Karl Fischer Water Titration—Principal Component Analysis Approach on Bread Products
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
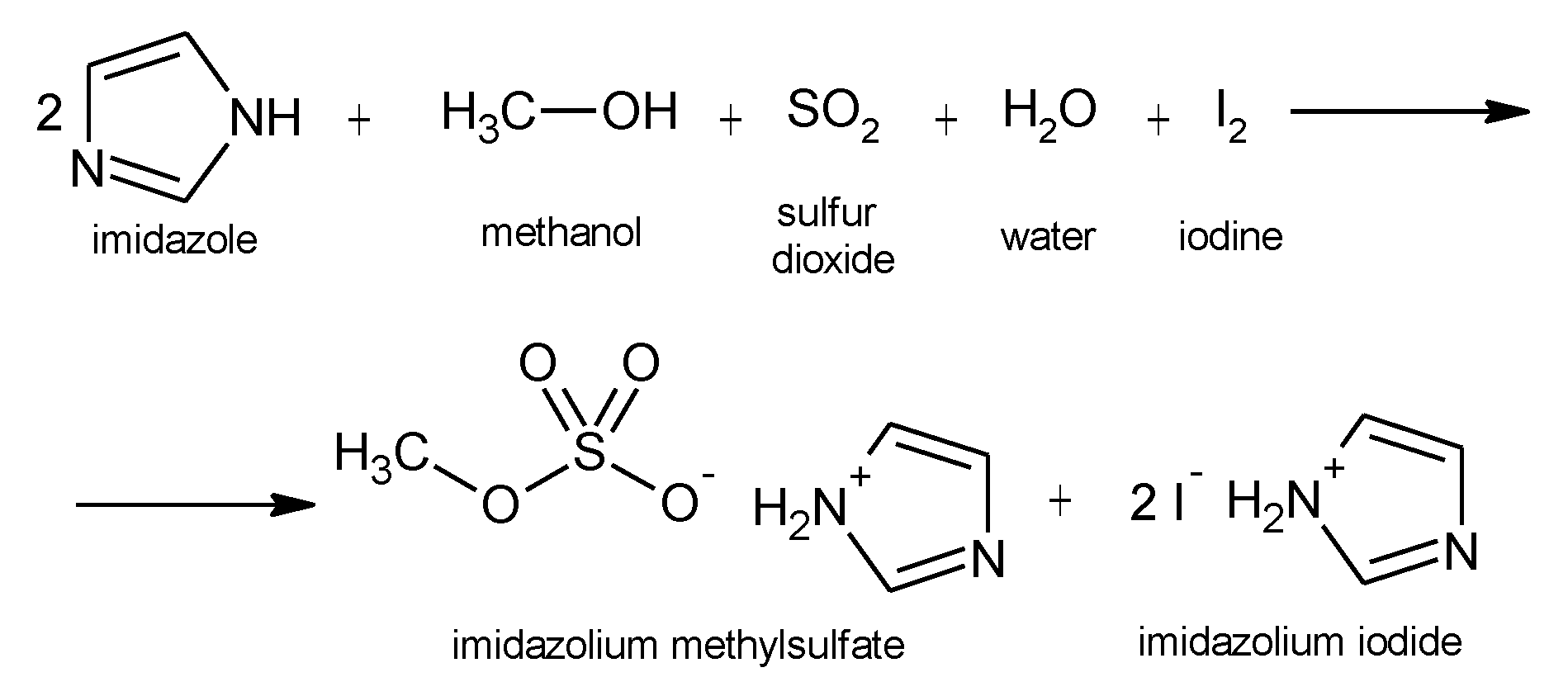
2.2. Volumetric Karl Fischer Water Titration (KFT)
2.3. Statistics and Principal Component Analysis (PCA)
3. Results
3.1. Karl Fischer Water Titration and Kinetics for Bread Samples
3.2. Principal Component Analysis on KFT Data for Bread Samples
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dewettinck, K.; Van Bockstaele, F.; Kühne, B.; Van de Walle, D.; Courtens, T.M.; Gellynck, X. Nutritional value of bread: Influence of processing, food interaction and consumer perception. J. Cereal Sci. 2008, 48, 243–257. [Google Scholar] [CrossRef]
- Goesaert, H.; Brijs, K.; Veraverbeke, W.S.; Courtin, C.M.; Gebruers, K.; Delcour, J.A. Wheat flour constituents: How they impact bread quality, and how to impact their functionality. Trends Food Sci. Technol. 2005, 16, 12–30. [Google Scholar] [CrossRef]
- Hădărugă, D.I.; Costescu, C.I.; Corpaş, L.; Hădărugă, N.G.; Isengard, H.-D. Differentiation of rye and wheat flour as well as mixtures by using the kinetics of Karl Fischer water titration. Food Chem. 2016, 195, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Mondal, A.; Datta, A.K. Bread baking—A review. J. Food Eng. 2008, 86, 465–474. [Google Scholar] [CrossRef]
- Lopes Almeida, E.; Chang, Y.K.; Joy Steel, C. Dietary fibre sources in bread: Influence on technological quality. LWT Food Sci. Technol. 2013, 50, 545–553. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Wang, W.; Li, Y. Dough properties, bread quality, and associated interactions with added phenolic compounds: A review. J. Funct. Foods 2019, 52, 629–639. [Google Scholar] [CrossRef]
- Birch, A.N.; Petersen, M.A.; Arneborg, N.; Hansen, A.S. Influence of commercial baker’s yeasts on bread aroma profiles. Food Res. Int. 2013, 52, 160–166. [Google Scholar] [CrossRef]
- Blandino, M.; Sovrani, V.; Marinaccio, F.; Reyneri, A.; Rolle, L.; Giacosa, S.; Locatelli, M.; Bordiga, M.; Travaglia, F.; Coïsson, J.D.; et al. Nutritional and technological quality of bread enriched with an intermediated pearled wheat fraction. Food Chem. 2013, 141, 2549–2557. [Google Scholar] [CrossRef]
- Bosmans, G.M.; Lagrain, B.; Fierens, E.; Delcour, J.A. The impact of baking time and bread storage temperature on bread crumb properties. Food Chem. 2013, 141, 3301–3308. [Google Scholar] [CrossRef]
- Caballero, P.A.; Gomez, M.; Rosell, C.M. Improvement of dough rheology, bread quality and bread shelf-life by enzymes combination. J. Food Eng. 2007, 81, 42–53. [Google Scholar] [CrossRef] [Green Version]
- Cho, I.H.; Peterson, D.G. Chemistry of Bread Aroma: A Review. Food Sci. Biotechnol. 2010, 19, 575–582. [Google Scholar] [CrossRef]
