Dental Condition as A Factor Modifying the Transmission of the Sound Vibration in the Skull Bones
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.3. Statistical Analysis
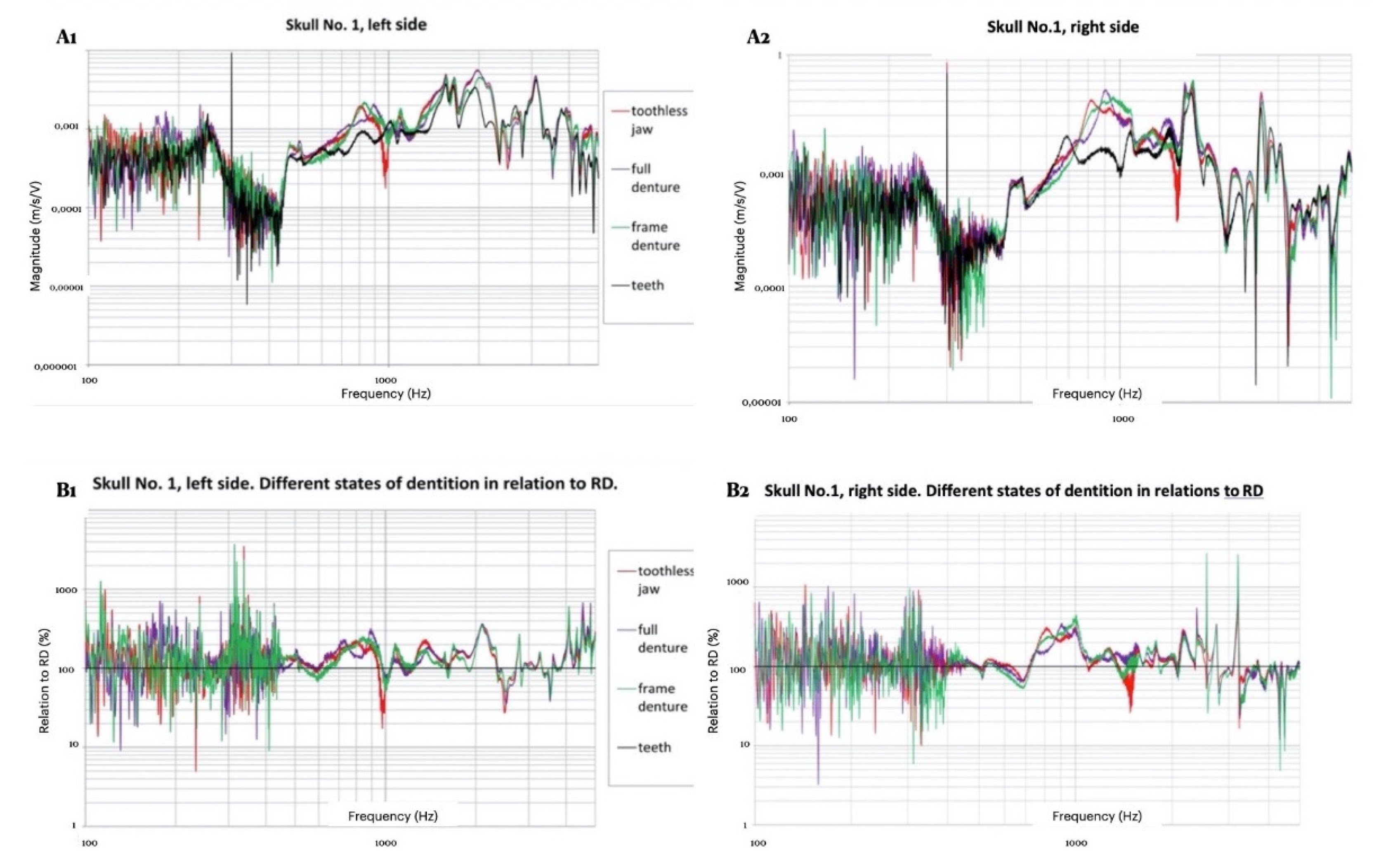
3. Results
3.1. Morphological Assessment of Recordings
3.2. Measurement Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Eeg-Olofsson, M.; Stenfelt, S.; Granström, G. Implications for contralateral bone-conducted transmission as measured by cochlear vibrations. Otol. Neurotol. 2011, 32, 192–198. [Google Scholar] [CrossRef] [Green Version]
- Stenfelt, S. Acoustic and physiologic aspects of bone conduction hearing. Adv. Otorhinolaryngol. 2011, 71, 10–21. [Google Scholar]
- Adelman, C.; Fraenkel, R.; Kriksunov, L.; Sohmer, H. Interactions in the cochlea between air conduction and osseous and non-osseous bone conduction stimulation. Eur. Arch. Otorhinolaryngol. 2012, 269, 425–429. [Google Scholar] [CrossRef]
- Chordekar, S.; Kriksunov, L.; Kishon-Rabin, L.; Adelman, C.; Sohmer, H. Mutual cancellation between tones presented by air conduction, by bone conduction, and by non-osseous (soft tissue) bone conduction. Hear. Res. 2012, 283, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Ozer, E.; Adelman, C.; Freeman, S.; Sohmer, H. Bone conduction hearing on the teeth of the lower jaw. J. Basic Clin. Physiol. Pharmacol. 2002, 13, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Behn, A.; Westerberg, B.D.; Zhang, H.; Riding, K.H.; Ludemann, J.P.; Kozak, F.K. Accuracy of the Weber and Rinne tuning fork tests in evaluation of children with otitis media with effusion. Otolaryngology 2007, 36, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Miodonski, J. The technique of the Weber test. J. Laryngol. Otol. 1960, 74, 125–127. (In Polish) [Google Scholar] [CrossRef] [PubMed]
- Brown, L.A. Dentaural hearing testing: Calibrating bone conduction through the teeth. Ann. Otol. Rhinol. Laryngol. 1969, 78, 1058–1061. [Google Scholar] [CrossRef]
- Dahlin, G.C.; Allen, F.G.; Collard, E.W. Bone-conduction thresholds of human teeth. J. Acoust. Soc. Am. 1973, 53, 1434–1437. [Google Scholar] [CrossRef] [PubMed]
- Semczuk, B. Studies on the role of the state of dentition in the physiopathology of the auditory organ. III. Experimental studies on the effect of the state of dentition on bone sound conductivity through the skull. Ann. Univ. Mariae Curie Sklodowska Med. 1967, 22, 165–172. [Google Scholar]
- Semczuk, B. Studies on the role of dentition in the physiopathology of the hearing organ. I. Clinical and statistical studies on the effect of dentition on the hearing organ and hearing in 5000 patients treated in an otolaryngologic clinic. Ann. Univ. Mariae Curie Sklodowska Med. 1966, 21, 167–174. [Google Scholar] [PubMed]
