Reduced Supply in the Organ Donor Market and How 3D Printing Can Address This Shortage: A Critical Inquiry into the Collateral Effects of Driverless Cars
Abstract
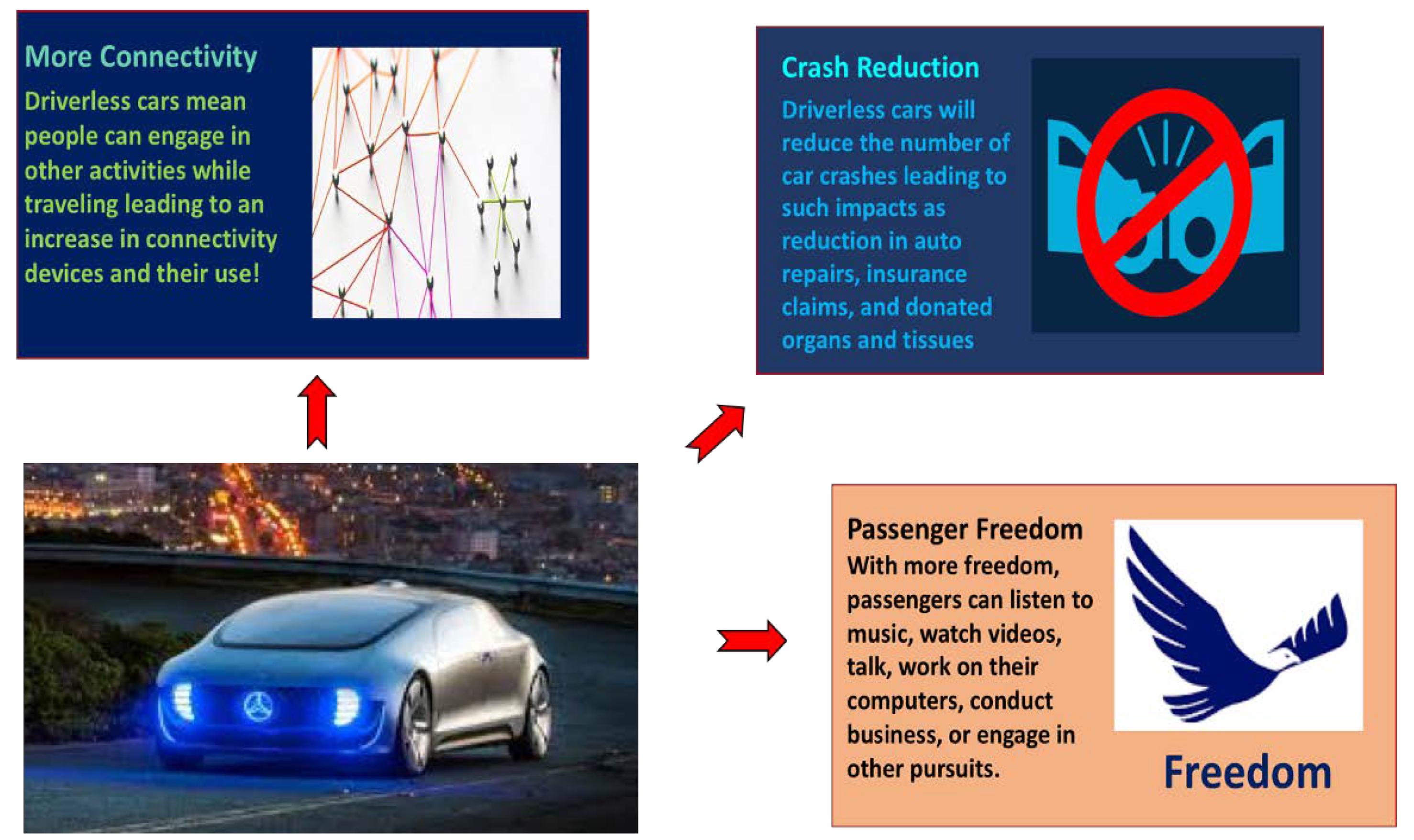
:1. Introduction
2. Driverless Car Technology and the Organ Donor Supply
2.1. Driverless Car Technology Explained
2.2. The Current State of Driverless Car Technology
2.3. Expected Dilution of Supply Following Introduction of Driverless Cars
3. Bioartificial Organs as a Solution to Organ Failure
3.1. Overview of Bioartificial Organ Technology
3.2. Current State of Bioartificial Organ Technology
4. Bioprinting as a Solution to Organ Failure
4.1. Current State of Bioprinting Technology
4.2. Opportunities Presented by Bioprinting
4.3. Commercial Challenges of Organ Replacement Technology
5. Legal Challenges of Organ Replacement Technology
6. Conclusions and Recommendations
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Advantages of Agile Work Strategies for Companies, Costs and Benefits. Available online: http://globalworkplaceanalytics.com/resources/costs-benefits (accessed on 5 March 2018).