- Corpaş, L.; Hădărugă, N.G.; Codina, G.G.; Riviş, A.; Guran, E.; Baliţa, E.-N.; Hădărugă, D.I. Phospholipids in homemade bread. J. Agroaliment. Process. Technol. 2012, 18, 336–340. [Google Scholar]
- Curti, E.; Carini, E.; Bonacini, G.; Tribuzio, G.; Vittadini, E. Effect of the addition of bran fractions on bread properties. J. Cereal Sci. 2013, 57, 325–332. [Google Scholar] [CrossRef]
- de Lamo, B.; Gómez, M. Bread enrichment with oilseeds. A Review. Foods 2018, 7, 191. [Google Scholar] [CrossRef] [Green Version]
- Goesaert, H.; Slade, L.; Levine, H.; Delcour, J.A. Amylases and bread firming—An integrated view. J. Cereal Sci. 2009, 50, 345–352. [Google Scholar] [CrossRef]
- Jiang, D.; Peterson, D.G. Identification of bitter compounds in whole wheat bread. Food Chem. 2013, 141, 1345–1353. [Google Scholar] [CrossRef]
- Moayedallaie, S.; Mirzaei, M.; Paterson, J. Bread improvers: Comparison of a range of lipases with a traditional emulsifier. Food Chem. 2010, 122, 495–499. [Google Scholar] [CrossRef]
- Shin, D.-J.; Kim, W.; Kim, Y. Physicochemical and sensory properties of soy bread made with germinated, steamed, and roasted soy flour. Food Chem. 2013, 141, 517–523. [Google Scholar] [CrossRef]
- Belz, M.C.E.; Ryan, L.A.M.; Arendt, E.K. The Impact of Salt Reduction in Bread: A Review. Crit. Rev. Food Sci. Nutr. 2012, 52, 514–524. [Google Scholar] [CrossRef]
- Ayub, M.; Wahab, S.; Durrani, Y. Effect of Water Activity (Aw) Moisture Content and Total Microbial Count on the Overall Quality of Bread. Int. J. Agric. Biol. 2003, 5, 274–278. [Google Scholar]
- Chen, G.; Öhgren, C.; Langton, M.; Lustrup, K.F.; Nydén, M.; Swenson, J. Impact of long-term frozen storage on the dynamics of water and ice in wheat bread. J. Cereal Sci. 2013, 57, 120–124. [Google Scholar] [CrossRef]
- Miś, A.; Krekora, M.; Niewiadomski, Z.; Dziki, D.; Nawrocka, A. Water redistribution between model bread dough components during mixing. J. Cereal Sci. 2020, 95, 103035. [Google Scholar] [CrossRef]
- Gil, M.J.; Callejo, M.J.; Rodriguez, G. Effect of water content and storage time on white pan bread quality: Instrumental evaluation. Z. Lebensm. Unters. Forsch. 1997, 205, 268–273. [Google Scholar] [CrossRef]
- Cappelli, A.; Oliva, N.; Cini, E. Stone milling versus roller milling: A systematic review of the effects on wheat flour quality, dough rheology, and bread characteristics. Trends Food Sci. Technol. 2020, 97, 147–155. [Google Scholar] [CrossRef]
- Ziobro, R.; Korus, J.; Juszczak, L.; Witczak, T. Influence of inulin on physical characteristics and staling rate of gluten-free bread. J. Food Eng. 2013, 116, 21–27. [Google Scholar] [CrossRef]
