Electrochemical Biosensors Based on Conducting Polymers: A Review
Abstract
1. Introduction
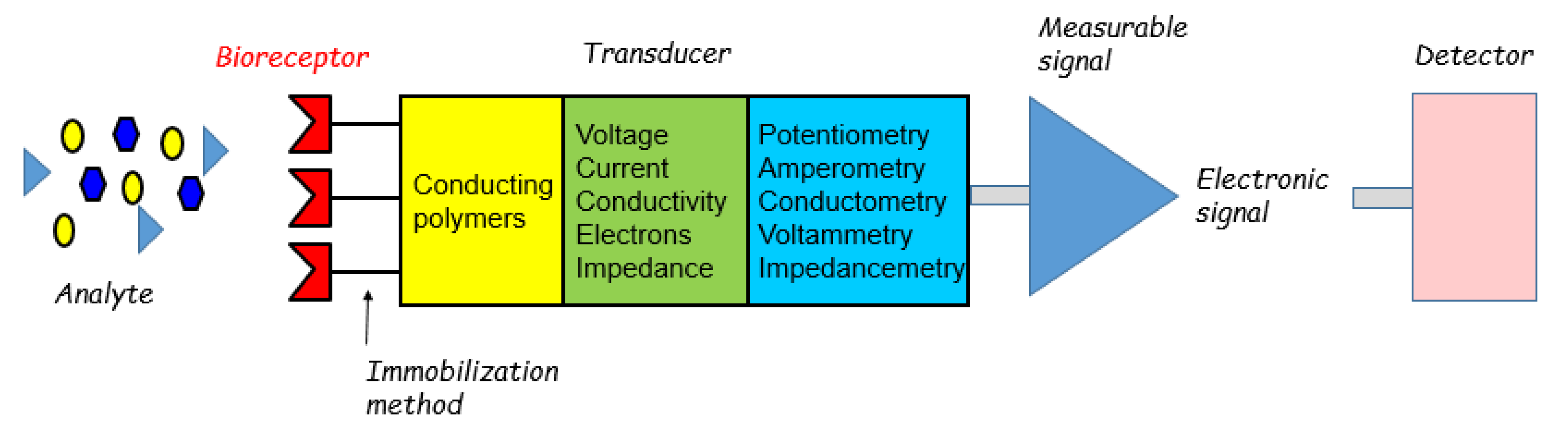
2. Preparation of Sensitive Materials
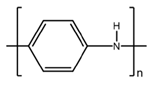
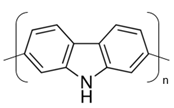
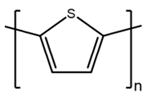
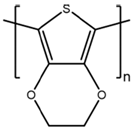
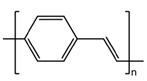
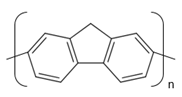
2.1. Preparation of Conducting Polymers
2.2. Strategies for Immobilizing Biological Sensing Elements into Conducting Polymers
3. Electroanalytical Methods
3.1. Potentiometry
3.2. Amperometry
3.3. Conductometry
3.4. Voltammetry
3.5. Impedancemetry
4. Conducting Polymer-Based Electrochemical Biosensors
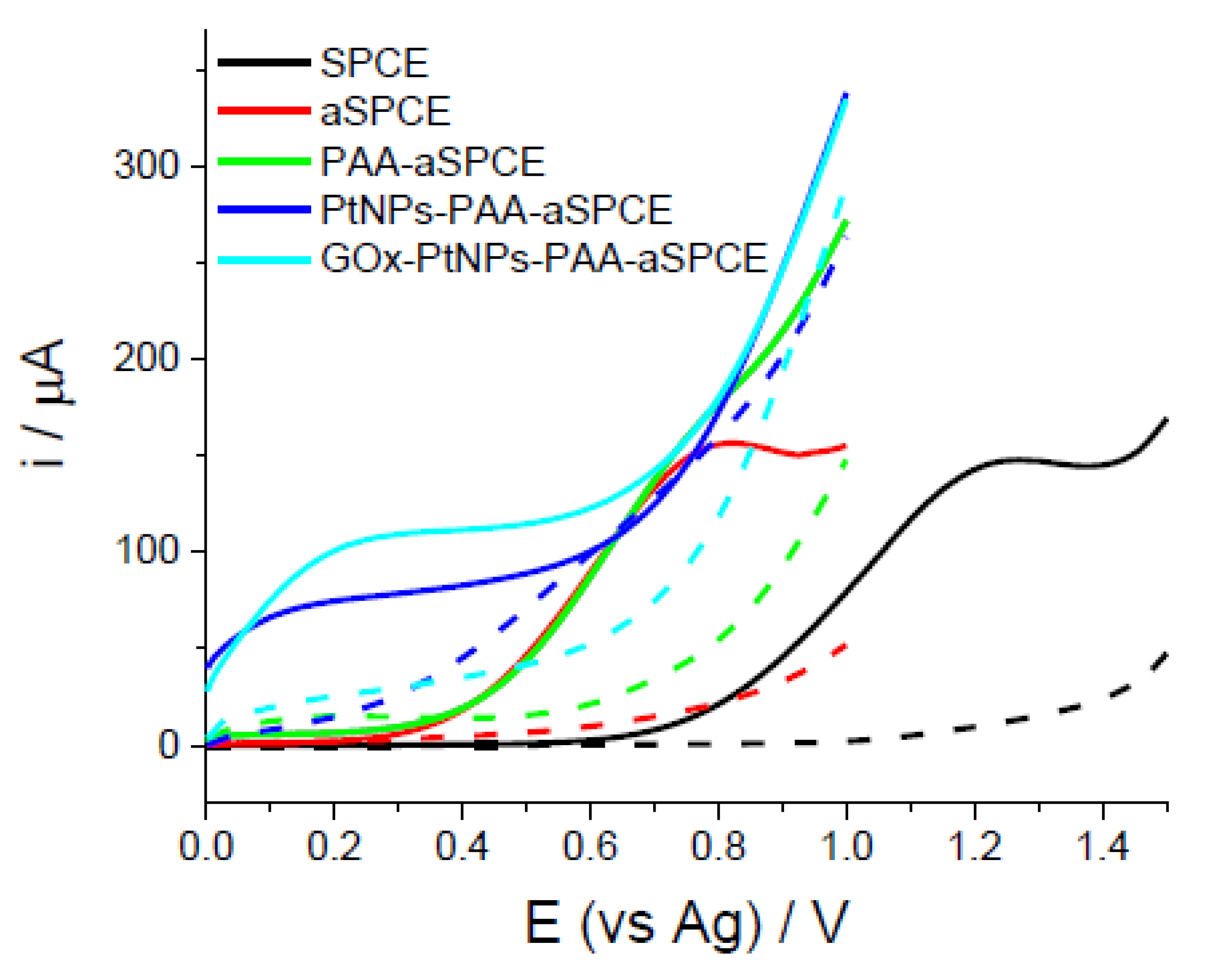
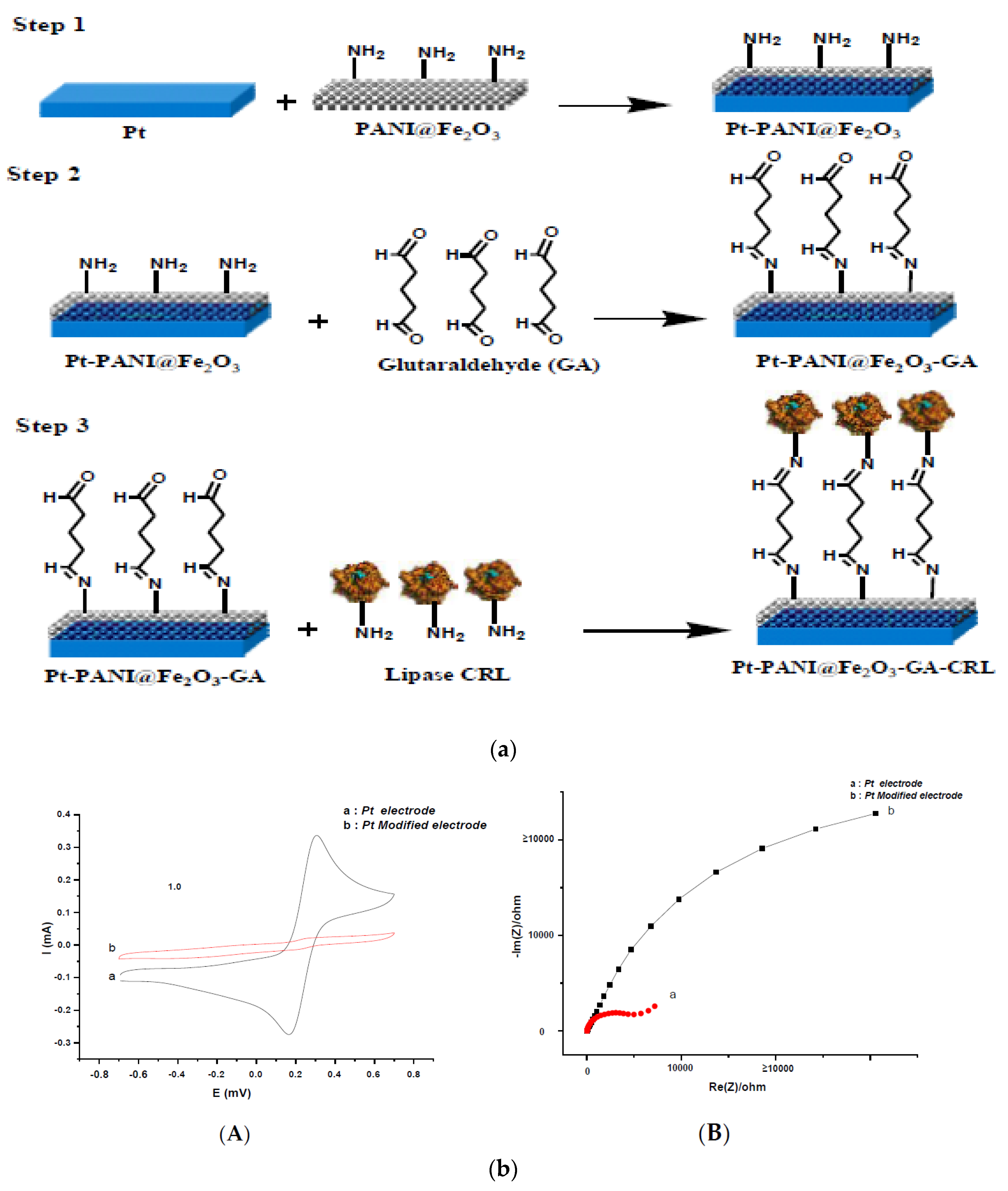
4.1. Conducting Polymer-Based Enzyme Biosensors
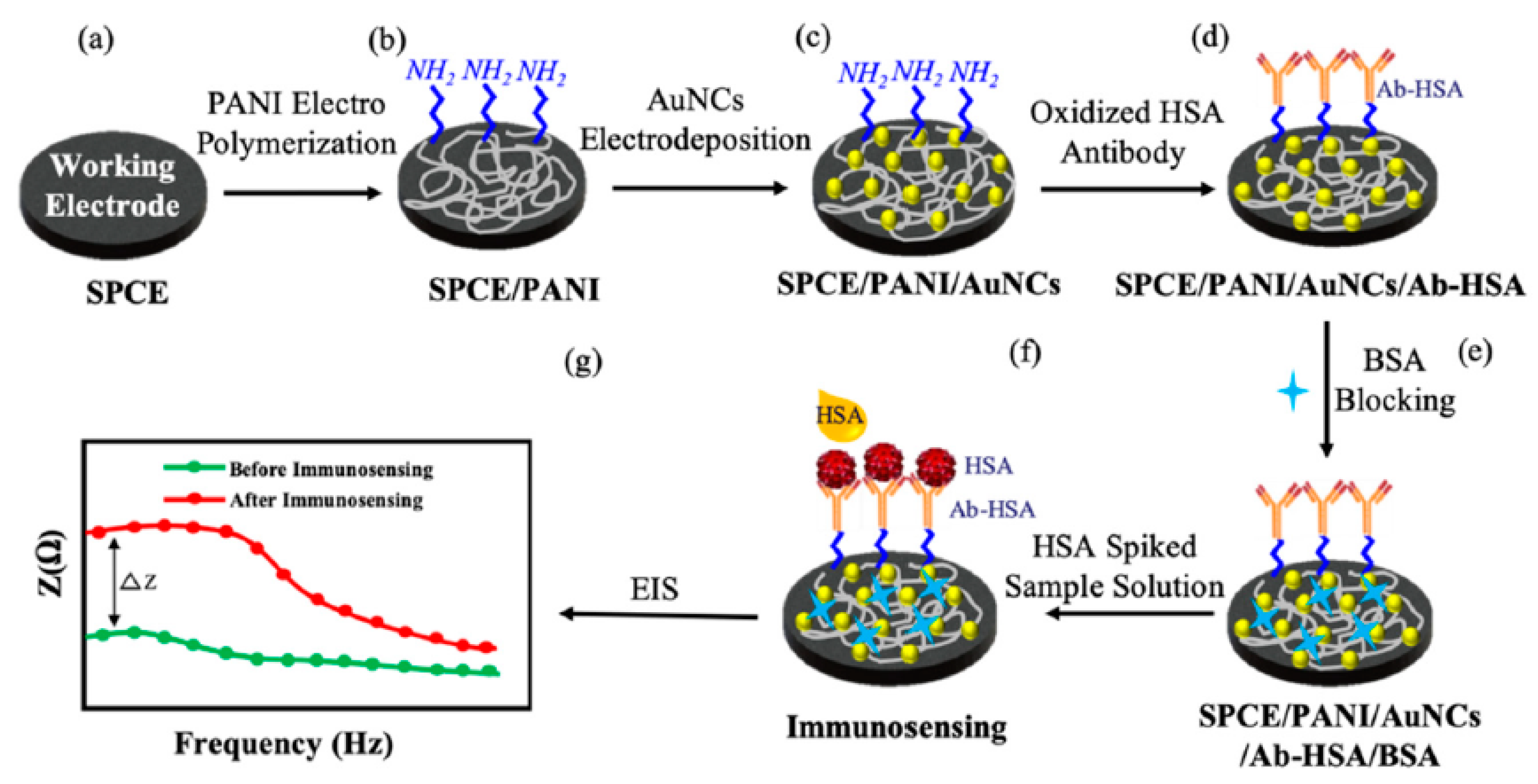
4.2. Conducting Polymer-Based Immunosensors
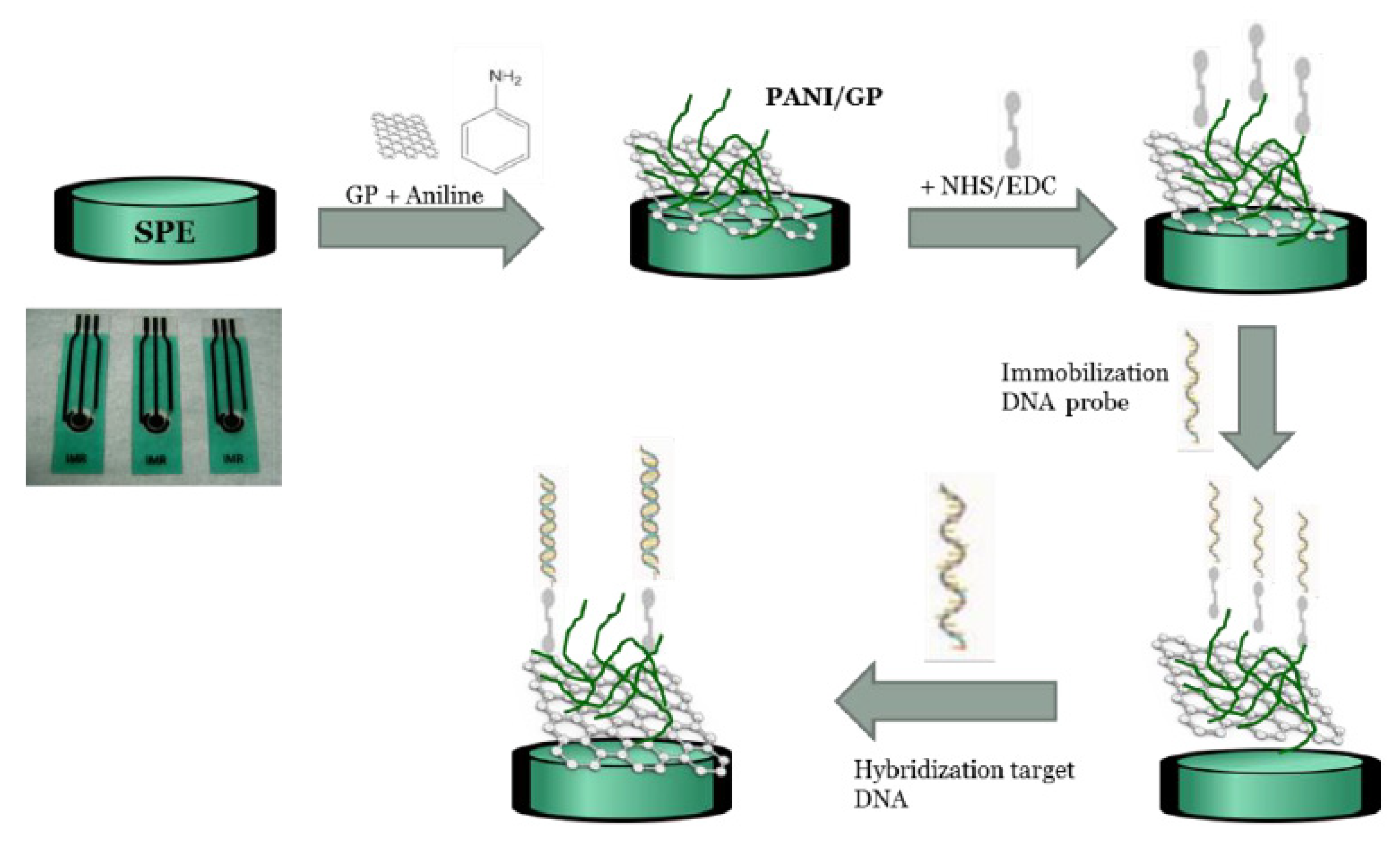
4.3. Conducting Polymer-Based DNA Biosensors
4.4. Conducting Polymer-Based Whole Cell Biosensors
4.5. Biosensors Based on Molecularly Imprinted Polymers
5. Conclusions
Funding
Conflicts of Interest
References
