Development of High-Resolution Simple Sequence Repeat Markers through Expression Profiling of Genes Associated with Pod Maturity of Soybean
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Field Tests
2.2. Analysis and Selection of Genes
2.3. Analysis of Genetic Polymorphisms
2.4. Profiling of Gene Expression
2.5. Agglomerative Clustering
2.6. Statistical Analysis
3. Results
3.1. Maturity Grouping
3.2. Development and Selection of SSR Markers
3.3. Analysis of Relationship Using the SSR Markers
4. Discussion
4.1. Large-Scale Genic SSR Marker Development in the Soybean
4.2. Expression Profiling Shows That Six Genes Were Linked to the Growth and Development of the Soybean Pod
4.3. Allelic Variations for the New SSR Markers Are Sufficient for Generating a Cluster and Distinguishing the Soybean Varieties by Ecotype Group
4.4. Agglomerative Clustering Shows That the Six New Genic SSR Markers Can Serve for the Genotyping and Fine QTL Mapping of Soybean Pod Maturity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Xu, M.; Xu, Z.; Liu, B.; Kong, F.; Tsubokura, Y.; Watanabe, S. Genetic variation in four maturity genes affects photoperiod insensitivity and PHYA-regulated post-flowering responses of soybean. BMC Plant Biol. 2013, 13, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, S.; Harada, K.; Abe, J. Genetic and molecular bases of photoperiod responses of flowering in soybean. Breed. Sci. 2012, 61, 531–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, D.; Takeshima, R.; Zhao, C.; Liu, B.; Abe, J.; Kong, F. Molecular mechanisms of flowering under long days and stem growth habit in soybean. J. Exp. Bot. 2017, 68, 1873–1884. [Google Scholar] [CrossRef] [Green Version]
- Scott, W.O.; Aldrich, S.R. Modern Soybean Production, 1st ed.; S & A Publ. Inc.: Champaign, IL, USA, 1970. [Google Scholar]
- Boerma, H.R.; Specht, J.E. (Eds.) Soybeans: Improvement, Production, and Uses, 3rd ed.; ASA, CSSA, and SSSA: Madison, WI, USA, 2004. [Google Scholar]
- Mourtzinis, S.; Conley, S.P. Delineating soybean maturity groups across the US. Agron. J. 2017, 109, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Ha, T.J.; Lim, S.G.; Shin, S.H.; Choi, K.J.; Baek, I.Y.; Lee, S.C.; Park, K.Y.; Shin, S.O. Maturity Grouping of Korean Soybean Cultivars and Character Relationships According to the Planting Date. Korean J. Crop Sci. 2009, 54, 104–118. [Google Scholar]
- Lee, J.E.; Jung, G.H.; Kim, S.K.; Kim, M.T.; Shin, S.H.; Jeon, W.T. Effects of Growth Period and Cumulative Temperature on Flowering, Ripening and Yield of Soybean by Sowing Times. Korean J. Crop Sci. 2019, 64, 406–413. [Google Scholar]
- Sun, F.; Xu, M.; Park, C.; Dwiyanti, M.S.; Nagano, A.J.; Zhu, J.; Watanabe, S.; Kong, F.; Liu, B.; Yamada, T.; et al. Characterization and quantitative trait locus mapping of late-flowering from a Thai soybean cultivar introduced into a photoperiod-insensitive genetic background. PLoS ONE 2019, 14, e0226116. [Google Scholar] [CrossRef] [Green Version]
- Jia, H.; Jiang, B.; Wu, C.; Lu, W.; Hou, W.; Sun, S.; Yan, H.; Han, T. Maturity group classification and maturity locus genotyping of early-maturing soybean varieties from high-latitude cold regions. PLoS ONE 2014, 9, e94139. [Google Scholar] [CrossRef]
- Thiel, T.; Michalek, W.; Varshney, R.K.; Graner, A. Exploiting EST databases for the development and characterization of gene-derived SSR-markers in barley (Hordeum vulgare L.). Theor. Appl. Genet. 2003, 106, 411–422. [Google Scholar] [CrossRef]
- Jiang, D.; Zhong, G.Y.; Hong, Q.B. Analysis of microsatellites in citrus unigenes. Acta Genet. Sin. 2006, 33, 345–353. [Google Scholar] [CrossRef]
- Gao, P.; Ma, H.; Luan, F.; Song, H. DNA fingerprinting of Chinese melon provides evidentiary support of seed quality appraisal. PLoS ONE 2012, 7, e52431. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Wang, P.; Su, Y.; Wang, R.; Li, Q.; Sun, K. Microsatellite diversity, population structure, and core collection formation in melon germplasm. Plant Mol. Biol. Rep. 2014, 33, 439–447. [Google Scholar] [CrossRef]
- Li, L.; Fang, Z.; Zhou, J.; Chen, H.; Hu, Z.; Gao, L.; Chen, L.; Ren, S.; Ma, H.; Lu, L.; et al. An accurate and efficient method for large-scale SSR genotyping and applications. Nucleic Acids Res. 2017, 45, e88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miah, G.; Rafii, M.Y.; Ismail, M.R.; Puteh, A.B.; Rahim, H.A.; Islam, K.; Latif, M.A. A review of microsatellite markers and their applications in rice breeding programs to improve blast disease resistance. Int. J. Mol. Sci. 2013, 14, 22499–22528. [Google Scholar] [CrossRef] [Green Version]
- Macaulay, M.; Ramsay, L.; Powell, W.; Waugh, R. A representative, highly informative “genotyping set” of barley SSRs. Theor. Appl. Genet. 2001, 102, 801–809. [Google Scholar] [CrossRef]
- SoyBase.org. Available online: https://www.soybase.org/ (accessed on 12 July 2017).