- Preliminary Statement of Policy Concerning Automated Vehicles. National Highway Traffic Safety Administration. Available online: https://www.nhtsa.gov/staticfiles/rulemaking/pdf/Automated_Vehicles_Policy.pdf (accessed on 26 February 2018).
- Tomita, H. Awaiting the Realization of Fully Automated Vehicles: Potential Economic Effects and the Need for a New Economic and Social Design 2017. Available online: https://voxeu.org/article/potential-economic-and-social-effects-driverless-cars (accessed on 26 February 2018).
- Godsmark, P. The Definitive Guide to the Levels of Automation in Driverless Cars 2017. Available online: https://driverless.wonderhowto.com/news/definitive-guide-levels-automation-for-driverless-cars-0176009/ (accessed on 2 March 2018).
- Fleetwood, J. Public health, ethics, and autonomous vehicles. Am. J. Public Health 2017, 107, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Holstein, T.; Dodig-Crnkovic, G.; Pelliccione, P. Ethical and social aspects of self-driving cars. arXiv 2018, arXiv:1802.04103. [Google Scholar]
- World Economic Forum. This is How Much Time Americans Spend Commuting to Work. Available online: https://www.weforum.org/agenda/2016/03/this-is-how-much-time-americans-spend-commuting-to-work (accessed on 5 March 2018).
- Clements, L.M.; Kockelman, K.M. Economic effects of automated vehicles. Transp. Res. Rec. J. Transp. Res. Board 2017, 2606, 106–114. [Google Scholar] [CrossRef]
- Fagnant, D.J.; Kockelman, K. Preparing a nation for autonomous vehicles: Opportunities, barriers and policy recommendations for capitalizing on self-driven vehicles. Transp. Res. Part A 2015, 77, 167–181. [Google Scholar]
- Mills, D.K. Future Medicine: The impact of 3D printing. J. Nanomater. Mol. Nanotechnol. 2015, 4, 1–3. [Google Scholar] [CrossRef]
- Mills, D.K.; Tappa, K.; Jammalamadaka, M.; Weisman, J.A. Chapter 8: Medical Applications for 3D Printing. In Advances in Manufacturing and Processing of Materials and Structures; Taylor & Francis Group: Abingdon-on-Thames, UK, 2018. [Google Scholar]
- Kang, H.-W.; Lee, S.J.; Ko, I.K.; Kengla, C.; Yoo, J.J.; Atala, A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat. Biotechol. 2016, 34, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Gross, B.C.; Erkal, J.L.; Lockwood, S.Y.; Chen, C.; Spence, D.M. Evaluation of 3D printing and Its potential impact on biotechnology and the chemical sciences. Anal. Chem. 2014, 86, 3240–3253. [Google Scholar] [CrossRef]
- Facts and Myths. Available online: https://www.americantransplantfoundation.org/about-transplant/facts-and-myths/ (accessed on 9 March 2018).
- Recommendation for Sustainable Kidney Care. Available online: http://ekha.eu/wp-content/uploads/2016/01/EKHA-Recs-for-Sustainable-Kidney-Care-25.08.2015.pdf (accessed on 8 September 2020).
- Yanklowitz, S. Give a Kidney, Get a Check 2015. Available online: https://www.wsj.com/articles/give-a-kidney-get-a-kidney-1470265583/ (accessed on 23 March 2018).
- USRDS. 2013 Annual Data Report: Atlas of End-Stage Renal Disease in the United States; National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases: Washington, DC, USA, 2014. [Google Scholar]
- Shimazono, Y. The state of the international organ trade: A provisional picture based on integration of available information. Bull. World Health Organ. 2012, 85, 901–980. [Google Scholar] [CrossRef] [PubMed]
- Small-Jordan, H. 2016 Organ Harvesting, Human Trafficking, and the Black Market. Available online: https://www.decodedscience.org/organ-harvesting-human-trafficking-black-market/56966 (accessed on 11 March 2018).