- Shirakawa, H.; Louis, E.J.; MacDiarmid, A.G.; Chiang, C.K.; Heeger, A.J. Synthesis of electrically conducting organic polymers: Halogen derivatives of polyacetylene, (CH)x. J. Chem. Soc. Chem. Commun. 1977, 578–580. [Google Scholar] [CrossRef]
- Diaz, A. Electrochemical preparation and characterisation of conducting polymers. Chem. Scr. 1981, 17, 145–148. [Google Scholar]
- Diaz, A.F.; Kanazawa, K.K. Electrochemical polymerisation of pyrrole. J. Chem. Soc. Chem. Commun. 1979, 635. [Google Scholar] [CrossRef]
- Kanazawa, K.K.; Diaz, A.F.; Geiss, R.H.; Gill, W.D.; Kwak, J.F.; Logan, J.A. ‘Organic metals’: Polypyrrole a stable synthetic ‘metallic’ polymer. J. Chem. Soc. Chem. Commun. 1979, 854. [Google Scholar] [CrossRef]
- Le, T.H.; Kim, Y.; Yoon, H. Electrical and Electrochemical Properties of Conducting Polymers. Polymers 2017, 9, 150. [Google Scholar] [CrossRef]
- Tsukamoto, J. Recent advances in highly conductive polyacetylene. Adv. Phys. 1992, 41, 509–546. [Google Scholar] [CrossRef]
- Tsukamoto, J.; Takahashi, A.; Kawasaki, K. Structure and electrical properties of polyacetylene yielding a conductivity of 105 S/cm. Jpn. J. Appl. Phys. 1990, 29, 125. [Google Scholar] [CrossRef]