- Wagner, M.J.; Lucas, T.; Le Ray, D.; Trystram, G. Water transport in bread during baking. J. Food Eng. 2007, 78, 1167–1173. [Google Scholar] [CrossRef]
- Margolis, S.A.; Huang, P.H.; Hădărugă, N.G.; Hădărugă, D.I. Water determination. In Encyclopedia of Analytical Science, 3rd ed.; Elsevier: Oxford, UK, 2019; Volume 10, pp. 382–390. [Google Scholar]
- Yazgan, S.; Bernreuther, A.; Ulberth, F.; Isengard, H.-D. Water—An important parameter for the preparation and proper use of certified reference materials. Food Chem. 2006, 96, 411–417. [Google Scholar] [CrossRef]
- Isengard, H.-D.; Kling, R.; Reh, C.T. Proposal of a new reference method to determine the water content of dried dairy products. Food Chem. 2006, 96, 418–422. [Google Scholar] [CrossRef]
- Isengard, H.-D.; Präger, H. Water determination in products with high sugar content by infrared drying. Food Chem. 2003, 82, 161–167. [Google Scholar] [CrossRef]
- Isengard, H.-D. Water content, one of the most important properties of food. Food Control. 2001, 12, 395–400. [Google Scholar] [CrossRef]
- Heinze, P.; Isengard, H.-D. Determination of the water content in different sugar syrups by halogen drying. Food Control. 2001, 12, 483–486. [Google Scholar] [CrossRef]
- Isengard, H.-D. Rapid water determination in foodstuffs. Trends Food Sci. Technol. 1995, 6, 155–162. [Google Scholar] [CrossRef]
- Sahin, A.W.; Wiertz, J.; Arendt, E.K. Evaluation of a new method to determine the water addition level in gluten-free bread systems. J. Cereal Sci. 2020, 93, 102971. [Google Scholar] [CrossRef]
- Zippenfening, S.E.; Hădărugă, D.I.; Negrea, I.; Velciov, A.; Hădărugă, N.G. Karl Fischer titration of salami products: Variation of the water content during ripening and kinetics of the KFT process. Chem. Bull. Politeh. Univ. Timişoara 2015, 64, 18–24. [Google Scholar]
- Corpaş, L.; Hădărugă, N.G.; David, I.; Pîrşan, P.; Hădărugă, D.I.; Isengard, H.-D. Karl Fischer water titration—Principal component analysis approach on wheat flour. Food Anal. Methods 2014, 7, 1353–1358. [Google Scholar] [CrossRef]
- Hădărugă, N.G.; Hădărugă, D.I.; Isengard, H.-D. Water content of natural cyclodextrins and their essential oil complexes: A comparative study between Karl Fischer titration and thermal methods. Food Chem. 2012, 132, 1741–1748. [Google Scholar] [CrossRef]
- Felgner, A.; Schlink, R.; Kirschenbuhler, P.; Faas, B.; Isengard, H.-D. Automated Karl Fischer titration for liquid samples—Water determination in edible oils. Food Chem. 2008, 106, 1379–1384. [Google Scholar] [CrossRef]
- Schmitt, K.; Isengard, H.-D. Karl Fischer titration. A method for determining the true water content of cereals. Fresenius’ J. Anal. Chem. 1998, 360, 465–469. [Google Scholar] [CrossRef]
