Abstract
As a reusable adsorbent to remove lead from water, a peptide-based magnetic adsorbent incorporating lead-binding peptide was constructed. First, a 7-mer lead-binding peptide (TNTLSNN) was covalently bonded onto the surface of a magnetic bead. Compared to the adsorption capacity of a bare magnetic bead (4.0 mg lead/g bead), the peptide-linked bead exhibited a capacity more than eight times higher than that of a bare bead (34.1 mg lead/g bead). The regenerated peptide bead, by desorbing the lead from the bead with EDTA, could be repeatedly used (tested over six cycles) for the following round of lead adsorption without any significant loss of adsorption capacity. The selective removal of lead in the presence of other interfering metals was demonstrated with the individual or the combinatory use of four metal ions, namely Pb(II), Ni(II), Co(II), and Cu(II), where the amount of adsorbed Pb(II) was remarkably higher than those of the other metal ions. The adsorption isotherm followed the Langmuir model well, with the maximum adsorption loading (qmax) of 70.4 mg lead/g bead.
1. Introduction
Heavy metal pollution in water environments has become a severe problem, since metals are generally refractory components and some are highly hazardous to the ecosystem, even at low concentrations [1]. Lead, which is principally discharged from industrial sources, has also been reported to be harmful to human health [2]. Various technologies (i.e., precipitation, filtration, ion exchange, and extraction) for removing heavy metals from aqueous effluents have been suggested, but many of those are only appropriate for the treatment of effluent, including high concentrations of metals [3,4,5]. Moreover, their removal efficiencies are not high enough to reduce the metal concentration down to the level of the water quality standard [6]. Therefore, a process capable of lowering the metal concentration from dilute solution is required. Adsorption is a relatively simple process for efficiently removing metals from dilute solution [7]. A wide range of adsorbents originating from various chemical to biological sources (i.e., activated carbon, cellulose, graphene oxide, and chitosan) has been explored as metal adsorbents [8,9,10,11]. However, most of the adsorbents explored have not shown the selective binding property for specific metal in complex environmental matrices. This means that excessive doses of the adsorbents need to be used for the removal of the target metal coexisting with a relatively greater amount of interfering pollutants.
A specific peptide sequence, an oligomer of amino acids, has been reported to have selective binding affinity for various inorganic materials, such as metal oxides, metal sulfides, and metals [12,13,14]. Therefore, peptides with an affinity to specific metals can be used as metal binding moieties from aqueous solution [15,16]. Various peptide sequences possessing an affinity to metals, such as nickel and cobalt, are summarized, where amino acid composition and theoretical pI was analyzed [15]. In a previous study, a lead-binding peptide sequence (TNTLSNN) was screened by chromatographic biopanning [17]. The 7-mer peptide showed a selective affinity to Pb(II) while displaying low binding affinities to different divalent metal ions, such as Ni(II), Cu(II), Co(II), and Fe(II). Moreover, there are nearly unlimited target materials (i.e., most options other than lead) that can be chosen from, and a peptide with affinity to a specific target can be identified through a biopanning procedure [15]. Therefore, the peptide-based adsorbent can potentially be applied for the selective removal of various target materials. Meanwhile, considering the high cost of synthesizing the peptide, it would be beneficial to construct a reusable adsorbent. To date, various frameworks for constructing reusable adsorbents for pollutant removal have been investigated [18,19,20], and it appears to be difficult to collect the adsorbent from an aqueous system. Magnetic particles have attracted substantial interest for this purpose, since solid–liquid separation can be facilely enabled by magnetic assistance [21]. To date, magnetic adsorbents for the removal of heavy metals have been developed as a composite of iron oxide and other compounds (i.e., biochar, chitosan, cellulose, graphene oxide, and titanate) [9,10,22,23,24]. Among them, only a few materials, such as magnetic ion-imprinted polymer and magnetic biomaterial coated with amino siloxane, have been reported as selective adsorbents for lead [25,26].
Here, the magnetic bead linked with a specific peptide is investigated as another promising reusable adsorbent with selective removal capability. We constructed a magnetic bead incorporating lead-binding peptide via covalent bonding on the bead’s surface. Then, the peptide bead was evaluated as a reusable adsorbent for the removal of lead under various aqueous conditions. In particular, its selective binding performance to lead was demonstrated in the presence of interfering substances like different metal ions (i.e., Ni(II), Cu(II), and Co(II)) and artificial wastewater. Finally, the variables in adsorption kinetic and isotherm relations were determined to characterize the constructed adsorbent.
2. Materials and Methods
2.1. Materials
First, 4-morpholineethanesulfonic acid (MES), N-(3-dimethyl aminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC), N-hydroxy-succinimide (NHS), ethylene diamine tetraacetic acid (EDTA), bovine serum albumin (BSA, A7906), anhydrous acetonitrile (ACN), and the nitrate salts of all of the cations (Pb(II), Cu(II), Co(II), and Ni(II)) were purchased from Sigma-Aldrich (St. Louis, MO, USA) and used without further purification. EDC, NHS, and EDTA were dissolved in 25 mM MES buffer. The lead-binding peptide (NH2-TNTLSNN-COOH) that was previously screened [17] and the reverse-sequence peptide (NH2-NNSLTNT-COOH) were custom synthesized by Bio-FD&C (Lugen Sci., Bucheon, Korea). Both sequences of the peptides were dissolved in 100 mM of MES buffer, to obtain 0.1 g/L, and then stored at 4 °C prior to use.
2.2. Adsorption on Bare Magnetic Bead
Depending on the surface characteristics of the magnetic bead, lead may be adsorbed onto the bare bead itself, which hinders the evaluation of the adsorption performance of the pure peptide. Thus, several types of commercially available magnetic beads were compared, to identify the bead adsorbing the lowest amount of lead on its bare surface. Four kinds of beads with a functional carboxyl group, namely DynabeadsTM M-270 Carboxylic Acid (Thermo Fisher Scientific, Oslo, Norway), AccuBeadTM COOH magnetic beads (Bioneer Inc, Daejeon, Korea), Cytodiagnostic ZeptoTM Mag Carboxyl Microspheres (CytoDiagnostics Inc., Burlington, ON, Canada), and BcMagTM long-arm carboxy-terminated magnetic beads (Bioclone Inc,. San Diego, CA, USA), were purchased and compared to each other. First, 3 mg of each bead was washed with 25 mM MES buffer twice and then incubated in 5 mL of 60 mg/L Pb(NO3)2 for 1 h. After magnetically separating the beads, the lead concentration remaining in each supernatant was measured.
2.3. Construction of Peptide-Linked Magnetic Bead
The AccuBeadTM COOH Magnetic Bead (diameter = 4.56 μm) was selected to be bonded with peptide. Initially, the bead was washed twice with 25 mM MES buffer. Then, the peptide was covalently bonded onto the bead surface via carbodiimide-mediated amide bond formation between N-terminus of peptides and the carboxylic acid groups on the bead surface, according to a slight modification of the Thermo Fisher Scientific protocol [27]. First, 3 mg of bead was mixed with 50 μL of EDC (50 g/L in cold 25 mM MES) and NHS (50 g/L in 25 mM MES), under slow tilt rotation, at room temperature, for 30 min. Then, the activated bead was collected, using magnets, before being washed with 25 mM MES. Next, 100 μL of peptide (100 mg/L) was incubated with the EDC/NHS-activated bead, at room temperature, for 1 h. After the peptide-linked bead was collected magnetically, the peptide concentration remaining in the supernatant was measured to determine the amount of peptide covalently bonded onto the bead. For comparison, the EDC/NHS-activated bead without further coating and the activated bead coated with albumin protein were prepared via the same protocol. Then, both beads lacking the peptide were incubated in 60 mg/L Pb(NO3)2 for 1 h, and the lead concentration in the supernatant after collecting the bead with a magnet was measured to determine the amount of lead adsorbed on the bead.