- Semczuk, B. Studies on the role of the state of dentition in the physiopathology of the auditory organ. II. Comparative clinical studies on the state of hearing and dentition in 600 healthy persons of the same age from 35-50 years. Ann. Univ. Mariae Curie Sklodowska Med. 1967, 22, 153–163. [Google Scholar] [PubMed]
- Schell, C.L.; Diehl, R.L.; Holmes, A.E.; Kubilis, P.S.; Loers, W.W.; Atchison, K.A.; Dolan, T.A. An association between dentate status and hearing acuity. Spec. Care Dent. 1999, 19, 208–213. [Google Scholar] [CrossRef]
- Lawrence, H.P.; Garcia, R.I.; Essick, G.K.; Hawkins, R.; Krall, E.A.; Spiro, A., 3rd; Vokonas, P.S.; Kong, L.; King, T.; Koch, G.G. A longitudinal study of the association between tooth loss and age-related hearing loss. Spec. Care Dent. 2001, 21, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Peeters, J.; Naert, I.; Carette, E.; Manders, E.; Jacobs, R. A potential link between oral status and hearing impairment: Preliminary observations. J. Oral Rehabil. 2004, 31, 306–310. [Google Scholar] [CrossRef]
- Kempf, H.G.; Roller, R.; Mühlbradt, L. Correlation between inner ear disorders and temporomandibular joint diseases. HNO 1993, 41, 7–10. [Google Scholar]
- Pihut, M.; Majewski, P.; Wisniewska, G.; Reron, E. Auriculo-vestibular symptoms related to structural and functional disorders of the stomatognathic system. J. Physiol. Pharmacol. 2011, 62, 251–256. [Google Scholar]
- King, W.H.; Burton, M.C.; Tucker, K.M. Clinical manifestations of dentaural hearing. J. Prosthet. Dent. 1974, 32, 130–140. [Google Scholar] [CrossRef]
- Lee, Y.-R.; Choi, J.-S.; Kim, H.-E. Unilateral chewing as a risk factor for hearing loss: Association between chewing habits and hearing acuity. Tohoku J. Exp. Med. 2018, 246, 45–50. [Google Scholar] [CrossRef] [Green Version]
- Nagasaka, H.; Matsukubo, T.; Takaesu, Y.; Kobayashi, Y.; Sato, T.; Ishikawa, T. Changes and equalization in hearing level induced by dental treatment and instruction in bilaterally equalized chewing: A clinical report. Bull. Tokyo Dent. Coll. 2002, 43, 243–250. [Google Scholar] [CrossRef]
- Shreedhar, S.; Raza, F.; Vaidyanathan, A.K.; Veeravalli, P.T. Effect of an implant-retained complete overdenture on the hearing ability of edentulous patients: A clinical pilot study. J. Prosthet. Dent. 2020. [Google Scholar] [CrossRef] [PubMed]
- Bochnia, M.; Porwolik, K.; Baliński, S.; Bolejko, R. A Device for Measuring Vibration of Human Skulls. Polish Patent PL W.122052, 13 January 2017. [Google Scholar]
