Structural and Magnetic Properties of NiZn Ferrite Nanoparticles Synthesized by a Thermal Decomposition Method
Abstract
:1. Introduction
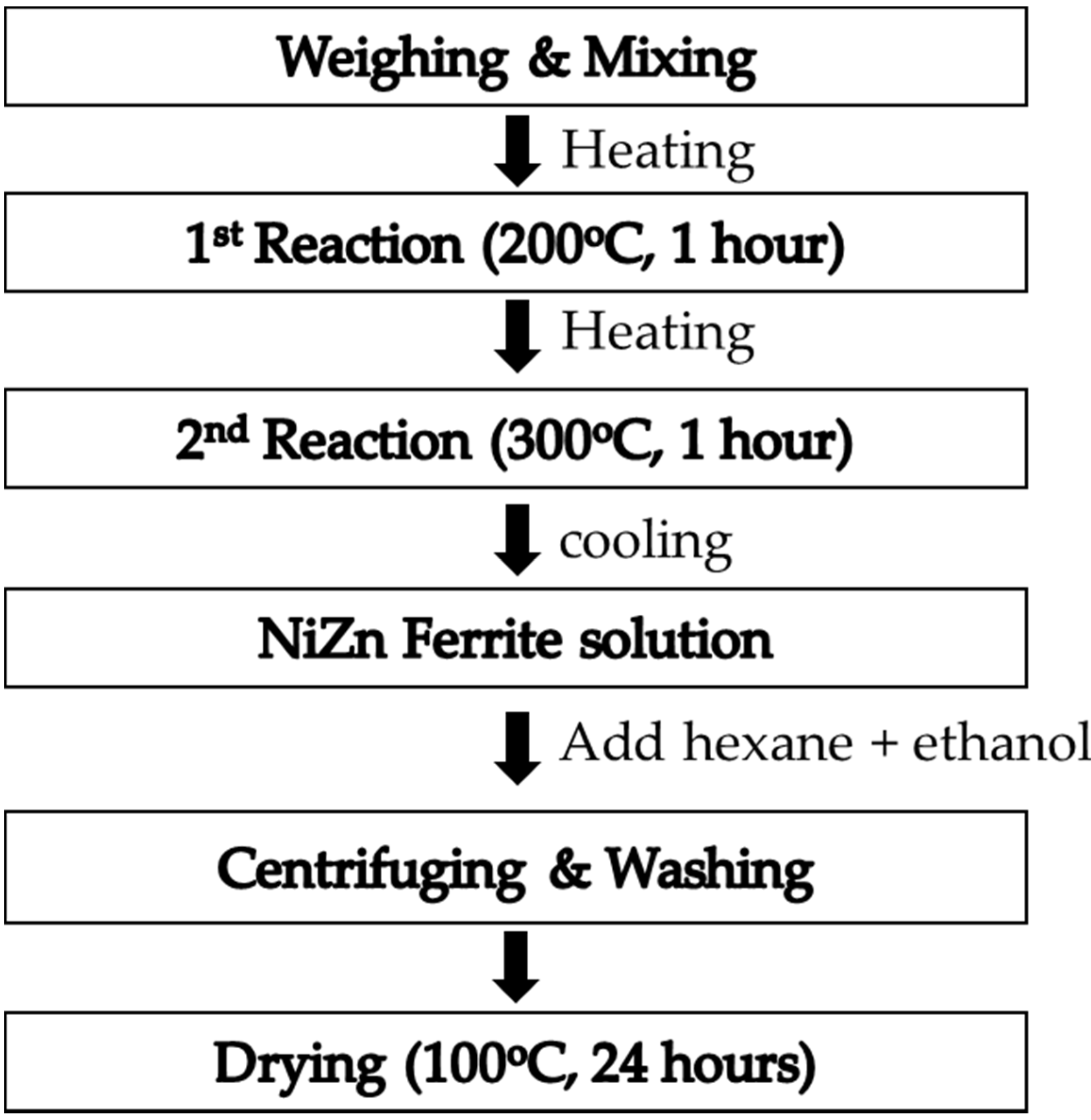
2. Materials and Methods
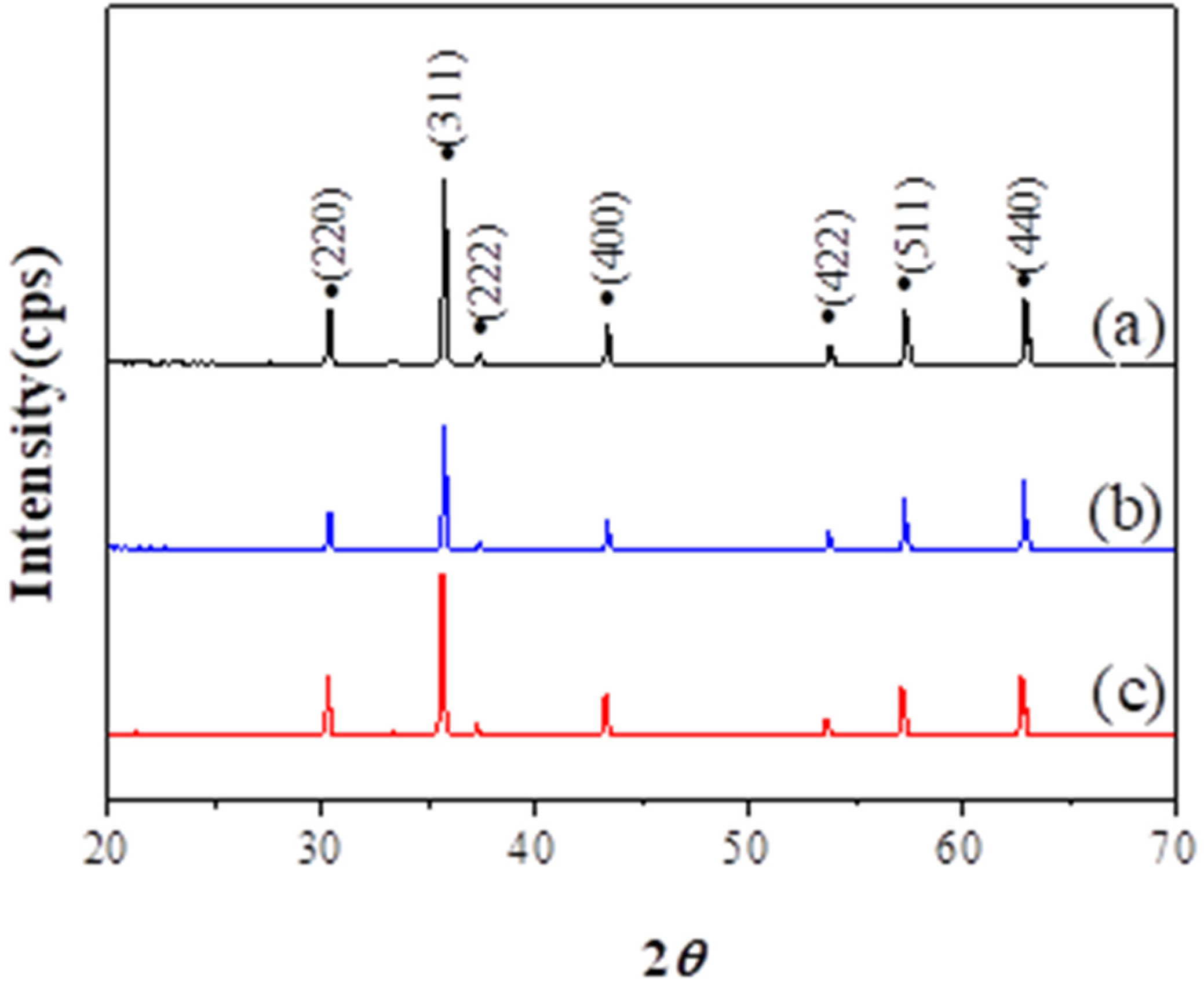
3. Results
| Zn2+(Fe23+)O4 | normal spinel |
| Fe3+(Ni2+Fe3+)O4 | inverse spinel |
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gubbala, S.; Nathani, H.; Koziol, K.; Misra, R.D.K. Magnetic properties of nanocrystalline Ni–Zn, Zn–Mn, and Ni–Mn ferrites synthesized by reverse micelle technique. Phys. B Condens. Mater. 2004, 348, 317. [Google Scholar] [CrossRef]
- Costa, A.C.F.M.; Morelli, M.R.; Kiminami, R.H.G.A. Microstructure and magnetic properties of Ni(1−x)Zx Fe2O4 synthesized by combustion reaction. J. Mater. Sci. 2007, 42, 779. [Google Scholar] [CrossRef]
- Goldman, M.; DeVitre, R.; Tenca, J. Materials Science Cement Distribution and Bond Strength in Cemented Posts. J. Dent. Res. 1984, 63, 582. [Google Scholar]
- Han, D.H.; Luo, H.I.; Yang, Z. Remanent and anisotropic switching field distribution of platelike Ba-ferrite and acicular particulate recording media. J. Magn. Magn. Mater. 1996, 161, 376. [Google Scholar] [CrossRef]
- Yaun, T.; Wei, Z.; Yuan, J.; Yan, L.; Liu, Q.; Wang, J. The microstructure and magnetic properties of Ni0.4Zn0.6Fe2O4 films prepared by spin-coating method. J. Sol-Gel Sci. Technol. 2011, 58, 501. [Google Scholar] [CrossRef]
