Osseointegration of Antimicrobial Acrylic Bone Cements Modified with Graphene Oxide and Chitosan
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Bone Cements
2.3. Setting Properties
2.4. Mechanical Properties
2.5. In Vitro Studies
2.5.1. Antibacterial Activity against Escherichia coli
2.5.2. Cell Viability
2.5.3. Cell Adhesion
2.6. In Vivo Studies
2.6.1. Subdermal Implantation
2.6.2. Bone Implantation Study
2.7. Statistical Analysis
3. Results and Discussion
3.1. Setting Properties
3.2. Mechanical Properties
3.3. In Vitro Studies
3.3.1. Antibacterial Activity against Escherichia coli
3.3.2. Cellular Behavior of Human Osteoblasts
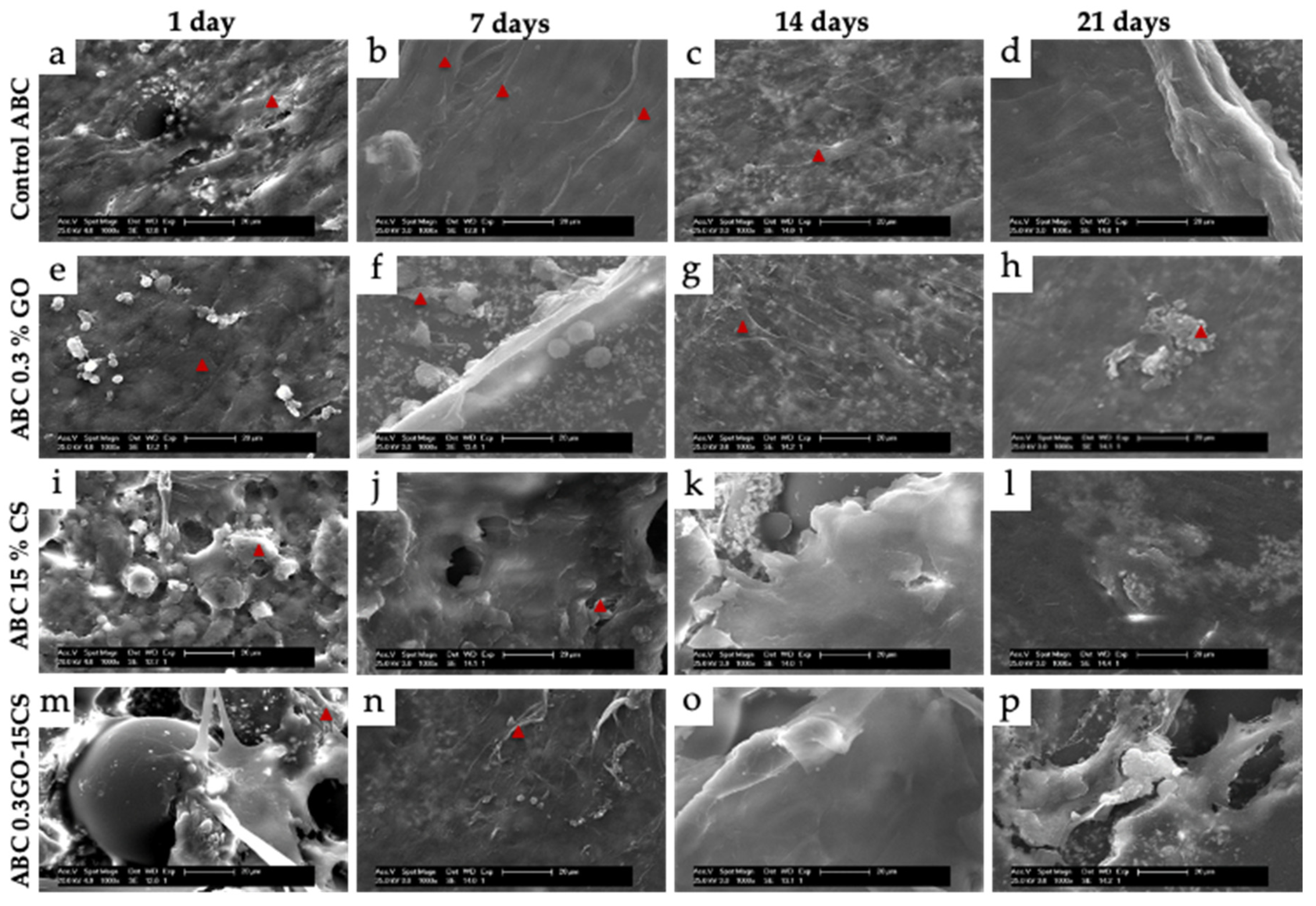
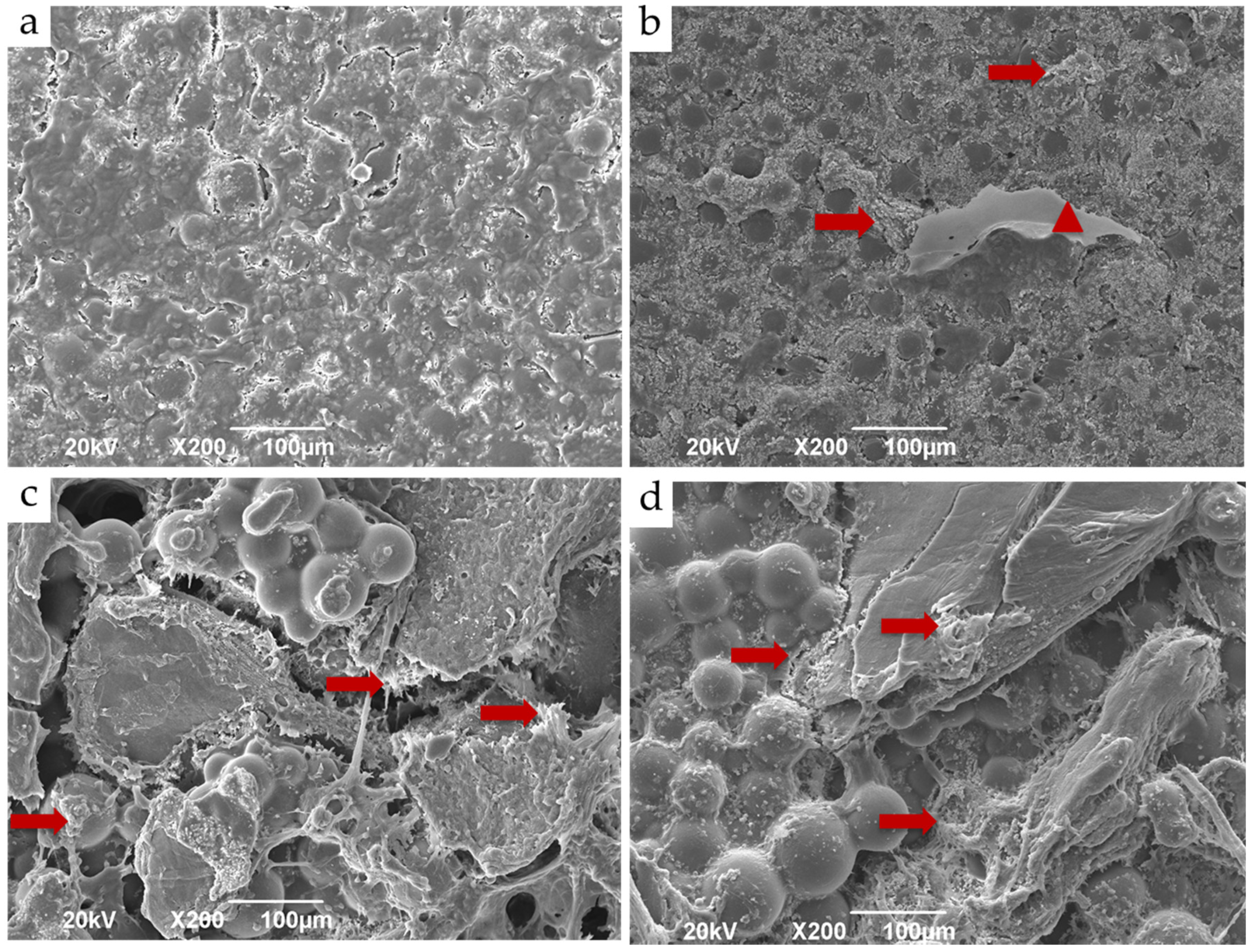
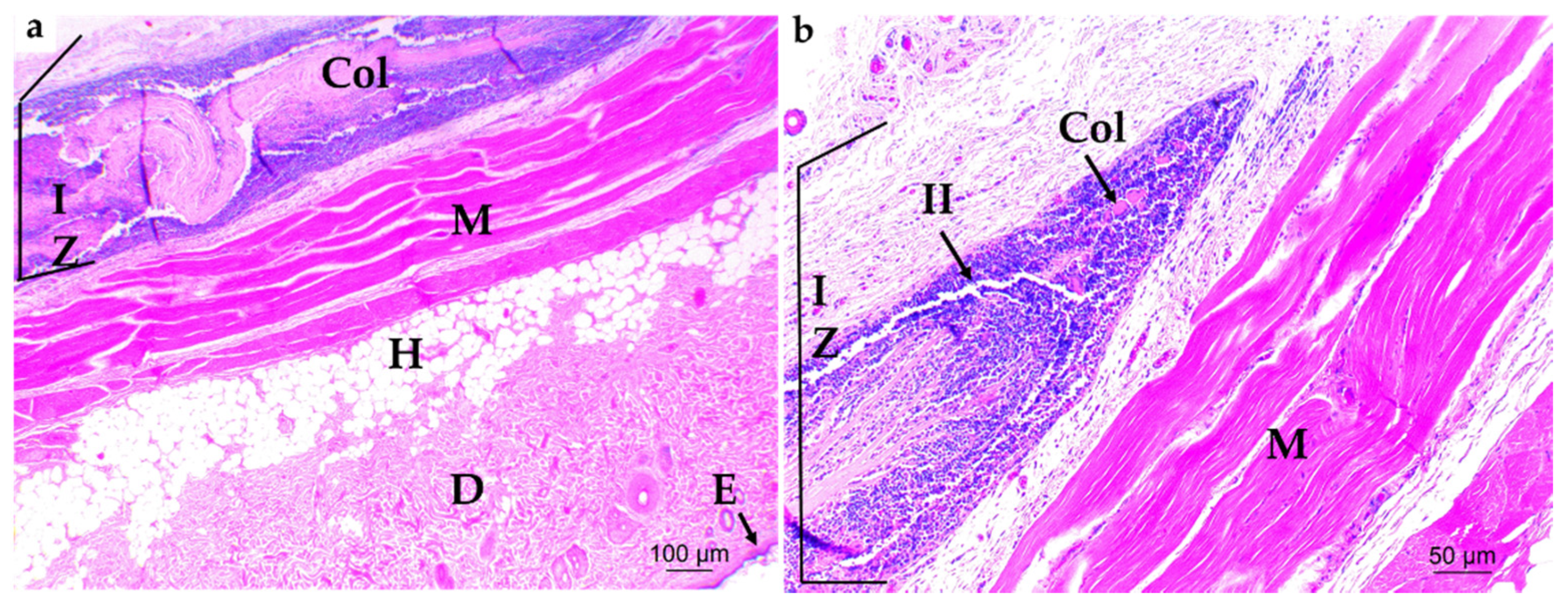
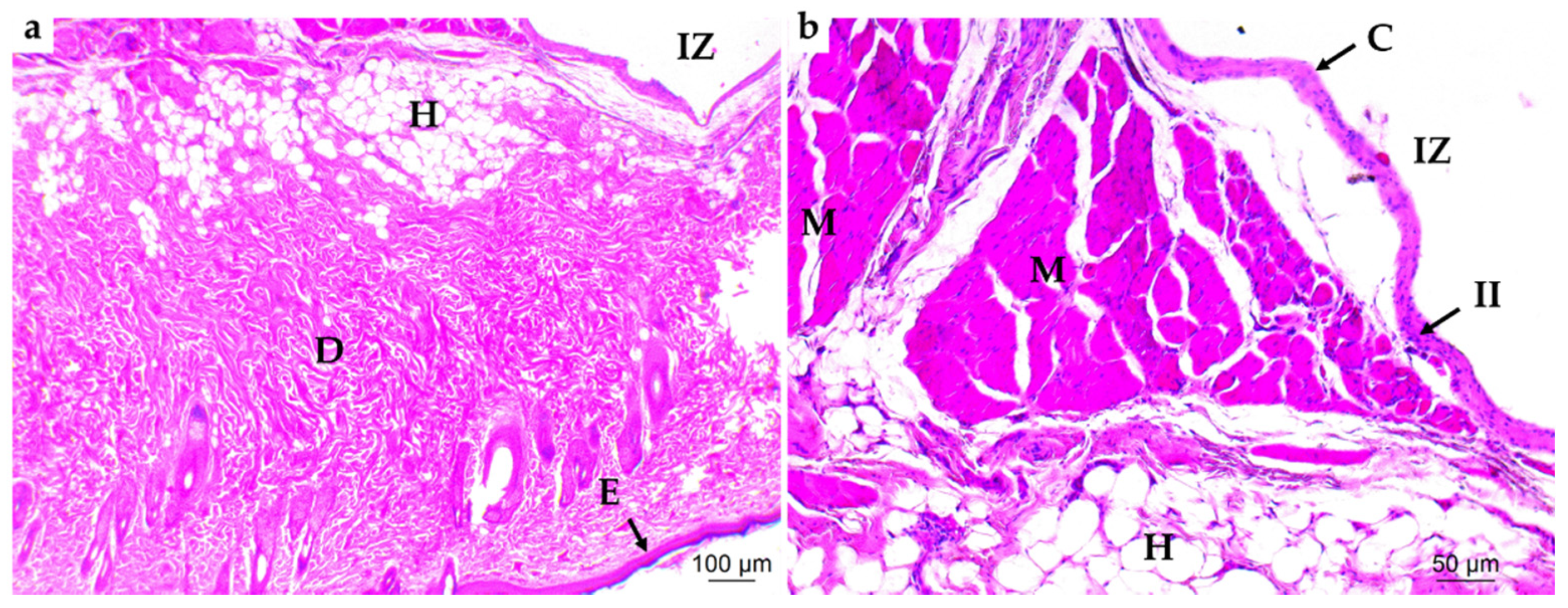
3.4. In Vivo Studies
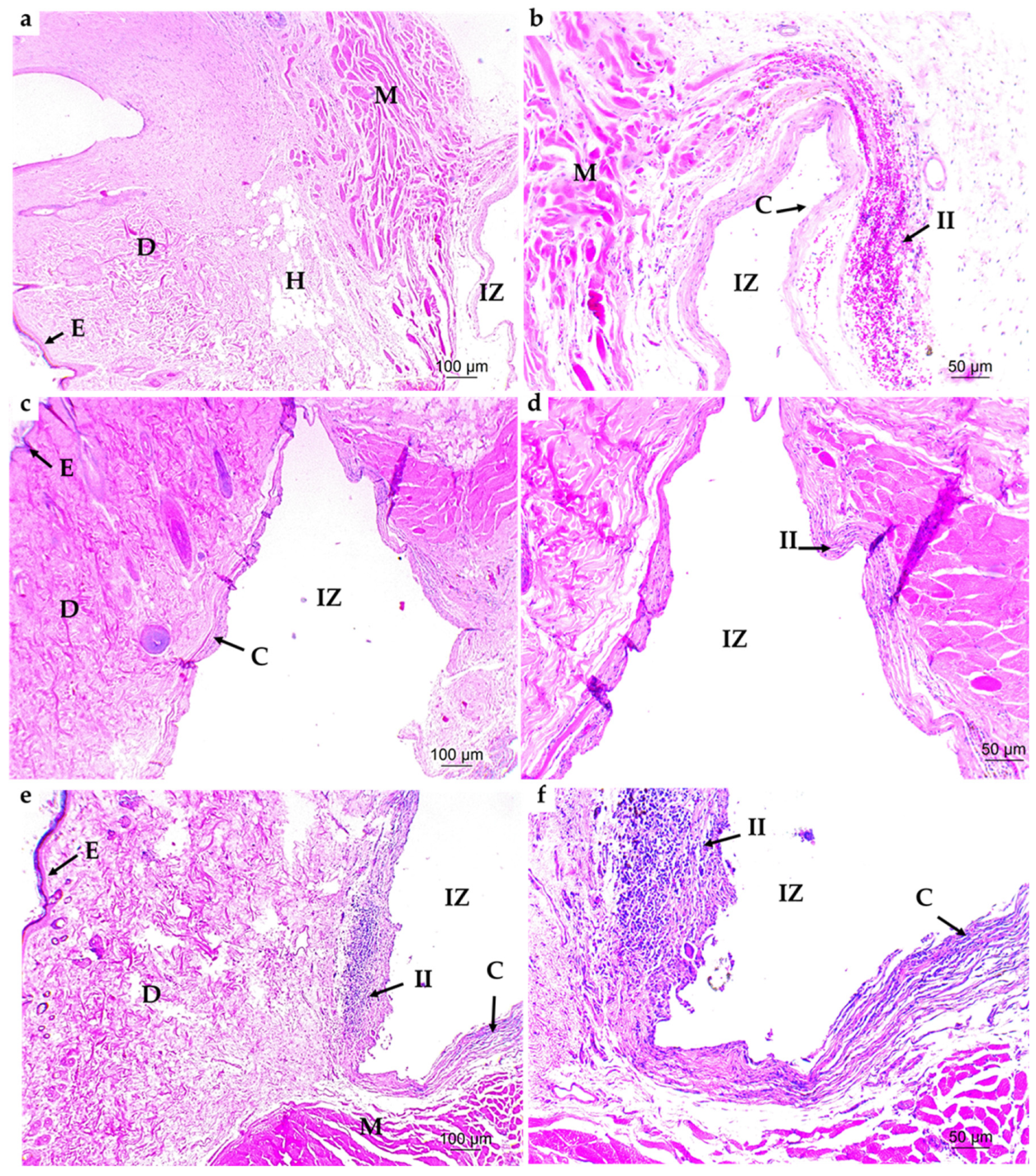
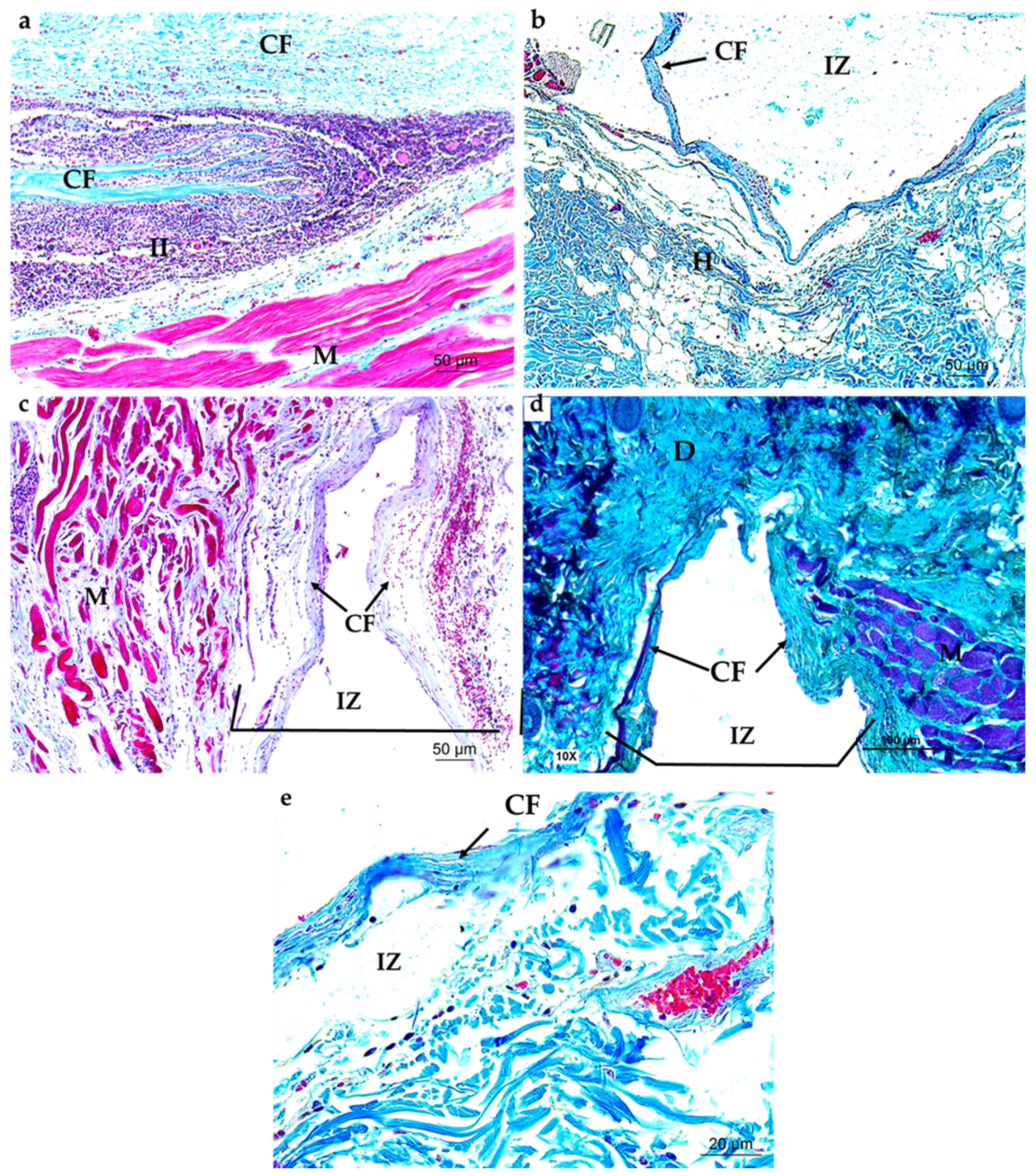
3.4.1. Subdermal Implantation
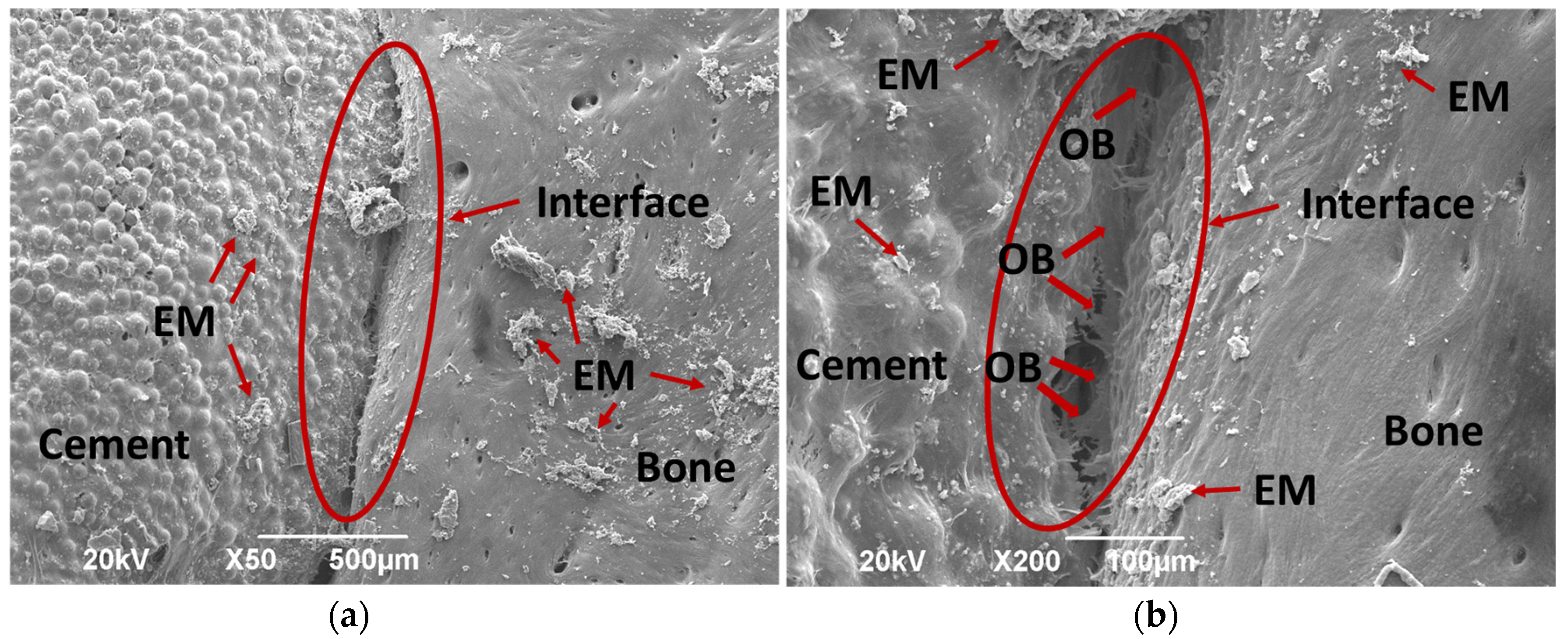
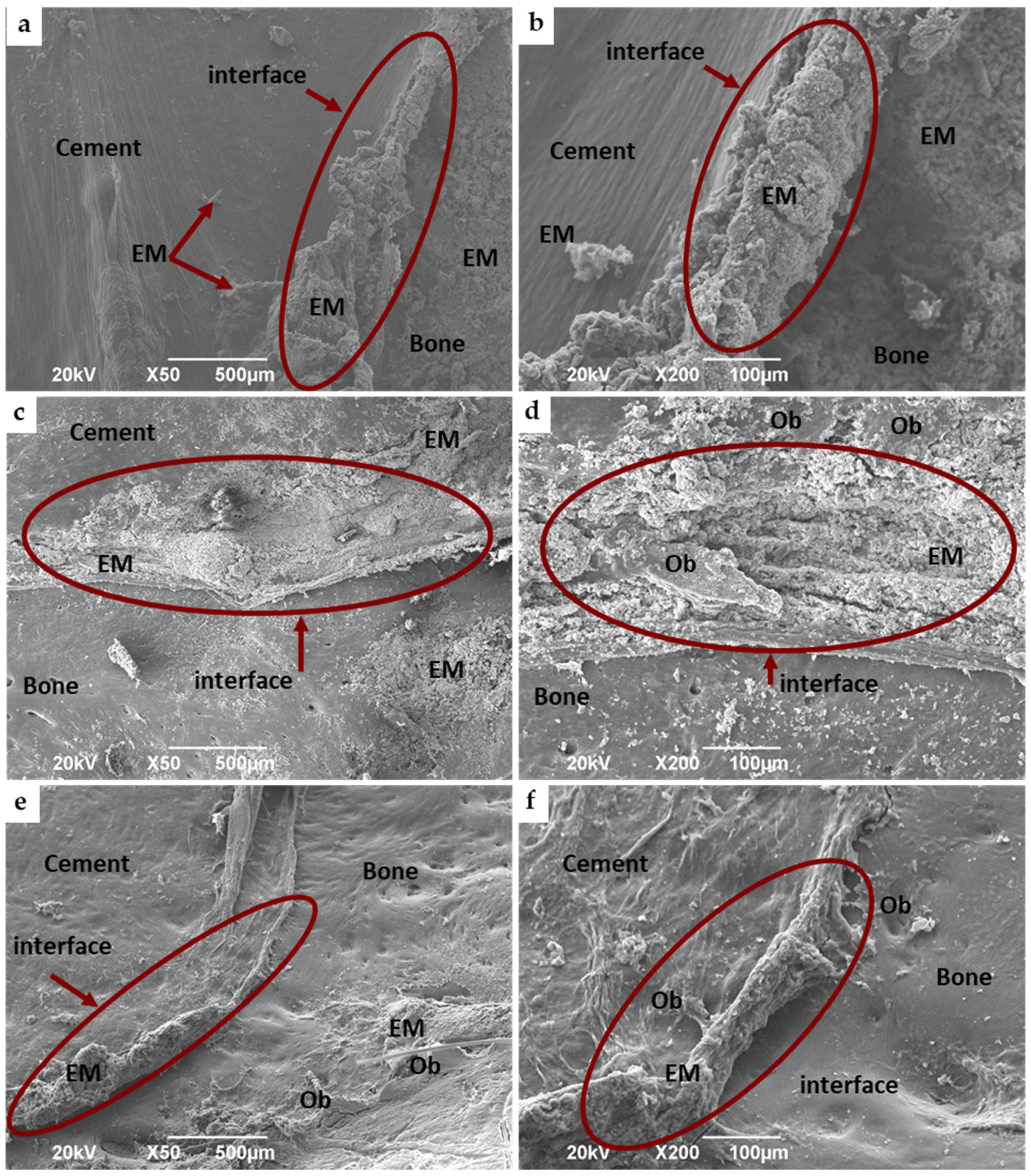
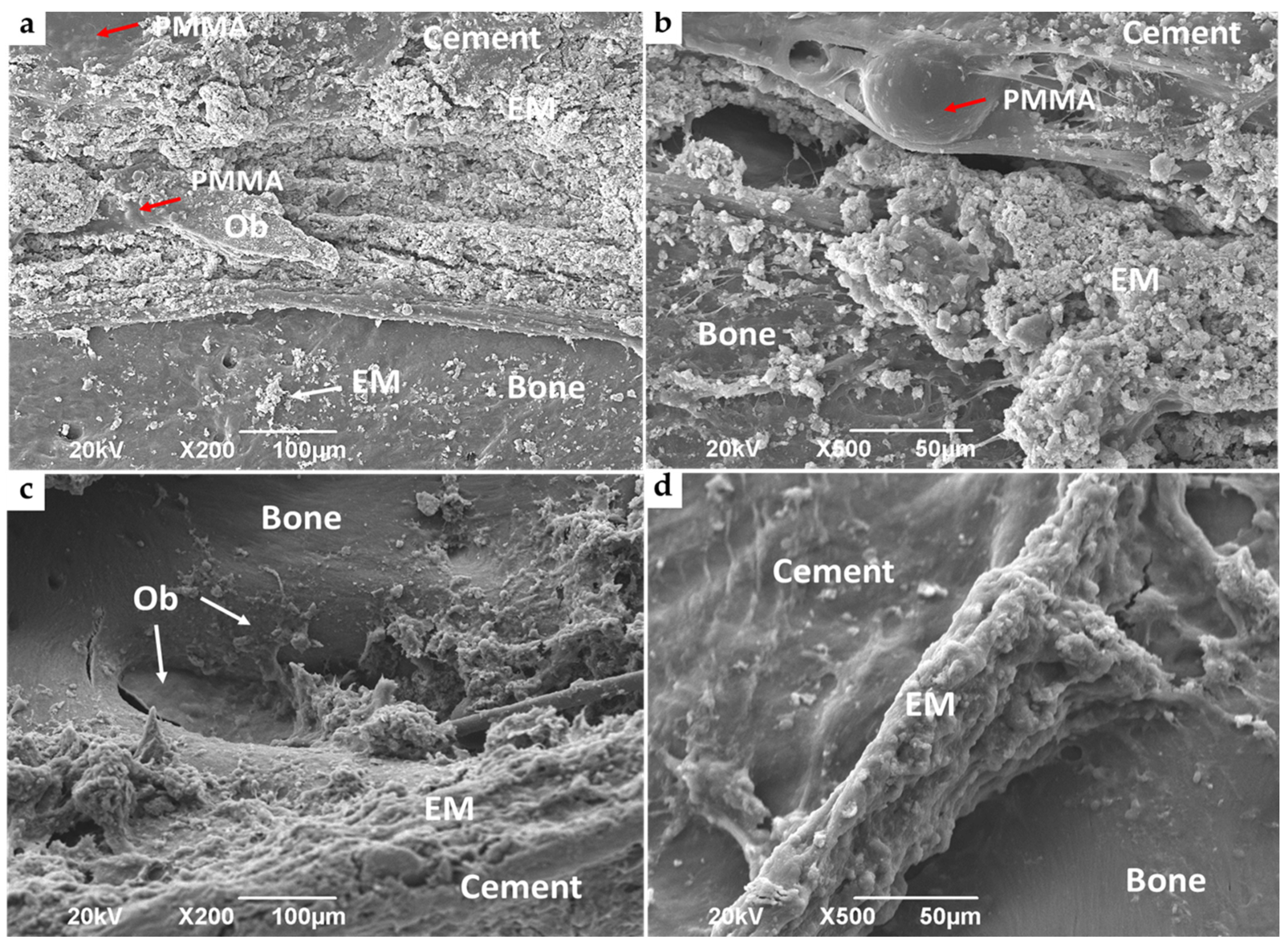
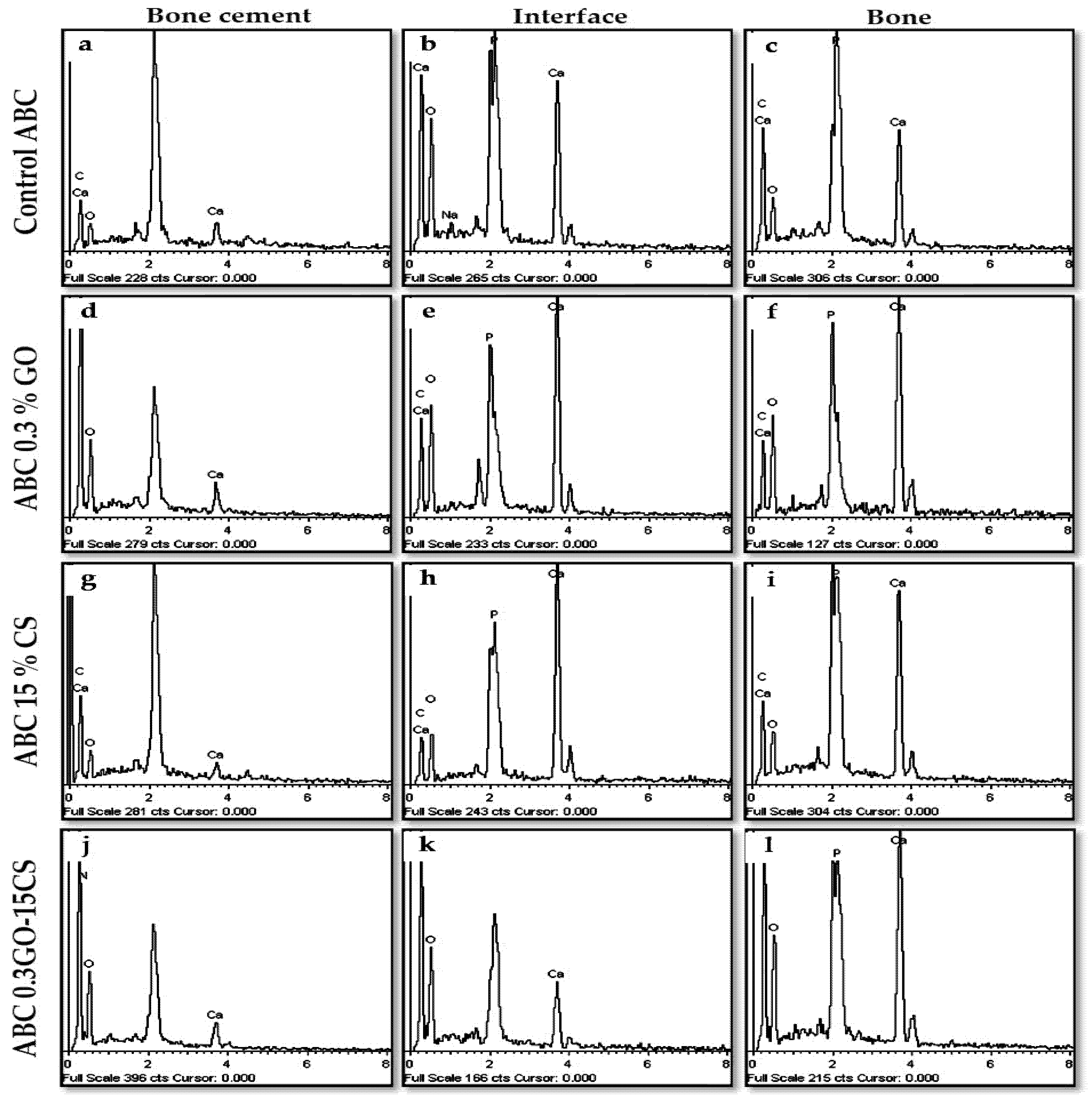
3.4.2. Bone Implantation of ABCs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Slane, J.; Vivanco, J.; Meyer, J.; Ploeg, H.L.; Squire, M. Modification of acrylic bone cement with mesoporous silica nanoparticles: Effects on mechanical, fatigue and absorption properties. J. Mech. Behav. Biomed. Mater. 2014, 29, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Lissarrague, M.H.; Fascio, M.L.; Goyanes, S.; D’Accorso, N.B. Acrylic Bone Cements: The Role of Nanotechnology in Mechanical Properties. J. Biomed. Nanotechnol. 2014, 10, 3536–3557. [Google Scholar] [CrossRef] [PubMed]
- Franco-Marquès, E.; Méndez, J.A.; Gironès, J.; Ginebra, M.P.; Pèlach, M.A. Evaluation of the influence of the addition of biodegradable polymer matrices in the formulation of self-curing polymer systems for biomedical purposes. Acta Biomater. 2009, 5, 2953–2962. [Google Scholar] [CrossRef] [PubMed]
- Endogan, T.; Kiziltay, A.; Kose, G.T.; Comunoglu, N.; Beyzadeoglu, T.; Hasirci, N. Acrylic bone cements: Effects of the poly(methyl methacrylate) powder size and chitosan addition on their properties. J. Appl. Polym. Sci. 2014, 131, 39662. [Google Scholar] [CrossRef]
- Webb, J.C.J.; Spencer, R.F. The role of polymethylmethacrylate bone cement in modern orthopaedic surgery. J. Bone Jt. Surg. 2007, 89, 851–857. [Google Scholar] [CrossRef]
