Liquid Nitrogen Efficiency in Treatment of Giant Cell Tumor of Bone and Prevention of Recurrence
Abstract
:1. Introduction
Cryotherapy
2. Materials and Methods
Surgical Procedure
3. Results
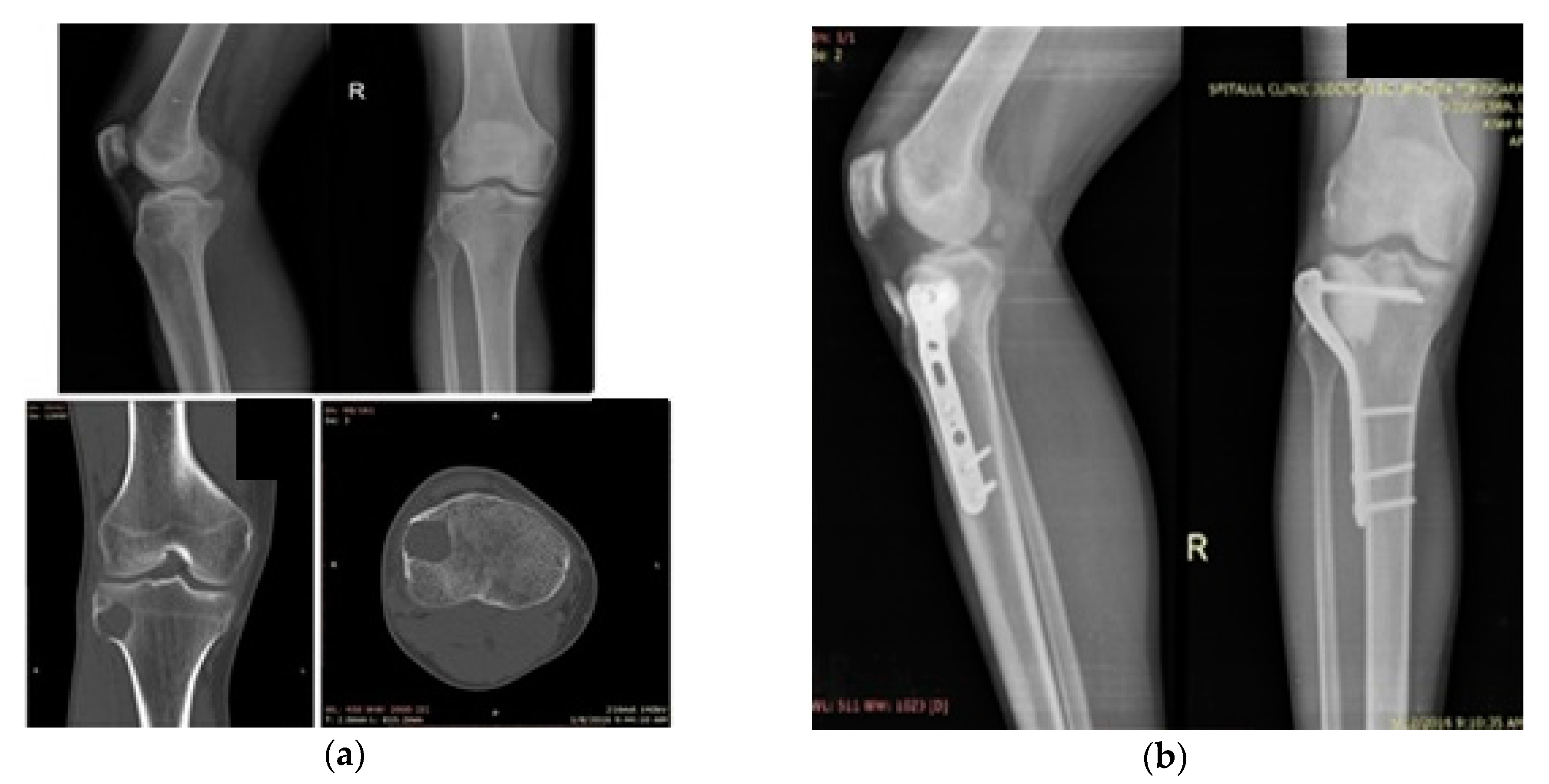
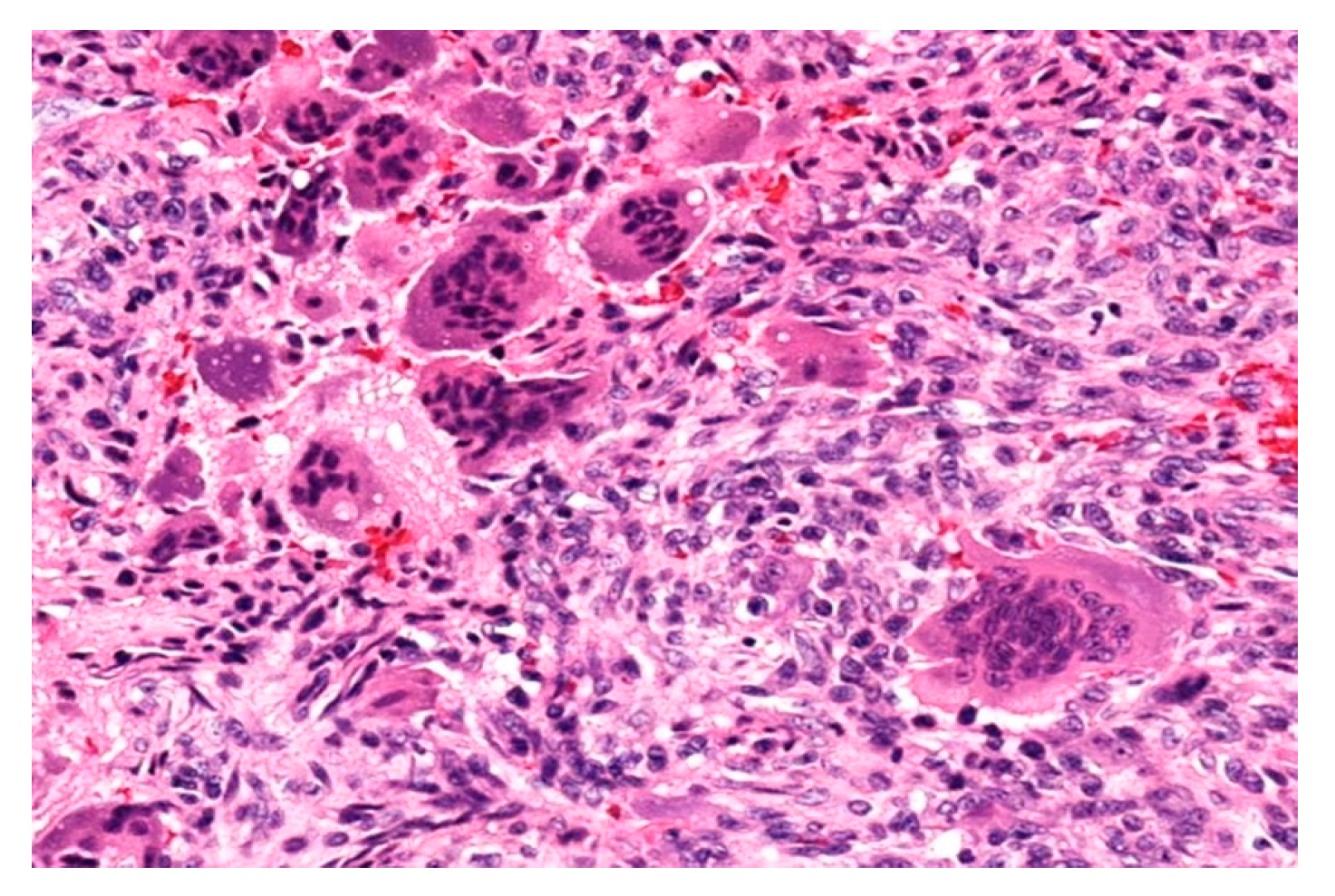
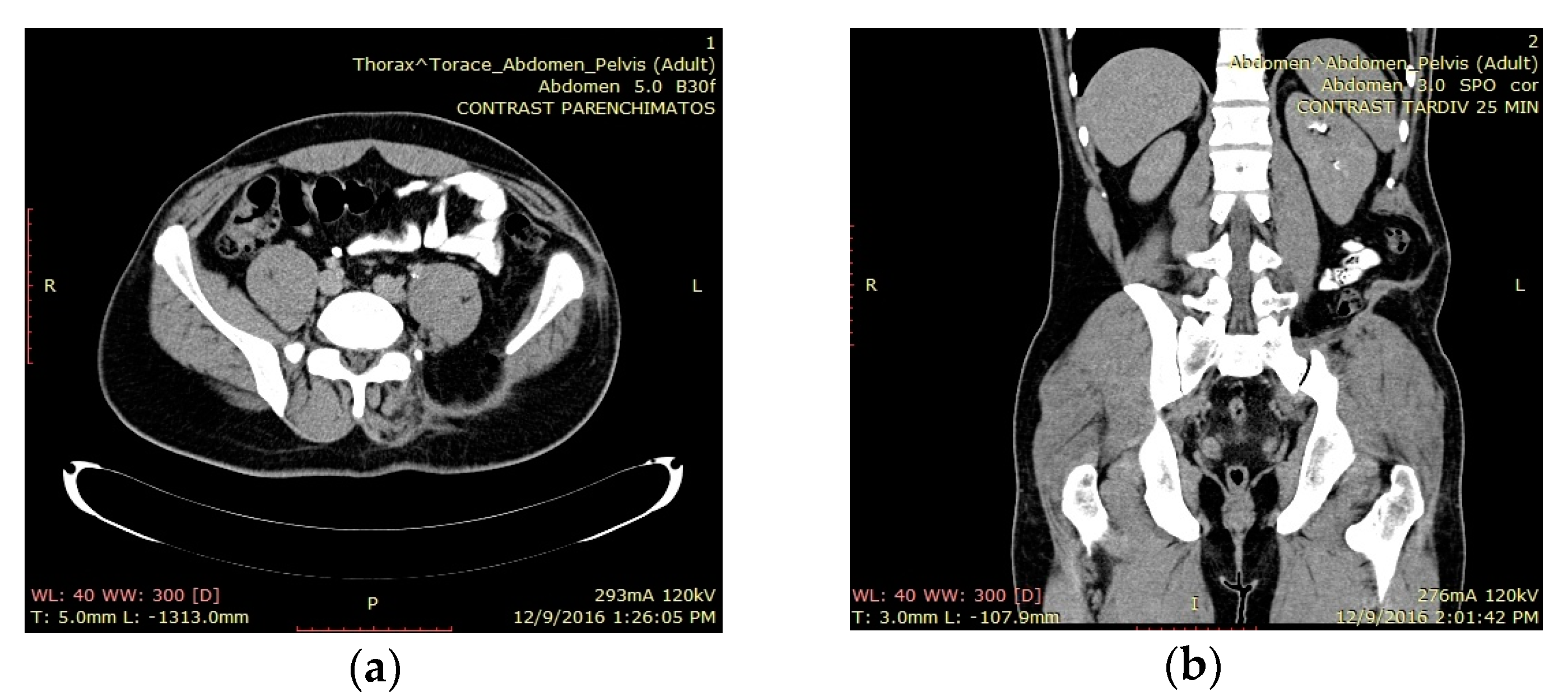
3.1. Case 1
3.2. Case 2
3.3. Case 3
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Klenke, F.M.; Wenger, D.E.; Inwards, C.Y.; Rose, P.S.; Sim, F.H. Giant Cell Tumor of Bone: Risk Factors for Recurrence. Clin. Orthop. Relat. Res. 2011, 469, 591–599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sobti, A.; Agrawal, P.; Agarwala, S.; Agarwal, M. Giant Cell Tumor of Bone-An Overview. Arch. Bone Jt. Surg. 2016, 4, 2–9. [Google Scholar]
- Thomas, D.M.; Skubitz, K.M. Giant cell tumor of the bone. Curr. Opin. Oncol. 2009, 21, 338–344. [Google Scholar] [CrossRef]
- Kim, Y.; Nizami, S.; Goto, H.; Lee, F.Y. Modern interpretation of giant cell tumor of bone: Predominantly osteoclastogenic stromal tumor. Clin. Orthop. Surg. 2012, 4, 107–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez-Pousa, A.; MartinBroto, J.; Garrido, T.; Vazquez, J. Giant cell tumor of bone: New treatments in development. Clin. Transl. Oncol. 2015, 17, 419–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knochentumoren, A.; Becker, W.T.; Dohle, J.; Bernd, L.; Braun, A.; Cserhati, M.; Enderle, A.; Hovy, L.; Matejovsky, Z.; Szendroi, M.; et al. Local recurrence of giant cell tumor of bone after intralesional treatment with and without adjuvant therapy. J. Bone Jt. Surg. Am. 2008, 90, 1060–1067. [Google Scholar] [CrossRef]