- Shangguan, W.F.; Teraoka, Y.; Kagaw, S. Promotion effect of potassium on the catalytic property of CuFe2O4 for the simultaneous removal of NOx and diesel soot particulate. Appl. Catal. B Environ. 1998, 16, 149. [Google Scholar] [CrossRef]
- Cui, H.-J.; Shi, J.-W.; Yuan, B.; Fu, M.-L. Synthesis of porous magnetic ferrite nanowires containing Mn and their application in water treatment. J. Mater. Chem. A 2013, 1, 5902. [Google Scholar] [CrossRef]
- Nowicka, A.; Kowalczyk, A.; Donten, M.; Krysinski, P.; Stojek, Z. Influence of a Magnetic Nanoparticle as a Drug Carrier on the Activity of Anticancer Drugs: Interactions of Double Stranded DNA and Doxorubicin Modified with a Carrier. Anal. Chem. 2009, 81, 7474–7483. [Google Scholar] [CrossRef]
- Sunkara, B.; Misra, R. Enhanced antibactericidal function of W4+-doped titania-coated nickel ferrite composite nanoparticles: A biomaterial system. Acta Biomater. 2008, 4, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Carta, D.; Loche, D.; Mountjoy, G.; Navarra, G.; Corrias, A. NiFe2O4Nanoparticles Dispersed in an Aerogel Silica Matrix: An X-ray Absorption Study. J. Phys. Chem. C 2008, 112, 15623–15630. [Google Scholar] [CrossRef]
- Kaur, B.; Arora, M.; Shankar, A.; Srivastava, A.K.; Pan, R.P. Induced Size Effects of Gd3+ ions Doping on Structural and Magnetic Properties of Ni-Zn Ferrite Nanoparticles. Adv. Mater. Lett. 2012, 3, 399–405. [Google Scholar] [CrossRef]
- Goswami, P.P.; Choudhury, H.A.; Chakma, S.; Moholkar, V.S. Sonochemical Synthesis and Characterization of Manganese Ferrite Nanoparticles. Ind. Eng. Chem. Res. 2013, 52, 17848–17855. [Google Scholar] [CrossRef]
