Dosimetric Issues Associated with Percutaneous Ablation of Small Liver Lesions with 90Y
Abstract
1. Introduction
2. Materials and Methods
2.1. Percutaneous Ablation with 90Y
2.2. Dosimetry with 90Y
- radiation released from 90Y within a given organ is fully absorbed by that organ. In most cases, this assumption is supported by the average 4 mm 90Y range in tissue.
- permanence of 90Y in the area where they have been delivered (i.e., no migration of the radiopharmaceutical outside the tumor region).
Monte Carlo Calculations
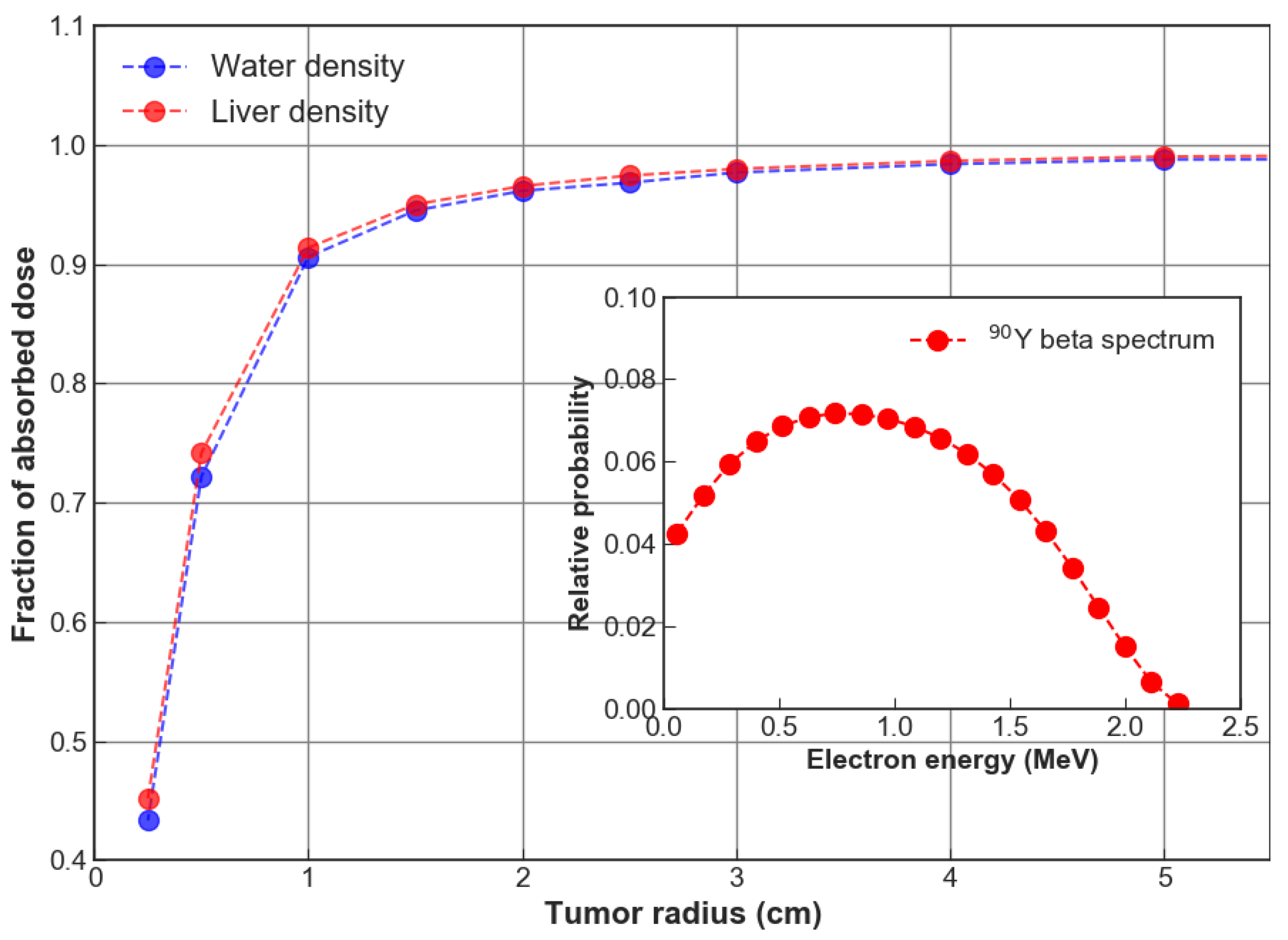
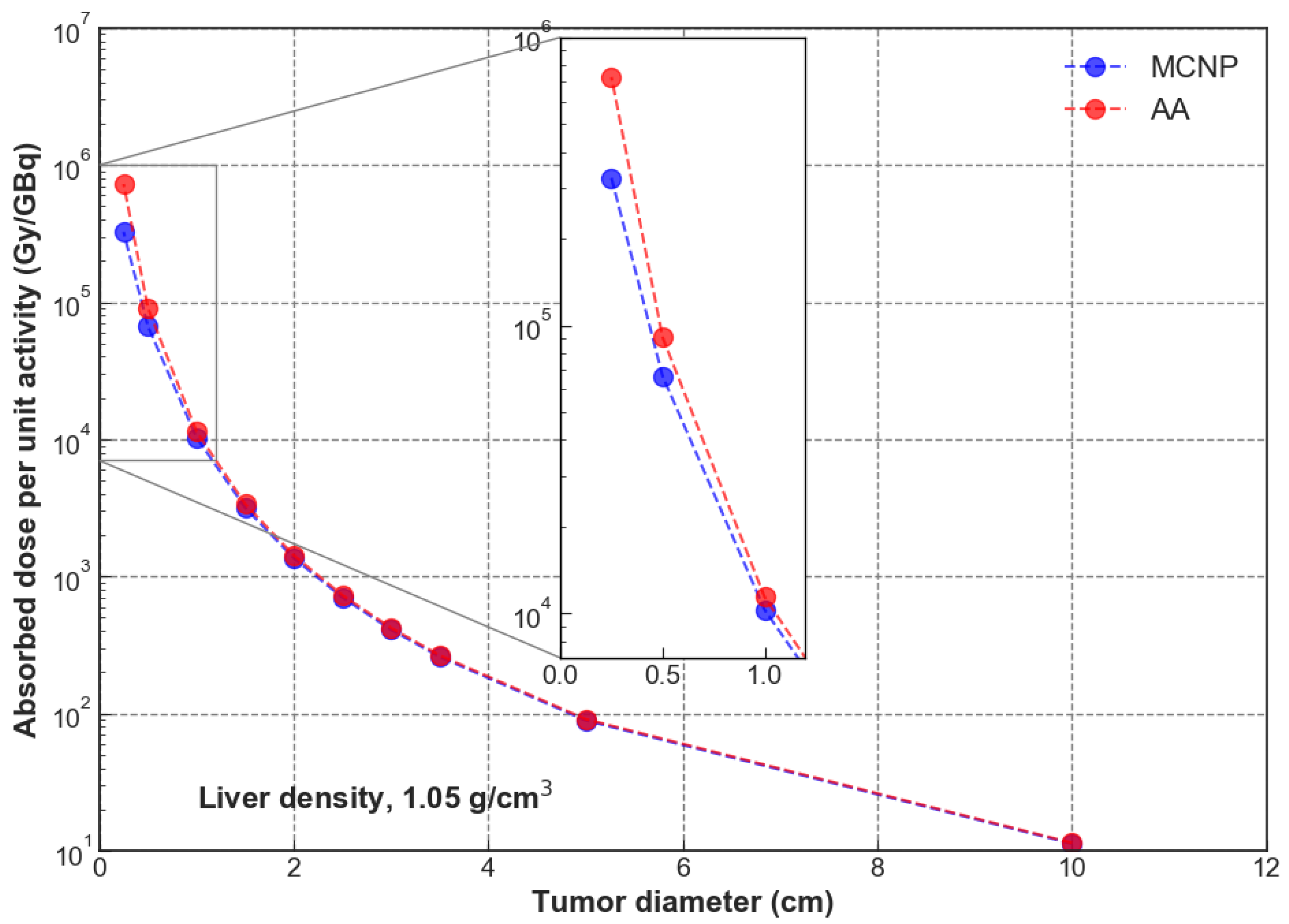
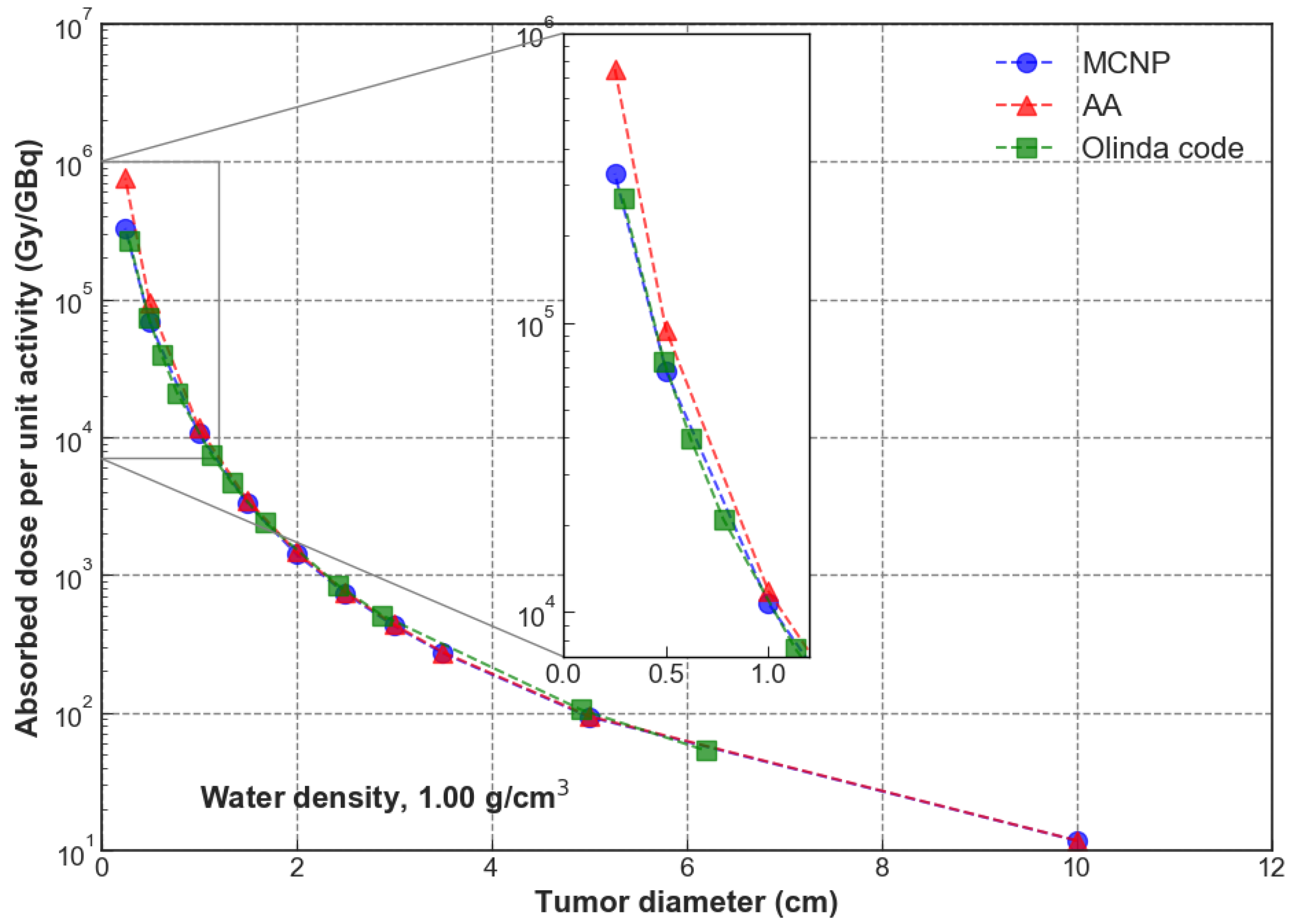
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Villanueva, A. Hepatocellular Carcinoma. N. Engl. J. Med. 2019, 380, 1450–1462. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.; Wu, Y.; Sun, S.; Yen, K.; Kao, C. Treating Hepatocellular Carcinoma With 90Y-Bearing Microspheres: A Review. BioMedicine 2016, 6, 19. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Stein, J.; Bellavia, R.; Broadwell, S. Treatment Options for Unresectable HCC with a Focus on SIRT with Yttrium-90 Resin Microspheres. Int. J. Clin. Pract. 2017, 71, e12972. [Google Scholar] [CrossRef] [PubMed]