- Patois, T.; Lakard, B.; Martin, N.; Fievet, P. Effect of various parameters on the conductivity of free standing electrosynthesized polypyrrole films. Synth. Met. 2010, 160, 2180–2185. [Google Scholar] [CrossRef]
- Zhang, Y.; de Boer, B.; Blom, P.W.M. Controllable molecular doping and charge transport in solution-processed polymer semiconducting layers. Adv. Funct. Mater. 2009, 19, 1901–1905. [Google Scholar] [CrossRef]
- Guimard, N.K.; Gomez, N.; Schmidt, C.E. Conducting polymers in biomedical engineering. Prog. Polym. Sci. 2007, 32, 876–921. [Google Scholar] [CrossRef]
- Ahlskog, M.; Reghu, M.; Heeger, A.J. The temperature dependence of the conductivity in the critical regime of the metal-insulator transition in conducting polymers. J. Phys. Condens. Matter 1997, 9, 4145–4156. [Google Scholar] [CrossRef]
- Aleshin, A.; Kiebooms, R.; Menon, R.; Wudl, F.; Heeger, A.J. Metallic conductivity at low temperatures in poly (3,4-ethylenedioxythiophene) doped with PF6. Phys. Rev. B 1997, 56, 3659–3663. [Google Scholar] [CrossRef]
