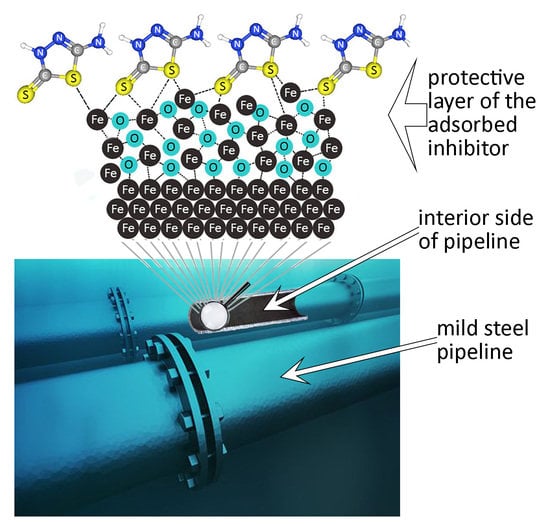
Corrosion Protection Evaluation of Mild Steel: The Role of Hybrid Materials Loaded with Inhibitors
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Substrates
2.3. Synthesis and Loading of Nanocontainers
2.4. Characterization
3. Results and Discussion
3.1. Characterization of Nanocontainers
3.1.1. Transmission Electron Microscopy and Energy Dispersive X-ray Spectroscopy
3.1.2. Thermogravimetric Analysis and Differential Scanning Calorimetry
3.1.3. Fourier Transform Infra-Red Spectroscopy
3.1.4. X-ray Diffraction Analysis
3.2. Corrosion Protection Evaluation
3.2.1. Scanning Electron Microscopy and Energy Dispersive X-ray Spectroscopy
3.2.2. RAMAN Spectroscopy
3.2.3. Electrochemical Studies
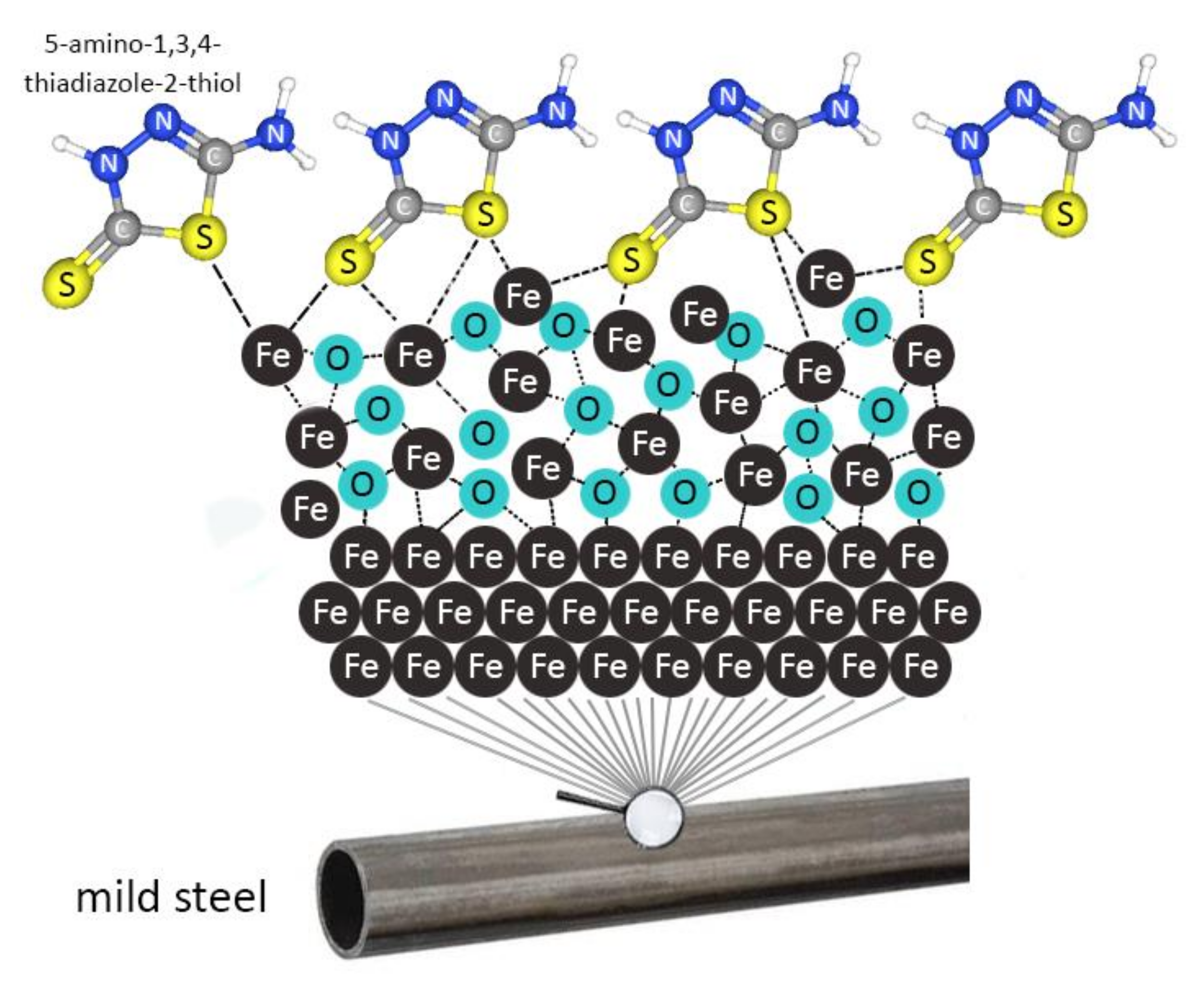
3.2.4. Corrosion Inhibition Mechanism
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Verma, C.; Ebenso, E.E.; Quraishi, M.A. Corrosion inhibitors for ferrous and non-ferrous metals and alloys in ionic sodium chloride solutions: A review. J. Mol. Liq. 2017, 248, 927–942. [Google Scholar] [CrossRef]
- Rouzmeh, S.S.; Naderi, R.; Mahdavian, M. A sulfuric acid surface treatment of mild steel for enhancing the protective properties of an organosilane coating. Prog. Org. Coat. 2017, 103, 156–164. [Google Scholar] [CrossRef]
- Khodair, Z.T.; Khadom, A.A.; Jasim, H.A. Corrosion protection of mild steel in different aqueous media via epoxy/nanomaterial coating: Preparation, characterization and mathematical views. J. Mater. Res. Technol. 2019, 8, 424–435. [Google Scholar] [CrossRef]
- Morozov, Y.; Calado, L.M.; Shakoor, R.A.; Raj, R.; Kahraman, R.; Taryba, M.G.; Montemor, M.F. Epoxy coatings modified with a new cerium phosphate inhibitor for smart corrosion protection of steel. Corros. Sci. 2019, 159, 108128. [Google Scholar] [CrossRef]
- Asami, K.; Waseda, Y.; Suzuki, S. (Eds.) Characterization of Rust Layers on a Plain-Carbon Steel and Weathering Steels Exposed to Industrial and Coastal Atmosphere for Years. In Characterization of Corrosion Products on Steel Surfaces, 1st ed.; Springer-Verlag: Berlin/Heidelberg, Germany, 2006; pp. 159–197. [Google Scholar] [CrossRef]
- Ovid’ko, I.A.; Valiev, R.Z.; Zhu, Y.T. Review on superior strength and enhanced ductility of metallic nanomaterials. Prog. Mater. Sci. 2018, 94, 462–540. [Google Scholar] [CrossRef]
- Prabakaran, M.; Kim, S.-H.; Mugila, N.; Hemapriya, V.; Parameswari, K.; Chitra, S.; Chung, I.-M. Aster koraiensis as nontoxic corrosion inhibitor for mild steel in sulfuric acid. J. Ind. Eng. Chem. 2017, 52, 235–242. [Google Scholar] [CrossRef]