- Salem, R.; Hunter, R. Yttrium-90 Microspheres for The Treatment of Hepatocellular Carcinoma: A Review. Int. J. Radiat. Oncol. Biol. Phys. 2006, 66, S83–S88. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M.; Cremonesi, M.; Bartolomei, M.; Bodei, L.; Chinol, M.; Fiorenza, M.; Tosi, G.; Paganelli, G. Dosimetric model for locoregional treatments of brain tumors with 90Y-conjugates: Clinical application with 90Y-DOTATOC. J. Nucl. Med. 2006, 47, 105–112. [Google Scholar] [PubMed]
- Fabbri, C.; Mattone, V.; Casi, M.; De Lauro, F.; Agostini, M.; Bartolini, N.; D’arienzo, M.; Marchi, G.; Bartolomei, M.; Sarti, G. Quantitative evaluation on [90Y] DOTATOC PET and SPECT imaging by phantom acquisitions and clinical applications in locoregional and systemic treatments. Q. J. Nucl. Med. Mol. Imaging 2012, 56, 522–528. [Google Scholar] [PubMed]
- Fabbri, C.; Mattone, V.; Sarti, G.; Casi, M.; De Lauro, F.; Agostini, M.; Bartolini, N.; Bartolomei, M. 90Y-based PET and SPECT/CT imaging in locoregional brain treatment for high-grade gliomas: Retrospective fusion with MRI. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 1822–1823. [Google Scholar] [CrossRef]
- Beta Glue – a loco-regional radio-therapy platform for tumors. Available online: https://www.betaglue.com/ (accessed on 1 May 2019).
- Arazi, L.; Cooks, T.; Schmidt, M.; Keisari, Y.; Kelson, I. Treatment of solid tumors by interstitial release of recoiling short-lived alpha emitters. Phys. Med. Biol. 2007, 52, 5025–5042. [Google Scholar] [CrossRef] [PubMed]
- Bult, W.; Kroeze, S.; Elschot, M.; Seevinck, P.; Beekman, F.; de Jong, H.; Uges, D.; Kosterink, J.; Luijten, P.; Hennink, W.; et al. Intratumoral Administration of Holmium-166 Acetylacetonate Microspheres: Antitumor Efficacy and Feasibility of Multimodality Imaging in Renal Cancer. PLoS ONE 2013, 8, e52178. [Google Scholar] [CrossRef] [PubMed]
- Siegel, J.A.; Stabin, M.G. Absorbed fractions for electrons and beta particles in spheres of various sizes. J. Nucl. Med. 1994, 35, 152–156. [Google Scholar] [PubMed]