- Behjati, S.; Tarpey, P.S.; Presneau, N.; Scheipl, S.; Pillay, N.; Van Loo, P.; Wedge, D.C.; Cooke, S.L.; Gundem, G.; Davies, H.; et al. Distinct H3F3A and H3F3B driver mutations define chondroblastoma and giant cell tumor of bone. Nat. Genet. 2013, 45, 1479–1482. [Google Scholar] [CrossRef]
- Marcove, R.C.; Miller, T.R. The treatment of primary and metastatic localized bone tumors by cryosurgery. Surg. Clin. N. Am. 1969, 49, 421–430. [Google Scholar] [CrossRef]
- Patrick, D.L.; Cleeland, C.S.; Von Moos, R.; Fallowfield, L.; Wei, R.; Öhrling, K.; Qian, Y. Pain outcomes in patients with bone metastases from advanced cancer: Assessment and management with bone-targeting agents. Support Care Cancer 2015, 23, 1157–1168. [Google Scholar] [CrossRef] [Green Version]
- Tsuchiya, H.; Wan, D.L.; Sakayama, K.; Yamamoto, N.; Nishida, H.; Tomita, K. Reconstruction using an autograft containing tumour treated by liquid nitrogen. J. Bone Jt. Surg. Br. 2005, 87, 218–225. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Garlich, J.; Vincent, K. Postoperative complications with cryotherapy in bone tumors. J. Bone Oncol. 2017, 7, 13–17. [Google Scholar] [CrossRef]