- Leitner, T.; Capitani, L. Investigations—Market for Black Market Organs Expands; NBC Chicago: Chicago, IL, USA, 2016. [Google Scholar]
- Munster, G. 2017 Here’s When Having a Self-Driving Car Will Be a Normal Thing. Available online: http://fortune.com/2017/09/13/gm-cruise-self-driving-driverless-autonomous-cars/ (accessed on 9 March 2018).
- Etherington, D. GM, Toyota Set to Push U.S. Regulators on Easing Self-Driving Restrictions 2017. Available online: https://techcrunch.com/2017/02/13/gm-toyota-set-to-push-u-s-regulators-on-easing-self-driving-restrictions/ (accessed on 13 February 2017).
- Whitwam, R. Honda May Team up with Waymo to Make Self-Driving Cars 2016. Available online: https://www.extremetech.com/extreme/241556-honda-may-team-waymo-make-self-driving-cars (accessed on 23 December 2016).
- Lynch, J.; Thibodeau, I. Filings: GM Plans 300 More Self-Driving Cars 2017. Available online: https://www.detroitnews.com/story/business/autos/general-motors/2017/04/14/gm-autonomous-fleet/100477110// (accessed on 14 April 2017).
- O’Kane, S. Ford’s New Autonomous Fusion Looks Freakishly Normal 2016. Available online: https://www.theverge.com/2016/12/28/14100278/ford-new-self-driving-car-fusion-hybrid-testing (accessed on 28 December 2016).
- Davies, A. Uber’s Self-Driving Truck Makes Its First Delivery: 50,000 Beers 2016. Available online: https://www.wired.com/2016/10/ubers-self-driving-truck-makes-first-delivery-50000-beers/ (accessed on 25 October 2016).
- Lambert, F. Uber Self-Driving Prototype Rolls Over after Crash in Arizona, Police Says Uber is Not at Fault 2017. Available online: https://electrek.co/2017/03/25/uber-self-driving-prototype-accident-arizona/ (accessed on 25 February 2017).
- Findling, D. CNBC Took BMW’s New Self-Driving Car out for a Spin 2017. Available online: https://www.cnbc.com/2017/01/13/cnbc-took-bmws-new-self-driving-car-out-for-a-spin-heres-what-it-was-like.html (accessed on 14 January 2017).
- Reuters. Forget Self-Driving Cars: Airbus will Test a Prototype for a Self-Flying Taxi by the End of This Year 2017. Available online: https://www.cnet.com/news/forget-self-driving-cars-how-about-a-self-flying-taxi/ (accessed on 16 January 2017).
- Lambert, F. Tesla Hints at Testing Self-Driving Car Prototypes outside of California 2017. Available online: https://electrek.co/2017/02/06/tesla-testing-self-driving-car-prototypes-outside-california// (accessed on 6 February 2017).
- Burgess, M. We Went Off-Road in Jaguar Land Rover’s Autonomous Car 2016. Available online: https://www.wired.co.uk/article/self-driving-autonomous-land-rover-jaguar-technology (accessed on 22 July 2016).
- McMahon, T. Long Term U.S. Inflation 2014. Available online: https://inflationdata.com/inflation/inflation_rate/long_term_inflation.asp (accessed on 1 April 2014).
- WHO (World Health Organization). GKT1 Activities and Practices. Available online: http://www.who.int/transplantation/gkt/statistics/en/ (accessed on 14 April 2018).
- Global Observatory on Donation and Transplantation. Available online: http://www.transplant-observatory.org (accessed on 14 April 2018).
- U.S. Renal Data System, USRDS.UC San Francisco Dept. of Bioengineering & Therapeutic Sciences. The Kidney Project. In 2013 Annual Data Report: Atlas of End-Stage Renal Disease in the United States; National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases: Washington, DC, USA, 2014; Available online: https://pharm.ucsf.edu/kidney/need/statistics (accessed on 4 April 2017).
- National Kidney Foundation Organ Donation and Transplantation Statistics. Available online: https://www.kidney.org/news/newsroom/factsheets/Organ-Donation-and-Transplantation-Stats (accessed on 14 April 2018).