- Lee, K.; Cho, S.; Heum Park, S.; Heeger, A.J.; Lee, C.W.; Lee, S.H. Metallic transport in polyaniline. Nature 2006, 441, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Wataru, T.; Shyam, S.P.; Masaki, F.; Keiichi, K. Cyclic step-voltammetric analysis of cation-driven and anion-driven actuation in polypyrrole films. Jpn. J. Appl. Phys. 2002, 41, 7532–7536. [Google Scholar]
- Paul, E.W.; Ricco, A.J.; Wrighton, M.S. Resistance of polyaniline films as a function of electrochemical potential and the fabrication of polyaniline-based microelectronic devices. J. Phys. Chem. 1985, 89, 1441–1447. [Google Scholar] [CrossRef]
- Saxena, V.; Malhotra, B.D.; Menon, R. Charge transport and electrical properties of doped conjugated polymers. In Handbook of Polymers in Electronics; Malhotra, B.D., Ed.; Rapra Technology Limited: Shrewsbury, Shropshire, UK, 2002; pp. 3–65. [Google Scholar]
- Wan, M. Conducting Polymers with Micro or Nanometer Structure; Springer: New York, NY, USA, 2008; pp. 1–13. [Google Scholar]
- Li, Y. Conducting polymer. In Organic Optoelectronic Materials; Li, Y., Ed.; Springer International Publishing: New York, NY, USA, 2015; pp. 23–50. [Google Scholar]
- Bredas, J.L.; Chance, R.R.; Silbey, R. Theoretical Studies of Charged Defect States in Doped Polyacetylene and Polyparaphenylene. Mol. Cryst. Liq. Cryst. 1981, 77, 319–332. [Google Scholar] [CrossRef]