- Eeg-Olofsson, M. Transmission of Bone-Conducted Sound in the Human Skull Based on Vibration and Perceptual Measures. Master’s Thesis, University of Gothenburg, Gothenburg, Sweden, 2012. [Google Scholar]
- Håkansson, B.; Brandt, A.; Carlsson, P.; Tjellström, A. Resonant frequencies of the human skull in vivo. J. Acoust. Soc. Am. 1994, 95, 1474–1481. [Google Scholar] [CrossRef] [PubMed]
- Khalil, T.B.; Viano, D.C.; Smith, D.L. Experimental analysis of the vibrational characteristics of the human skull. J. Sound. Vib. 1979, 63, 351–376. [Google Scholar] [CrossRef]
- Bochnia, M. Studies of the Dynamics of General Vibration on the Organ of Corti; Oficyna Wydawnicza Politechniki Wroclawskiej: Wroclaw, Poland, 2001; pp. 307–311. (In Polish) [Google Scholar]
- Stenfelt, S.; Goode, R.L. Bone-conducted sound: Physiological and clinical aspects. Otol. Neurotol. 2005, 26, 1245–1261. [Google Scholar] [CrossRef]
- Stenfelt, S.; Goode, R.L. Transmission properties of bone-conducted sound: Measurements in cadaver heads. J. Acoust. Soc. Am. 2005, 118, 2373–2391. [Google Scholar] [CrossRef]
- Eeg-Olofsson, M.; Stenfelt, S.; Taghavi, H.; Reinfeldt, S.; Håkansson, B.; Tengstrand, T.; Finizia, C. Transmission of bone-conducted sound—Correlation between hearing perception and cochlear vibration. Hear. Res. 2013, 306, 11–20. [Google Scholar] [CrossRef]
- Franke, E.K. Response of the human skull to mechanical vibrations. J. Acoust. Soc. Am. 1956, 28, 1277–1284. [Google Scholar] [CrossRef]
- Gurdjian, E.S.; Hodgson, V.R.; Thomas, I.M. Studies on mechanical impedance of the human skull: Preliminary report. J. Biomech. 1970, 3, 239–247. [Google Scholar] [CrossRef]
- Håkansson, B.; Carlsson, P.; Tjellström, A. The mechanical point impedance of the human head, with and without skin penetration. J. Acoust. Soc. Am. 1986, 80, 1065–1075. [Google Scholar] [CrossRef]
- Eeg-Olofsson, M.; Stenfelt, S.; Tjellström, A.; Granström, G. Transmission of bone-conducted sound in the human skull measured by cochlear vibrations. Int. J. Audiol. 2008, 47, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Semczuk, B. Studies on the role of the state of dentition in the physiopathology of the auditory organ. IV. Studies on the effect of dentition on the etiology of acoustic trauma. Ann. Univ. Mariae Curie Sklodowska Med. 1967, 22, 173–178. [Google Scholar] [PubMed]
- Ceypek, T.; Kuźniar, Z.J. The significance of limited frequency bands for understanding Polish speech. Otolaryngol. Pol. 1970, 24, 429–433. (In Polish) [Google Scholar] [PubMed]
- Nowicki, J. Polish speech intelligibility depending on the frequency band. Otolaryng. Pol. 1972, 26, 7–14. (In Polish) [Google Scholar]