- Yan, F.; Liu, Z.; Zhang, T.; Zhang, Q.; Chen, Y.; Xie, Y.; Lei, J.; Cai, L. Biphasic Injectable Bone Cement with Fe3O4/GO Nanocomposites for the Minimally Invasive Treatment of Tumor-Induced Bone Destruction. ACS Biomater. Sci. Eng. 2019, 5, 5833–5843. [Google Scholar] [CrossRef]
- Pahlevanzadeh, F.; Bakhsheshi-Rad, H.R.; Hamzah, E. In-vitro biocompatibility, bioactivity, and mechanical strength of PMMA-PCL polymer containing fluorapatite and graphene oxide bone cements. J. Mech. Behav. Biomed. Mater. 2018, 82, 257–267. [Google Scholar] [CrossRef]
- Ni, G.X.; Chiu, K.Y.; Lu, W.W.; Wang, Y.; Zhang, Y.G.; Hao, L.B.; Li, Z.Y.; Lam, W.M.; Lu, S.B.; Luk, K.D.K. Strontium-containing hydroxyapatite bioactive bone cement in revision hip arthroplasty. Biomaterials 2006, 27, 4348–4355. [Google Scholar] [CrossRef]
- De Mori, A.; Di Gregorio, E.; Kao, A.P.; Tozzi, G.; Barbu, E.; Sanghani-Kerai, A.; Draheim, R.R.; Roldo, M. Antibacterial PMMA Composite Cements with Tunable Thermal and Mechanical Properties. ACS Omega 2019, 4, 19664–19675. [Google Scholar] [CrossRef] [Green Version]
- Shi, Z.; Neoh, K.G.; Kang, E.T.; Wang, W. Antibacterial and mechanical properties of bone cement impregnated with chitosan nanoparticles. Biomaterials 2006, 27, 2440–2449. [Google Scholar] [CrossRef]
- Kowalski, R.; Schmaehling, R. Chapter 6. Commercial aspects and delivery systems of bone cements. In Orthopaedic Bone Cements; Deb, S., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2008; pp. 113–139. ISBN 978-1-84569-517-0. [Google Scholar]
- Boesel, L.F.; Cachinho, S.C.P.; Fernandes, M.H.V.; Reis, R.L. The in vitro bioactivity of two novel hydrophilic, partially degradable bone cements. Acta Biomater. 2007, 3, 175–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, A.A.; Mirza, E.H.; Mohamed, B.A.; Alharthi, N.H.; Abdo, H.S.; Javed, R.; Alhur, R.S.; Vallittu, P.K. Physical, mechanical, chemical and thermal properties of nanoscale graphene oxide-poly methylmethacrylate composites. J. Compos. Mater. 2018, 52, 2803–2813. [Google Scholar] [CrossRef]
- Lozano, K.; Mina, J.; Zuluaga, F.; Valencia, C.; Valencia, M. Influencia de la incorporación de un co-monómero alcalino e hidroxiapatita en las propiedades de cementos óseos acrílicos. DYNA 2013, 80, 153–162. [Google Scholar]
- Espigares, I.; Elvira, C.; Mano, J.F.; Vázquez, B.; San Román, J.; Reis, R.L. New partially degradable and bioactive acrylic bone cements based on starch blends and ceramic fillers. Biomaterials 2002, 23, 1883–1895. [Google Scholar] [CrossRef]