- JGI Genome Portal Tutorial. Available online: https://mycocosm.jgi.doe.gov/Tutorial/tutorial/kog.html (accessed on 12 July 2017).
- Pfam. Available online: https://pfam.xfam.org/search#tabview=tab1 (accessed on 12 July 2017).
- PANTHER—Gene List Analysis. Available online: http://www.pantherdb.org/ (accessed on 12 July 2017).
- SoyCyc Database. Available online: https://soycyc.soybase.org/ (accessed on 12 July 2017).
- SoyCyc 7.0. Plant Metabolic Network. Available online: https://www.plantcyc.org/databases/soycyc/7.0 (accessed on 12 July 2017).
- Available online: https://www.arabidopsis.org/download_files/Pathways/BLAST_sets/soycyc_enzymes.fasta (accessed on 12 July 2017).
- Temnykh, S.; DeClerck, G.; Lukashova, A.; Lipovich, L.; Cartinhour, S.; McCouch, S. Computational and experimental analysis of microsatellites in rice (Oryza sativa L.): Frequency, length variation, transposon associations, and genetic marker potential. Genome Res. 2001, 11, 1441–1452. [Google Scholar] [CrossRef] [Green Version]
- Botstein, D.; White, R.L.; Skolnick, M.; Davis, R.W. Construction of a genetic linkage map in man using restriction fragment length polymorphisms. Am. J. Hum. Genet. 1980, 32, 314–331. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using realtime quantitative PCR and the 2ΔΔC(T) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef] [Green Version]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef]
- Tamura, K.; Dudley, J.; Nei, V.; Kumar, S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol. Biol. Evol. 2007, 24, 1596–1599. [Google Scholar] [CrossRef] [PubMed]
- Mansur, L.M.; Orf, J.H.; Chase, K.; Jarvik, T.; Cregan, P.B.; Lark, K.G. Genetic mapping of agronomic traits using recombinant inbred lines of soybean. Crop Sci. 1996, 36, 1327–1336. [Google Scholar] [CrossRef]
- Orf, J.H.; Chase, K.; Jarvik, T.; Mansur, L.M.; Cregan, P.B.; Adler, F.R.; Lark, K.G. Genetics of soybean agronomic traits: I. Comparison of three related recombinant inbred populations. Crop Sci. 1999, 39, 1642–1651. [Google Scholar] [CrossRef] [Green Version]
- Specht, J.E.; Chase, K.; Macrander, M.; Graef, G.L.; Chung, J.; Markwell, J.P.; Germann, M.; Orf, J.H.; Lark, K.G. Soybean response to water: A QTL analysis of drought tolerance. Crop Sci. 2001, 41, 493–509. [Google Scholar] [CrossRef]
- Wang, D.; Graef, G.L.; Procopiuk, A.M.; Diers, B.W. Identification of putative QTL that underlie yield in interspecific soybean backcross populations. Theor. Appl. Genet. 2004, 108, 458–467. [Google Scholar] [CrossRef]
- Li, W.; Zheng, D.H.; Van, K.; Lee, S.H. QTL Mapping for major agronomic traits across two years in soybean (Glycine max L. Merr.). J. Crop Sci. Biot. 2008, 11, 171–190. [Google Scholar]