- Ren, X.; Ott, H.C. Pflugers on the road to bioartificial organs. Arch. Eur. J. Physiol. 2014, 466, 1847. [Google Scholar] [CrossRef]
- Atala, A.; Kasper, F.K.; Mikos, A.G. Engineering complex tissues. Sci. Transl. Med. 2012, 4, 160rv112. [Google Scholar] [CrossRef] [Green Version]
- Iwamuro, M.; Shiraha, H.; Nakaji, S.; Yamamoto, K. Prospects for creating bioartificial liver system with induced pluripotent stem cell technology. Biotechnol. Biomater. 2013, 3, 157. [Google Scholar] [CrossRef]
- Carpentier, B.; Gautier, A.; Legallais, C. Artificial and bioartificial liver devices: Present and future. Gut 2009, 58, 1690–1702. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.; Herrero, P.; El Sharkawy, M.; Pesl, P.; Jugnee, N.; Thomson, H.; Pavitt, D.; Toumazou, C.; Johnston, D.; Georgiou, P.; et al. Feasibility study of a bio-inspired artificial pancreas in adults with Type 1 diabetes. Diabetes Tech. Ther. 2014, 16, 550–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The Kideny Project. Available online: https://pharm.ucsf.edu/kidney (accessed on 14 March 2018).
- Weymann, A.; Patil, N.P.; Sabashnikov, A.; Jungebluth, P.; Korkmaz, S.; Li, S.; Veres, G.; Soós, P.; Ishtok, R.; Chaimow, N.; et al. Bioartificial heart: A human-sized porcine model—The way ahead. PLoS ONE 2014, 9, e111591. [Google Scholar] [CrossRef] [Green Version]
- Jessop, Z.M.; Al-Sabah, A.; Gardiner, M.D.; Combellack, E.; Hawkins, K.; Whitaker, I.S. 3D bioprinting for reconstructive surgery: Principles, applications and challenges. J. Plast. Reconstr. Aesthetic Surg. 2017, 70, 1155–1170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef]
- Ringeisen, B.R.; Othon, C.M.; Barron, J.A.; Wu, P.K.; Spargo, B.J. The evolution of cell printing. In Fundamentals of Tissue Engineering and Regenerative Medicine; Meyer, U., Handschel, J., Wiesmann, P.H., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 613–631. [Google Scholar]
- Guillemot, F.; Mironov, V.; Nakamura, M. Bioprinting is coming of age: Report from the International Conference on Bioprinting and Biofabrication in Bordeaux (3B’09). Biofabrication 2010, 2, 1–7. [Google Scholar] [CrossRef]
- Groll, J.; Boland, T.; Blunk, T.; Burdick, J.A.; Cho, D.W.; Dalton, P.D.; Derby, B.; Forgacs, G.; Li, Q.; Mironov, V.A. Biofabrication: Reappraising the definition of an evolving field. Biofabrication 2016, 8, 13001. [Google Scholar] [CrossRef] [Green Version]
- Bajaj, P.; Schweller, R.M.; Khademhosseini, A.; West, J.L.; Bashir, R. 3D biofabrication strategies for tissue engineering and regenerative medicine. Annu. Rev. Biomed. Eng. 2014, 16, 247–276. [Google Scholar] [CrossRef] [Green Version]
- Derakhshanfar, S.; Mbeleck, R.; Xu, K.; Zhang, X.; Zhong, W.; Xing, M. 3D bioprinting for biomedical devices and tissue engineering: A review of recent trends and advances. Bioact. Mater. 2018, 3, 144–156. [Google Scholar] [CrossRef]
- Bioprinting is Coming of Age. Available online: https://www.shapingtomorrow.com/home/alert/225863-Bioprinting-is-coming-of-age (accessed on 8 September 2020).