- Fernandes, C.M.; Ferreira Fagundes, T.D.S.; Escarpini dos Santos, N.; de Shewry, M.; Rocha, T.; Garrett, R.; Borges, R.M.; Muricy, G.; Valverde, A.L.; Ponzio, E.A. Ircinia strobilina crude extract as corrosion inhibitor for mild steel in acid medium. Electrochim. Acta 2019, 312, 137–148. [Google Scholar] [CrossRef]
- Gong, W.; Yin, X.; Liu, Y.; Chen, Y.; Yang, W. 2-Amino-4-(4-methoxyphenyl)-thiazole as a novel corrosion inhibitor for mild steel in acidic medium. Prog. Org. Coat. 2019, 126, 150–161. [Google Scholar] [CrossRef]
- Xu, D.; Lou, C.; Huang, J.; Lu, X.; Xin, Z.; Zhou, C. Effect of inhibitor-loaded halloysite nanotubes on active corrosion protection of polybenzoxazine coatings on mild steel. Prog. Org. Coat. 2019, 134, 126–133. [Google Scholar] [CrossRef]
- Alibakhshi, E.; Akbarian, M.; Ramezanzadeh, M.; Ramezanzadeh, B.; Mahdavian, M. Evaluation of the corrosion protection performance of mild steel coated with hybrid sol-gel silane coating in 3.5 wt.% NaCl solution. Prog. Org. Coat. 2018, 123, 190–200. [Google Scholar] [CrossRef]
- Luo, X.; Ci, C.; Li, J.; Lin, K.; Du, S.; Zhang, H.; Li, X.; Cheng, Y.F.; Zang, J.; Liu, Y. 4-aminoazobenzene modified natural glucomannan as a green eco-friendly inhibitor for the mild steel in 0.5 M HCl solution. Corros. Sci. 2019, 151, 132–142. [Google Scholar] [CrossRef]
- Gholamhosseinzadeh, M.R.; Aghaie, H.; Zandi, M.S.; Giahi, M. Rosuvastatin drug as a green and effective inhibitor for corrosion of mild steel in HCl and H2SO4 solutions. J. Mater. Res. Technol. 2019, 8, 5314–5324. [Google Scholar] [CrossRef]
- Habeeb, H.J.; Luaibi, H.M.; Abdullah, T.A.; Dakhil, R.M.; Kadhum, A.A.H.; Al-Amiery, A.A. Case study on thermal impact of novel corrosion inhibitor on mild steel. Case Stud. Therm. Eng. 2018, 12, 64–68. [Google Scholar] [CrossRef]
- Tsoeunyane, M.G.; Makhatha, M.E.; Arotiba, O.A. Corrosion Inhibition of Mild Steel by Poly(butylene succinate)-l-histidine Extended with 1,6-diisocynatohexane Polymer Composite in 1 M HCl. Int. J. Corros. 2019, 2019, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Kartsonakis, I.A.; Stamatogianni, P.; Karaxi, E.K.; Charitidis, C.A. Comparative Study on the Corrosion Inhibitive Effect of 2-Mecraptobenzothiazole and Na2HPO4 on Industrial Conveying API 5L X42 Pipeline Steel. Appl. Sci. 2019, 10, 290. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Song, W.; Ji, J.; Zhu, X.; Sun, Y.; Yang, R.; Zhang, Z. pH-dependent 2-amino-5-mercapto-1,3,4-thiadiazole monolayers at the silver surface: Surface enhanced Raman scattering spectroscopic and electrochemical observations. Appl. Surf. Sci. 2008, 255, 2994–2999. [Google Scholar] [CrossRef]