- McLeod, R.W.J.; Roberts, W.H.; Perry, I.A.; Richardson, B.E.; Culling, J.F. Scanning laser Doppler vibrometry of the cranium when stimulated by a B71 bone transduce. Appl. Acoust. 2018, 142, 53–58. [Google Scholar] [CrossRef]
- Dobrev, I.; Stenfelt, S.; Röösli, C. Influence of stimulation position on the sensitivity for bone conduction hearing aids without skin penetration. Int. J. Audiol. 2016, 55, 439–446. [Google Scholar] [CrossRef]
- Stenfelt, S.; Håkansson, B.; Tjellström, A. Vibration characteristics of bone conducted sound in vitro. J. Acoust. Soc. Am. 2000, 107, 422–431. [Google Scholar] [CrossRef]
- Eeg-Olofsson, M.; Granstrom, G.; Håkansson, B.; Stenfelt, S.; Tjellstrom, A. Implanterbar BAHA. Acta Soc. Med. Suec. 2002, 111, 265. [Google Scholar]
- Hoffman, B.; Lorens, G. Dental problems cause hearing loss. Sluch 2010, 72, 8. (In Polish) [Google Scholar]




| Skull N° | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Sex | M | W | W | M | M | W |
| Mass (g) | 518 | 402 | 442 | 583 | 639 | 438 |
| Circumference (mm) | 491 | 458 | 481 | 504 | 511 | 480 |
| Skull N° | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Left side | R: 470 Hz R: 1546 Hz | R: 234 Hz R: 956 Hz | R: 426 Hz R: 1354 Hz | AR: 501 Hz R: 1616 Hz | AR: 507 Hz R: 1819 Hz | R: 425 Hz AR: 1334 Hz |
| Right side | R: 504 Hz R: 1635 Hz | R: 237 Hz R: 917 Hz | R: 426 Hz AR: 1373 Hz | AR: 505 Hz R: 1678 Hz | AR: 508 Hz R: 1818 Hz | R: 421 Hz AR: 1251 Hz |
| Skull N° | RESONANCE (R) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RD | TJ | CD | FD | |||||||||||||
| Full Range | Range of Differences | Full Range | Range of Differences | Full Range | Range of Differences | Full Range | Range of Differences | |||||||||
| l | r | l | r | l | r | l | r | l | r | l | r | l | r | l | r | |
| 1 | 14 | 17 | 2 | 4 | 13 | 17 | 2 | 2 | 13 | 16 | 2 | 2 | 14 | 16 | 2 | 2 |
| 2 | 19 | 18 | 4 | 3 | 20 | 21 | 5 | 5 | 20 | 19 | 3 | 3 | 19 | 16 | 4 | 4 |
| 3 | 16 | 14 | 2 | 3 | 17 | 18 | 3 | 5 | 15 | 15 | 2 | 3 | 12 | 16 | 2 | 2 |
| 4 | 12 | 11 | 2 | 1 | 11 | 13 | 3 | 2 | 11 | 11 | 3 | 2 | 11 | 12 | 3 | 2 |
| 5 | 13 | 12 | 1 | 1 | 15 | 12 | 1 | 1 | 14 | 11 | 2 | 1 | 14 | 11 | 1 | 1 |
| 6 | 16 | 11 | 2 | 2 | 12 | 12 | 3 | 2 | 14 | 10 | 2 | 1 | 16 | 11 | 2 | 2 |
| 1–6 | 90 | 83 | 13 | 14 | 88 | 93 | 17 | 17 | 87 | 82 | 14 | 12 | 86 | 82 | 14 | 13 |
| Mean for A Skull | 14.4 | 2.25 | 15.1 | 2.8 | 14.1 | 2.1 | 14 | 2.25 | ||||||||
| Skull N° | ANTIRESONANCE (AR) | |||||||||||||||
| RD | TJ | CD | FD | |||||||||||||
| Full Range | Range of Differences | Full range | Range of Differences | Full Range | Range of Differences | Full Range | Range of Differences | |||||||||