- Van der Heijden, L.; Van de Sande, M.A.; Heineken, A.C.; Fiocco, M.; Nelissen, R.G.; Dijkstra, P.D. Mid-term outcome after curettage with polymethylmethacrylate for giant cell tumor around the knee: Higher risk of radiographic osteoarthritis? J. Bone Jt. Surg. Am. 2013, 95, e1591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabel, M.S. Cryo-immunology: A review of the literature and proposed mechanisms for stimulatory versus suppressive immune responses. Cryobiol 2009, 58, 1–11. [Google Scholar] [CrossRef]
- Goel, R.; Anderson, K.; Slaton, J.; Schmidlin, F.; Vercellotti, G.; Belcher, J.; Bischof, J.C. Adjuvant approaches to enhance cryosurgery. J. Biomech. Eng. 2009, 131, 074003. [Google Scholar]
- Balke, M.; Schremper, L.; Gebert, C.; Ahrens, H.; Streitbuerger, A.; Koehler, G.; Hardes, J.; Gosheger, G. Giant cell tumor of bone: Treatment and outcome of 214 cases. J. Cancer Res. Clin. Oncol. 2008, 134, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Pop, D.L.; Nodiţi, G.; Abu-Awwad, A.; Maliţa, D.C.; Zamfir, C.L.; Grigoraş, M.L.; Vermeşan, D.; Prejbeanu, R.; Hărăguş, H.G.; Boşcu, A.L.; et al. Alveolar rhabdomyosarcoma in an adolescent male patient - case report and current perspectives. Rom. J. Morphol. Embryol. 2018, 59, 1247–1252. [Google Scholar] [PubMed]
- Meller, I.; Weinbroum, A.; Bickels, J.; Dadia, S.; Nirkin, A.; Merimsky, O.; Issakov, J.; Flusser, G.; Marouani, N.; Cohen, N.; et al. Fifteen years of bone tumor cryosurgery: A single-center experience of 440 procedures and long-term follow-up. Eur. J. Surg. Oncol. 