- Panthee, D.R.; Pantalone, V.R.; Saxton, A.M.; West, D.R.; Sams, C.E. Quantitative trait loci for agronomic traits in soybean. Plant Breed. 2007, 126, 51–57. [Google Scholar] [CrossRef]
- Watanabe, S.; Hideshima, R.; Xia, Z.J.; Tsubokura, Y.; Sato, S.; Nakamoto, Y.; Yamanaka, N.; Takahashi, R.; Ishimoto, M.; Anai, T.; et al. Map-based cloning of the gene associated with the soybean maturity locus E3. Genetics 2009, 182, 1251–1262. [Google Scholar] [CrossRef] [Green Version]
- Mason, A.S. SSR genotyping. Methods Mol. Biol. 2015, 1245, 77–89. [Google Scholar] [CrossRef]
- Akkaya, M.G.; Bhawat, A.; Cregan, P.B. Length polymorphisms of simple sequence repeat DNA in soybean. Genetics 1992, 132, 1131–1139. [Google Scholar]
- Song, Q.J.; Quigley, C.V.; Nelson, R.L.; Carter, T.E.; Boerma, H.R.; Strachan, J.R.; Cregan, P.B. A selected set of trinucleotide simple sequence repeat markers for soybean cultivar identification. Plant Var. Seeds 1999, 12, 207–220. [Google Scholar]
- Narvel, J.M.; Fehr, W.R.; Chu, W.S.; Grant, D.; Shoemaker, R.C. Simple sequence repeat diversity among soybean plant introductions and elite genotypes. Crop Sci. 2000, 40, 1452–1458. [Google Scholar] [CrossRef]
- Song, Q.J.; Marek, L.F.; Shoemaker, R.C.; Lark, K.G.; Concibido, V.C.; Delannay, X.; Specht, J.E.; Cregan, P.B. A new integrated genetic linkage map of the soybean. Theor. Appl. Genet. 2004, 109, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Hisano, H.; Sato, S.; Isobe, S.; Sasamoto, S.; Wada, T.; Matsuno, A.; Fujishiro, T.; Yamada, M.; Nakayama, S.; Nakamura, Y.; et al. Characterization of the soybean genome using EST-derived microsatellite markers. DNA Res. 2007, 14, 271–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varshney, R.K.; Graner, A.; Sorrells, M.E. Genic microsatellite markers in plants: Features and applications. Trends Biotechnol. 2005, 23, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Yi, G.; Lee, J.M.; Lee, S.; Choi, D.; Kim, B.D. Exploitation of pepper EST–SSRs and an SSR-based linkage map. Theor. Appl. Genet. 2006, 114, 113–130. [Google Scholar] [CrossRef]
- Simko, I. Development of EST–SSR markers for the study of population structure in lettuce (Lactuca sativa L.). J. Hered. 2009, 100, 256–262. [Google Scholar] [CrossRef] [Green Version]
- Izzah, N.K.; Lee, J.; Jayakodi, M.; Perumal, S.; Jin, M.; Park, B.S.; Ahn, K.; Yang, T.J. Transcriptome sequencing of two parental lines of cabbage (Brassica oleracea L. var. capitata L.) and construction of an EST-based genetic map. BMC Genom. 2014, 15, 149. [Google Scholar] [CrossRef] [Green Version]
- Dillon, N.L.; Innes, D.J.; Bally, I.S.; Wright, C.L.; Devitt, L.C.; Dietzgen, R.G. Expressed sequence tag-simple sequence repeat (EST–SSR) marker resources for diversity analysis of mango (Mangifera indica L.). Diversity 2014, 6, 72–87. [Google Scholar] [CrossRef] [Green Version]