| l | r | l | r | l | r | l | r | l | r | l | r | l | r | l | r | |
| 1 | 12 | 16 | 3 | 5 | 12 | 16 | 2 | 1 | 12 | 15 | 2 | 2 | 13 | 15 | 2 | 2 |
| 2 | 20 | 19 | 3 | 3 | 20 | 21 | 6 | 6 | 20 | 18 | 4 | 4 | 20 | 16 | 5 | 5 |
| 3 | 16 | 14 | 3 | 3 | 16 | 18 | 4 | 5 | 14 | 15 | 3 | 3 | 11 | 16 | 3 | 5 |
| 4 | 12 | 11 | 2 | 1 | 13 | 13 | 3 | 2 | 11 | 12 | 3 | 2 | 11 | 12 | 2 | 2 |
| 5 | 14 | 13 | 1 | 1 | 16 | 13 | 2 | 1 | 15 | 12 | 2 | 1 | 15 | 11 | 1 | 1 |
| 6 | 16 | 10 | 2 | 1 | 13 | 12 | 2 | 2 | 14 | 9 | 2 | 1 | 16 | 10 | 2 | 2 |
| 1–6 | 90 | 83 | 14 | 14 | 90 | 93 | 19 | 17 | 86 | 81 | 16 | 13 | 86 | 80 | 15 | 17 |
| Mean for A Skull | 14.4 | 2.33 | 15.25 | 3 | 13.9 | 2.4 | 3.8 | 2.7 | ||||||||
| Frequency Band [kHz] | Statistics | Dentition | p-Value | |||
|---|---|---|---|---|---|---|
| Toothless Jaw | Complete Denture | Frame Denture | Teeth | |||
| 0.5 ÷ 2.0 | Me (Q1 ÷ Q3) | 4.72 (3.26; 5.49) | 4.67 (3.38; 5.56) | 4.17 (3.27; 4.64) | 3.33 (2.5; 3.97) | 0.038 |
| 2.0 ÷ 3.5 | Me (Q1 ÷ Q3) | 1.67 (1.13; 2.21 | 1.65 (0.95; 2.31) | 1.61 (1.13; 2.16) | 1.49 (0.99; 2.02) | 0.940 |
| 3.5 ÷ 5.0 | Me (Q1 ÷ Q3) | 0.86 (0.53; 1.14) | 1.00 (0.74; 1.19) | 0.96 (0.69; 1.11) | 0.82 (0.42; 0.99) | 0.466 |
| TJ | CD | FD | RD | |
|---|---|---|---|---|
| Me = 4.72 | Me = 4.67 | Me = 4.17 | Me = 3.33 | |
| TJ | p = 1.000 | p = 0.866 | p = 0.028 | |
| CD | p = 1.000 | p = 0.273 | p = 0.018 | |
| FD | p = 0.866 | p = 0.273 | p = 0.033 | |
| RD | p = 0.028 | p = 0.018 | p = 0.033 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balinski, S.; Morawska-Kochman, M.; Bolejko, R.; Dudek, K.; Bochnia, M. Dental Condition as A Factor Modifying the Transmission of the Sound Vibration in the Skull Bones. Appl. Sci. 2020, 10, 6478. https://doi.org/10.3390/app10186478
Balinski S, Morawska-Kochman M, Bolejko R, Dudek K, Bochnia M. Dental Condition as A Factor Modifying the Transmission of the Sound Vibration in the Skull Bones. Applied Sciences. 2020; 10(18):6478. https://doi.org/10.3390/app10186478
Chicago/Turabian StyleBalinski, Slawomir, Monika Morawska-Kochman, Romuald Bolejko, Krzysztof Dudek, and Marek Bochnia. 2020. "Dental Condition as A Factor Modifying the Transmission of the Sound Vibration in the Skull Bones" Applied Sciences 10, no. 18: 6478. https://doi.org/10.3390/app10186478
APA StyleBalinski, S., Morawska-Kochman, M., Bolejko, R., Dudek, K., & Bochnia, M. (2020). Dental Condition as A Factor Modifying the Transmission of the Sound Vibration in the Skull Bones. Applied Sciences, 10(18), 6478. https://doi.org/10.3390/app10186478