- Dalby, M.J.; Di Silvio, L.; Harper, E.J.; Bonfield, W. In vitro evaluation of a new polymethylmethacrylate cement reinforced with hydroxyapatite. J. Mater. Sci. Mater. Med. 1999, 10, 793–796. [Google Scholar] [CrossRef]
- Lopes, P.P.; Garcia, M.P.; Fernandes, M.H.; Fernandes, M.H.V. Acrylic formulations containing bioactive and biodegradable fillers to be used as bone cements: Properties and biocompatibility assessment. Mater. Sci. Eng. C 2013, 33, 1289–1299. [Google Scholar] [CrossRef] [PubMed]
- Fini, M.; Giavaresi, G.; Nicoli Aldini, N.; Torricelli, P.; Botter, R.; Beruto, D.; Giardino, R. A bone substitute composed of polymethylmethacrylate and α-tricalcium phosphate: Results in terms of osteoblast function and bone tissue formation. Biomaterials 2002, 23, 4523–4531. [Google Scholar] [CrossRef]
- García-Enriquez, S.; Guadarrama, H.E.R.; Reyes-González, I.; Mendizábal, E.; Jasso-Gastinel, C.F.; García-Enriquez, B.; Rembao-Bojórquez, D.; Pane-Pianese, C. Mechanical performance and in vivo tests of an acrylic bone cement filled with bioactive sepia officinalis cuttlebone. J. Biomater. Sci. Polym. Ed. 2010, 21, 113–125. [Google Scholar] [CrossRef]
- He, Q.; Chen, H.; Huang, L.; Dong, J.; Guo, D.; Mao, M.; Kong, L.; Li, Y.; Wu, Z.; Lei, W. Porous Surface Modified Bioactive Bone Cement for Enhanced Bone Bonding. PLoS ONE 2012, 7, e42525. [Google Scholar] [CrossRef] [Green Version]
- Lewis, G. Alternative acrylic bone cement formulations for cemented arthroplasties: Present status, key issues, and future prospects. J. Biomed. Mater. Res. B Appl. Biomater. 2008, 84, 301–319. [Google Scholar] [CrossRef]
- Rodríguez-Vázquez, M.; Vega-Ruiz, B.; Ramos-Zúñiga, R.; Saldaña-Koppel, D.A.; Quiñones-Olvera, L.F. Chitosan and Its Potential Use as a Scaffold for Tissue Engineering in Regenerative Medicine. Biomed. Res. Int. 2015, 2015, 821279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oryan, A.; Sahvieh, S. Effectiveness of chitosan scaffold in skin, bone and cartilage healing. Int. J. Biol. Macromol. 2017, 104, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Park, S.J.; Yang, D.H.; Chun, H.J. Chitosan for Tissue Engineering. In Advances in Experimental Medicine and Biology; Springer: Singapore, 2018; Volume 1077, pp. 475–485. ISBN 9789811309472. [Google Scholar]