- Varshney, R.K.; Thiel, T.; Stein, N.; Langridge, P.; Graner, A. In Silico analysis on frequency and distribution of microsatellites in ESTs of some cereal species. Cell. Mol. Biol. Lett. 2002, 7, 537–546. [Google Scholar] [PubMed]
- Weingartner, M.; Subert, C.; Sauer, N. LATE, a C2H2 zinc-finger protein that acts as floral repressor. Plant J. 2011, 68, 681–692. [Google Scholar] [CrossRef]
- Close, T.J. Dehydrins: Emergence of a biochemical role of a family of plant dehydration proteins. Physiol. Plant. 1996, 97, 795–803. [Google Scholar] [CrossRef]
- Close, T.J. Dehydrins: A commonalty in the response of plants to dehydration and low temperature. Physiol. Plant. 1997, 100, 291–296. [Google Scholar] [CrossRef]
- Campbell, S.A.; Close, T.J. Dehydrins: Genes, proteins, and associations with phenotypic traits. New Phytol. 1997, 137, 611–674. [Google Scholar] [CrossRef]
- Teixeira, R.N.; Ligterink, W.; França-Neto, J.B.; Hilhorst, H.W.; da Silva, E.A. Gene expression profiling of the green seed problem in soybean. BMC Plant Biol. 2016, 16, 37. [Google Scholar] [CrossRef] [Green Version]
- Gu, Y.; Li, W.; Jiang, H.; Wang, Y.; Gao, H.; Liu, M.; Chen, Q.; Lai, Y.; He, C. Differential expression of a WRKY gene between wild and cultivated soybeans correlates to seed size. J. Exp. Bot. 2017, 68, 2717–2729. [Google Scholar] [CrossRef]
- Dunwell, J.M.; Purvis, A.; Khuri, S. Cupins: The most functionally diverse protein superfamily? Phytochemistry 2004, 65, 7–17. [Google Scholar] [CrossRef]
- Song, B.; An, L.; Han, Y.; Gao, H.; Ren, H.; Zhao, X.; Wei, X.; Krishnan, H.B.; Liu, S. Transcriptome profile of near-isogenic soybean lines for β-conglycinin α-subunit deficiency during seed maturation. PLoS ONE 2016, 11, e0159723. [Google Scholar] [CrossRef]
- Wang, L.; Yu, S.; Tong, C.; Zhao, Y.; Liu, Y.; Song, C.; Zhang, Y.; Zhang, X.; Wang, Y.; Hua, W.; et al. Genome sequencing of the high oil crop sesame provides insight into oil biosynthesis. Genome Biol. 2014, 15, 2014–2015. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Zhang, X.; Lu, C.; Zeng, X.; Li, Y.; Fu, D.; Wu, G. Non-specific lipid transfer proteins in plants: Presenting new advances and an integrated functional analysis. J. Exp. Bot. 2015, 66, 5663–5681. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Chang, C.; Tucker, M.L. To grow old: Regulatory role of ethylene and jasmonic acid in senescence. Front. Plant Sci. 2015, 6, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Y.; Fukushige, H.; Hildebrand, D.F.; Gan, S. Evidence supporting a role of jasmonic acid in Arabidopsis leaf senescence. Plant Physiol. 2002, 128, 8761–8884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danisman, S.; van der Wal, F.; Dhondt, S.; Waites, R.; de Folter, S.; Bimbo, A.; van Dijk, A.D.; Muino, J.M.; Cutri, L.; Dornelas, M.C.; et al. Arabidopsis class I and class II TCP transcription factors regulate jasmonic acid metabolism and leaf development antagonistically. Plant Physiol. 2012, 159, 1511–1523. [Google Scholar] [CrossRef] [Green Version]