- Merkh, G.; Pfaff, R.; Isengard, H.-D. Capabilities of automated Karl Fischer titration combined with gas extraction for water determination in selected dairy products. Food Chem. 2012, 132, 1736–1740. [Google Scholar] [CrossRef]
- Isengard, H.-D.; Kerwin, H. Proposal of a new reference method for determining water content in butter oil. Food Chem. 2003, 82, 117–119. [Google Scholar] [CrossRef]
- Tomassetti, M.; Campanella, L.; Aureli, T. Thermogravimetric analysis of some spices and commercial food products. Comparison with other analytical methods for moisture content determination (Part 3). Thermochim. Acta 1989, 143, 15–26. [Google Scholar] [CrossRef]
- Isengard, H.-D.; Haschka, E.; Merkh, G. Development of a method for water determination in lactose. Food Chem. 2012, 132, 1660–1663. [Google Scholar] [CrossRef]
- Kestens, V.; Conneely, P.; Bernreuther, A. Vaporisation coulometric Karl Fischer titration: A perfect tool for water content determination of difficult matrix reference materials. Food Chem. 2008, 106, 1454–1459. [Google Scholar] [CrossRef]
- Rückold, S.; Grobecker, K.H.; Isengard, H.-D. The effects of drying on biological matrices and the consequences for reference materials. Food Control. 2001, 12, 401–407. [Google Scholar] [CrossRef]
- Kestens, V.; Charoud-Got, J.; Bau’, A.; Bernreuther, A.; Emteborg, H. Online measurement of water content in candidate reference materials by acousto-optical tuneable filter near-infrared spectrometry (AOTF-NIR) using pork meat calibrants controlled by Karl Fischer titration. Food Chem. 2008, 106, 1359–1365. [Google Scholar] [CrossRef]
- Isengard, H.-D.; Färber, J.-M. ‘Hidden parameters’ of infrared drying for determining low water contents in instant powders. Talanta 1999, 50, 239–246. [Google Scholar] [CrossRef]
- Belitz, H.-D.; Grosch, W.; Schieberle, P. Food Chemistry; Springer: Berlin, Germany, 2009; pp. 670–745. [Google Scholar] [CrossRef]
- Bhatt, C.M.; Nagaraju, J. Studies on electrical properties of wheat bread as a function of moisture content during storage. Sens. Instrum. Food Qual. Saf. 2010, 4, 61–66. [Google Scholar] [CrossRef]
- Isengard, H.-D.; Heinze, P. Determination of total water and surface water in sugars. Food Chem. 2003, 82, 169–172. [Google Scholar] [CrossRef]
- Ureta, M.M.; Diascorn, Y.; Cambert, M.; Flick, D.; Salvadori, V.O.; Lucas, T. Water transport during bread baking: Impact of the baking temperature and the baking time. Food Sci. Technol. Int. 2018, 25, 187–197. [Google Scholar] [CrossRef]
- Hădărugă, D.I.; Birău (Mitroi), C.L.; Gruia, A.T.; Păunescu, V.; Bandur, G.N.; Hădărugă, N.G. Moisture evaluation of β-cyclodextrin/fish oils complexes by thermal analyses: A data review on common barbel (Barbus barbus L.), Pontic shad (Alosa immaculata Bennett), European wels catfish (Silurus glanis L.), and common bleak (Alburnus alburnus L.) living in Danube river. Food Chem. 2017, 236, 49–58. [Google Scholar] [CrossRef]