- Silva, F.G.D.; Depeyrot, J.; Campos, A.F.C.; Aquino, R.; Fiorani, D.D. PeddisStructural and magnetic properties of spinel ferrite nanoparticles. J. Nanosci. Nanotechnol. 2019, 19, 4888–4902. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.J.; Nadeem, M.; Javaid, S.; Atif, M. Cation distribution in nanocrystalline ZnFe2O4 investigated using X-ray absorption fine structure spectroscopy. J. Phys. Condens. Matter 2009, 21, 405303. [Google Scholar] [CrossRef] [PubMed]
- Cullity, B.D.; Graham, C.D. Introduction to Magnetic Materials, 2nd ed.; John Wiley & Sons Inc.: New York, NY, USA, 2009. [Google Scholar]
- Nakashima, S.; Fujita, K.; Tanaka, K.; Hirao, K.; Yamamoto, T.; Tanaka, I. First-principles XANES simulations of spinel zinc ferrite with a disordered cation distribution. Phys. Rev. B 2007, 75. [Google Scholar] [CrossRef] [Green Version]
- Hasirci, C.; Karaagac, O.; Köçkar, H. Superparamagnetic zinc ferrite: A correlation between high magnetizations and nanoparticle sizes as a function of reaction time via hydrothermal process. J. Magn. Magn. Mater. 2019, 474, 282–286. [Google Scholar] [CrossRef]
- Rameshbabu, R.; Ramesh, R.; Kanagesan, S.; Karthigeyan, A.; Ponnusamy, S. Synthesis of superparamagnetic ZnFe2O4 nanoparticle by surfactant assisted hydrothermal method. J. Mater. Sci. Mater. Electron. 2013, 24, 4279–4283. [Google Scholar] [CrossRef]