- Aguilar, A.; Zein, N.; Harmouch, E.; Hafdi, B.; Bornert, F.; Damien, O.; Clauss, F.; Fioretti, F.; Huck, O.; Benkirane-jessel, N.; et al. Application of Chitosan in Bone and Dental Engineering. Molecules 2019, 24, 3009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamburaci, S.; Tihminlioglu, F. Chitosan-hybrid poss nanocomposites for bone regeneration: The effect of poss nanocage on surface, morphology, structure and in vitro bioactivity. Int. J. Biol. Macromol. 2020, 142, 643–657. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, V.; Yuan, Y.; Rigney, D.A.; Chesnutt, B.M.; Puckett, A.D.; Ong, J.L.; Yang, Y.; Haggard, W.O.; Elder, S.H.; Bumgardner, J.D. Bone cell attachment and growth on well-characterized chitosan films. Polym. Int. 2006, 55, 641–647. [Google Scholar] [CrossRef]
- Lin, M.C.; Chen, C.C.; Wu, I.T.; Ding, S.J. Enhanced antibacterial activity of calcium silicate-based hybrid cements for bone repair. Mater. Sci. Eng. C 2020, 110, 110727. [Google Scholar] [CrossRef]
- Palla-Rubio, B.; Araujo-Gomes, N.; Fernandez-Gutierrez, M.; Rojo, L.; Suay, J.; Gurruchaga, M.; Goni, I. Synthesis and characterization of silica-chitosan hybrid materials as antibacterial coatings for titanium implants. Carbohydr. Polym. 2019, 203, 331–341. [Google Scholar] [CrossRef]
- Rojo, L.; Deb, S. Polymer Therapeutics in Relation to Dentistry. Front. Oral Biol. 2015, 17, 13–21. [Google Scholar] [CrossRef]
- Valencia Zapata, M.E.; Mina Hernandez, J.H.; Grande Tovar, C.D.; Valencia Llano, C.H.; Diaz Escobar, J.A.; Vázquez-Lasa, B.; San Román, J.; Rojo, L.; Rojo, L. Novel Bioactive and Antibacterial Acrylic Bone Cement Nanocomposites Modified with Graphene Oxide and Chitosan. Int. J. Mol. Sci. 2019, 20, 2938. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Khun, N.W.; Tang, X.Z.; Liu, E.; Khor, K.A. Mechanical, tribological and biological properties of novel 45S5 Bioglass® composites reinforced with in situ reduced graphene oxide. J. Mech. Behav. Biomed. Mater. 2017, 65, 77–89. [Google Scholar] [CrossRef]
- Kurapati, R.; Bonachera, F.; Russier, J.; Sureshbabu, A.R.; Ménard-Moyon, C.; Kostarelos, K.; Bianco, A. Covalent chemical functionalization enhances the biodegradation of graphene oxide. 2D Mater. 2018, 5, 015020. [Google Scholar] [CrossRef]
- Palmieri, V.; Papi, M.; Conti, C.; Ciasca, G.; Maulucci, G.; De Spirito, M.; Palmieri, V.; Papi, M.; Conti, C.; Ciasca, G.; et al. The future development of bacteria fighting medical devices: The role of graphene oxide. Expert Rev. Med. Devices 2016, 13, 1013–1019. [Google Scholar] [CrossRef]
- Mirza, E.H.; Khan, A.A.; Al-Khureif, A.A.; Saadaldin, S.A.; Mohamed, B.A.; Fareedi, F.; Khan, M.M.; Alfayez, M.; Al-Fotawi, R.; Vallittu, P.K.; et al. Characterization of osteogenic cells grown over modified graphene-oxide-biostable polymers. Biomed. Mater. 2019, 14, 65004. [Google Scholar] [CrossRef]