- Vinothkumar, K.; Sethuraman, M.G. Corrosion inhibition ability of electropolymerised composite film of 2-amino-5-mercapto-1,3,4-thiadiazole/TiO2 deposited over the copper electrode in neutral medium. Mater. Today Commun. 2018, 14, 27–39. [Google Scholar] [CrossRef]
- Ouici, H.; Tourabi, M.; Benali, O.; Selles, C.; Jama, C.; Zarrouk, A.; Bentiss, F. Adsorption and corrosion inhibition properties of 5-amino 1,3,4-thiadiazole-2-thiol on the mild steel in hydrochloric acid medium: Thermodynamic, surface and electrochemical studies. J. Electroanal. Chem. 2017, 803, 125–134. [Google Scholar] [CrossRef]
- Solmaz, R.; Kardaş, G.; Yazıcı, B.; Erbil, M. Adsorption and corrosion inhibitive properties of 2-amino-5-mercapto-1,3,4-thiadiazole on mild steel in hydrochloric acid media. Colloids Surf. A Physicochem. Eng. Asp. 2008, 312, 7–17. [Google Scholar] [CrossRef]
- Kartsonakis, I.A.; Stanciu, S.G.; Matei, A.A.; Hristu, R.; Karantonis, A.; Charitidis, C.A. A comparative study of corrosion inhibitors on hot-dip galvanized steel. Corros. Sci. 2016, 112, 289–307. [Google Scholar] [CrossRef]
- Matsushima, I. Carbon Steel—Atmospheric Corrosion. In R. Winston Revie, E.U.s., Corrosion Handbook; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008; pp. 515–528. [Google Scholar]
- Mekeridis, E.D.; Kartsonakis, I.A.; Pappas, G.S.; Kordas, G.C. Release studies of corrosion inhibitors from cerium titanium oxide nanocontainers. J. Nanopart. Res. 2010, 13, 541–554. [Google Scholar] [CrossRef]
- Karaxi, E.K.; Kartsonakis, I.A.; Charitidis, C.A. Assessment of Self-Healing Epoxy-Based Coatings Containing Microcapsules Applied on Hot Dipped Galvanized Steel. Front. Mater. 2019, 6. [Google Scholar] [CrossRef] [Green Version]
- Kartsonakis, I.A.; Danilidis, I.L.; Pappas, G.S.; Kordas, G.C. Encapsulation and release of corrosion inhibitors into titania nanocontainers. J. Nanosci. Nanotechnol. 2010, 10, 5912–5920. [Google Scholar] [CrossRef]
- Hsu, C.H.; Mansfeld, F. Technical Note:Concerning the Conversion of the Constant Phase Element Parameter Y0into a Capacitance. Corrosion 2001, 57, 747–748. [Google Scholar] [CrossRef]
- Kong, G.; Lingyan, L.; Lu, J.; Che, C.; Zhong, Z. Corrosion behavior of lanthanum-based conversion coating modified with citric acid on hot dip galvanized steel in aerated 1M NaCl solution. Corros. Sci. 2011, 53, 1621–1626. [Google Scholar] [CrossRef]