- Rostamnejadi, A.; Salamati, H.; Kameli, P.; Ahmadvand, H. Superparamagnetic behavior of La0.67Sr0.33MnO3 nanoparticles prepared via sol–gel method. J. Magn. Magn. Mater. 2009, 321, 3126–3131. [Google Scholar] [CrossRef]
- Zheng, Z.; Zhong, X.; Zhang, Y.; Yu, H.; Zeng, D. Synthesis, structure and magnetic properties of nanocrystalline ZnxMn1−xFe2O4 prepared by ball milling. J. Alloys Compd. 2008, 466, 377–382. [Google Scholar] [CrossRef]
- Gao, R.-R.; Zhang, Y.; Yu, W.; Xiong, R.; Shi, J. Superparamagnetism and spin-glass like state for the MnFe2O4 nano-particles synthesized by the thermal decomposition method. J. Magn. Magn. Mater. 2012, 324, 2534–2538. [Google Scholar] [CrossRef]
- Kozlov, D.A.; Shcherbakov, A.B.; Kozlova, T.O.; Angelov, B.; Kopitsa, G.P.; Garshev, A.V.; Baranchikov, A.E.; Ivanova, O.S.; Ivanov, V.K. Photochromic and Photocatalytic Properties of Ultra-Small PVP-Stabilized WO3 Nanoparticles. Molecules 2019, 25, 154. [Google Scholar] [CrossRef] [Green Version]
- Padmapriya, G.; Manikandan, A.; Krishnasamy, V.; Jaganathan, S.K.; Antony, S.A. Spinel NixZn1−xFe2O4 (0.0 ≤ x ≤ 1.0) nano-photocatalysts: Synthesis, characterization and photocatalytic degradation of methylene blue dye. J. Mol. Struct. 2016, 1119, 39–47. [Google Scholar] [CrossRef]
- Slatineanu, T.; Iordan, A.; Palamaru, M.N.; Caltun, O.F.; Gafton, V.; Leontie, L. Synthesis and characterization of nanocrystalline Zn ferrites substituted with Ni. Mater. Res. Bull. 2011, 46, 1455–1460. [Google Scholar] [CrossRef]