- Mukherjee, S.P.; Gliga, A.R.; Lazzaretto, B.; Brandner, B.; Fielden, M.; Vogt, C.; Newman, L.; Rodrigues, A.F.; Shao, W.; Fournier, P.M.; et al. Graphene oxide is degraded by neutrophils and the degradation products are non-genotoxic. Nanoscale 2018, 10, 1180–1188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Girish, C.M.; Sasidharan, A.; Gowd, G.S.; Nair, S.; Koyakutty, M. Confocal raman imaging study showing macrophage mediated biodegradation of graphene in vivo. Adv. Healthc. Mater. 2013, 2, 1489–1500. [Google Scholar] [CrossRef] [PubMed]
- Kotchey, G.P.; Allen, B.L.; Vedala, H.; Yanamala, N.; Kapralov, A.A.; Tyurina, Y.Y.; Klein-Seetharaman, J.; Kagan, V.E.; Star, A. The enzymatic oxidation of graphene oxide. ACS Nano 2011, 5, 2098–2108. [Google Scholar] [CrossRef]
- Wright, Z.M.; Arnold, A.M.; Holt, B.D.; Eckhart, K.E.; Sydlik, S.A. Functional Graphenic Materials, Graphene Oxide, and Graphene as Scaffolds for Bone Regeneration. Regen. Eng. Transl. Med. 2019, 5, 190–209. [Google Scholar] [CrossRef]
- Holt, B.D.; Arnold, A.M.; Sydlik, S.A. In It for the Long Haul: The Cytocompatibility of Aged Graphene Oxide and Its Degradation Products. Adv. Healthc. Mater. 2016, 5, 3056–3066. [Google Scholar] [CrossRef]
- International Standard ISO 5833: Implants for Surgery—Acrylic Resin Cements; ISO: Geneva, Switzerland, 2002; pp. 1–22.
- International Organization for Standardization ISO 10993-6:2016: Biological Evaluation of Medical Devices—Part 6: Tests for Local Effects after Implantation; ISO: Geneva, Switzerland, 2016.
- Sharma, R.; Kapusetti, G.; Bhong, S.Y.; Roy, P.; Singh, S.K.; Singh, S.; Balavigneswaran, C.K.; Mahato, K.K.; Ray, B.; Maiti, P.; et al. Osteoconductive Amine-Functionalized Graphene-Poly(methyl methacrylate) Bone Cement Composite with Controlled Exothermic Polymerization. Bioconjug. Chem. 2017, 28, 2254–2265. [Google Scholar] [CrossRef]
- Paz, E.; Forriol, F.; del Real, J.C.; Dunne, N. Graphene oxide versus graphene for optimisation of PMMA bone cement for orthopaedic applications. Mater. Sci. Eng. C 2017, 77, 1003–1011. [Google Scholar] [CrossRef]
