, 10, 9028–9037. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.; Lee, S.; Kim, G.-U.; Lee, W.; Kim, B.J. Recent Advances, Design Guidelines, and Prospects of All-Polymer Solar Cells. Chem. Rev. 2019, 119, 8028–8086. [Google Scholar] [CrossRef] [PubMed]
- Tamilavan, V.; Liu, Y.; Lee, J.; Shin, I.; Jung, Y.K.; Lee, B.R.; Jeong, J.H.; Park, S.H. Efficient Polymeric Donor for Both Visible and Near-Infrared-Absorbing Organic Solar Cells. ACS Appl. Energy Mater. 2019, 2, 4284–4291. [Google Scholar] [CrossRef]
- Ding, F.; Zhan, Y.; Lu, X.; Sun, Y. Recent advances in near-infrared II fluorophores for multifunctional biomedical imaging. Chem. Sci. 2018, 9, 4370–4380. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.-M.; Conde, J.; Lipinski, T.; Bednarkiewicz, A.; Huang, C.-C. Revisiting the classification of NIR-absorbing/emitting nanomaterials for in vivo bioapplications. Npg Asia Mater. 2016, 8, e295. [Google Scholar] [CrossRef]
- Guo, Z.; Park, S.; Yoon, J.; Shin, I. Recent progress in the development of near-infrared fluorescent probes for bioimaging applications. Chem. Soc. Rev. 2014, 43, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Swamy, P.C.A.; Sivaraman, G.; Priyanka, R.N.; Raja, S.O.; Ponnuvel, K.; Shanmugpriya, J.; Gulyani, A. Near Infrared (NIR) absorbing dyes as promising photosensitizer for photodynamic therapy. Coord. Chem. Rev. 2020, 411, 213233. [Google Scholar] [CrossRef]
- Fabian, J.; Nakazumi, H.; Matsuoka, M. Near infrared absorbing dyes. Chem. Rev. 1992, 92, 1197–1226. [Google Scholar] [CrossRef]
- Freidus, L.G.; Pradeep, P.; Kumar, P.; Choonara, Y.E.; Pillay, V. Alternative fluorophores designed for advanced molecular imaging. Drug Discov. Today 2018, 23, 115–133. [Google Scholar] [CrossRef]
- Luo, S.; Zhang, E.; Su, Y.; Cheng, T.; Shi, C. A review of NIR dyes in cancer targeting and imaging. Biomaterials 2011, 32, 7127–7138. [Google Scholar] [CrossRef]
- Wu, D.; Chen, L.; Lee, W.; Ko, G.; Yin, J.; Yoon, J. Recent progress in the development of organic dye based near-infrared probes for metal ions. Coord. Chem. Rev. 2018, 354, 74–97. [Google Scholar] [CrossRef]
- Mishra, A.; Behera, R.K.; Behera, P.K.; Mishra, B.K.; Behera, G.P. Cyanines during the 1990s: A Review. Chem. Rev. 2000, 100, 1973–2011. [Google Scholar] [CrossRef] [PubMed]
- Shindy, H.A. Fundamentals in the chemistry of cyanine dyes: A review. Dye. Pigment. 2017, 145, 505–513. [Google Scholar] [CrossRef]
- Ruppel, M.; Lungerich, D.; Sturm, S.; Lippert, R.; Hampel, F.; Jux, N. A Comprehensive Study on Tetraryltetrabenzoporhyrins. Chem. Eur. J. 2020, 26, 3287–3296. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zheng, B.-D.; Peng, X.-H.; Li, S.-Z.; Ying, J.-W.; Zhao, Y.; Huang, J.-D.; Yoon, J. Phtalocyanines as medicinal photosensitizers: Developments in the last five years. Coord. Chem. Rev. 2019, 379, 147–160. [Google Scholar] [CrossRef]
- Demchenko, A.P. Photobleaching of organic fluorophores: Quantitative characterization, mechanisms, protection. Methods Appl. Fluoresc. 2020, 8, 022001. [Google Scholar] [CrossRef]
- Bañuelos, J. BODIPY Dye, the Most Versatile Fluorophore Ever? Chem. Rec. 2016, 16, 335–348. [Google Scholar] [CrossRef]