- Gubanova, N.; Baranchikov, A.E.; Kopitsa, G.P.; Almasy, L.; Angelov, B.; Ivanov, V.K.; Rosta, L.; Ivanov, V.K. Combined SANS and SAXS study of the action of ultrasound on the structure of amorphous zirconia gels. Ultrason. Sonochem. 2015, 24, 230–237. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.K.; Goel, T.C.; Mendiratta, R.G. Low-temperature synthesis of Mn0.2Ni0.2Zn0.6Fe2O4 ferrites by citrate precursor method and study of their properties. Phys. Status Solidi (a) 2004, 201, 1453–1457. [Google Scholar] [CrossRef]
- Pujar, R.B.; Kulkarni, S.N.; Bellad, C.B.; Chougule, B.K. Compositional, temperature and frequency dependence of initial permeability in Zr4+ substituted Mg–Zn ferrites. J. Mater. Sci. Lett. 1997, 16, 1668–1699. [Google Scholar] [CrossRef]
- Velmurugana, K.; Venkatachalapathyb, V.S.K.; Sendhilnathanc, S. Thermogravimetric and Magnetic Properties of Ni1−xZnxFe2O4 Nanoparticles Synthesized by Coprecipitation. Mater. Res. 2009, 12, 529–534. [Google Scholar] [CrossRef] [Green Version]
- Verma, A.; Goel, T.; Mendiratta, R.; Alam, M. Dielectric properties of NiZn ferrites prepared by the citrate precursor method. Mater. Sci. Eng. B 1999, 60, 156–162. [Google Scholar] [CrossRef]
- Stoner, E.C. Introduction to Magnetic Materials; Addison-Wesley: Boston, MA, USA, 1972; pp. 183–187. [Google Scholar]
- Guillaud, C. Properties Manetiques des Ferrites. J. Phys. Radium 1951, 12, 239–248. [Google Scholar] [CrossRef]