- Shifler, D.A.; Aylor, D.M. Testing in environments-seawater. In Corrosion Tests and Standards Application and Interpretation; Baboian, R., Ed.; ASTM International: Baltimore, MD, USA, 2005; pp. 362–379. [Google Scholar]
- Cole, K.S.; Cole, R.H. Dispersion and Absorption in Dielectrics II. Direct Current Characteristics. J. Chem. Phys. 1942, 10, 98–105. [Google Scholar] [CrossRef]
- Takeuchi, M.; Martra, G.; Coluccia, S.; Anpo, M. Investigations of the structure of H2O clusters adsorbed on TiO2 surfaces by near-infrared absorption spectroscopy. J. Phys. Chem. B 2005, 109, 7387–7391. [Google Scholar] [CrossRef]
- Kartsonakis, I.A.; Liatsi, P.; Danilidis, I.; Bouzarelou, D.; Kordas, G. Synthesis, characterization and antibacterial action of hollow titania spheres. J. Phys. Chem. Solids 2008, 69, 214–221. [Google Scholar] [CrossRef]
- Nakamoto, K. (Ed.) Infrared and Raman Spectra of Inorganic and Coordination Compounds Part B: Applications in Coordination, Organometallic, and Bioinorganic Chemistry, 6th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009. [Google Scholar]
- Socrates, G. (Ed.) Amines, Imines, and Their Hydrohalides. In Infrared and Raman Characteristic Group Frequencies, Tables and Charts, 2nd ed.; John Wiley & Sons Ltd.: Baffins Lane, Chichester, West Sussex, UK, 2001; pp. 107–114. [Google Scholar]
- Socrates, G. (Ed.) Sulfur and Selinium compounds. In Infrared and Raman Characteristic Group Frequencies, Tables and Charts, 2nd ed.; John Wiley & Sons Ltd: Baffins Lane, Chichester, West Sussex, UK, 2001; pp. 209–228. [Google Scholar]
- Dwivedi, D.; Lepková, K.; Becker, T. Carbon steel corrosion: A review of key surface properties and characterization methods. RSC Adv. 2017, 7, 4580–4610. [Google Scholar] [CrossRef] [Green Version]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurrences and Uses, 2nd ed.; Wiley-VCH Verlag GmbH & Co.; KGaA: Weinheim, Germany, 2003. [Google Scholar]
- Raman, A.; Nasrazadani, S.; Sharma, L. Morphology of rust phases formed on weathering steels in various laboratory corrosion tests. Metallography 1989, 22, 79–96. [Google Scholar] [CrossRef]
- Refait, P.; Génin, J.M.R. The transformation of chloride-containing green rust one into sulphated green rust two by oxidation in mixed Cl− and SO42− aqueous media. Corros. Sci. 1994, 36, 55–65. [Google Scholar] [CrossRef]