- Bishop, E.S.; Mostafa, S.; Pakvasa, M.; Luu, H.H.; Lee, M.J.; Wolf, J.M.; Ameer, G.A.; He, T.-C.; Reid, R.R. 3-D bioprinting technologies in tissue engineering and regenerative medicine: Current and future trends. Genes Disease 2017, 4, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.; Miao, S.; Zhong, J.; Zhang, Z.; Mills, D.K.; Zhang, L.G. Bio-based polymers for 3D printing of bioscaffolds. Poly Rev. 2018, 58, 668–687. [Google Scholar] [CrossRef] [PubMed]
- Faramarzi, N.; Yazdi, I.K.; Nabavinia, M.; Gemma, A.; Fanelli, A.; Caizzone, A.; Ptaszek, L.M.; Sinha, I.; Khademhosseini, A.; Ruskin, J.N.; et al. Patient—Specific bioinks for 3D bioprinting of tissue engineering scaffolds. Adv. Healthc. Mater. 2018, 7, 1701347. [Google Scholar] [CrossRef] [PubMed]
- Chimene, D.; Lennox, K.K.; Kaunas, R.; Gaharwar, A.K. Advanced bioinks for 3D printing: A materials science perspective. Ann. Biomed. Eng. 2016, 44, 2090–2102. [Google Scholar] [CrossRef]
- Mironov, V.; Trusk, T.; Kasyanov, V.; Little, S.; Swaja, R.; Markwald, R. Biofabrication: A 21st century manufacturing paradigm. Biofabrication 2009, 1, 22001. [Google Scholar] [CrossRef] [Green Version]
- Shim, J.-H.; Lee, J.-S.; Kim, J.Y.; Cho, D.-W. (2012) Bioprinting of a mechanically enhanced three-dimensional dual cell-laden construct for osteochondral tissue engineering using a multihead tissue/organ building system. J. Micromech. Microeng. 2012, 22, 85014. [Google Scholar] [CrossRef]
- Mobaraki, M.; Maryam, M.; Yazdanpanah, A.; Luo, Y.; Mills, D.K. Bioinks and bioprinting: A focused review. Bioprinting 2020, 18, e00080. [Google Scholar] [CrossRef]
- Gopinathan, J.; Noh, I. Recent trends in bioinks for 3D printing. Biomater. Res. 2018, 22, 11. [Google Scholar] [CrossRef] [Green Version]
- Gungor-Ozkerim, P.S.; Inci, I.; Zhang, Y.S.; Khademhosseini, A.; Dokmeci, M.R. Bioinks for 3D bioprinting: An overview. Biomater. Sci. 2018, 6, 915–946. [Google Scholar] [CrossRef] [Green Version]
- Shim, J.-H.; Kim, J.Y.; Park, M.; Park, J.; Cho, D.-W. Development of a hybrid scaffold with synthetic biomaterials and hydrogel using solid freeform fabrication technology. Biofabrication 2011, 3, 34102. [Google Scholar] [CrossRef]
- Cheng, R.Y.; Eylert, G.; Gariepy, J.M.; He, S.; Ahmad, H.; Gao, Y.; Priore, S.; Hakimi, N.; Jeschke, M.; Günther, A. Handheld instrument for wound-conformal delivery of skin precursor sheets improves healing in full-thickness burns. Biofabrication 2020, 12, 25002. [Google Scholar] [CrossRef]
- Feildena, E.; Tuñón Blancaa, E.-G.; Giuliania, F.; Saiza, E.; Vandeperrea, L. Robocasting of structural ceramic parts with hydrogel inks. J. Eur. Ceram. Soc. March 2016, 32, 2525–2533. [Google Scholar] [CrossRef]
- Peng, W.; Datta, P.; Ayan, B.; Ozbolat, V.; Sosnoski, D.; Ozbolat, I.T. 3D bioprinting for drug discovery and development in pharmaceutics. Acta Biomater. 2017, 57, 26–46. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Liu, J.; Zhu, W.; Tang, M.; Lawrence, N.; Yu, C.; Gou, M.; Chen, S. 3D bioprinting of functional tissue models for personalized drug screening and in vitro disease modeling. Adv. Drug Deliv. Rev. 2018, 132, 235–251. [Google Scholar] [CrossRef] [PubMed]
- Daimond vs. Charbartgy. Available online: https://www.oyez.org/cases/1979/79-136 (accessed on 20 May 2020).
- H.R.1249—Leahy-Smith America Invents Act. Available online: https://www.congress.gov/bill/112th-congress/house-bill/1249 (accessed on 9 June 2020).