- Fütterer, J.; Hohn, T. Translation in plants—Rules and exceptions. Plant Mol. Biol. 1996, 32, 159–189. [Google Scholar] [CrossRef]
- Gallie, D.R. Translational control of cellular and viral mRNAs. Plant Mol. Biol. 1996, 32, 145–158. [Google Scholar] [CrossRef]
- Gray, N.K.; Wickens, M. Control of translation initiation in animals. Annu. Rev. Cell Dev. Biol. 1998, 14, 399–458. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez, R.A.; MacIntosh, G.C.; Green, P.J. Current perspectives on mRNA stability in plants: Multiple levels and mechanisms of control. Trends Plant Sci. 1999, 4, 429–438. [Google Scholar] [CrossRef]
- McClure, B.A.; Guilfoyle, T. Rapid redistribution of auxin-regulated RNAs during gravitropism. Science 1989, 243, 91–93. [Google Scholar] [CrossRef]
- Portis, E.; Portis, F.; Valente, L.; Moglia, A.; Barchi, L.; Lanteri, S.; Acquadro, A. A genome-wide survey of the microsatellite content of the globe artichoke genome and the development of a web-based database. PLoS ONE 2016, 11, e0162841. [Google Scholar] [CrossRef]
- Mathi Thumilan, B.; Sajeevan, R.S.; Biradar, J.; Madhuri, T.; Nataraja, N.K.; Sreeman, S.M. Development and characterization of genic SSR markers from indian mulberry transcriptome and their transferability to related species of Moraceae. PLoS ONE 2016, 11, e0162909. [Google Scholar] [CrossRef] [PubMed]
- Saha, D.; Rana, R.S.; Chakraborty, S.; Datta, S.; Kumar, A.A.; Chakraborty, A.K.; Karmakar, P.G. Development of a set of SSR markers for genetic polymorphism detection and interspecific hybrid jute breeding. Crop J. 2017, 5, 416–429. [Google Scholar] [CrossRef]
- Cloutier, S.; Niu, Z.; Datla, R.; Duguid, S. Development and analysis of EST-SSRs for flax (Linum usitatissimum L.). Theor. Appl. Genet. 2009, 119, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Leigh, F.; Lea, V.; Law, J.; Wolters, P.; Powell, W.; Donini, P. Assessment of EST-and genomic microsatellite markers for variety discrimination and genetic diversity studies in wheat. Euphytica 2003, 133, 359–366. [Google Scholar] [CrossRef]
- Wen, M.F.; Wang, H.Y.; Xia, Z.Q.; Zou, M.L.; Lu, C.; Wang, W.Q. Development of EST-SSR and genomic-SSR markers to assess genetic diversity in Jatropha curcas L. BMC Res. Notes 2010, 3, 42. [Google Scholar] [CrossRef] [Green Version]
- Ting, N.C.; Zaki, N.M.; Rosli, R.; Low, E.T.; Ithnin, M.; Cheah, S.C.; Tan, S.G.; Singh, R. SSR mining in oil palm EST database: Application in oil palm germplasm diversity studies. J. Genet. 2010, 89, 135–145. [Google Scholar] [CrossRef]