- Hădărugă, N.G.; Bandur, G.N.; David, I.; Hădărugă, D.I. A review on thermal analyses of cyclodextrins and cyclodextrin complexes. Environ. Chem. Lett. 2019, 17, 349–373. [Google Scholar] [CrossRef]
- Hădărugă, N.G.; Szakal, R.N.; Chirilă, C.A.; Lukinich-Gruia, A.T.; Păunescu, V.; Muntean, C.; Rusu, G.; Bujancă, G.; Hădărugă, D.I. Complexation of Danube common nase (Chondrostoma nasus L.) oil by β-cyclodextrin and 2-hydroxypropyl-β-cyclodextrin. Food Chem. 2020, 303, 125419. [Google Scholar] [CrossRef] [PubMed]
- Curti, E.; Carini, E.; Tribuzio, G.; Vittadini, E. Effect of bran on bread staling: Physico-chemical characterization and molecular mobility. J. Cereal Sci. 2015, 65, 25–30. [Google Scholar] [CrossRef]
- Curti, E.; Carini, E.; Diantom, A.; Vittadini, E. The use of potato fibre to improve bread physico-chemical properties during storage. Food Chem. 2016, 195, 64–70. [Google Scholar] [CrossRef]
- Chiavaro, E.; Vittadini, E.; Musci, M.; Bianchi, F.; Curti, E. Shelf-life stability of artisanally and industrially produced durum wheat sourdough bread (“Altamura bread”). LWT Food Sci. Technol. 2008, 41, 58–70. [Google Scholar] [CrossRef]
- Marzec, A.; Lewicki, P.P. Antiplasticization of cereal-based products by water. Part I. Extruded flat bread. J. Food Eng. 2006, 73, 1–8. [Google Scholar] [CrossRef]
- Kobus-Cisowska, J.; Szymanowska-Powałowska, D.; Szymandera-Buszka, K.; Rezler, R.; Jarzębski, M.; Szczepaniak, O.; Marciniak, G.; Jędrusek-Golińska, A.; Kobus-Moryson, M. Effect of fortification with calcium from eggshells on bioavailability, quality, and rheological characteristics of traditional Polish bread spread. J. Dairy Sci. 2020, 103, 6918–6929. [Google Scholar] [CrossRef]
- Primo-Martín, C.; de Beukelaer, H.; Hamer, R.J.; Van Vliet, T. Fracture behaviour of bread crust: Effect of ingredient modification. J. Cereal Sci. 2008, 48, 604–612. [Google Scholar] [CrossRef]
- Purlis, E.; Salvadori, V.O. Modelling the browning of bread during baking. Food Res. Int. 2009, 42, 865–870. [Google Scholar] [CrossRef]
- Primo-Martín, C.; van de Pijpekamp, A.; van Vliet, T.; de Jongh, H.H.J.; Plijter, J.J.; Hamer, R.J. The role of the gluten network in the crispness of bread crust. J. Cereal Sci. 2006, 43, 342–352. [Google Scholar] [CrossRef]
- Pico, J.; Reguilón, M.P.; Bernal, J.; Gómez, M. Effect of rice, pea, egg white and whey proteins on crust quality of rice flour-corn starch based gluten-free breads. J. Cereal Sci. 2019, 86, 92–101. [Google Scholar] [CrossRef]
- Lind, I.; Rask, C. Sorption isotherms of mixed minced meat, dough, and bread crust. J. Food Eng. 1991, 14, 303–315. [Google Scholar] [CrossRef]