- Tsutaoka, T. Frequency dispersion of complex permeability in Mn–Zn and Ni–Zn spinel ferrites and their composite materials. J. Appl. Phys. 2003, 93, 2789–2796. [Google Scholar] [CrossRef] [Green Version]
- Su, H.; Zhang, H.; Tang, X.; Jing, Y. Influence of microstructure on permeability dispersion and power loss of NiZn ferrite. Appl. Phys. 2008, 103, 093903. [Google Scholar] [CrossRef]
- Nakamura, T. Snoek’s limit in high-frequency permeability of polycrystalline Ni–Zn, Mg–Zn, and Ni–Zn–Cu spinel ferrites. J. Appl. Phys. 2000, 88, 348. [Google Scholar] [CrossRef]
- Lebourgeois, R.; Coillot, C. Mn–Zn ferrites for magnetic sensor in space applications. J. Appl. Phys. 2008, 103, 07E510. [Google Scholar] [CrossRef]
- Verma, A.; Chatterjee, R. Effect of zinc concentration on the structural, electrical and magnetic properties of mixed Mn–Zn and Ni–Zn ferrites synthesized by the citrate precursor technique. J. Magn. Magn. Mater. 2006, 306, 313–320. [Google Scholar] [CrossRef]
- Shaikh, P.A.; Kambale, R.C.; Rao, A.V.; Kolekar, Y.D. Effect of Ni doping on structural and magnetic properties of Co1−xNixFe1.9Mn0.1O4. J. Magn. Magn. Mater. 2010, 322, 718. [Google Scholar] [CrossRef]
- Byun, T.Y.; Bycon, S.C.; Hong, K.S. Factors affectinginitial permeability of Co-substituted Ni-Zn-Cu ferrites. IEEE Trans. Magn. 1999, 35, 3445. [Google Scholar] [CrossRef]
- Guyot, M.; Globus, A. Wall displacement and bulgingin magnetization mechanisms of the hysteresis loop. Phys. Status Solidi B 1972, 52, 427. [Google Scholar]




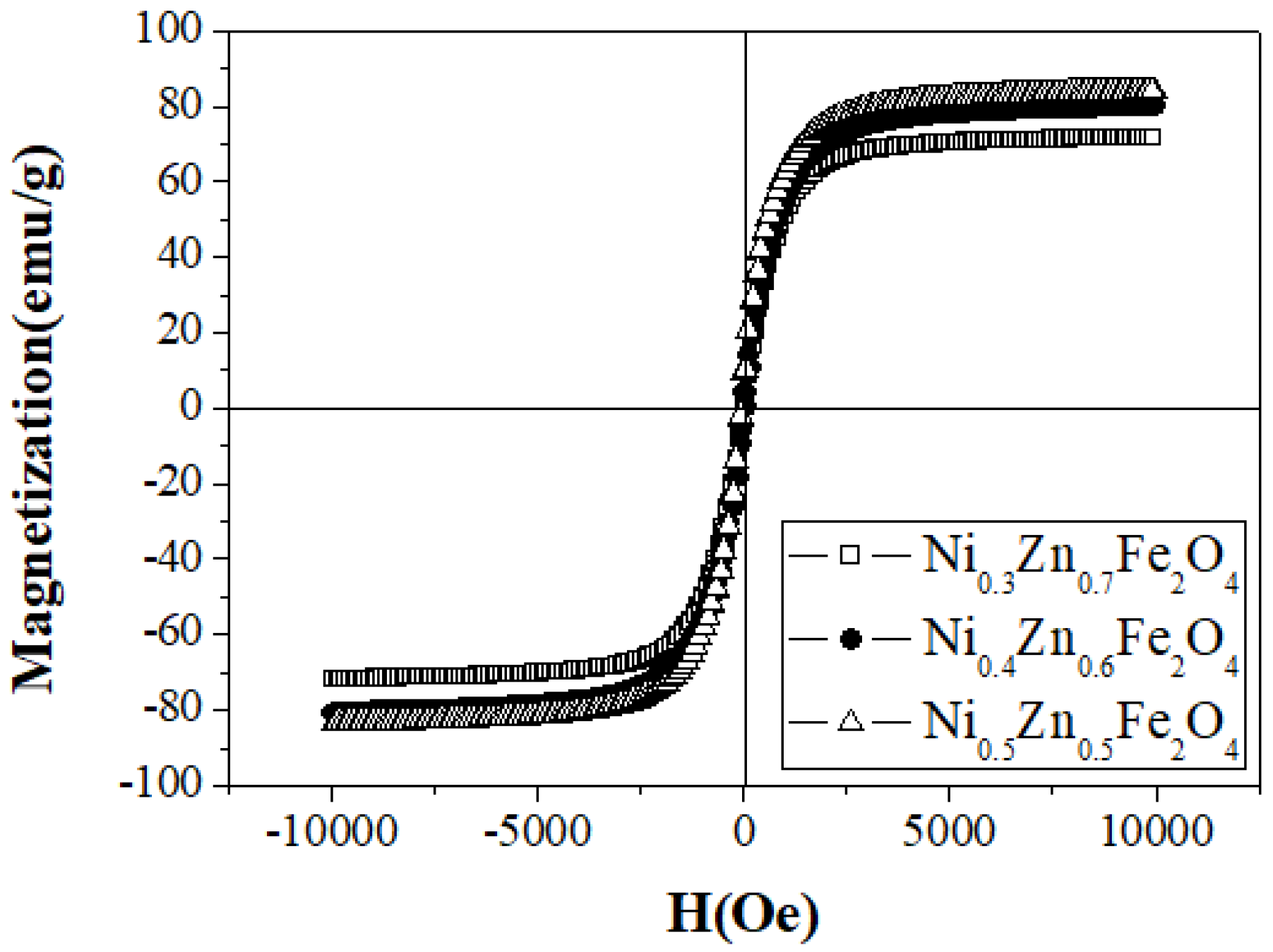