- Poddar, M.; Misra, R. Recent advances of BODIPY based derivatives for optoelectronic applications. Coord. Chem. Rev. 2020, 421, 213462. [Google Scholar] [CrossRef]
- Boens, N.; Verbelen, B.; Ortiz, M.J.; Jiao, L.; Dehaen, W. Synthesis of BODIPY dyes through postfunctionalization of the boron dipyrromethene core. Coord. Chem. Rev. 2019, 399, 213024. [Google Scholar] [CrossRef]
- Lu, H.; Mack, J.; Yang, Y.; Shen, Z. Structural modifications strategies for the rational design of red/NIR region BODIPYs. Chem. Soc. Rev. 2014, 43, 4778–4823. [Google Scholar] [CrossRef] [Green Version]
- Martin, A.; Long, C.; Forster, R.J.; Keyes, T.E. Near IR emitting BODIPY fluorophores with mega-stokes shift. Chem. Commun. 2012, 48, 5617–5619. [Google Scholar] [CrossRef]
- Ni, Y.; Kannadorai, R.K.; Yu, S.W.-K.; Chang, Y.-T.; Wu, J. Push-pull type meso-ester substituted BODIPY near-infrared dyes as contrast agents for photoacoustic imaging. Org. Biomol. Chem. 2017, 21, 4531–4535. [Google Scholar] [CrossRef]
- Umezawa, K.; Matsui, A.; Nakamura, Y.; Citterio, D.; Suzuki, K. Bright, Color-Tunable Fluorescent Dyes in the Vis/NIR Region: Establishment of New “Tailor-Made” Multicolor Fluorophores Based on Borondipyrromethene. Chem. Eur. J. 2009, 15, 1096–1106. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Qiao, Z.; Li, T.; Zeika, O.; Leo, K. Highly Efficient Deep-Red- to Near-Infrared-Absorbing and Emissive Benzo/Naphthol[b]furan-Fused Boron Dipyrromethene (BODIPY). ChemPhotoChem 2018, 2, 1017–1021. [Google Scholar] [CrossRef]
- Gómez-Durán, C.F.A.; Esnal, I.; Valois-Escamilla, I.; Urías-Benavides, A.; Bañuelos, J.; López-Arbeloa, I.; García-Moreno, I.; Peña-Cabrera, E. Near-IR BODIPY Dyes á la Carte—Programmed Orthogonal Functionalization of Rationally Designed Building Blocks. Chem. Eur. J. 2016, 22, 1048–1061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verbelen, B.; Boodts, S.; Hofkens, J.; Boens, N.; Dehaen, W. Radical C-H Arylation of the BODIPY Core with Aryldiazonium Salts: Synthesis of Highly Fluorescent Red-Shifted Dyes. Angew. Chem. Int. Ed. 2015, 54, 4612–4616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bröring, M.; Krüger, R.; Link, S.; Kleeberg, C.; Köhler, S.; Xie, X.; Ventura, B.; Flamigni, L. Bis(BF2)-2,2′-Bidipyrrins (BisBODIPYs): Highly Fluorescent BODIPY dimers with Large Stokes Shifts. Chem. Eur. J. 2008, 14, 2976–2983. [Google Scholar] [CrossRef] [PubMed]
- Patalag, L.J.; Ho, L.P.; Jones, P.G.; Werz, D.B. Ethylene-bridged Oligo-BODIPYs: Access to Intramolecular J-Aggregates and Superfluorophores. J. Am. Chem. Soc. 2017, 139, 15104–15113. [Google Scholar] [CrossRef]
- Nakamura, M.; Tahara, H.; Takahashi, K.; Nagata, T.; Uoyama, H.; Kuzuhara, D.; Mori, S.; Okujima, T.; Yamada, H.; Uno, H. π-Fused bis-BODIPY as candidate for NIR dyes. Org. Biomol. Chem. 2012, 10, 6840–6849. [Google Scholar] [CrossRef]
- Yu, C.; Jiao, L.; Li, T.; Wu, Q.; Miao, W.; Wang, J.; Wei, Y.; Mu, X.; Hao, E. Fusion and planarization of bisBODIPY: A new family of photostable near infrared dyes. Chem. Commun. 2015, 51, 16852–16855. [Google Scholar] [CrossRef]
- Ni, Y.; Lee, S.; Son, M.; Aratani, N.; Ishida, M.; Samanta, A.; Yamada, H.; Chang, Y.-T.; Furuta, H.; Kim, D.; et al. A Diradical Approach towards BODIPY-Based Dyes with Intense Near-Infrared Absorption around λ = 1100 nm. Angew. Chem. Int. Ed. 2016, 55, 2815–2819. [Google Scholar] [CrossRef]