- Bryant, D.; Moulton, V. Neighbor Net: An agglomerative algorithm for the construction of planar phylogenetic networks. Mol. Biol. Evol. 2004, 21, 255–265. [Google Scholar] [CrossRef]


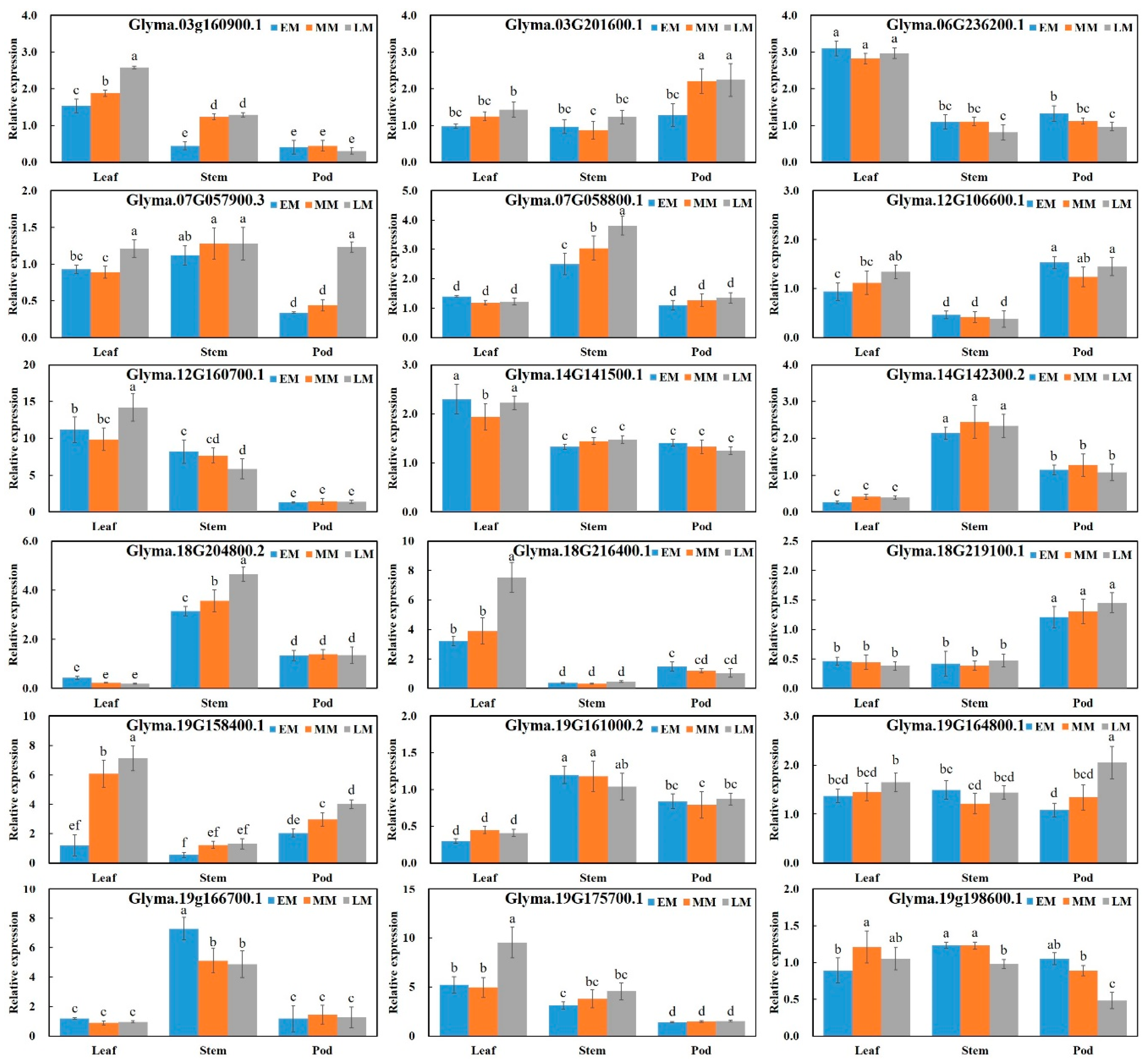
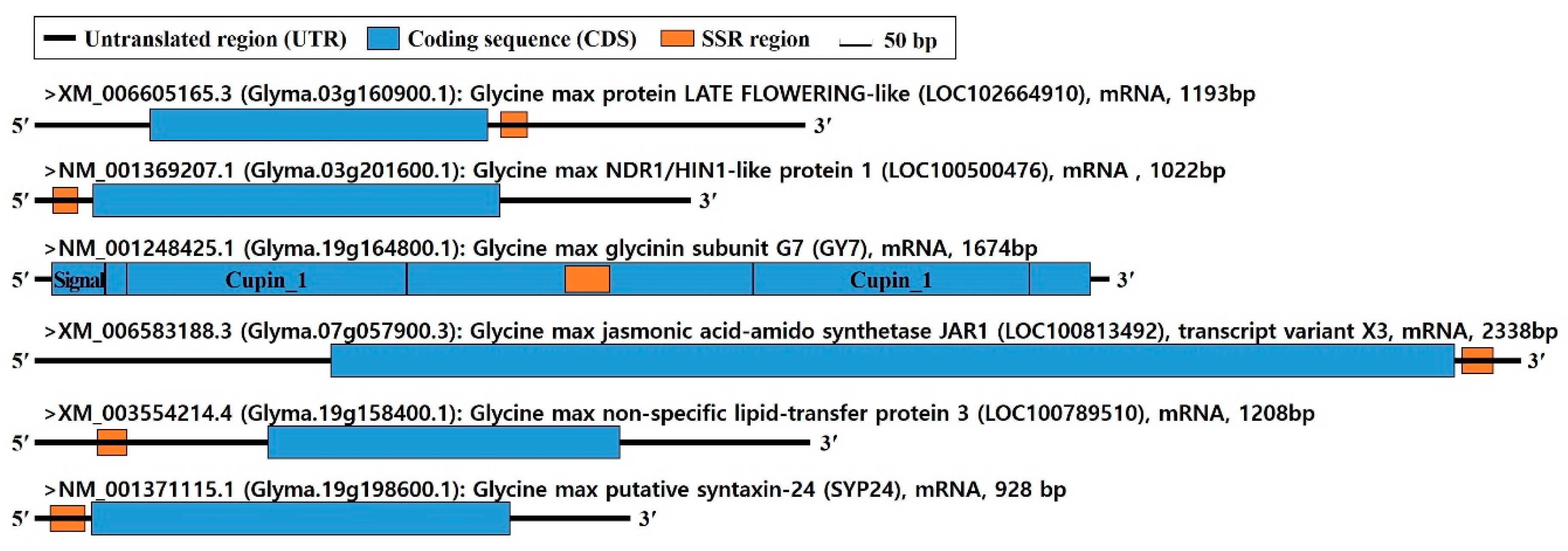

| Maturity Group | Days to Maturity (From Sowing) | Varieties | No. of Varieties | Maturity Ecotype |
|---|---|---|---|---|
| 0 | ≤79 | OT89-06 | 1 | Early Maturity (EM) |
| I | 80–84 | Dongnong13-6522, Dongnong44, Dongnong59, Dongnong66, Dongnongdou117, Dongnongdou118, Geomjeongol, OT89-05, OT93-37, OT94-51 | 10 | |
| II | 85–89 | Dongdou641, Dongnong13-6010, Dongnong13-6271, Dongnong13-6334, Dongnong13-6590, Dongnong13-6622, Dongnong55, Dongnong67, Heinong48, Jilidou4, Jiyu201, Jiyu401, Liaodou21, Liaodou32, OT93-26, OT93-28, OT94-39, Yandou2, Yandou4 | 19 | |
| III | 90–94 | 211B, Dongnong13-6004, Dongnong56, Heihe43, Heinong44, Heinong52, IT213198, IT263300, IT252691, Kenfeng20, Kenfeng22, L62-667, Liaodou46, Nongqingdou1, Yandou1, Yandou3, Zaoshu1 | 17 | Middle Maturity (MM) |
| IV | 95–99 | Dandou12, Dongdou339, Dongdou9, IT142772, IT273835, IT284565, Liaodou14, Liaowanshu, Liaozhongshu, Ludou10, Mengdou1001, Shanning16, Xudou9, Xudou14, Xudou18, Xudou20, Yandou5, Zhonghuang13, Zhonghuang35 | 19 | |
| V | 100–104 | Cangdou4, Cheonga, Dandou14, Duyoukong, Gangil, Gaofeng1, Haessal, Hedou21, IT142770, IT212835, IT213177, Keumgangkong, Liaodou47, Liaozaoshu, Seonyu, Shennong8, Tiefeng31, Yongpoong, Zhonghuang37, Zhonghuang57 | 20 | Late Maturity (LM) |
| VI | 105–109 | Daechan, IT213175, Jinpung, Pungwon, Shinhwa, Taekwangkong | 6 | |
| VII | ≥110 | Daepung, Daepung2, Daewonkong, Haepum, IT213173, Pungsannamul, Seonpung, Uram | 8 | |
| Total | 100 varieties | 100 | ||
| Marker (Loci) | QTL Name | Chr. No. | SSR Pattern | Forward Primer (5′–3′) | Reverse Primer (5′–3′) | References |
|---|---|---|---|---|---|---|
| Satt079 | Pod mat 4-1 | Chr.06 | (ATT)13 | AGTCGAAGATACACAATTAGAT | CTTTTAGACACAAATTTATCACT | [32] |
| Satt150 | Pod mat 8-2 | Chr.07 | (ATT)20 | AAGCTTGAGGTTATTCGAAAATGAC | TGCCATCAGGTTGTGTAAGTGT | [33] |
| Satt429 | Pod mat 13-1 | Chr.08 | (ATT)25 | GCGACCATCATCTAATCACAATCTACTA | TCCCCATCATTTATCGAAAATAATAATT | [34] |
| Satt481 | Pod mat 14-1, Pod mat 15-4 | Chr.19 | (ATT)14 | GGGTTAACCGTCCACACATCTATT | GACGGTTTTAAACGGTAAGAAAAT | [35] |
| Sat_038 | Pod mat 26-3 | Chr.10 | (AT)20 | GCGTCGCAACTTTTTCATTTTTCTTACT | GCGAGTTCTTTTAACAACACTCACTTTT | [36] |
| Satt292 | Pod mat 31-4, Pod mat 37-4 | Chr.20 | (ATT)16 | GCGGAATTAGAACTCCAGTAAAGA | GCGAGGCCAACATTGAAAAGT | [37] |
| Group | Sub-Group | No. of Genes | Annotation ID of Genes |
|---|---|---|---|
| Flower | enzyme | 15 | AT5G11530.1, AT1G29750.2, AT2G48010.1 |
| pollen | 134 | GO:0048544 | |