- Scrosati, B. Electrochemical Properties of Conducting Polymers. Prog. Solid State Chem. 1988, 18, 1–77. [Google Scholar] [CrossRef]
- Beaujuge, P.M.; Reynolds, J.R. Color Control in π—Conjugated Organic Polymers for Use in Electrochromic Devices. Chem. Rev. 2010, 110, 268–320. [Google Scholar] [CrossRef]
- Moliton, A.; Hiorns, R.C. Review of Electronic and Optical Properties of Semiconducting π-Conjugated Polymers: Applications in Optoelectronics. Polym. Int. 2004, 53, 1397–1412. [Google Scholar] [CrossRef]
- Bharti, M.; Singh, A.; Samanta, S.; Aswal, D.K. Conductive Polymers for Thermoelectric Power Generation. Prog. Mater. Sci. 2018, 93, 270–310. [Google Scholar] [CrossRef]
- Fan, Z.; Ouyang, J. Thermoelectric Properties of PEDOT: PSS. Adv. Electron. Mater. 2019, 5, 1800769. [Google Scholar] [CrossRef]
- Guerfi, A.; Trottier, J.; Boyano, I.; De Meatza, I.; Blazquez, J.; Brewer, S.; Ryder, K.; Vijh, A.; Zaghib, K. High cycling stability of zinc-anode/conducting polymer rechargeable battery with non-aqueous electrolyte. J. Power Sources 2014, 248, 1099–1104. [Google Scholar] [CrossRef]