- Wolter, A.; Hager, A.-S.; Zannini, E.; Czerny, M.; Arendt, E.K. Influence of dextran-producing Weissella cibaria on baking properties and sensory profile of gluten-free and wheat breads. Int. J. Food Microbiol. 2014, 172, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Grünke, S.; Wünsch, G. Kinetics and stoichiometry in the Karl Fischer solution. Fresenius’ J. Anal. Chem. 2000, 368, 139–147. [Google Scholar]
- Yap, W.T.; Cummings, A.L.; Margolis, S.A.; Schaffer, R. Estimation of water content by kinetic method in Karl Fischer titration. Anal. Chem. 1979, 51, 1595–1596. [Google Scholar] [CrossRef]
- Hădărugă, D.I.; Hădărugă, N.G.; Bandur, G.N.; Isengard, H.-D. Water content of flavonoid/cyclodextrin nanoparticles: Relationship with the structural descriptors of biologically active compounds. Food Chem. 2012, 132, 1651–1659. [Google Scholar] [CrossRef]
- Hădărugă, N.G.; Hădărugă, D.I.; Isengard, H.-D. “Surface water” and “strong-bonded water” in cyclodextrins: A Karl Fischer titration approach. J. Incl. Phenom. Macrocycl. Chem. 2013, 75, 297–302. [Google Scholar] [CrossRef]
- Ünlüsayin, M.; Hădărugă, N.G.; Rusu, G.; Gruia, A.T.; Păunescu, V.; Hădărugă, D.I. Nano-encapsulation competitiveness of omega-3 fatty acids and correlations of thermal analysis and Karl Fischer water titration for European anchovy (Engraulis encrasicolus L.) oil/β-cyclodextrin complexes. LWT Food Sci. Technol. 2016, 68, 135–144. [Google Scholar] [CrossRef]



| No. | Code | Sample Mass (g) | KFT Volume (mL) | KFT Volume/Sample Mass Ratio (mL/g) 1 | Water Content (%) 1 |
|---|---|---|---|---|---|
| 1 | HC1 * | 0.0768 (±0.0149) | 3.223 (±0.498) | 42.16 (±1.71) a | 43.12 (±6.66) a |
| 2 | HC2 | 0.0572 (±0.0070) | 2.554 (±0.291) | 44.66 (±0.84) a | 39.56 (±0.74) a |
| 3 | HC3 | 0.0656 (±0.0178) | 2.902 (±0.860) | 43.16 (±0.18) a | 39.04 (±1.00) a |
| 4 | HC4 | 0.4192 (±0.5131) | 3.002 (±0.799) | 44.05 (±1.85) a | 39.37 (±1.47) a |
| 5 | HC5 * | 0.0690 (±0.0141) | 2.894 (±0.566) | 41.69 (±0.71) a | 41.06 (±0.34) a |
| 6 | GC | 0.1288 (±0.0600) | 5.328 (±2.491) | 40.57 (±1.49) a | 36.50 (±1.32) b |
| 7 | MC | 0.0850 (±0.0032) | 3.884 (±0.187) | 46.45 (±0.97) a | 44.23 (±1.14) a |
| 8 | BC1 | 0.0837 (±0.0018) | 3.777 (±0.135) | 44.06 (±0.59) a | 44.11 (±0.61) a |
| 9 | BC2 | 0.0631 (±0.0376) | 2.297 (±1.370) | 37.86 (±1.70) ab | 35.15 (±0.35) b |
| 10 | MCd * | 0.0851 | 2.731 | 32.43 b | 31.06 c |
| 11 | BCd | 0.0851 | 2.752 | 32.74 b | 31.30 c |
| 12 | MS | 0.0909 (±0.0014) | 2.154 (±0.081) | 22.95 (±1.01) c | 22.94 (±1.05) d |
| 13 | MSd * | 0.0915 | 0.099 | 23.61 c | 1.05 e |
| 14 | BS | 0.0811 (±0.0084) | 1.656 (±0.572) | 17.56 (±4.04) d | 19.44 (±4.72) d |
| 15 | BSd * | 0.0926 | 0.452 | 5.35 e | 4.72 e |