2.4. Adsorption on Peptide-Linked Magnetic Bead
First, 3 mg of the as-prepared peptide bead was incubated in 5 mL of 60 mg/L metal solution (distilled water), at 25 °C, with gentle shaking, if not specifically mentioned otherwise. The amount of adsorbed metal onto the bead was determined based on the difference in the metal concentrations before and after the adsorption; the data were averaged from three independent trials. In isotherm experiments, the bead was incubated with Pb(NO3)2 solutions ranging from 20 to 600 mg lead/L for 1 h. In experiments investigating bead reusability, the bead after the adsorption was treated with 5 mL of 1 mM EDTA for 5 min, then washed with 25 mM MES buffer. The amount of desorbed lead was determined from the lead concentration in EDTA solution. The restored bead was successively used for the next round of adsorption. To verify the lead selectivity of the peptide bead in a metal mixture solution, the bead was incubated with either 5 mL of single-metal solution for each of the metal nitrates (Pb(NO3)2, Co(NO3)2, Cu(NO3)2, and Ni(NO3)2) or 5 mL of a mixture composed of the four metal nitrates at the same concentrations (60 mg/L each). Finally, the synthetic wastewater [28] containing lead was used to verify if the lead selectivity of the peptide bead was maintained in complex environmental matrices.
2.5. Analysis
The peptide concentration was measured by High Performance Liquid Chromatography (HPLC) (UV 220 nm, Agilent 1200, Agilent Technologies, Inc. Santa Clara, CA, USA), using a C18 column (Kromasil 100–5-C18, Kromasil, Bohus, Sweden), at 25 °C. Acetonitrile and water with 0.1% (v/v) trifluoroacetic acid were used for gradient elution from 0 to 70% (v/v) of acetonitrile, at a flow rate of 1 mL/min. The metal concentrations including lead were measured, using an atomic absorption spectrophotometer (AAS, Shimadzu AA-7000, Shimadzu Corporation, Kyoto, Japan).
3. Results
3.1. Construction of Peptide-Linked Magnetic Bead
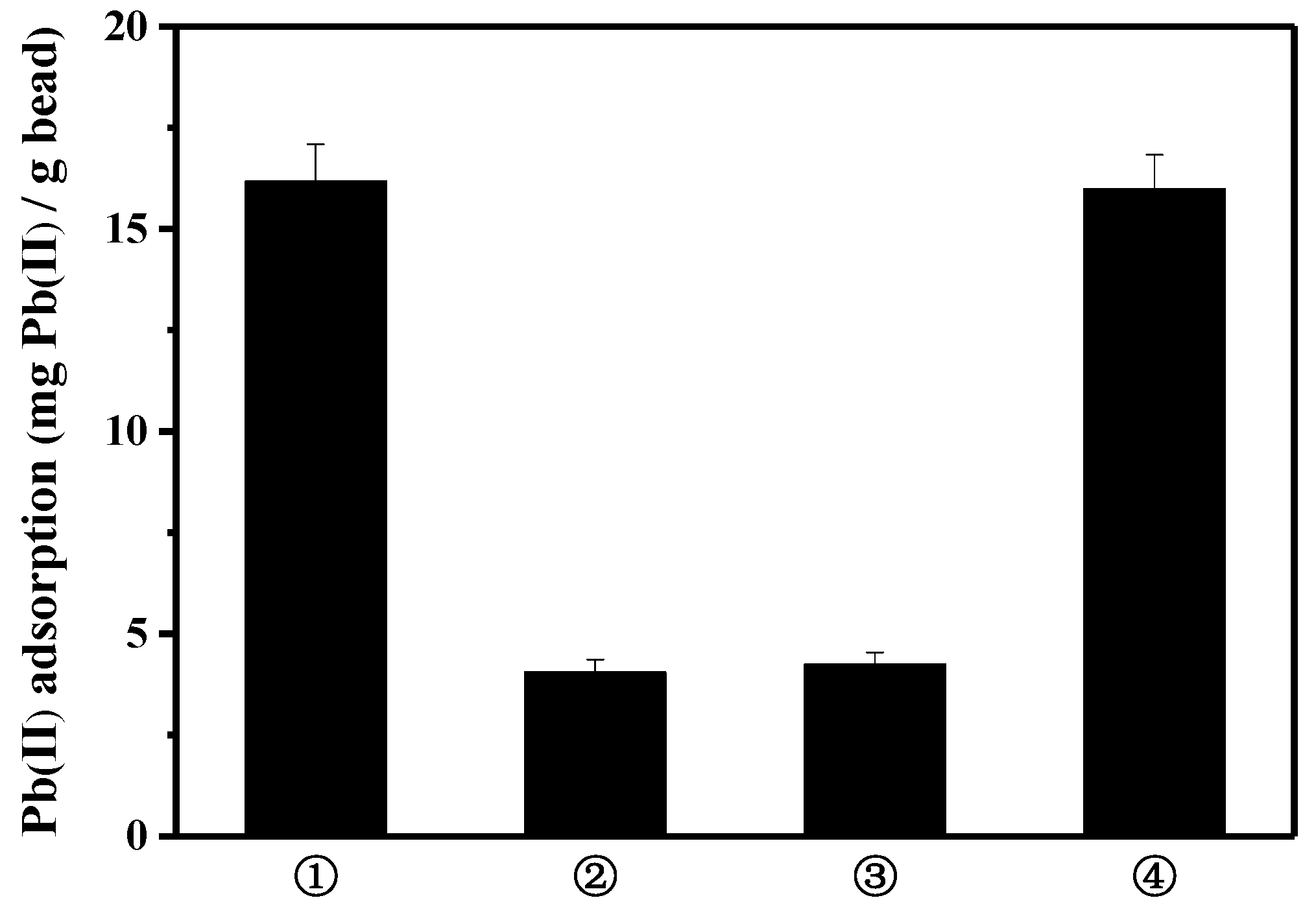
To construct a reusable adsorbing system, the magnetic-core bead enabling magnetic separation from water was used as a basic frame to bind the peptide on the bead surface. Since the peptide has an amine and a carboxylic group on both ends, the lead-binding peptide can be linked via EDC/NHS chemistry to the bead functionalized with NH2/COOH on its surface. Meanwhile, depending on the surface characteristic of the bead, the lead ion can be adsorbed to the bare surface of the bead that is not occupied with the peptide. To examine the lead removal performance that is mainly caused by the lead-binding peptide, and not by the raw bead, various kinds of commercially available magnetic beads were tested to select the bead with the lowest affinity to lead. Among the four types of beads shown in Figure 1, the AccuBeadTM COOH magnetic bead and the Cytodiagnostic ZeptoTM Mag bead adsorbed less lead than the two other types of bead. Since the AccBeadTM COOH magnetic bead was cheaper to produce and separated better by magnet, it was selected as the frame with which to bind peptide.