| time control | 19 | AT5G24860.1, AT1G25540.1, AT3G48430.1 | |
| Hormone synthesis | ABA 1 | 19 | SoyCyc: PWY-695 |
| AUX/IAA 2 | 151 | AT3G02260.1, AT1G75310.1, PFAM: PF02309, Panther: PTHR22950:SF3, SoyCyc7: PWYDQC-4, AT2G14960.1, AT2G46370.1, AT2G46370.4, AT3G02260.1, AT3G07390.1, AT3G25290.1, AT3G26810.1, AT3G59900.1, AT4G03400.1, AT4G12980.1, AT4G27260.1, AT4G37390.1, AT5G13320.1, AT5G13360.1, AT5G37020.1, AT5G37020.2, AT5G47530.1, AT5G49980.1, AT5G54510.1, AT5G57420.1, AT5G60450.1, PFAM: PF02309, PFAM: PF02519, PFAM: PF06507, Panther: PTHR22950:SF3, SoyCyc: PWYDQC-4 | |
| ethylene | 62 | AT1G05010.1, AT1G05010.1, SoyCyc7: ETHYL-PWY, AT3G15210.1, AT3G20310.1, AT3G23240.1, AT4G17490.1, AT4G17500.1, AT5G05740.2, AT5G47220.1, AT5G47230.1, PFAM: PF04873 | |
| GA 3 | 73 | AT1G02400.1, AT5G51810.1, AT5G07200.1, AT1G30040.1, AT1G47990.1, AT1G02400.1, AT4G21200.1, AT1G15550.1 | |
| JA 4 | 67 | SoyCyc7: PWY-735 | |
| Seed | embryo | 279 | AT1G01470.1, AT1G13120.1, AT1G21390.1, AT1G49510.1, AT1G52330.1, AT1G52330.2, AT1G52690.1, AT1G56200.1, AT1G60870.1, AT1G64065.1, AT1G71830.1, AT2G01735.1, AT2G02955.1, AT2G15890.1, AT2G25660.1, AT2G31340.1, AT2G31340.1, AT2G34090.1, AT2G34090.2, AT2G34780.1, AT2G35460.1, AT2G35980.1, AT2G42560.1, AT2G46150.1, AT3G05680.1, AT3G05680.2, AT3G20600.1, AT3G23440.1, AT3G44380.1, AT3G48470.1, AT3G56990.1, AT3G60360.1, AT3G61780.1, AT4G01410.1, AT4G13230.1, AT4G13560.1, AT4G14590.1, AT4G19350.1, AT4G21020.1, AT4G24270.2, AT4G28210.1, AT4G29660.1, AT4G35170.1, AT4G36600.1, AT4G37300.1, AT5G05950.1, AT5G06240.1, AT5G21140.1, AT5G38760.1, AT5G40480.1, AT5G44310.2, AT5G53820.1, AT5G53860.2, AT5G53860.5, AT5G54370.1, AT5G60520.1, AT5G63050.1, GO:0009790, KOG2717, KOG4443, PFAM: PF02987, PFAM: PF03168, PFAM: PF03242, PFAM: PF10714, Panther: PTHR23241 |
| metabolite | 11 | AT4G26740.1, SoyCyc7-rxn: GN7V-64549, SoyCyc7-rxn: GN7V-65361, SoyCyc7-rxn: GN7V-65494, SoyCyc7-rxn: GN7V-66171, SoyCyc7-rxn: GN7V-66188, SoyCyc7-rxn: GN7V-66278, SoyCyc7-rxn: GN7V-66349, SoyCyc7-rxn: GN7V-66447, SoyCyc7-rxn: GN7V-66925, SoyCyc7-rxn: GN7V-67046 | |
| maturation | 5 | PFAM: PF04927 | |
| storage protein | 195 | AT1G49320.1, AT1G62500.1, AT1G62790.1, AT2G15325.1, AT2G44300.1, AT2G45180.1, AT2G45690.1, AT2G48140.1, AT3G18280.1, AT3G22142.1, AT3G22600.1, AT3G53980.1, AT4G08670.1, AT4G12520.1, AT4G14805.1, AT4G30880.1, AT4G33355.1, AT4G33550.2, AT5G05960.1, AT5G13900.1, AT5G44265.1, AT5G46890.1, AT5G46900.1, AT5G62065.1, PFAM: PF00234, PFAM: PF00477 | |
| Senescence | 117 | AT1G17020.1, AT1G20780.1, AT1G66330.1, AT1G66580.1, AT1G71190.1, AT2G29350.1, AT3G02040.1, AT3G10985.1, AT5G14930.2, AT5G45890.1, PFAM: PF04520, PFAM: PF06911 | |
| Total | 1147 | ||
| Transcript | Classification | Sub-Classification | Chromosome No. | SSR Pattern | By Use | Forward Primer (5′–3′) | Reverse Primer (5′–3′) | Ta 1 (°C) |