- Kelly, E. FDA regulations of 3D printed organs and associated ethical challenges. Univ. Pa. Law Rev. 2018, 166, 516–545. [Google Scholar]
- Galarraga, J.H.; Kwon, M.Y.; Burdick, J.A. 3D bioprinting via an in situ crosslinking technique towards engineering cartilage tissue. Sci. Rep. 2019, 9, 19987. [Google Scholar] [CrossRef]
- Tang, D.; Tare, R.; Yang, L.-Y.; Williams, D.F.; Ou, K.-L.; Oreffo, R.O. Biofabrication of bone tissue: Approaches, challenges and translation for bone regeneration. Biomaterials 2018, 83, 363–382. [Google Scholar] [CrossRef] [PubMed]
- Mussi, E.; Furferi, R.; Volpe, Y.; Facchini, F.; McGreevy, K.S.; Uccheddu, F. Ear reconstruction simulation: From handcrafting to 3D printing. Bioengineering 2019, 6, 14. [Google Scholar] [CrossRef] [Green Version]
- Jana, S.; Lerman, A. Bioprinting a cardiac valve. Biotechnol. Adv. 2015, 33, 1503–1521. [Google Scholar] [CrossRef]
- Atala, A.; Bauer, S.B.; Soker, S.; Yoo, J.J.; Retik, A.B. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet 2006, 367, 1241–1246. [Google Scholar] [CrossRef]



| Level 0 | No Automation—the human driver performs the entire dynamic driving task. This level includes vehicles with warning or intervention systems such as lane departure warning or braking assistance because this assistance is not sustained and, therefore, does not control the human driver’s basic role. All traditional cars from the advent of the automobile until today, including most current vehicle models fall into this category. |
| Level 1 | Driver Assistance—one or more driving modes exist that the human driver can engage that will execute certain aspects of steering or acceleration/deceleration by employing information about the driving environment via the utilization of a system of onboard sensors. Within this sort of vehicle, the human driver remains responsible for all driving tasks other than that performed by the vehicle’s limited driving mode. Jaguar Land Rover’s experimental Range Rover Sport that utilizes an off-road cruise control system is an example of a Level 1 autonomous vehicle. |
| Level 2 | Partial Automation—one or more driving modes exist that the human driver can engage that will execute ALL aspects of BOTH steering and acceleration/deceleration by employing information about the driving environment. Within this system, the human driver is still required to monitor the driving environment—the tactical driving task—and to serve as a fallback performer (manual intervention). Some experts consider Tesla’s Autopilot system to be a Level 2 autonomous driving system. |
| Level 3 | Conditional Automation—one or more driving modes exist that perform all aspects of the dynamic driving task, including monitoring the driving environment. Like Level 2 automobiles, Level 3 automation systems still require the human driver to serve as a fallback performer should the system request manual intervention. Other experts consider Tesla’s Autopilot system to be a Level 3 autonomous driving system. |
| Level 4 | High Automation—one or more driving modes exist that perform all aspects of the dynamic driving task as well as providing fallback performance should the human driver fail to respond to a request to intervene. In other words, when a problem occurs, the car can resolve the issue without human assistance. The fleet of self-driving taxis currently being developed by Ford for release in the US market by 2021 qualify as Level 4 autonomous vehicles. |
| Level 5 | Full Automation—ALL driving modes are fully automated meaning the vehicle maintains control over the dynamic driving task under all roadway and environmental conditions. Only strategic tasks such as picking a destination remain in the hands of the human driver or, in the case of a driverless taxi, the human passenger. The vehicle that Google is developing through its Waymo subsidiary is expected to utilize Level 5 automation. |