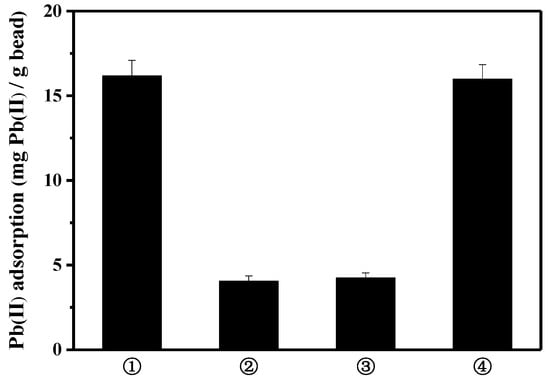
Figure 1.
Pb(II) adsorption onto bare bead (mg Pb(II)/g bead): ① Dynabeads® M-270 Carboxylic Acid; ② AccuBeadTM COOH magnetic bead; ③ Cytodiagnostic ZeptoTM Mag Carboxyl Microspheres; and ④ BcMagTM long-arm carboxy-terminated magnetic bead.
The amine group of peptide was linked to the carboxylic group on the beads, where the previously screened lead-binding peptide (NH2-TNTLSNN-COOH, [17]) and the reverse sequence of the peptide (NH2-NNSLTNT-COOH) were used to examine the effect of the spatial position of the peptide sequence on the lead binding performance. After each peptide was linked to the magnetic beads, the remaining peptide concentration of each supernatant was measured to determine the amount of peptide bound to bead. Slightly more peptide (0.89 ± 0.05 mg peptide/g bead for NH2-TNTLSNN-COOH, 0.79 ± 0.05 mg peptide/g bead for NH2-NNSLTNT-COOH) was bound to the bead in the case of the originally screened peptide sequence (NH2-TNTLSNN-COOH), where the NH2 group of threonine (T) reacted with the COOH group of the bead. The amine groups of threonine (T) and asparagine (N) in each peptide involved the binding reaction with the bead. Each amino acid has a similarly polar uncharged side group, but asparagine has a longer side chain than threonine. Thus, the higher peptide binding efficiency may be attributable to the smaller steric hindrance of threonine during the EDC/NHS reaction. The amount of adsorbed lead was 34.1 ± 1.3 mg lead/mg bead for NH2-TNTLSNN-COOH and 30.9 ± 1.6 mg lead/mg bead for NH2-NNSLTNT-COOH. The lead binding efficiency in each peptide sequence was similar in terms of the amount of adsorbed lead per peptide mass on the bead. Thus, the adsorption capacity of the peptide bead would increase if more peptide were further attached on the bead surface.
3.2. Lead Adsorption Using Peptide-Linked Magnetic Bead
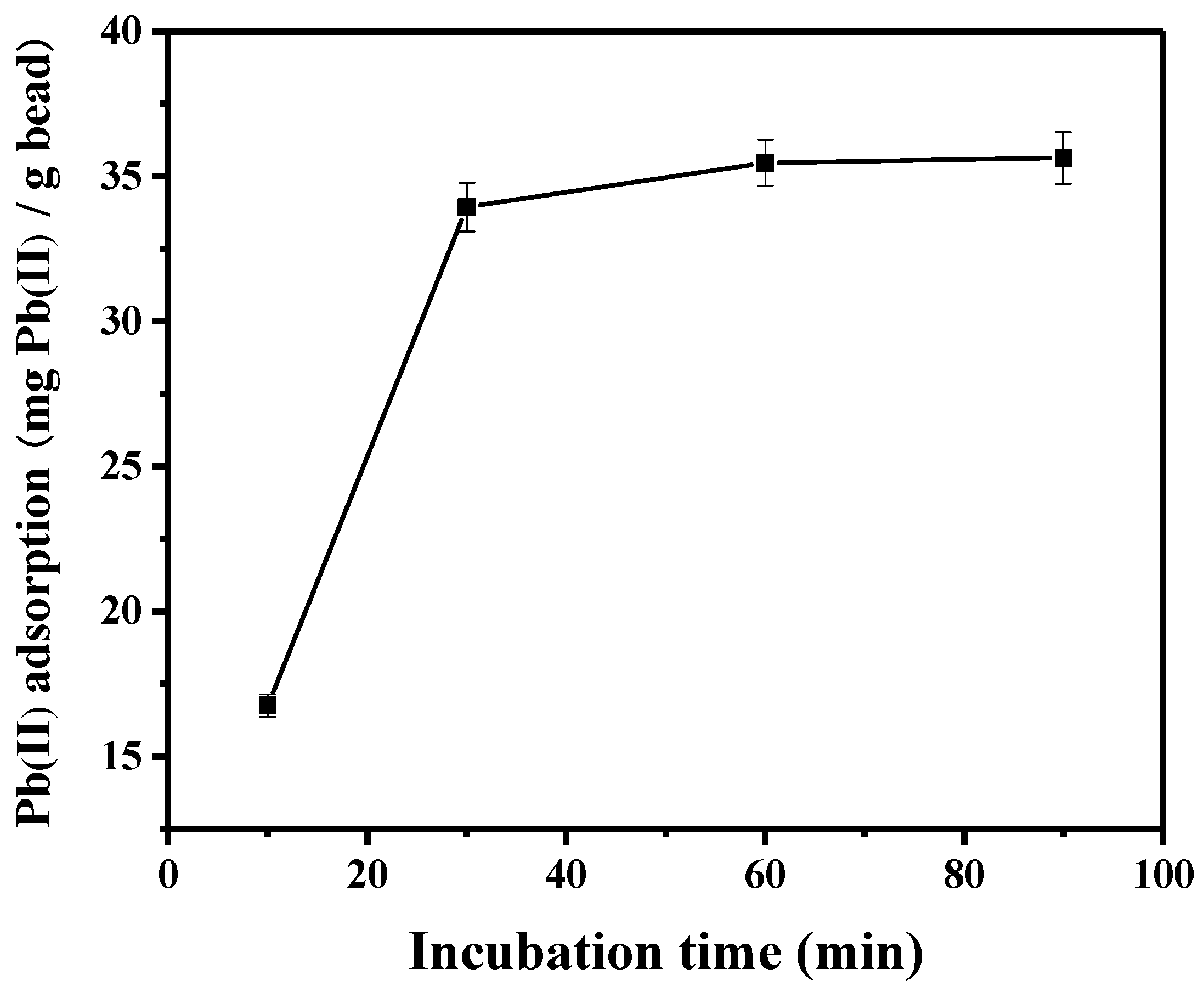
To determine the contact time for lead adsorption, the time profile of lead adsorption onto the peptide-linked (TNTLSNN) bead was monitored, and the results are shown in Figure 2. A contact time within approximately 30 min was required for the peptide bead to be saturated with lead. The increase in lead adsorption was not significant after 1 h, so the time for each of the following adsorption experiments was determined to be 1 h, if not specifically mentioned otherwise. Previous research has shown that the experimental pH can affect the adsorptive uptake of lead ion [29,30,31]. For example, lead can precipitate at pH values equal to or greater than 7, due to the formation of Pb(OH)2 in the solution. Meanwhile, the extent of protonation from sorbent materials interacting electrostatically with Pb(II) ion is determined by the solution pH. The adsorptive uptake of lead decreased at lower pH due to the protonation of sorbents. For the polymeric adsorbent [31], the adsorption in distilled water led to the highest adsorption amount of Pb(II) ion. In this study, the pH effect was not examined in detail (pH around 5–6 provided similar results; data not shown). Each metal nitrate was solubilized in distilled water without any further pH adjustment.