- Simard, S.; Odziemkowski, M.; Irish, D.E.; Brossard, L.; Ménard, H. In situ micro-Raman spectroscopy to investigate pitting corrosion product of 1024 mild steel in phosphate and bicarbonate solutions containing chloride and sulfate ions. J. Appl. Electrochem. 2001, 31, 913–920. [Google Scholar] [CrossRef]
- Alcántara, J.; Chico, B.; Simancas, J.; Díaz, I.; de la Fuente, D.; Morcillo, M. An attempt to classify the morphologies presented by different rust phases formed during the exposure of carbon steel to marine atmospheres. Mater. Charact. 2016, 118, 65–78. [Google Scholar] [CrossRef]
- Dhaiveegan, P.; Elangovan, N.; Nishimura, T.; Rajendran, N. Weathering Steel in Industrial-Marine-Urban Environment: Field Study. Mater. Trans. 2016, 57, 148–155. [Google Scholar] [CrossRef] [Green Version]
- Tamilselvi, M.; Kamaraj, P.; Arthanareeswari, M.; Devikala, S.; Selvi, J.A. Development of nano SiO2 incorporated nano zinc phosphate coatings on mild steel. Appl. Surf. Sci. 2015, 332, 12–21. [Google Scholar] [CrossRef]
- Feng, Y.; Chen, S.; Zhang, H.; Li, P.; Wu, L.; Guo, W. Characterization of iron surface modified by 2-mercaptobenzothiazole self-assembled monolayers. Appl. Surf. Sci. 2006, 253, 2812–2819. [Google Scholar] [CrossRef]
- Sandhyarani, N.; Skanth, G.; Berchmans, S.; Yegnaraman, V.V.; Pradeep, T. A Combined Surface-Enhanced Raman-X-Ray Photoelectron Spectroscopic Study of 2-mercaptobenzothiazole Monolayers on Polycrystalline Au and Ag Films. J. Colloid Interface Sci. 1999, 209, 154–161. [Google Scholar] [CrossRef] [Green Version]
- Neff, D.; Bellot-Gurlet, L.; Dillmann, P.; Reguer, S.; Legrand, L. Raman imaging of ancient rust scales on archaeological iron artefacts for long-term atmospheric corrosion mechanisms study. J. Raman Spectrosc. 2006, 37, 1228–1237. [Google Scholar] [CrossRef]
- Ohtsuka, T.; Tanaka, S. Monitoring the development of rust layers on weathering steel using in situ Raman spectroscopy under wet-and-dry cyclic conditions. J. Solid State Electrochem. 2015, 19, 3559–3566. [Google Scholar] [CrossRef]
- Thierry, D. In-Situ Raman Spectroscopy Combined with X-Ray Photoelectron Spectroscopy and Nuclear Microanalysis for Studies of Anodic Corrosion Film Formation on Fe-Cr Single Crystals. J. Electrochem. Soc. 1988, 135, 305. [Google Scholar] [CrossRef]
- Ohtsuka, T. Raman Spectra of Passive Films of Iron in Neutral Borate Solution. Mater. Trans. Jim 1996, 37, 67–69. [Google Scholar] [CrossRef] [Green Version]