- Katz, H.E.; Searson, P.C.; Poehler, T.O. Batteries and Charge Storage Devices Based on Electronically Conducting Polymers. J. Mater. Res. 2010, 25, 1561–1574. [Google Scholar] [CrossRef]
- Ehsani, A.; Shiri, H.M.; Kowsari, E.; Safari, R.; Torabian, J.; Hajghani, S. High performance electrochemical pseudocapacitors from ionic liquid assisted electrochemically synthesized p-type conductive polymer. J. Colloid Interface Sci. 2017, 490, 91–96. [Google Scholar] [CrossRef]
- Kao, P.; Best, A.S. Conducting-polymer-based supercapacitor devices and electrodes. J. Power Sources 2011, 196, 1–12. [Google Scholar]
- Lee, J.; Kang, H.; Kee, S.; Lee, S.H.; Jeong, S.Y.; Kim, G.; Kim, J.; Hong, S.; Back, H.; Lee, K. Long-Term Stable Recombination Layer for Tandem Polymer Solar Cells Using Self-Doped Conducting Polymers. ACS Appl. Mater. Interfaces 2016, 8, 6144–6151. [Google Scholar] [CrossRef] [PubMed]
- Mengistie, D.A.; Ibrahem, M.A.; Wang, P.C.; Chu, C.W. Highly conductive PEDOT: PSS treated with formic acid for ITO-free polymer solar cells. ACS Appl. Mater. Interfaces 2014, 6, 2292–2299. [Google Scholar] [CrossRef]