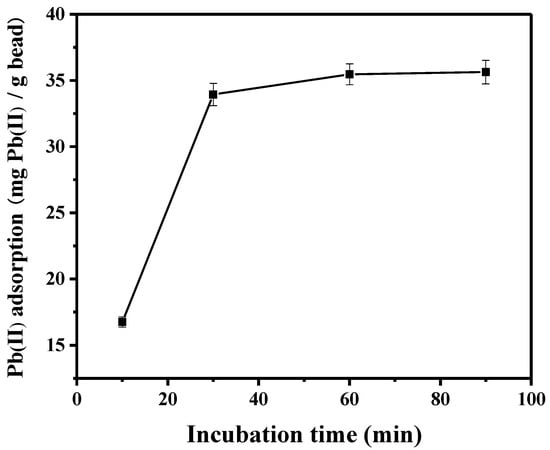
Figure 2.
Effect of contact time on Pb(II) adsorption onto peptide-linked bead.
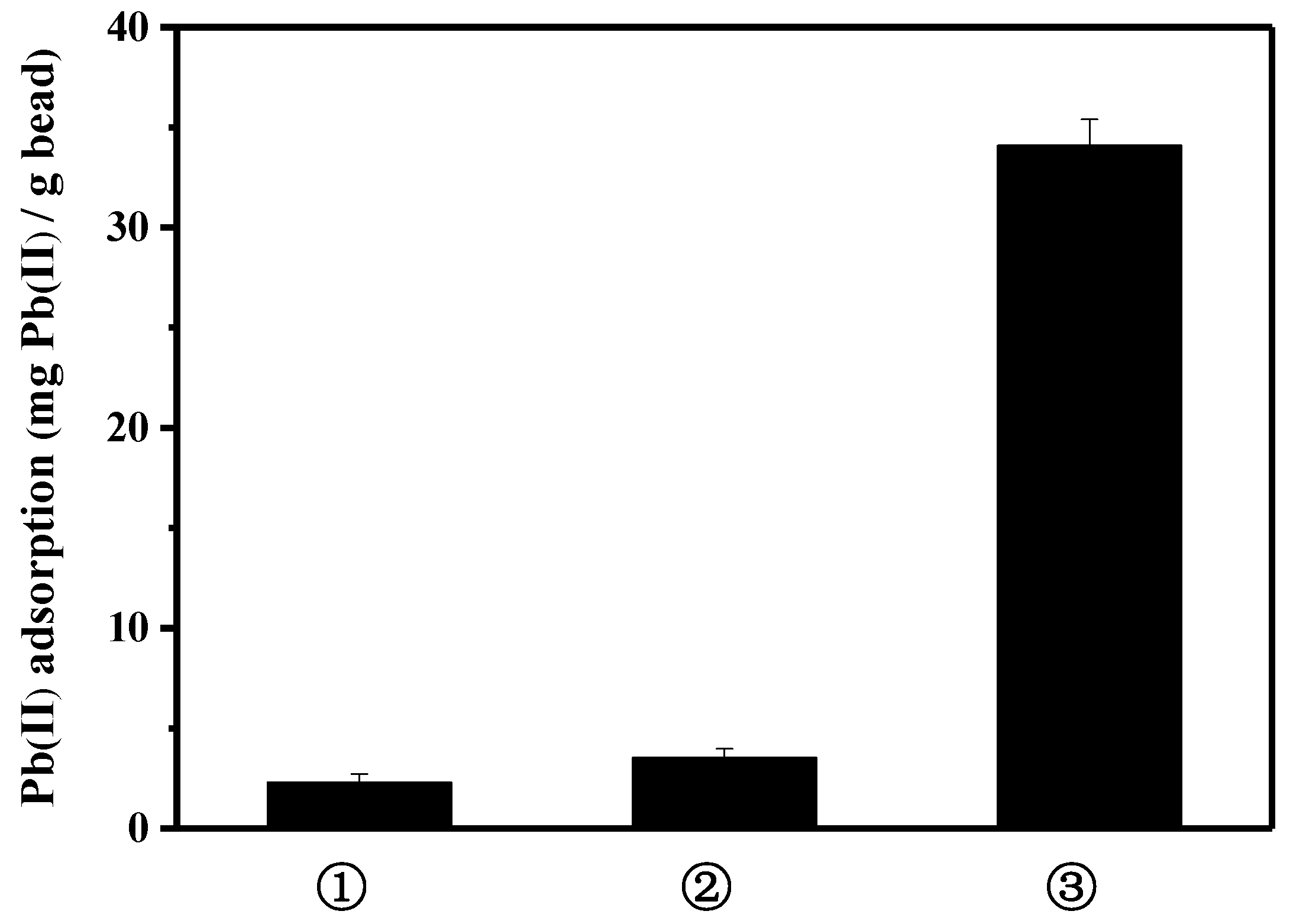
To further explore the contribution of peptide on the lead binding, three kinds of bead were compared: activated bare bead for EDC/NHS reaction, albumin(BSA)-coated bead using the activated bare bead, and peptide-linked bead using the activated bare bead (Figure 3). Bovine serum albumin is frequently used as a blocking agent, to prevent the nonspecific binding of antibodies in biological research. Compared to the lead binding capacity of the peptide bead, the albumin bead showed significantly lower lead binding (3.5 mg lead/g bead). A slight increase in lead binding, as compared to that of bare bead, may be caused by the exposed functional group of albumin surface interacting with lead. Thus, it can be presumed that the specific lead-binding capacity was substantially enhanced by the function of the lead-binding peptide.
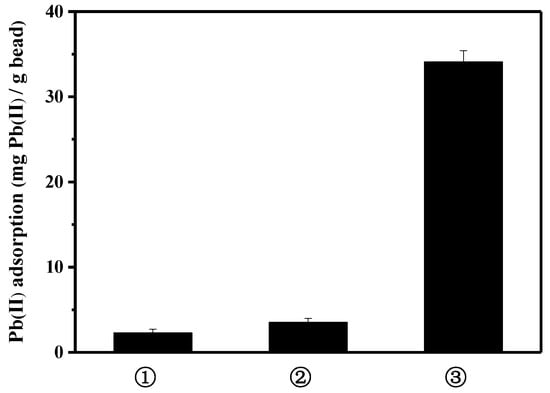
Figure 3.
Comparison of lead adsorption capacity: ① bare bead, ② albumin-coated bead, and ③ peptide-linked bead.