- Bardies, M.; Chatal, J. Absorbed doses for internal radiotherapy from 22 beta-emitting radionuclides: Beta dosimetry of small spheres. Phys. Med. Biol. 1994, 39, 961–981. [Google Scholar] [CrossRef] [PubMed]
- Stabin, M.G.; Konijnenberg, M.W. Re-evaluation of absorbed fractions for photons and electrons in spheres of various sizes. J. Nucl. Med. 2000, 41, 149–160. [Google Scholar] [PubMed]
- Amato, E.; Lizio, D.; Baldari, S. Absorbed fractions for photons in ellipsoidal volumes. Phys. Med. Biol. 2009, 56, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Chao, H.; Torchiana, D. BioGlue: Albumin/Glutaraldehyde Sealant in Cardiac Surgery. J. Card. Surg. 2003, 18, 500–503. [Google Scholar] [CrossRef] [PubMed]
- Bé, M.-M.; Chisté, V.; Dulieu, C.; Browne, E.; Baglin, C.; Chechev, V.; Kuzmenko, N.; Helmer, R.; Kondev, F.; MacMahon, D.; et al. Table of Radionuclides. Monographie BIPM-5, 2006; 3 Bureau International des Poids et Mesures, Pavillon de Breteuil, F-92310 Sèvres, France. Available online: http://www.bipm.org/utils/common/pdf/monographieRI/Monographie_BIPM-5_Tables_Vol3.pdf (accessed on 1 May 2019).