| No. | Code | Variation of the KFT Volume/Sample Mass on Time [Δ(V/m)1/Δt1, mL/g/s] 1 | Variation of the KFT Volume/Sample Mass on Time [ΔV/m)2/Δt2, mL/g/s] 1 | Mean KFT Reaction Rate v2 (mM/s) 1 | Mean KFT Reaction Rate v2 (mM/s) 1 |
|---|---|---|---|---|---|
| 1 | HC1 * | 0.434 (±0.078) a | 0.0039 (±0.0027) a | 2.42 (±0.43) a | 0.022 (±0.015) a |
| 2 | HC2 | 0.581 (±0.066) a | 0.0090 (±0.0008) a | 3.23 (±0.37) a | 0.050 (±0.004) a |
| 3 | HC3 | 0.503 (±0.124) a | 0.0192 (±0.0093) a | 2.80 (±0.69) a | 0.107 (±0.052) a |
| 4 | HC4 | 0.495 (±0.112) a | 0.0127 (±0.0067) a | 2.75 (±0.62) a | 0.071 (±0.037) a |
| 5 | HC5 * | 0.483 (±0.107) a | 0.0139 (±0.0015) a | 2.68 (±0.59) a | 0.077 (±0.008) a |
| 6 | GC | 0.301 (±0.156) ab | 0.0111 (±0.0093) a | 1.67 (±0.87) ab | 0.062 (±0.052) a |
| 7 | MC | 0.762 (±0.024) c | 0.0380 (±0.0056) b | 4.24 (±0.13) c | 0.211 (±0.031) b |
| 8 | BC1 | 0.389 (±0.004) a | 0.0175 (±0.0034) a | 2.17 (±0.02) a | 0.097 (±0.019) a |
| 9 | BC2 | 0.708 (±0.083) c | 0.0848 (±0.0086) c | 3.94 (±0.46) c | 0.472 (±0.048) c |
| 10 | MCd * | 0.717 c | 0.0557 d | 3.99 c | 0.310 d |
| 11 | BCd * | 0.682 c | 0.0857 cd | 3.79 c | 0.476 cd |
| 12 | MS | 0.380 (±0.015) a | 0.0571 (±0.0013) d | 2.12 (±0.09) a | 0.318 (±0.007) d |
| 13 | MSd * | 0.136 d | 0.0785 c | 0.76 d | 0.436 c |
| 14 | BS * | 0.180 d | 0.0533 d | 1.00 d | 0.296 d |
| 15 | BSd * | 0.205 d | 0.0108 a | 1.14 d | 0.060 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popescu, G.; Radulov, I.; Iordănescu, O.A.; Orboi, M.D.; Rădulescu, L.; Drugă, M.; Bujancă, G.S.; David, I.; Hădărugă, D.I.; , C.A.L.; et al. Karl Fischer Water Titration—Principal Component Analysis Approach on Bread Products. Appl. Sci. 2020, 10, 6518. https://doi.org/10.3390/app10186518
Popescu G, Radulov I, Iordănescu OA, Orboi MD, Rădulescu L, Drugă M, Bujancă GS, David I, Hădărugă DI, CAL, et al. Karl Fischer Water Titration—Principal Component Analysis Approach on Bread Products. Applied Sciences. 2020; 10(18):6518. https://doi.org/10.3390/app10186518
Chicago/Turabian StylePopescu, Gabriela, Isidora Radulov, Olimpia A. Iordănescu, Manuela D. Orboi, Laura Rădulescu, Mărioara Drugă, Gabriel S. Bujancă, Ioan David, Daniel I. Hădărugă, Christine A. Lucan (Banciu), and et al. 2020. "Karl Fischer Water Titration—Principal Component Analysis Approach on Bread Products" Applied Sciences 10, no. 18: 6518. https://doi.org/10.3390/app10186518
APA StylePopescu, G., Radulov, I., Iordănescu, O. A., Orboi, M. D., Rădulescu, L., Drugă, M., Bujancă, G. S., David, I., Hădărugă, D. I., , C. A. L., Hădărugă, N. G., & Riviş, M. (2020). Karl Fischer Water Titration—Principal Component Analysis Approach on Bread Products. Applied Sciences, 10(18), 6518. https://doi.org/10.3390/app10186518