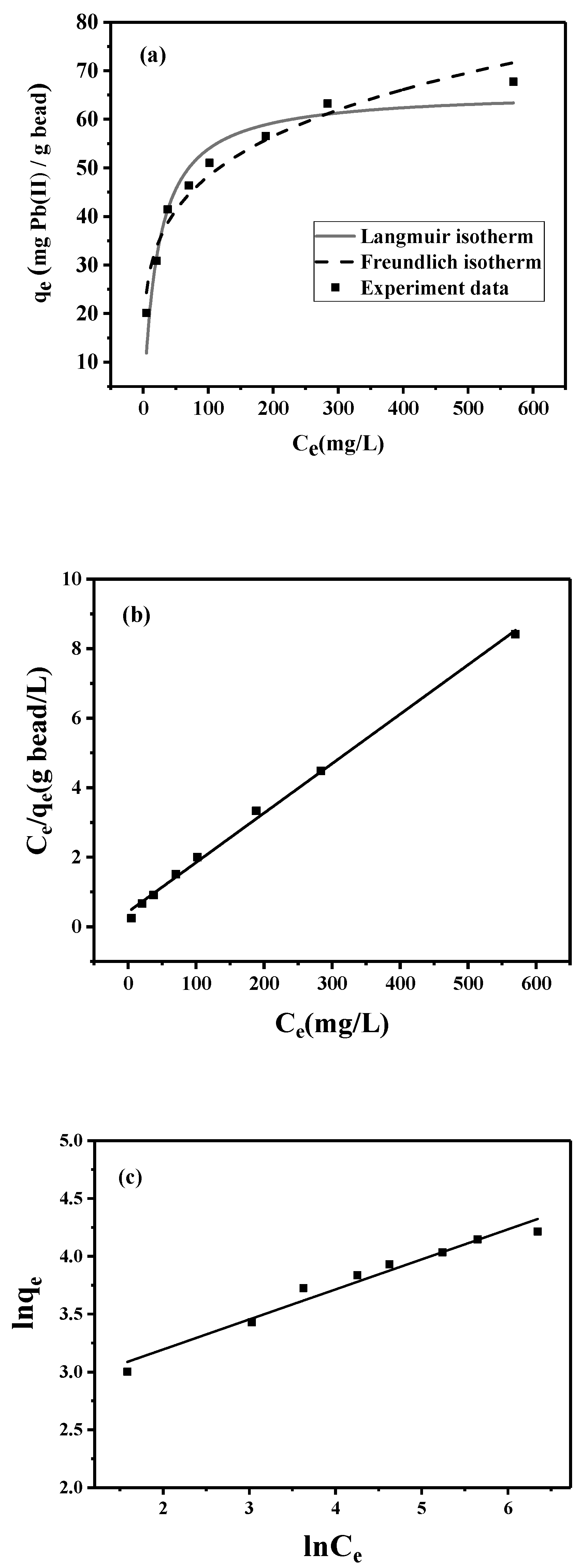
To characterize the lead adsorption by the peptide bead, adsorption isotherm experiments were conducted. The experimental data shown in Figure 4a comprise an L-shaped isotherm, meaning that the peptide bead is expected to have a high affinity toward lead. The representative model for this type of adsorption is the Langmuir model (Equation (1)), which describes the homogeneous distribution of the adsorbate onto the uniform surface of the adsorbent. To analyze the model parameter, the linearized model (Equation (2)) was applied (Figure 4b).
where qe (mg/g of adsorbent) is the equilibrium lead-adsorption capacity of the peptide bead and Ce (mg/L) is the equilibrium lead concentration in solution. The two empirical parameters of qmax (mg/g) and KL (L/mg) represent the theoretical maximum adsorption capacities of the adsorbent and the affinity constant, respectively. Based on the linear regression analysis (R2 = 0.997) presented in Figure 4b, qmax was determined to be 70.4 mg/g, while KL was 0.03 L/mg.
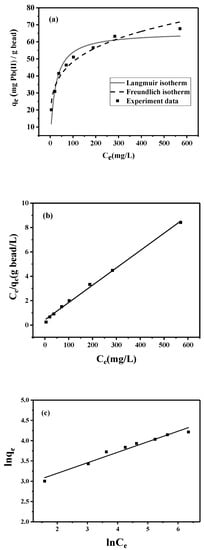
Figure 4.
Adsorption isotherms of lead onto peptide bead: (a) nonlinear fitting by Langmuir and Freundlich isotherms; (b) linear Langmuir isotherm; and (c) linear Freundlich isotherm.
The Freundlich model (Equation (3)) known to describe multilayer adsorption behavior on the heterogeneous surface was also fitted to the experimental data.
where kf is the Freundlich constant and 1/n is the heterogeneity factor. Based on the linear regression analysis (R2 = 0.967) presented in Figure 4c, kf was determined to be 14.5, while 1/n was 3.85. Considering the range of regression coefficients (R2), both isotherm models could fit the experimental data, while the Langmuir model slightly outperformed the Freundlich model.
3.3. Reusability of Peptide-Linked Bead
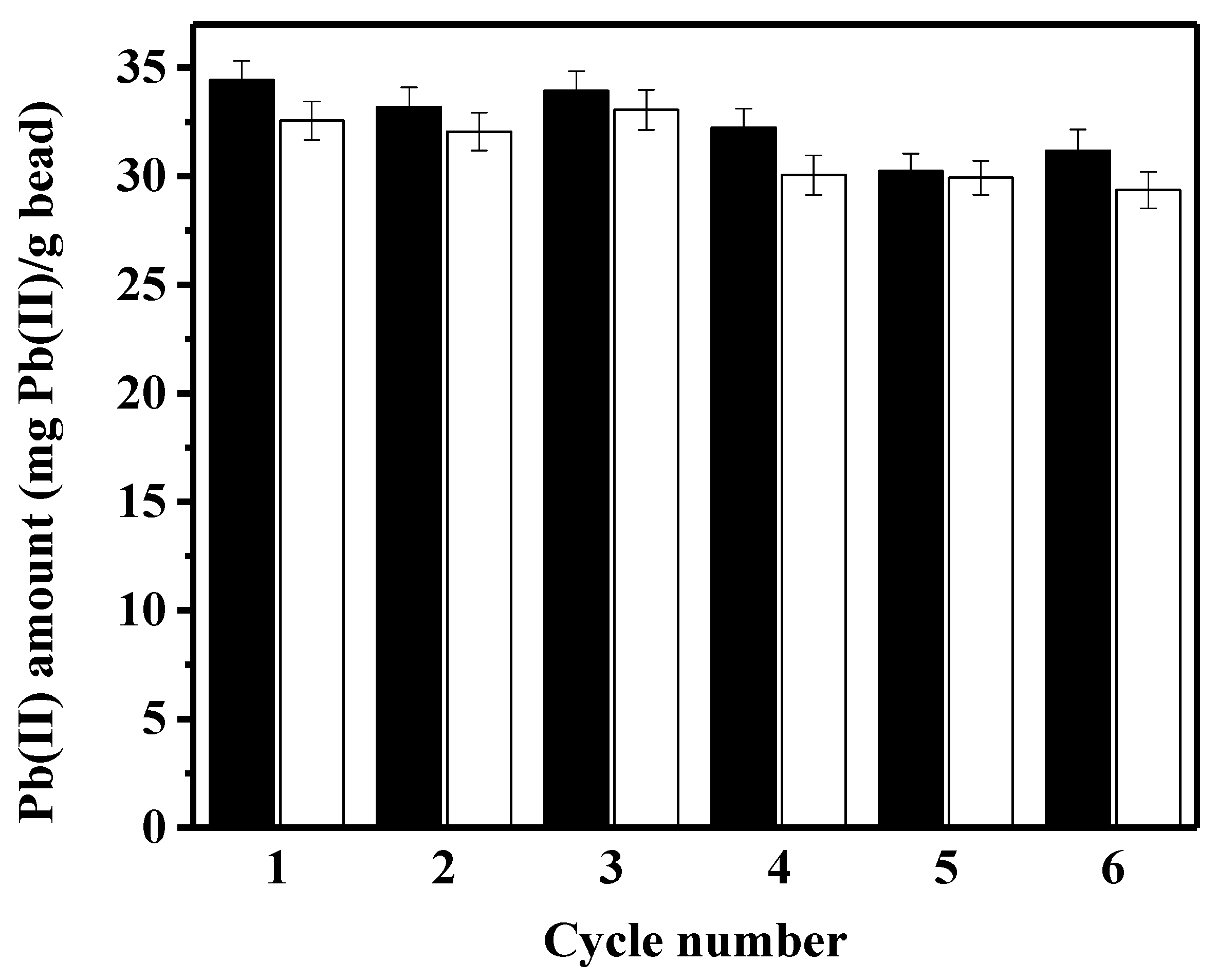
To evaluate whether the peptide-linked bead is reusable, the amounts of adsorbed/desorbed lead were quantified during six adsorption–desorption cycles. Although the exact mass balance between the adsorbed/desorbed amounts of lead was not observed in Figure 5, small differences in mass balance may be attributed to the incomplete desorbing operation by EDTA. The adsorbed amount of lead, and eventually the desorbed amount, gradually decreased during the successive use of the peptide bead. Compared to the first adsorption capacity (33.4 mg/g bead), the regenerated capacity in the sixth cycle (31.8 mg/g bead) decreased by less than 5%. By optimizing the desorbing process to regenerate the used adsorbent, as has been previously demonstrated by using