- McKinney, J.; Pasciak, A.; Bradley, Y. Handbook of Radioembolization, 1st ed.; CRC Press-Taylor &Francis Group: Boca Raton, FL, USA, 2017. [Google Scholar]
- Dezarn, W.; Cessna, J.; DeWerd, L.; Feng, W.; Gates, V.; Halama, J.; Kennedy, A.; Nag, S.; Sarfaraz, M.; Sehgal, V.; et al. Recommendations of the American Association of Physicists in Medicine on dosimetry, imaging, and quality assurance procedures for 90Y microsphere brachytherapy in the treatment of hepatic malignancies. Med. Phys. 2011, 38, 4824–4845. [Google Scholar] [CrossRef]
- Dieudonné, A.; Hobbs, R.; Sanchez-Garcia, M.; Lebtahi, R. Absorbed-dose calculation for treatment of liver neoplasms with 90Y-microspheres. Clin. Transl. Imaging 2016, 4, 273–282. [Google Scholar] [CrossRef]
- Ho, S.; Lau, W.; Leung, T.; Chan, M.; Ngar, Y.; Johnson, P.; Li, A. Partition model for estimating radiation doses from yttrium-90 microspheres in treating hepatic tumours. Eur. J. Nucl. Med. 1996, 23, 947–952. [Google Scholar] [CrossRef]
- Ho, S.; Lau, W.; Leung, T.; Chan, M.; Johnson, P.; Li, A. Clinical evaluation of the partition model for estimating radiation doses from yttrium-90 microspheres in the treatment of hepatic cancer. Eur. J. Nucl. Med. 1997, 24, 293–298. [Google Scholar]
- Gulec, S.A.; Mesoloras, G.; Stabin, M. Dosimetric techniques in 90Y-microsphere therapy of liver cancer: The MIRD equations for dose calculations. J. Nucl. Med. 2006, 47, 1209–1211. [Google Scholar]
- Briesmeister, J.F. MCNP-A General Monte Carlo N-Particle Transport Code Version 4C; Los Alamos National Laboratory: Los Alamos, NM, USA, 2000. [Google Scholar]
- Storm, L.; Israel, H. Photon Cross Sections From 1 Kev To 100 Mev For Elements Z = 1 To Z = 100. At. Data Nucl. Data Tables 1970, 7, 565–681. [Google Scholar] [CrossRef]
- Hubbell, J.; Veigele, W.; Briggs, E.; Brown, R.; Cromer, D.; Howerton, R. Atomic Form Factors, Incoherent Scattering Functions, And Photon Scattering Cross Sections. J. Phys. Chem. Ref. Data 1975, 4, 471–538. [Google Scholar] [CrossRef]
- Stabin, M.G.; Sparks, R.B.; Crowe, E. OLINDA/EXM: The second-generation personal computer software for internal dose assessment in nuclear medicine. J. Nucl. Med. 2005, 46, 1023–1027. [Google Scholar] [PubMed]
- Kennedy, A.; Nutting, C.; Coldwell, D.; Gaiser, J.; Drachenberg, C. Pathologic response and microdosimetry of 90Y microspheres in man: Review of four explanted whole livers. Int. J. Radiat. Oncol. Biol. Phys. 2004, 60, 1552–1563. [Google Scholar] [CrossRef] [PubMed]
- Strigari, L.; Sciuto, R.; Rea, S.; Carpanese, L.; Pizzi, G.; Soriani, A.; Iaccarino, G.; Benassi, M.; Ettorre, G.; Maini, C. Efficacy and toxicity related to treatment of hepatocellular carcinoma with 90Y-SIR spheres: Radiobiologic considerations. J. Nucl. Med. 2010, 51, 1377–1385. [Google Scholar] [CrossRef] [PubMed]
- Ariel, I. Cure of an embryonal rhabdomyosarcoma of the nose of an infant by interstitial 90Yttrium microspheres: A case report. Int. J. Nucl. Med. Biol. 1978, 5, 37–41. [Google Scholar] [CrossRef]