- Dubois, F.; Mendibide, C.; Pagnier, T.; Perrard, F.; Duret, C. Raman mapping of corrosion products formed onto spring steels during salt spray experiments. A correlation between the scale composition and the corrosion resistance. Corros. Sci. 2008, 50, 3401–3409. [Google Scholar] [CrossRef]
- Demoulin, A.; Trigance, C.; Neff, D.; Foy, E.; Dillmann, P.; L’Hostis, V. The evolution of the corrosion of iron in hydraulic binders analysed from 46- and 260-year-old buildings. Corros. Sci. 2010, 52, 3168–3179. [Google Scholar] [CrossRef]
- De la Fuente, D.; Alcántara, J.; Chico, B.; Díaz, I.; Jiménez, J.A.; Morcillo, M. Characterisation of rust surfaces formed on mild steel exposed to marine atmospheres using XRD and SEM/Micro-Raman techniques. Corros. Sci. 2016, 110, 253–264. [Google Scholar] [CrossRef]
- Camí, G.E.; Chufán, E.E.; Pedregosa, J.C.; Varetti, E.L. Infrared and Raman spectra of 5-amino-1,3,4-thiadiazole-2-sulfonamide (Hats). Experimental data and quantum chemistry calculations. J. Mol. Struct. 2001, 570, 119–127. [Google Scholar] [CrossRef]
- Weber, I.; Böttger, U.; Pavlov, S.G.; Hübers, H.W.; Hiesinger, H.; Jessberger, E.K. Laser alteration on iron sulfides under various environmental conditions. J. Raman Spectrosc. 2017, 48, 1509–1517. [Google Scholar] [CrossRef]
- Chufán, E.E.; Pedregosa, J.C.; Borrás, J. Spectroscopic behaviour of metal–drug complexes. Infrared spectra of Cu(II) complexes with 5-amino-1,3,4-thiadiazole-2-thiol (Hatm). Vib. Spectrosc. 1997, 15, 191–199. [Google Scholar] [CrossRef]
- Sherif, E.-S.M.; Erasmus, R.M.; Comins, J.D. In situ Raman spectroscopy and electrochemical techniques for studying corrosion and corrosion inhibition of iron in sodium chloride solutions. Electrochim. Acta 2010, 55, 3657–3663. [Google Scholar] [CrossRef]
- Shen, S.; Zuo, Y.; Zhao, X. The effects of 8-hydroxyquinoline on corrosion performance of a Mg-rich coating on AZ91D magnesium alloy. Corros. Sci. 2013, 76, 275–283. [Google Scholar] [CrossRef]
- Khan, G.; Basirun, W.J.; Kazi, S.N.; Ahmed, P.; Magaji, L.; Ahmed, S.M.; Khan, G.M.; Rehman, M.A.; Badry, A. Electrochemical investigation on the corrosion inhibition of mild steel by Quinazoline Schiff base compounds in hydrochloric acid solution. J. Colloid Interface Sci. 2017, 502, 134–145. [Google Scholar] [CrossRef]
- Kaesche, H. (Ed.) Rusting of Iron and Steel. In Corrosion of Metals: Physicochemicl Principles and Current Problems, 1st ed.; Springer-Verlag: Berlin/Heidelberg, Germany, 2003; pp. 193–203. [Google Scholar] [CrossRef]