various chemicals [30,32], the regeneration efficiency of the constructed peptide bead may improve further. For the reusable gelatin–bentonite adsorbent, 0.1 N nitric acid restored the lead-adsorption capacity to more than 92% of initial capacity after ten adsorption–desorption cycles [32]. For the biochar-based adsorbent used for lead removal, a combination of 0.1 M HCl + 0.1 M CaCl2 was the best desorbing eluent [30]. The activated carbon is a commonly reported adsorbent for lead removal, and HCl has been shown to be an efficient chemical for desorbing lead from the used adsorbent. However, the repeated use of activated carbon for lead removal seems to not be a good strategy, since the adsorption capacity significantly decreased by 20–27% after the third or the fourth cycle of adsorption–desorption [7,33].
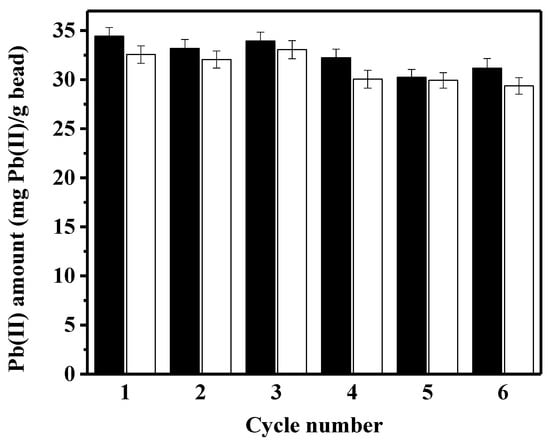
Figure 5.
The amounts of lead adsorbed and desorbed during six cycles of successive adsorption-desorption batches (black bar: adsorption, white bar: desorption).
3.4. Selective Lead Adsorption
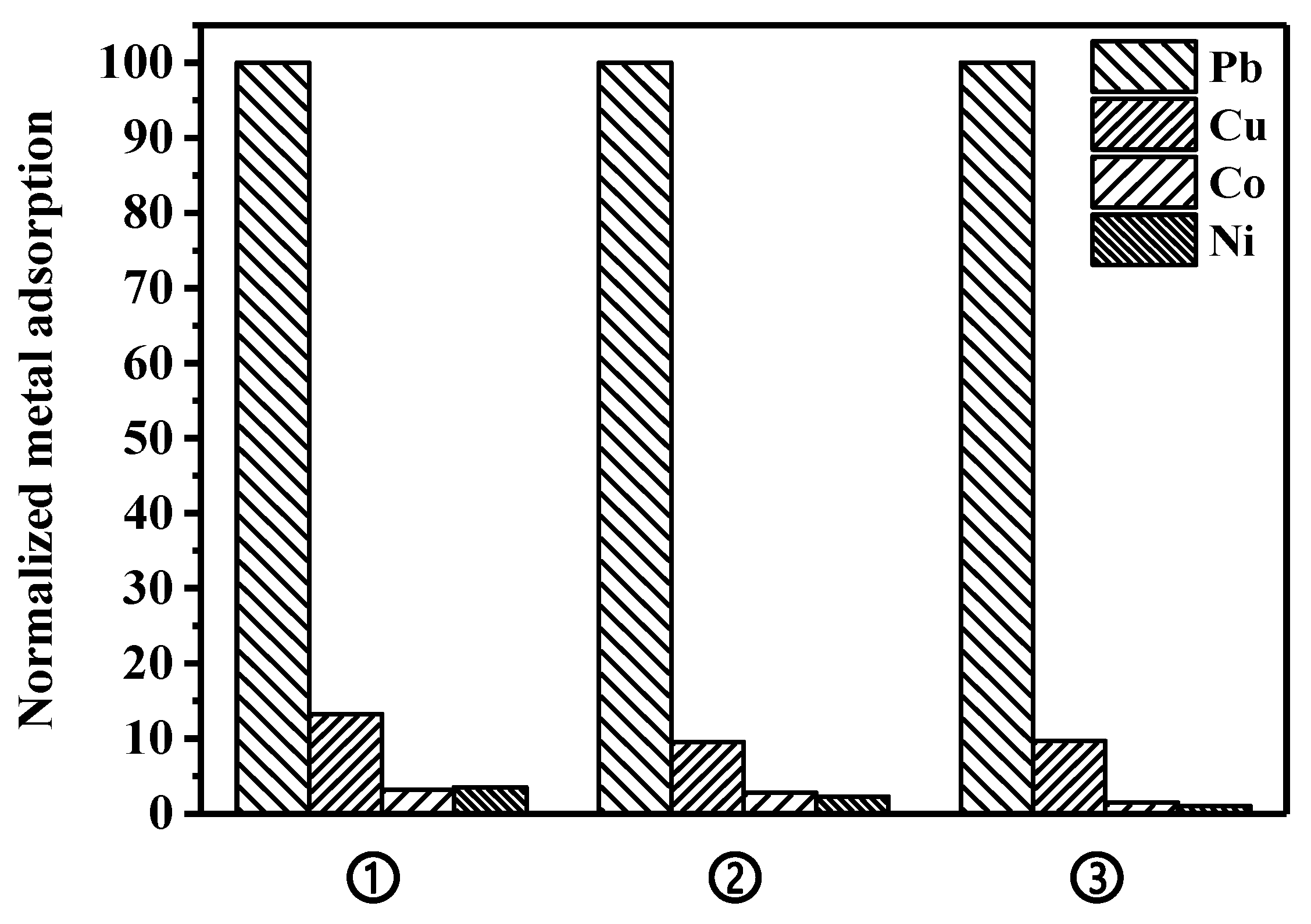
Adsorption studies using different divalent metal ions were conducted to evaluate if the selectivity of the peptide toward lead, which has previously been demonstrated on the surface of a microorganism [34], is maintained on the surface of the magnetic bead. Copper, cobalt, and nickel were tested as interferences against lead binding in a single-metal solution, as well as in a metal-mixture solution. To simulate lead removal in a real water environment, an adsorption experiment was also conducted in synthetic wastewater containing a metal mixture. As shown in Figure 6, the constructed peptide bead shows the highest binding performance toward lead, while the adsorptions of nickel and cobalt were significantly low. Compared to nickel and cobalt, copper showed a relatively higher binding property onto the peptide bead across all experimental conditions, which is consistent with the results of a previous study investigating a microorganism-based peptide adsorbent [34]. The bare bead not possessing the peptide showed adsorption capacities of 4.5 and 2.7 mg/g bead toward lead and copper, respectively, which means that the bare bead has not only low binding property toward both metals, but also insignificant selectivity toward lead over copper. Meanwhile, compared to the bare bead examined in this study, relatively much higher adsorptions of lead and copper onto wild-type microorganisms not possessing the peptide were observed, and this was attributed to nonspecific binding onto various surface peptides/proteins of the microorganism.