- Ormsby, R.W.; Modreanu, M.; Mitchell, C.A.; Dunne, N.J. Carboxyl functionalised MWCNT/polymethyl methacrylate bone cement for orthopaedic applications. J. Biomater. Appl. 2014, 29, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Gonalves, G.; Cruz, S.M.A.; Ramalho, A.; Grácio, J.; Marques, P.A.A.P. Graphene oxide versus functionalized carbon nanotubes as a reinforcing agent in a PMMA/HA bone cement. Nanoscale 2012, 4, 2937–2945. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Chang, S.; Kuo, S.M.; Chen, S.H.U.F.E.N. Evaluation of chitosan/β-tricalcium phosphate microspheres as a constituent to PMMA cement. J. Mater. Sci. Mater. Med. 2005, 16, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Zamora Lagos, S.I.; Murillo Salas, J.; Valencia Zapata, M.E.; Mina Hernandez, J.H.; Valencia, C.H.; Rojo, L.; Grande Tovar, C.D. Influence of the chitosan morphology on the properties of acrylic cements and their biocompatibility. RSC Adv. 2020, 10, 31156–31164. [Google Scholar] [CrossRef]
- Ruiz, S.; Tamayo, J.A.; Ospina, J.D.; Navia Porras, D.P.; Valencia Zapata, M.E.; Mina Hernandez, J.H.; Valencia, C.H.; Zuluaga, F.; Grande Tovar, C.D. Antimicrobial Films Based on Nanocomposites of Chitosan/Poly (vinyl alcohol)/Graphene Oxide for Biomedical Applications. Biomolecules 2019, 9, 109. [Google Scholar] [CrossRef] [Green Version]
- Valencia, C.; Valencia, C.; Zuluaga, F.; Valencia, M.; Mina, J.; Grande-Tovar, C. Synthesis and Application of Scaffolds of Chitosan-Graphene Oxide by the Freeze-Drying Method for Tissue Regeneration. Molecules 2018, 23, 2651. [Google Scholar] [CrossRef] [Green Version]
- Tavakoli, M.; Bakhtiari, S.S.E.; Karbasi, S. Incorporation of chitosan/graphene oxide nanocomposite in to the PMMA bone cement: Physical, mechanical and biological evaluation. Int. J. Biol. Macromol. 2020, 149, 783–793. [Google Scholar] [CrossRef]
- Moon, C.; Seo, D.J.; Song, Y.S.; Jung, W.J. Antibacterial activity of various chitosan forms against Xanthomonas axonopodis pv. glycines. Int. J. Biol. Macromol. 2020, 156, 1600–1605. [Google Scholar] [CrossRef]
- Maleki Dizaj, S.; Mennati, A.; Jafari, S.; Khezri, K.; Adibkia, K. Antimicrobial activity of carbon-based nanoparticles. Adv. Pharm. Bull. 2015, 5, 19–23. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E. Toxicity of graphene and graphene oxide nanowalls against bacteria. ACS Nano 2010, 4, 5731–5736. [Google Scholar] [CrossRef]