- Génin, J.M.R.; Refait, P.; Olowe, A.A.; Abdelmoula, M.; Fall, I.; Drissi, S.H. Identification of Green Rust Compounds in the Aqueous Corrosion Processes of Steels; the Case of Microbially Induced Corrosion and Use of 78 K CEMS. Hyperfine Interact. 1998, 112, 47–51. [Google Scholar] [CrossRef]
- Jafari, H.; Akbarzade, K.; Danaee, I. Corrosion inhibition of carbon steel immersed in a 1 M HCl solution using benzothiazole derivatives. Arab. J. Chem. 2019, 12, 1387–1394. [Google Scholar] [CrossRef] [Green Version]
- Mahdavian, M.; Ashhari, S. Corrosion inhibition performance of 2-mercaptobenzimidazole and 2-mercaptobenzoxazole compounds for protection of mild steel in hydrochloric acid solution. Electrochim. Acta 2010, 55, 1720–1724. [Google Scholar] [CrossRef]
- Amar, H.; Tounsi, A.; Makayssi, A.; Derja, A.; Benzakour, J.; Outzourhit, A. Corrosion inhibition of Armco iron by 2-mercaptobenzimidazole in sodium chloride 3% media. Corros. Sci. 2007, 49, 2936–2945. [Google Scholar] [CrossRef]
- Gece, G.; Bilgiç, S. Quantum chemical study of some cyclic nitrogen compounds as corrosion inhibitors of steel in NaCl media. Corros. Sci. 2009, 51, 1876–1878. [Google Scholar] [CrossRef]
- Ortega-Luoni, P.; Vera, L.; Astudillo, C.; Guzmán, M.; Ortega-López, P. Synthesis of Metallic Azoderivatives of 2-Amino-5-Mercapto-1,3,4-Thiadiazole. J. Chil. Chem. Soc. 2007, 52, 1120–1122. [Google Scholar] [CrossRef]
- Guzmán, M.; Lara, R.; Vera, L. 5-Amino-1,3,4-Thiadiazole-2-Thiol Corrosion Current Density and Adsorption Thermodynamics on Astm A-890-1b Stainless Steel in A 3.5% NaC1 Solution. J. Chil. Chem. Soc. 2009, 54. [Google Scholar] [CrossRef] [Green Version]









| Composition | C | Mn | S | P | Si | Ni | Cr | Mo | Cu | Al | N | V + Ti + Nb | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (wt.%) | 0.15 | 0.56 | 0.002 | 0.12 | 0.21 | 0.07 | 0.04 | 0.01 | 0.22 | 0.02 | 0.009 | 0.004 | as remainder |
| Element | C | O | Na | Si | S | K | Ti | Ce |
|---|---|---|---|---|---|---|---|---|
| (wt.%) | 24.11 | 2.93 | 0.24 | 0.48 | 4.16 | 0.28 | 60.17 | 7.63 |
| Sample | Spectrum at | wt.% Element Concentration | ||||
|---|---|---|---|---|---|---|
| O | Fe | C | S | N | ||
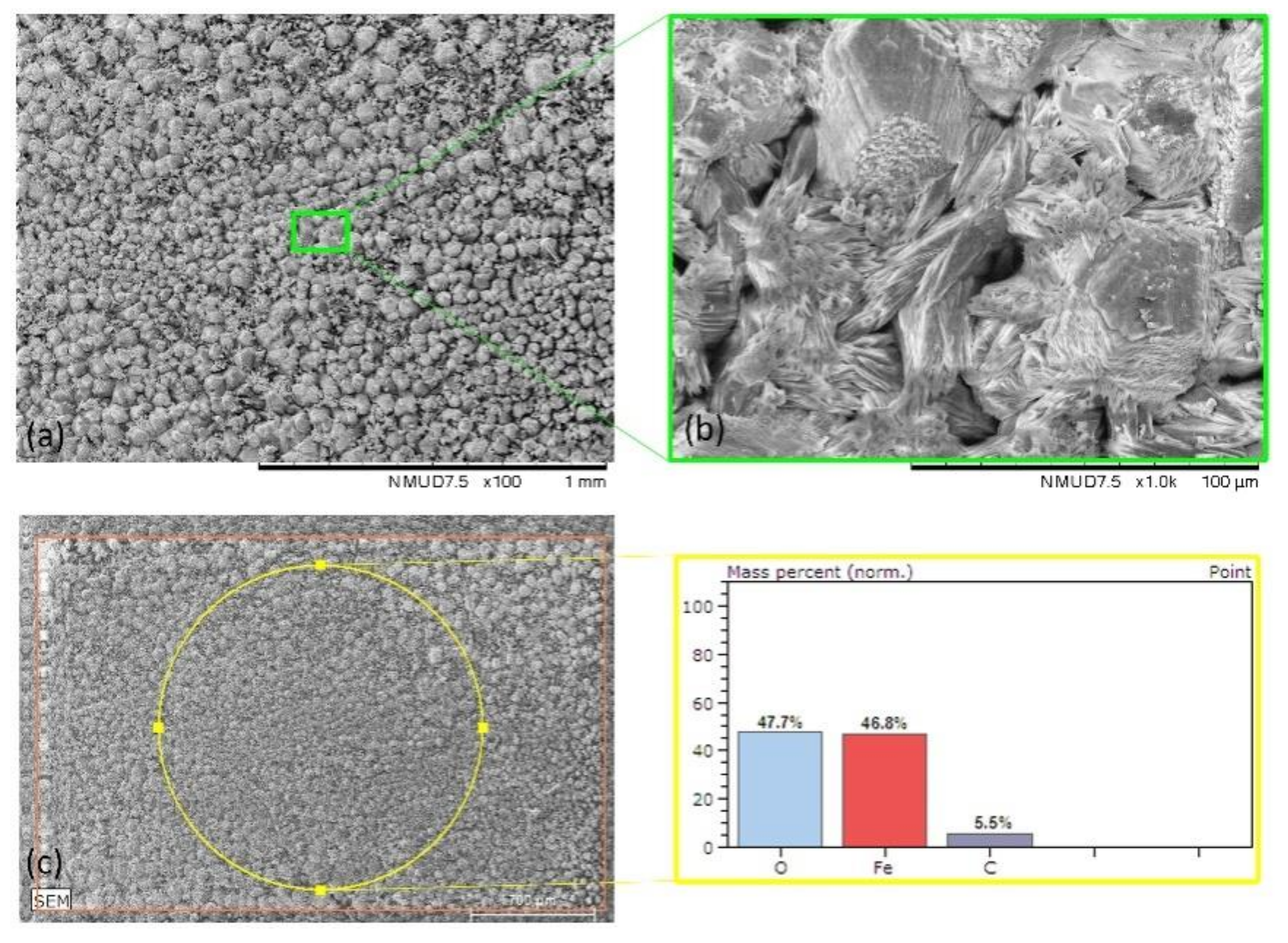
| Mild Steel-blank | Figure 5 | 1.7 | 96.1 | 3.1 | - | - |
| Mild Steel-3.5%NaCl | Figure 6 | 47.7 | 46.8 | 5.5 | - | - |
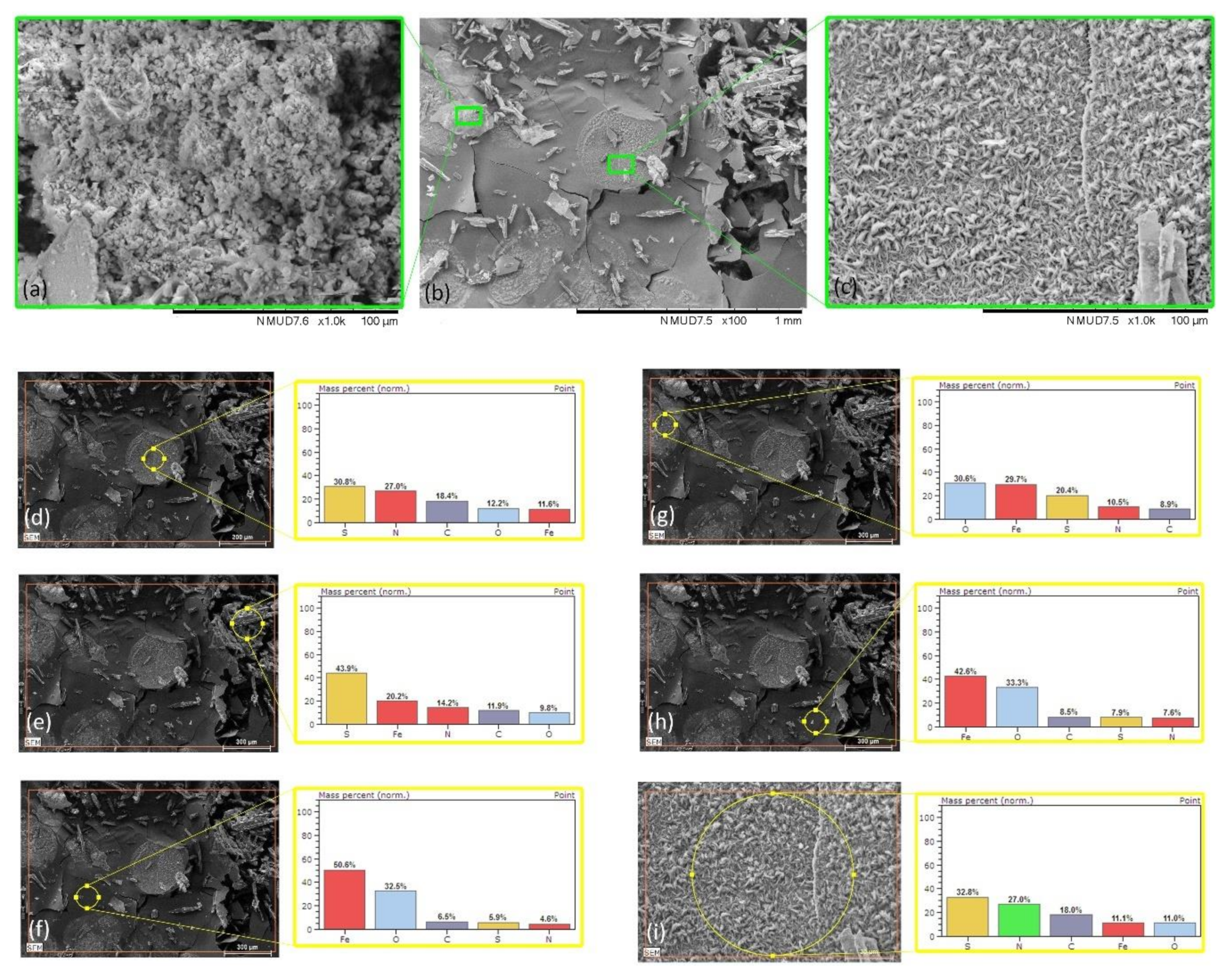
| Mild Steel-3.5%NaCl-ATT | Figure 7d | 12.2 | 11.6 | 18.4 | 30.8 | 27.0 |
| Mild Steel-3.5%NaCl-ATT | Figure 7e | 9.8 | 20.2 | 11.9 | 43.9 | 14.2 |
| Mild Steel-3.5%NaCl-ATT | Figure 7f | 32.5 | 50.6 | 6.5 | 5.9 | 4.6 |