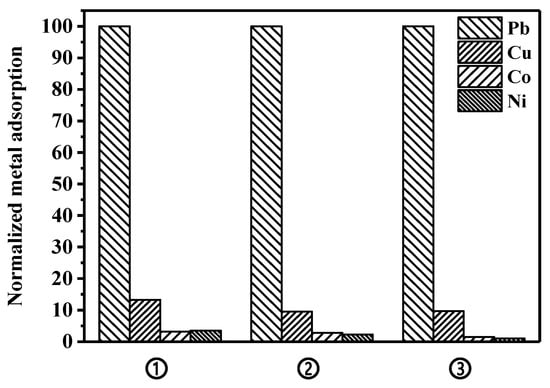
Figure 6.
Comparison of the metal adsorption capacity in various conditions: ① single-metal solution; ② four-metal mixture solution; and ③ synthetic wastewater including four metal mixture.
To further verify the specificity of the constructed peptide bead, adsorption experiments were conducted with a four-metal mixture, at the same concentration (60 mg/L each metal). Compared to the adsorption preference in single-metal solutions, a slight decrease in the amount of lead adsorption was observed due to the overall increase in metal concentration (240 mg metal/L). However, the selectivity of lead over copper (adsorbed mass of Pb over adsorbed mass of Cu per mass of bead) increased from 7.5 to 9.7, implying that the preferential selectivity of peptide toward lead was further highlighted in a competitive situation. Finally, a synthetic wastewater including the four-metal mixture described above was tested to simulate a real water environment. Compared to the pure metal solutions presented above, a slight decrease in the lead adsorption amount was observed, but the selectivity of lead over copper was maintained at 8.6, suggesting that the peptide bead can possibly be used as a selective adsorbent in various environments.
4. Conclusions
This study shows the potential of the peptide-linked magnetic bead as a highly selective adsorbent for the removal of lead from environment matrix. The peptide, previously verified to have selective binding affinity to lead, was fused to the surface of a magnetic bead, to construct an easily separable and reusable adsorbent. When the experimental adsorption data were assayed by isotherm curve, the maximum adsorption capacity was found to be relatively high (70.4 mg lead/g bead). The bead could be regenerated by using EDTA, to desorb lead, and then reused for six cycles, without any significant loss in adsorption performance. A selective removal of lead in the presence of interfering metals or synthetic wastewater was demonstrated, where the amount of adsorbed lead was substantially higher than those of the other metal ions. However, the weakness of this adsorbent is the high cost of synthesizing the peptide, so future research should focus on reducing the costs of peptide synthesis, using genetic-engineering tools (i.e., protein-fused peptide synthesis).
Author Contributions
This study was carried out by Y.X. under the supervision of I.-K.Y.; I.-K.Y. initiated the idea and Y.X. planned the experiments; Y.X. wrote the initial drat, which was reviewed and edited by I.-K.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the 2018 Research Fund of University of Ulsan.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Azarudeen, R.S.; Riswan Ahamed, M.A.; Subha, R.; Burkanudeen, A.R. Heavy and toxic metal ion removal by a novel polymeric ion-exchanger: Synthesis, characterization, kinetics and equilibrium studies. J. Chem. Technol. Biotechnol. 2015, 90, 2170–2179. [Google Scholar] [CrossRef]
- Joseph, L.; Jun, B.M.; Flora, J.R.V.; Park, C.M.; Yoon, Y. Removal of heavy metals from water sources in the developing world using low-cost materials: A review. Chemosphere 2019, 229, 142–159. [Google Scholar] [CrossRef] [PubMed]
- Bashir, A.; Malik, L.A.; Ahad, S.; Manzoor, T.; Bhat, M.A.; Dar, G.N.; Pandith, A.H. Removal of heavy metal ions from aqueous system by ion-exchange and biosorption methods. Environ. Chem. Lett. 2018, 17, 729–754. [Google Scholar] [CrossRef]
- Kazemipour, M.; Ansari, M.; Tajrobehkar, S.; Majdzadeh, M.; Kermani, H.R. Removal of lead, cadmium, zinc, and copper from industrial wastewater by carbon developed from walnut, hazelnut, almond, pistachio shell, and apricot stone. J. Hazard. Mater. 2008, 150, 322–327. [Google Scholar] [CrossRef]
- Manawi, Y.; McKay, G.; Ismail, N.; Kayvani Fard, A.; Kochkodan, V.; Atieh, M.A. Enhancing lead removal from water by complex-assisted filtration with acacia gum. Chem. Eng. 2018, 352, 828–836. [Google Scholar] [CrossRef]
- Brower, J.B.; Ryan, R.L.; Pazirandeh, M. Comparison of ion-exchange resins and biosorbents for the removal of heavy metals from plating factory wastewater. Environ. Sci. Technol. 1997, 31, 2910–2914. [Google Scholar] [CrossRef]
- Cechinel, M.A.P.; Ulson de Souza, S.M.A.G.; Ulson de Souza, A.A. Study of lead (II) adsorption onto activated carbon originating from cow bone. J. Clean. Prod. 2014, 65, 342–349. [Google Scholar] [CrossRef]
- Aguayo-Villarreal, I.A.; Bonilla-Petriciolet, A.; Muñiz-Valencia, R. Preparation of activated carbons from pecan nutshell and their application in the antagonistic adsorption of heavy metal ions. J. Mol. Liq. 2017, 230, 686–695. [Google Scholar] [CrossRef]
- Huang, Q.; Chen, Y.; Yu, H.; Yan, L.; Zhang, J.; Wang, B.; Du, B.; Xing, L. Magnetic graphene oxide/MgAl-layered double hydroxide nanocomposite: One-pot solvothermal synthesis, adsorption performance and mechanisms for Pb2+, Cd2+, and Cu2+. Chem. Eng. 2018, 341, 1–9. [Google Scholar] [CrossRef]
- Luo, X.; Lei, X.; Cai, N.; Xie, X.; Xue, Y.; Yu, F. Removal of Heavy Metal Ions from Water by Magnetic Cellulose-Based Beads with Embedded Chemically Modified Magnetite Nanoparticles and Activated Carbon. ACS Sustain. Chem. Eng. 2016, 4, 3960–3969. [Google Scholar] [CrossRef]
- Zhang, L.; Zeng, Y.; Cheng, Z. Removal of heavy metal ions using chitosan and modified chitosan: A review. J. Mol. Liq. 2016, 214, 175–191. [Google Scholar] [CrossRef]