- Zhao, W.; Carreira, E.M. Conformationally Restrcited Aza-BODIPY: Highly Fluorescent, Stable Near-Infrared Absorbing Dyes. Chem. Eur. J. 2006, 12, 7254–7263. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; O’Shea, D.F. Azadipyrromethenes: From traditional dye chemistry to leading edge applications. Chem. Soc. Rev. 2016, 45, 3846–3864. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Sheng, W.; Wu, Q.; Yu, C.; Hao, E.; Bobadova-Parvanova, P.; Storer, M.; Asiri, A.M.; Marwani, H.M.; Jiao, J. Synthesis, Structure, and Properties of Near-Infrared [b]Phenanthrene-Fused BF2 Azadipyrromethenes. Chem. Asian J. 2017, 12, 2486–2493. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, J.K.G.; Harriman, A. Origin of the Red-Shifted Optical Spectra recorded for Aza-BODIPY Dyes. J. Phys. Chem. A 2016, 120, 2537–2546. [Google Scholar] [CrossRef] [PubMed]
- Kuehne, A.J.C.; Gather, M.C. Organic Lasers: Recent Developments of Materials, Device Geometries, and Fabrication Techniques. Chem. Rev. 2016, 116, 12823–12864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Y.; Liu, Y.-Y.; Liu, X.; Lin, H.; Gao, K.; Lai, W.-Y.; Huang, W. Organic solid-state lasers: A materials view and future developments. Chem. Soc. Rev. 2020. [Google Scholar] [CrossRef]
- Belmonte-Vázquez, J.L.; Avellanal-Zaballa, E.; Enríquez-Palacios, E.; Cerdán, L.; Esnal, I.; Bañuelos, J.; Villegas-Gómez, C.; López-Arbeloa, I.; Peña-Cabrera, E. Synthetic Approach to Readily Accessible Benzofuran-Fused Borondipyrromethenes as Red-Emitting Laser Dyes. J. Org. Chem. 2019, 84, 2523–2541. [Google Scholar] [CrossRef]
- Prieto-Castañeda, A.; Avellanal-Zaballa, E.; Gartzia-Rivero, L.; Cerdán, L.; Agarrabeitia, A.R.; García-Moreno, I.; Bañuelos, J.; Ortiz, M.J. Tailoring the Molecular Skeleton of Aza-BODIPYs to Design Photostable Red-Light-Emitting Laser Dyes. ChemPhotoChem 2019, 3, 75–85. [Google Scholar] [CrossRef]
- Lin, Z.; Kohn, A.W.; Voorhis, T.V. Toward Prediction of Nonradiative Decay Pathways in Organic Compounds II: Two Internal Conversion Channels in BODIPYs. J. Phys. Chem. C 2020, 124, 3925–3938. [Google Scholar] [CrossRef]
- Mei, J.; Leung, N.L.C.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission: Together We Shine, United We Soar! Chem. Rev. 2015, 115, 11708–11940. [Google Scholar] [CrossRef]
- Cerdán, L.; Martínez-Martínez, V.; García-Moreno, I.; Costela, A.; Pérez-Ojeda, M.E.; López-Arbeloa, I.; Wu, L.; Burgess, K. Naturally Asembled Excimers in Xanthenes as Singular and Highly Efficient Laser Dyes in Liquid and Solid Media. Adv. Opt. Mater. 2013, 1, 984–990. [Google Scholar] [CrossRef]
- Durán-Sampedro, G.; Agarrabeitia, A.R.; Arbeloa López, T.; Bañuelos, J.; López-Arbeloa, I.; Chiara, J.L.; Ortiz, M.J. Increased laser action in commercial dyes form fluorination regardless of their skeleton. Laser Phys. Lett. 2014, 11, 115818. [Google Scholar] [CrossRef] [Green Version]
- López-Arbeloa, F.; Bañuelos Prieto, J.; Martínez Martínez, V.; Arbeloa López, T.; López Arbeloa, I. Intramolecular Charge Transfer in Pyrromethene Laser Dyes: Photophysical behaviour of PM650. ChemPhysChem 2004, 5, 1762–1771. [Google Scholar] [CrossRef]