| % Car Accidents. | 25% Adoption | 50% Adoption | 75% Adoption | 90% Adoption |
| 12.5% | −3.125% | −6.25% | −9.375% | −11.25% |
| 15% | −3.75% | −7.5% | −11.25% | −13.5% |
| 17.5% | −4.375% | −8.75% | −13.125% | −15.75% |
| 20% | −5% | −10% | −15% | −18% |
| DIMINUTION in KIDNEYS 1 (Based on WHO data for 2015 in United States). | ||||
| % Car Accidents | 25% Adoption | 50% Adoption | 75% Adoption | 90% Adoption |
| 12.5% | −811 | −1621 | −2432 | −2918 |
| 15% | −973 | −1945 | −2918 | −3502 |
| 17.5% | −1135 | −2270 | −3404 | −4085 |
| 20% | −1297 | −2594 | −3891 | −4669 |
| DIMINUTION in LIVERS (Based on WHO data for 2015 in United States). | ||||
| % Car Accidents | 25% Adoption | 50% Adoption | 75% Adoption | 90% Adoption |
| 12.5% | −212 | −423 | −635 | −761 |
| 15% | −254 | −508 | −761 | −914 |
| 17.5% | −296 | −592 | −888 | −1066 |
| 20% | −338 | −677 | −1015 | −1218 |
| DIMINUTION in HEARTS (Based on WHO data for 2015 in United States). | ||||
| % Car Accidents | 25% Adoption | 50% Adoption | 75% Adoption | 90% Adoption |
| 12.5% | −88 | −176 | −264 | −317 |
| 15% | −106 | −211 | −317 | −381 |
| 17.5% | −123 | −247 | −370 | −444 |
| 20% | −141 | −282 | −423 | −507 |
| DIMINUTION in LUNGS (Based on WHO data for 2015 in United States). | ||||
| % Car Accidents | 25% Adoption | 50% Adoption | 75% Adoption | 90% Adoption |
| 12.5% | −65 | −130 | −194 | −233 |
| 15% | −78 | −155 | −233 | −280 |
| 17.5% | −91 | −181 | −272 | −326 |
| 20% | −104 | −207 | −311 | −373 |
| DIMINUTION in PANCREAS (Based on WHO data for 2015 in United States). | ||||
| % Car Accidents | 25% Adoption | 50% Adoption | 75% Adoption | 90% Adoption |
| 12.5% | −30 | −59 | −89 | −107 |
| 15% | −36 | −71 | −107 | −128 |
| 17.5% | −41 | −83 | −124 | −149 |
| 20% | −47 | −95 | −142 | −170 |
| ONE | YEAR CHART | ||||
| Medical Costs | 25% Adoption | 50% Adoption | 75% Adoption | 90% Adoption | YEAR CHART |
| 12.5% | $72,140,063 | $144,280,125 | $216,420,188 | $259,704,225 | |
| 15% | $86,568,075 | $173,136,150 | $259,704,225 | $311,645,070 | |
| 17.5% | $100,996,088 | $201,992,175 | $302,988,263 | $363,585,915 | |
| 20% | $115,424,100 | $230,848,200 | $346,272,300 | $415,526,760 | |
| TWO | |||||
| Medical Costs | 25% Adoption | 50% Adoption | 75% Adoption | 90% Adoption | YEAR CHART |
| 12.5% | $216,420,187 | $432,840,375 | $649,260,562 | $779,112,675 | |
| 15% | $259,704,225 | $519,408,450 | $779,112,675 | $934,935,210 | |
| 17.5% | $302,988,262 | $605,976,525 | $908,964,787 | $1,090,757,745 | |
| 20% | $346,272,300 | $692,544,600 | $1,038,816,900 | $1,246,580,280 | |
| THREE | |||||
| Medical Costs | 25% Adoption | 50% Adoption | 75% Adoption | 90% Adoption | |
| 12.5% | $432,840,375 | $865,680,750 | $1,298,521,125 | $1,558,225,350 | |
| 15% | $519,408,450 | $1,038,816,900 | $1,558,225,350 | $1,869,870,420 | |
| 17.5% | $605,976,525 | $1,211,953,050 | $1,817,929,575 | $2,181,515,490 | |
| 20% | $692,544,600 | $1,385,089,200 | $2,077,633,800 | $2,493,160,560 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mills, P.A.S.; Mills, D.K. Reduced Supply in the Organ Donor Market and How 3D Printing Can Address This Shortage: A Critical Inquiry into the Collateral Effects of Driverless Cars. Appl. Sci. 2020, 10, 6400. https://doi.org/10.3390/app10186400
Mills PAS, Mills DK. Reduced Supply in the Organ Donor Market and How 3D Printing Can Address This Shortage: A Critical Inquiry into the Collateral Effects of Driverless Cars. Applied Sciences. 2020; 10(18):6400. https://doi.org/10.3390/app10186400
Chicago/Turabian StyleMills, Patrick A. S., and David K. Mills. 2020. "Reduced Supply in the Organ Donor Market and How 3D Printing Can Address This Shortage: A Critical Inquiry into the Collateral Effects of Driverless Cars" Applied Sciences 10, no. 18: 6400. https://doi.org/10.3390/app10186400