- Chen, C.L.; Rosi, N.L. Peptide-based methods for the preparation of nanostructured inorganic materials. Angew. Chem. Int. Ed. Engl. 2010, 49, 1924–1942. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Liu, Z.; Huang, W.; Zhao, Y.; Dong, S.; Zeng, M. Purification and characterisation of a zinc-binding peptide from oyster protein hydrolysate. J. Funct. Foods 2013, 5, 689–697. [Google Scholar] [CrossRef]
- Shen, W.Z.; Cetinel, S.; Sharma, K.; Borujeny, E.R.; Montemagno, C. Peptide-functionalized iron oxide magnetic nanoparticle for gold mining. J. Nanopart. Res. 2017, 19, 74. [Google Scholar] [CrossRef]
- Braun, R.; Bachmann, S.; Schonberger, N.; Matys, S.; Lederer, F.; Pollmann, K. Peptides as biosorbents - Promising tools for resource recovery. Res. Microbiol. 2018, 169, 649–658. [Google Scholar] [CrossRef]
- Yang, T.; Zhang, X.X.; Yang, J.Y.; Wang, Y.T.; Chen, M.L. Screening arsenic(III)-binding peptide for colorimetric detection of arsenic(III) based on the peptide induced aggregation of gold nanoparticles. Talanta 2018, 177, 212–216. [Google Scholar] [CrossRef]
- Nian, R.; Kim, D.S.; Nguyen, T.; Tan, L.; Kim, C.W.; Yoo, I.K.; Choe, W.S. Chromatographic biopanning for the selection of peptides with high specificity to Pb2+ from phage displayed peptide library. J. Chromatogr. A 2010, 1217, 5940–5949. [Google Scholar] [CrossRef]
- Gaber, D.; Abu Haija, M.; Eskhan, A.; Banat, F. Graphene as an Efficient and Reusable Adsorbent Compared to Activated Carbons for the Removal of Phenol from Aqueous Solutions. Water Air Soil Pollut. 2017, 228. [Google Scholar] [CrossRef]
- Yang, Y.; Yan, Z.; Wang, L.; Meng, Q.; Yuan, Y.; Zhu, G. Constructing synergistic groups in porous aromatic frameworks for the selective removal and recovery of lead(ii) ions. J. Mater. Chem. A 2018, 6, 5202–5207. [Google Scholar] [CrossRef]
- Zhao, R.; Ma, T.; Li, S.; Tian, Y.; Zhu, G. Porous Aromatic Framework Modified Electrospun Fiber Membrane as a Highly Efficient and Reusable Adsorbent for Pharmaceuticals and Personal Care Products Removal. ACS Appl. Mater. Interfaces 2019, 11, 16662–16673. [Google Scholar] [CrossRef]
- Pan, S.; Zhang, Y.; Shen, H.; Hu, M. An intensive study on the magnetic effect of mercapto-functionalized nano-magnetic Fe3O4 polymers and their adsorption mechanism for the removal of Hg(II) from aqueous solution. Chem. Eng. 2012, 210, 564–574. [Google Scholar] [CrossRef]
- Karunanayake, A.G.; Todd, O.A.; Crowley, M.; Ricchetti, L.; Pittman, C.U.; Anderson, R.; Mohan, D.; Mlsna, T. Lead and cadmium remediation using magnetized and nonmagnetized biochar from Douglas fir. Chem. Eng. 2018, 331, 480–491. [Google Scholar] [CrossRef]
- Liu, F.; Jin, Y.; Liao, H.; Cai, L.; Tong, M.; Hou, Y. Facile self-assembly synthesis of titanate/Fe3O4 nanocomposites for the efficient removal of Pb2+ from aqueous systems. J. Mater. Chem. A 2013, 1, 805–813. [Google Scholar] [CrossRef]
- Liu, T.; Han, X.; Wang, Y.; Yan, L.; Du, B.; Wei, Q.; Wei, D. Magnetic chitosan/anaerobic granular sludge composite: Synthesis, characterization and application in heavy metal ions removal. J. Colloid Interface Sci. 2017, 508, 405–414. [Google Scholar] [CrossRef]
- Guo, B.; Deng, F.; Zhao, Y.; Luo, X.; Luo, S.; Au, C. Magnetic ion-imprinted and –SH functionalized polymer for selective removal of Pb(II) from aqueous samples. Appl. Surf. Sci. 2014, 292, 438–446. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, Y.; Zhou, X.; Ren, J.; Zhong, C. Magnetic multi-porous bio-adsorbent modified with amino siloxane for fast removal of Pb(II) from aqueous solution. Appl. Surf. Sci. 2018, 427, 976–985. [Google Scholar] [CrossRef]
- Fischer, M.J. Amine coupling through EDC/NHS: A practical approach. Methods Mol. Biol. 2010, 627, 55–73. [Google Scholar] [CrossRef]
- Kositzi, M.; Poulios, I.; Malato, S.; Caceres, J.; Campos, A. Solar photocatalytic treatment of synthetic municipal wastewater. Water Res. 2004, 38, 1147–1154. [Google Scholar] [CrossRef]
- Huang, M.R.; Peng, Q.Y.; Li, X.G. Rapid and effective adsorption of lead ions on fine poly(phenylenediamine) microparticles. Chemistry 2006, 12, 4341–4350. [Google Scholar] [CrossRef]
- Mahdi, Z.; Yu, Q.J.; El Hanandeh, A. Removal of lead(II) from aqueous solution using date seed-derived biochar: Batch and column studies. Appl. Water Sci. 2018, 8. [Google Scholar] [CrossRef]
- Xu, Y.; Yoo, I.K.; Lee, H.; Ryu, K. Adsorptive removal of heavy metal ions in water using poly(m-phenylenediamine) synthesized by laccase. Chem. Pap. 2019, 73, 1705–1711. [Google Scholar] [CrossRef]
- Pal, P.; Syed, S.S.; Banat, F. Gelatin-bentonite composite as reusable adsorbent for the removal of lead from aqueous solutions: Kinetic and equilibrium studies. J. Water Process. Eng. 2017, 20, 40–50. [Google Scholar] [CrossRef]
- Tang, C.; Shu, Y.; Zhang, R.; Li, X.; Song, J.; Li, B.; Zhang, Y.; Ou, D. Comparison of the removal and adsorption mechanisms of cadmium and lead from aqueous solution by activated carbons prepared from Typha angustifolia and Salix matsudana. RSC. Adv. 2017, 7, 16092–16103. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Lee, H.R.; Hong, S.H.; Jang, J.R.; Choe, W.S.; Yoo, I.K. Selective lead adsorption by recombinant Escherichia coli displaying a lead-binding peptide. Appl. Biochem. Biotechnol. 2013, 169, 1188–1196. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).