- Gurunathan, S.; Han, J.W.; Abdal Dayem, A.; Eppakayala, V.; Kim, J.H. Oxidative stress-mediated antibacterial activity of graphene oxide and reduced graphene oxide in Pseudomonas aeruginosa. Int. J. Nanomed. 2012, 7, 5901–5914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mangadlao, J.D.; Santos, C.M.; Felipe, M.J.L.; de Leon, A.C.C.; Rodrigues, D.F.; Advincula, R.C. On the antibacterial mechanism of graphene oxide (GO) Langmuir–Blodgett films. Chem. Commun. 2015, 51, 2886–2889. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zeng, T.H.; Hofmann, M.; Burcombe, E.; Wei, J.; Jiang, R.; Kong, J.; Chen, Y. Antibacterial activity of graphite, graphite oxide, graphene oxide, and reduced graphene oxide: Membrane and oxidative stress. ACS Nano 2011, 5, 6971–6980. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Peng, H.; Wang, X.; Shao, F.; Yuan, Z.; Han, H. Graphene oxide exhibits broad-spectrum antimicrobial activity against bacterial phytopathogens and fungal conidia by intertwining and membrane perturbation. Nanoscale 2014, 6, 1879–1889. [Google Scholar] [CrossRef]
- Ahmed, S.; Annu; Ali, A.; Sheikh, J. A review on chitosan centred scaffolds and their applications in tissue engineering. Int. J. Biol. Macromol. 2018, 116, 849–862. [Google Scholar] [CrossRef]
- Khandaker, M.; Vaughan, M.B.; Morris, T.L.; White, J.J.; Meng, Z. Effect of additive particles on mechanical, thermal, and cell functioning properties of poly(methyl methacrylate) cement. Int. J. Nanomed. 2014, 9, 2699–2712. [Google Scholar] [CrossRef] [Green Version]
- Richards, R.G. The effect of surface roughness on fibroblast adhesion in vitro. Injury 1996, 27, S/C38–S/C43. [Google Scholar] [CrossRef]
- Wirth, C.; Comte, V.; Lagneau, C.; Exbrayat, P.; Lissac, M.; Jaffrezic-Renault, N.; Ponsonnet, L. Nitinol surface roughness modulates in vitro cell response: A comparison between fibroblasts and osteoblasts. Mater. Sci. Eng. C 2005, 25, 51–60. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, Z.; Ding, N.; Zheng, D. Effect of airborne-particle abrasion of presintered zirconia on surface roughness and bacterial adhesion. J. Prosthet. Dent. 2015, 113, 448–452. [Google Scholar] [CrossRef]
- Rosqvist, E.; Niemelä, E.; Venu, A.P.; Kummala, R.; Ihalainen, P.; Toivakka, M.; Eriksson, J.E.; Peltonen, J. Human dermal fibroblast proliferation controlled by surface roughness of two-component nanostructured latex polymer coatings. Colloids Surf. B Biointerfaces 2019, 174, 136–144. [Google Scholar] [CrossRef]
- Andrukhov, O.; Behm, C.; Blufstein, A.; Wehner, C.; Gahn, J.; Pippenger, B.; Wagner, R.; Rausch-Fan, X. Effect of implant surface material and roughness to the susceptibility of primary gingival fibroblasts to inflammatory stimuli. Dent. Mater. 2020, 36, e194–e205. [Google Scholar] [CrossRef]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign body reaction to biomaterials. Semin. Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef] [Green Version]