- Tian, J.H.; Xu, B.X.; Zhang, J.M.; Dong, B.W.; Liang, P.; Wang, X.D. Ultrasound-guided internal radiotherapy using yttrium-90-glass microspheres for liver malignancies. J. Nucl. Med. 1996, 37, 958–963. [Google Scholar] [PubMed]
- Bakker, R.; Lam, M.; van Nimwegen, S.; Rosenberg, A.; van Es, R.; Nijsen, J. Intratumoral treatment with radioactive beta-emitting microparticles: A systematic review. J. Radiat. Oncol. 2017, 6, 323–341. [Google Scholar] [CrossRef] [PubMed]

| Parameter | ValueW | urelW/% | ValueL | urelL/% |
|---|---|---|---|---|
| −11.883 | 1.10 | −12.999 | 1.00 | |
| A | 4.416 | 1.15 | 4.654 | 1.10 |
| a | 2.190 | 0.90 | 1.909 | 1.00 |
| B | 56.820 | 0.05 | 57.701 | 0.05 |
| b | 0.290 | 0.15 | 0.271 | 0.10 |
| Lesion Diameter | Mass (kg) | Dose/Particle (Gy/p) | MCNP (Gy/GBq) | AA (Gy/GBq) | |
|---|---|---|---|---|---|
| 20 cm | 4.40 | 3.38 | −1.8% | ||
| 10 cm | −2.1% | ||||
| 8.0 cm | −2.5% | ||||
| 6.0 cm | −3.1% | ||||
| 5.0 cm | −3.7% | ||||
| 4.0 cm | −4.2% | ||||
| 3.0 cm | −6.2% | ||||
| 2.0 cm | −9.6% | ||||
| 1.0 cm | −26.7% | ||||
| 0.5 cm | −55.4% |
| Lesion Diameter | Mass (kg) | Dose/Particle (Gy/p) | MCNP (Gy/GBq) | AA (Gy/GBq) | |
|---|---|---|---|---|---|
| 20 cm | 4.19 | 3.54 | 1.18 | 0.0% | |
| 10 cm | 5.23 | 2.81 | 9.33 | 9.44 | −1.2% |
| 8.0 cm | 2.68 | 5.47 | 1.81 | 1.84 | −1.5% |
| 6.0 cm | 1.13 | 1.29 | 4.27 | 4.37 | −2.3% |
| 5.0 cm | 6.54 | 2.20 | 7.31 | 7.55 | −3.2% |
| 4.0 cm | 3.35 | 4.27 | 1.42 | 1.47 | −3.4% |
| 3.0 cm | 1.41 | 9.95 | 3.30 | 3.49 | −5.4% |
| 2.0 cm | 4.19 | 3.22 | 1.07 | 1.18 | −9.3% |
| 1.0 cm | 5.23 | 2.05 | 6.82 | 9.44 | −27.8% |
| 0.5 cm | 6.54 | 9.86 | 3.27 | 7.55 | −56.7% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Arienzo, M.; Sarnelli, A.; Mezzenga, E.; Chiacchiararelli, L.; Amato, A.; Romanelli, M.; Cianni, R.; Cremonesi, M.; Paganelli, G. Dosimetric Issues Associated with Percutaneous Ablation of Small Liver Lesions with 90Y. Appl. Sci. 2020, 10, 6605. https://doi.org/10.3390/app10186605
D’Arienzo M, Sarnelli A, Mezzenga E, Chiacchiararelli L, Amato A, Romanelli M, Cianni R, Cremonesi M, Paganelli G. Dosimetric Issues Associated with Percutaneous Ablation of Small Liver Lesions with 90Y. Applied Sciences. 2020; 10(18):6605. https://doi.org/10.3390/app10186605
Chicago/Turabian StyleD’Arienzo, Marco, Anna Sarnelli, Emilio Mezzenga, Laura Chiacchiararelli, Antonino Amato, Massimo Romanelli, Roberto Cianni, Marta Cremonesi, and Giovanni Paganelli. 2020. "Dosimetric Issues Associated with Percutaneous Ablation of Small Liver Lesions with 90Y" Applied Sciences 10, no. 18: 6605. https://doi.org/10.3390/app10186605
APA StyleD’Arienzo, M., Sarnelli, A., Mezzenga, E., Chiacchiararelli, L., Amato, A., Romanelli, M., Cianni, R., Cremonesi, M., & Paganelli, G. (2020). Dosimetric Issues Associated with Percutaneous Ablation of Small Liver Lesions with 90Y. Applied Sciences, 10(18), 6605. https://doi.org/10.3390/app10186605