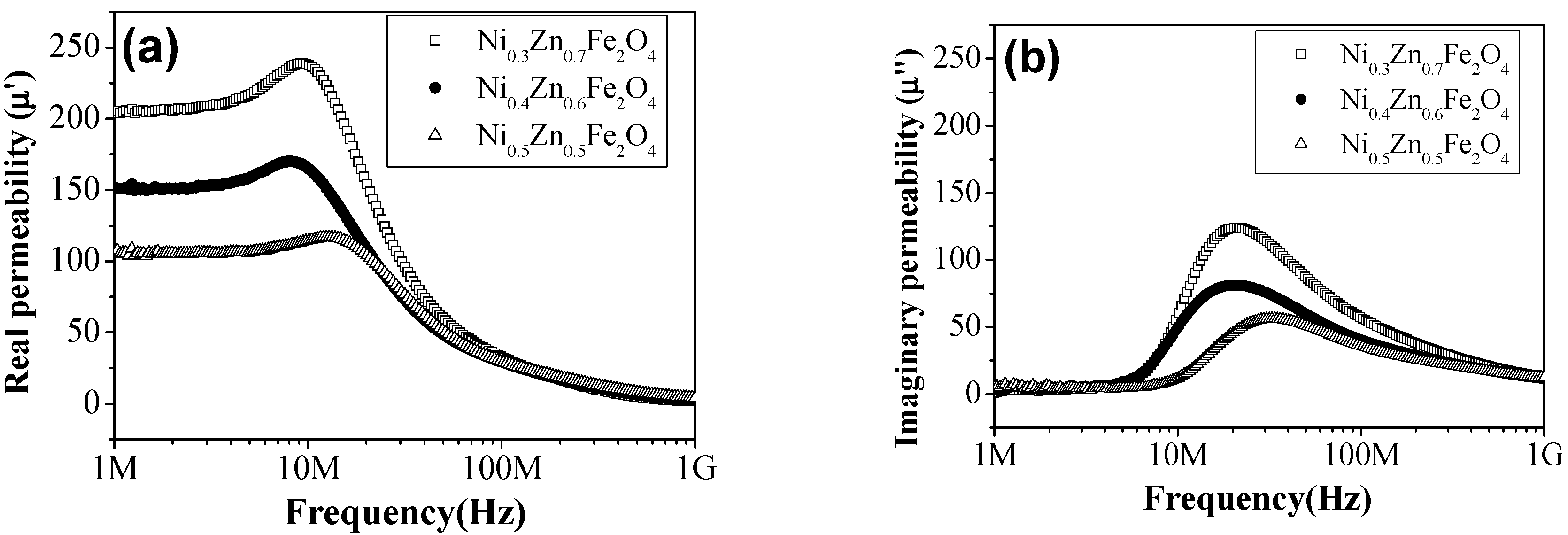
| Sample Name | Saturation Magnetization Ms (emu/g) | Lattice Parameter a (A) | Crystallite Size (nm) | Permeability μ′ (5MHz) | Coercivity Hc (Oe) | Anisotropic Constant K1 (erg/cm3) |
|---|---|---|---|---|---|---|
| Ni0.5Zn0.5Fe2O4 | 84.44 | 8.340 | 49 | 106 | 18.54 | 1631 |
| Ni0.4Zn0.6Fe2O4 | 83.97 | 8.346 | 51 | 150 | 17.56 | 1536 |
| Ni0.3Zn0.7Fe2O4 | 71.84 | 8.358 | 46 | 217 | 16.48 | 1233 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, J.; Choi, M.; Shin, H.-S.; Ju, B.-K.; Chun, M. Structural and Magnetic Properties of NiZn Ferrite Nanoparticles Synthesized by a Thermal Decomposition Method. Appl. Sci. 2020, 10, 6279. https://doi.org/10.3390/app10186279
Hwang J, Choi M, Shin H-S, Ju B-K, Chun M. Structural and Magnetic Properties of NiZn Ferrite Nanoparticles Synthesized by a Thermal Decomposition Method. Applied Sciences. 2020; 10(18):6279. https://doi.org/10.3390/app10186279
Chicago/Turabian StyleHwang, JinAh, Moonhee Choi, Hyo-Soon Shin, Byeong-Kwon Ju, and MyoungPyo Chun. 2020. "Structural and Magnetic Properties of NiZn Ferrite Nanoparticles Synthesized by a Thermal Decomposition Method" Applied Sciences 10, no. 18: 6279. https://doi.org/10.3390/app10186279
APA StyleHwang, J., Choi, M., Shin, H.-S., Ju, B.-K., & Chun, M. (2020). Structural and Magnetic Properties of NiZn Ferrite Nanoparticles Synthesized by a Thermal Decomposition Method. Applied Sciences, 10(18), 6279. https://doi.org/10.3390/app10186279