- Maiborodin, I.V.; Shevela, A.I.; Morozov, V.V.; Novikova, Y.V.; Matveeva, V.A.; Drovosekov, M.N.; Barannik, M.I. Reaction of the rat tissues to implantation of polyhydroxyalkanoate films and ultrafine fibers. Bull. Exp. Biol. Med. 2013, 154, 379–384. [Google Scholar] [CrossRef]
- Lorenz, J.; Barbeck, M.; Sader, R.A.; Kirkpatrick, C.J.; Russe, P.; Choukroun, J.; Ghanaati, S. Foreign Body Giant Cell-Related Encapsulation of a Synthetic Material Three Years after Augmentation. J. Oral Implantol. 2016, 42, 273–277. [Google Scholar] [CrossRef]

| Formulation | SP | LP | ||||||
|---|---|---|---|---|---|---|---|---|
| PMMA | BaSO4 | BPO | CS | MMA | Comonomers | DMPT | GO | |
| Control ABC | 88 | 10 | 2 | 0 | 95.5 | 2 | 2.5 | 0 |
| ABC 0.3% GO | 88 | 10 | 2 | 0 | 95.2 | 2 | 2.5 | 0.3 |
| ABC 15% CS | 73 | 10 | 2 | 15 | 95.5 | 2 | 2.5 | 0 |
| ABC 0.3GO-15CS | 73 | 10 | 2 | 15 | 95.2 | 2 | 2.5 | 0.3 |
| Formulation | Tmax (°C) | Tset (min) |
|---|---|---|
| Control ABC | 59 ± 3 | 355 ± 30 |
| ABC 0.3% GO | 51 ± 4 | 420 ± 25 |
| ABC 15% CS | 55 ± 4 | 460 ± 20 * |
| ABC 0.3GO-15CS | 46 ± 3 * | 440 ± 10 * |
| Formulation | C (MPa) [31] | B (MPa) | E (MPa) |
|---|---|---|---|
| Control ABC | 93.3 ± 2.2 | 48.7 ± 4.2 | 2593 ± 178 |
| ABC 0.3% GO | 94.0 ± 0.5 | 51.8 ± 0.4 | 2599 ± 154 |
| ABC 15% CS | 62.6 ± 1.1 ** | 32.8 ± 2.9 ** | 2141 ± 212 ** |
| ABC 0.3GO-15CS | 77.2 ± 2.6 ** | 44.6 ± 4.1 | 2379 ± 132 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zapata, M.E.V.; Hernandez, J.H.M.; Grande Tovar, C.D.; Valencia Llano, C.H.; Vázquez-Lasa, B.; San Román, J.; Rojo, L. Osseointegration of Antimicrobial Acrylic Bone Cements Modified with Graphene Oxide and Chitosan. Appl. Sci. 2020, 10, 6528. https://doi.org/10.3390/app10186528
Zapata MEV, Hernandez JHM, Grande Tovar CD, Valencia Llano CH, Vázquez-Lasa B, San Román J, Rojo L. Osseointegration of Antimicrobial Acrylic Bone Cements Modified with Graphene Oxide and Chitosan. Applied Sciences. 2020; 10(18):6528. https://doi.org/10.3390/app10186528
Chicago/Turabian StyleZapata, Mayra Eliana Valencia, José Herminsul Mina Hernandez, Carlos David Grande Tovar, Carlos Humberto Valencia Llano, Blanca Vázquez-Lasa, Julio San Román, and Luis Rojo. 2020. "Osseointegration of Antimicrobial Acrylic Bone Cements Modified with Graphene Oxide and Chitosan" Applied Sciences 10, no. 18: 6528. https://doi.org/10.3390/app10186528