2008, 34, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Boons, H.W.; Keijser, L.C.; Schreuder, H.W.; Pruszczynski, M.; Lemmens, J.A.; Veth, R.P. Oncologic and functional results after treatment of giant cell tumors of bone. Arch. Orthop. Trauma Surg. 2002, 122, 17–23. [Google Scholar] [CrossRef]
- Abdelrahman, M.; Bassiony, A.A.; Shalaby, H.; Assal, M.K. Cryosurgery and impaction subchondral bone graft for the treatment of giant cell tumor around the knee. HSS J. 2009, 5, 123–128. [Google Scholar] [CrossRef] [Green Version]
- Popken, F.; Michael, J.W.; Zarghooni, K.; Sobottke, R.; Kasper, H.U.; Blaecker, D.; Niehoff, A.; Emrich, F.; Eysel, P. Stability changes after cryosurgery in long tubular bones in correlation to histological results: An animal trial. Arch. Orthop. Trauma Surg. 2009, 129, 857–862. [Google Scholar] [CrossRef]
- Hanahan, D.; Coussens, L.M. Accessories to the crime: Functions of cells recruited to the tumor microenvironment. Cancer Cell 2012, 21, 309–322. [Google Scholar] [CrossRef] [Green Version]
- Baust, J.G.; Gage, A.A.; Bjerklund, Johansen, T.E.; Baust, J.M. Mechanisms of cryoablation: Clinical consequences on malignant tumors. Cryobiology 2014, 68, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, B.L.; Pogrel, M.A. Neurosensory changes after liquid nitrogen cryotherapy. J. Oral Maxillofac. Surg. 2004, 62, 1183–1187. [Google Scholar] [CrossRef]