|---|---|---|---|---|---|---|---|---|
| Glyma.03g160900.1 | Flower | Time | Chr.03 | (AT)21 | Polymorphism | ATTAAAGATAAGTTTGAAGAGA | CTTCAAGCCATCTCTATACAA | 50 |
| Expression | CAAGCCTCTCATCCACTGCTC | TCTTGCTCAAACACGTGATGAC | 58 | |||||
| Glyma.03g201600.1 | Seed | Embryo | Chr.03 | (GGT)13 | Polymorphism | CCTTGTGATTTGCTCACAAAAC | AGATGCGTCTCCAGAGCTTGT | 50 |
| Expression | GCTTTCAGGTCACGCTTTCCTC | TTGTGACCTTGGTATGAGGGT | 58 | |||||
| Glyma.07g057900.3 | Hormone | JA | Chr.07 | (AT)24 | Polymorphism | GCGATACACAAGCTCACGCAA | TTTTTATTCAATTGAGAAAGGGA | 52 |
| Expression | GTATGGAGCCTAAGCCTGTGGG | TGAATTATGGAACCCCATAACCTT | 58 | |||||
| Glyma.19g158400.1 | Seed | Storage | Chr.19 | (AAG)15 | Polymorphism | GGCTCAATCTGATCAATCAAGT | TAACAACCTCACATAGGCATAGT | 52 |
| Expression | GTGGTGAGGTCACAGCCACTAT | AGTGCCAAAGCTCGAGTCTGAT | 56 | |||||
| Glyma.19G164800.1 | Seed | Storage | Chr.19 | (GAA)12 | Polymorphism | AGAAGCATCGAACGTGAAGGTG | CGTGTTCTCCTATGTGGTGCTT | 50 |
| Expression | AGAAGCATCGAACGTGAAGGTG | CGTGTTCTCCTATGTGGTGCTT | 58 | |||||
| Glyma.19g198600.1 | Seed | Embryo | Chr.19 | (AGC)18 | Polymorphism | TCATTTCCCACTCCCGCGTTTCC | AACACGAACCCGGCGATGCC | 52 |
| Expression | TCATTTCCCACTCCCGCGTTTCC | AACACGAACCCGGCGATGCC | 58 |
| Marker | No. of Alleles | Amplified Size of Allele | PIC 1 |
|---|---|---|---|
| Satt079 | 4 | 133–154 | 0.74 |
| Satt150 | 3 | 189–210 | 0.66 |
| Satt429 | 4 | 241–274 | 0.74 |
| Satt481 | 5 | 124–169 | 0.77 |
| Sat_038 | 4 | 99–131 | 0.68 |
| Satt292 | 3 | 215–248 | 0.63 |
| Sub-average | 3.8 | 0.70 | |
| Glyma.03g160900.1 | 1 | 212 | 0.59 |
| Glyma.03g201600.1 | 3 | 114–144 | 0.62 |
| Glyma.07g057900.3 | 4 | 285–315 | 0.63 |
| Glyma.19g158400.1 | 4 | 135–168 | 0.71 |
| Glyma.19G164800.1 | 3 | 178–192 | 0.52 |
| Glyma.19g198600.1 | 4 | 232–253 | 0.59 |
| Sub-average | 3.2 | 0.61 | |
| Average | 3.5 | 0.66 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, M.R.; Lee, I.; Seo, M.-J.; Yun, H.-T. Development of High-Resolution Simple Sequence Repeat Markers through Expression Profiling of Genes Associated with Pod Maturity of Soybean. Appl. Sci. 2020, 10, 6363. https://doi.org/10.3390/app10186363
Park MR, Lee I, Seo M-J, Yun H-T. Development of High-Resolution Simple Sequence Repeat Markers through Expression Profiling of Genes Associated with Pod Maturity of Soybean. Applied Sciences. 2020; 10(18):6363. https://doi.org/10.3390/app10186363
Chicago/Turabian StylePark, Myoung Ryoul, Inhye Lee, Min-Jung Seo, and Hong-Tae Yun. 2020. "Development of High-Resolution Simple Sequence Repeat Markers through Expression Profiling of Genes Associated with Pod Maturity of Soybean" Applied Sciences 10, no. 18: 6363. https://doi.org/10.3390/app10186363