- Englman, R.; Jortner, J. The energy gap law for non-radiative decay in large molecules. J. Lumin. 1970, 1, 134–142. [Google Scholar] [CrossRef]
- Cerdán, L.; Enciso, E.; Martin, V.; Bañuelos, J.; López-Arbeloa, I.; Costela, A.; García-Moreno, I. FRET-assisted laser emission in colloidal suspensions of dye-doped latex nanoparticles. Nat. Photon. 2012, 6, 621–626. [Google Scholar] [CrossRef] [Green Version]
- Durán-Sampedro, G.; Agarrabeitia, A.R.; Cerdán, L.; Pérez-Ojeda, M.E.; Costela, A.; García-Moreno, I.; Esnal, I.; Bañuelos, J.; López-Arbeloa, I.; Ortiz, M.J. Carboxylates versus Fluorines: Boosting the Emission Properties of Commercial BODIPYs in Liquid and Solid Media. Adv. Funct. Mater. 2013, 23, 4195–4205. [Google Scholar]
- Durán-Sampedro, G.; Esnal, I.; Agarrabeitia, A.R.; Bañuelos Prieto, J.; Cerdán, L.; García-Moreno, I.; Costela, A.; López-Arbeloa, I.; Ortiz, M.J. First Highly Efficient and Photostable E and C Derivatives of 4,4-Difluoro-4-bora-3a,4a-diaza-s-indacene (BODIPY) as Dye Lasers in the Liquid Phase, Thin Films, and Solid-State Rods. Chem. Eur. J. 2014, 20, 2646–2653. [Google Scholar] [CrossRef]
- Bodio, W.; Goze, C. Investigation of B-F substitution on BODIPY and aza-BODIPY dyes: Development of B-O and B-C BODIPYs. Dye. Pigment. 2019, 160, 700–710. [Google Scholar] [CrossRef]



| R | λab (nm) | εmax·10−4 (M−1·cm−1) | λfl (nm) | φ | λla (nm) | % Eff | |
|---|---|---|---|---|---|---|---|
 | |||||||
| F | 614 | 3.2 | 639 | 0.32 | 647 | 13 | |
| CN | 604 | 5.5 | 638 | 0.22 | 644 | 13 | |
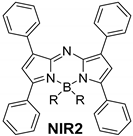 | F | 645 | 8.6 | 668 | 0.12 | 681 | 16 |
| OCOCF3 | 646 | 5.6 | 671 | 0.47 | 689 | 19 | |
| CN | 652 | 7.3 | 670 | 0.46 | 686 | 12 | |
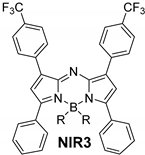 | F | 654 | 8.6 | 682 | 0.15 | 690 | 20 |
| OCOCF3 | 655 | 6.7 | 687 | 0.44 | 696 | 16 | |
| CN | 654 | 7.3 | 677 | 0.12 | 692 | 16 | |
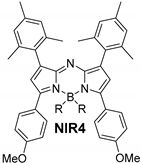 | F | 669 | 7.8 | 696 | 0.46 | 708 | 23 |
| OCOCF3 | 678 | 7.9 | 708 | 0.49 | 720 | 33 | |
| CN | 667 | 5.6 | 703 | 0.31 | 717 | 29 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avellanal-Zaballa, E.; Gartzia-Rivero, L.; Bañuelos, J.; García-Moreno, I.; R. Agarrabeitia, A.; Peña-Cabrera, E.; Ortiz, M.J. A Palette of Efficient and Stable Far-Red and NIR Dye Lasers. Appl. Sci. 2020, 10, 6206. https://doi.org/10.3390/app10186206
Avellanal-Zaballa E, Gartzia-Rivero L, Bañuelos J, García-Moreno I, R. Agarrabeitia A, Peña-Cabrera E, Ortiz MJ. A Palette of Efficient and Stable Far-Red and NIR Dye Lasers. Applied Sciences. 2020; 10(18):6206. https://doi.org/10.3390/app10186206
Chicago/Turabian StyleAvellanal-Zaballa, Edurne, Leire Gartzia-Rivero, Jorge Bañuelos, Inmaculada García-Moreno, Antonia R. Agarrabeitia, Eduardo Peña-Cabrera, and Maria Jose Ortiz. 2020. "A Palette of Efficient and Stable Far-Red and NIR Dye Lasers" Applied Sciences 10, no. 18: 6206. https://doi.org/10.3390/app10186206
APA StyleAvellanal-Zaballa, E., Gartzia-Rivero, L., Bañuelos, J., García-Moreno, I., R. Agarrabeitia, A., Peña-Cabrera, E., & Ortiz, M. J. (2020). A Palette of Efficient and Stable Far-Red and NIR Dye Lasers. Applied Sciences, 10(18), 6206. https://doi.org/10.3390/app10186206