- Han, B.; Bischof, J.C. Direct cell injury associated with eutectic crystallization during freezing. Cryobiology 2004, 48, 8–21. [Google Scholar] [CrossRef]
- Rock, M. Curettage of giant cell tumor of bone. Factors influencing local recurrences and metastasis. Chir. Organi Mov. 1990, 75, 204–205. [Google Scholar]
- Trieb, K.; Bitzan, P.; Lang, S.; Dominkus, M.; Kotz, R. Recurrence of curetted and bone-grafted giant- cell tumours with and without adjuvant phenol therapy. Eur. J. Surg. Oncol. 2001, 27, 200–202. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ioan Faur, C.; Abu-Awwad, A.; Pop, D.L.; Zamfir, C.L.; Gurgus, D.; Hoinoiu, T.; Motoc, A.; Haivas, C.; Grigoraș, M.L.; Folescu, R. Liquid Nitrogen Efficiency in Treatment of Giant Cell Tumor of Bone and Prevention of Recurrence. Appl. Sci. 2020, 10, 6310. https://doi.org/10.3390/app10186310
Ioan Faur C, Abu-Awwad A, Pop DL, Zamfir CL, Gurgus D, Hoinoiu T, Motoc A, Haivas C, Grigoraș ML, Folescu R. Liquid Nitrogen Efficiency in Treatment of Giant Cell Tumor of Bone and Prevention of Recurrence. Applied Sciences. 2020; 10(18):6310. https://doi.org/10.3390/app10186310
Chicago/Turabian StyleIoan Faur, Cosmin, Ahmed Abu-Awwad, Daniel Laurențiu Pop, Carmen Lăcrămioara Zamfir, Daniela Gurgus, Teodora Hoinoiu, Andrei Motoc, Carmen Haivas, Mirela Loredana Grigoraș, and Roxana Folescu. 2020. "Liquid Nitrogen Efficiency in Treatment of Giant Cell Tumor of Bone and Prevention of Recurrence" Applied Sciences 10, no. 18: 6310. https://doi.org/10.3390/app10186310