| Mild Steel-3.5%NaCl-ATT | Figure 7g | 30.6 | 29.7 | 8.9 | 20.4 | 10.5 |
| Mild Steel-3.5%NaCl-ATT | Figure 7h | 33.3 | 42.6 | 8.5 | 7.9 | 7.6 |
| Mild Steel-3.5%NaCl-ATT | Figure 7i | 11.0 | 11.1 | 18.0 | 32.8 | 27.0 |
| Main Raman Peaks (cm−1) | Assignment | Samples |
|---|---|---|
| 154 | symmetric stretch of interlayer chloride ions in green rust | Mild Steel-3.5%NaCl |
| 215 | hematite | |
| 273 | goethite | |
| 393 | lepidocrocide | |
| 683 | magnetite | |
| 715 | maghemite | |
| 776 | maghemite | |
| 1362 | ferrihydrite | |
| 1613 | ferrihydrite | |
| 185 | symmetric stretch of interlayer chloride ions in green rust | Mild Steel-3.5%NaCl-ATT |
| 341 | Fe-S due to ATT | |
| 406 | NCS of the ring due to ATT | |
| 469 | Fe-S due to ATT | |
| 788 | N-H out of plane due to ATT | |
| 1037 | N-N due to ATT | |
| 1146 | C-N due to ATT | |
| 1391 | C-N due to ATT |
| 24 h into 3.5 wt.%NaCl | Mild Steel-3.5%NaCl | Mild Steel-3.5%NaCl-ATT |
| CPEinh-T (μF cm−2 s−n) | - | 26.333 |
| CPEinh-P | - | 0.73644 |
| Rinh(kohm cm2) | - | 46.019 |
| Cinh (mF cm−2) | - | 0.33420 |
| CPEdl-T (mF cm−2 s−n) | 12.358 | 0.13117 |
| CPEdl-P | 0.78496 | 0.48632 |
| Rct(kohm cm2) | 0.184 | 82.241 |
| Cdl (mF cm−2) | 15.476 | 1.6175 |
| η% | - | 99.77 |
| 144 h into 3.5 wt.%NaCl | Mild Steel-3.5%NaCl | Mild Steel-3.5%NaCl-ATT |
| CPEinh-T (μF cm−2 s−n) | - | 94.663 |
| CPEinh-P | - | 0.71006 |
| Rinh(kohm cm2) | - | 52.700 |
| Cinh (mF cm−2) | - | 3.0632 |
| CPEdl-T (mF cm−2 s−n) | 10.619 | 0.73004 |
| CPEdl-P | 0.74194 | 0.99998 |
| Rct(kohm cm2) | 0.154 | 14.845 |
| Cdl (mF cm−2) | 12.600 | 0.73007 |
| η% | - | 98.96 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kartsonakis, I.A.; Charitidis, C.A. Corrosion Protection Evaluation of Mild Steel: The Role of Hybrid Materials Loaded with Inhibitors. Appl. Sci. 2020, 10, 6594. https://doi.org/10.3390/app10186594
Kartsonakis IA, Charitidis CA. Corrosion Protection Evaluation of Mild Steel: The Role of Hybrid Materials Loaded with Inhibitors. Applied Sciences. 2020; 10(18):6594. https://doi.org/10.3390/app10186594
Chicago/Turabian StyleKartsonakis, Ioannis A., and Costas A. Charitidis. 2020. "Corrosion Protection Evaluation of Mild Steel: The Role of Hybrid Materials Loaded with Inhibitors" Applied Sciences 10, no. 18: 6594. https://doi.org/10.3390/app10186594
APA StyleKartsonakis, I. A., & Charitidis, C. A. (2020). Corrosion Protection Evaluation of Mild Steel: The Role of Hybrid Materials Loaded with Inhibitors. Applied Sciences, 10(18), 6594. https://doi.org/10.3390/app10186594