- Zhang, J.; Hao, Y.; Yang, L.; Mohammadi, H.; Vlachopoulos, N.; Sun, L.; Hagfeldt, A.; Sheibani, E. Electrochemically polymerized poly (3, 4-phenylenedioxythiophene) as efficient and transparent counter electrode for dye sensitized solar cells. Electrochim. Acta 2019, 300, 482–488. [Google Scholar] [CrossRef]
- Boudreault, P.L.T.; Najari, A.; Leclerc, M. Processable Low-Bandgap Polymers for Photovoltaic Applications. Chem. Mater. 2011, 23, 456–469. [Google Scholar] [CrossRef]
- Kim, Y.H.; Lee, J.; Hofmann, S.; Gather, M.C.; Müller-Meskamp, L.; Leo, K. Achieving high efficiency and improved stability in ITO free transparent organic light-emitting diodes with conductive polymer electrodes. Adv. Funct. Mater. 2013, 23, 3763–3769. [Google Scholar] [CrossRef]
- Bhuvana, K.P.; Joseph Bensingh, R.; Abdul Kader, M.; Nayak, S.K. Polymer Light Emitting Diodes: Materials, Technology and Device. Polym. Plast. Technol. Eng. 2018, 57, 1784–1800. [Google Scholar] [CrossRef]
- Dutta, K.; Das, S.; Rana, D.; Kundu, P.P. Enhancements of Catalyst Distribution and Functioning Upon Utilization of Conducting Polymers as Supporting Matrices in DMFCs: A Review. Polym. Rev. 2015, 55, 1–56. [Google Scholar] [CrossRef]
- Baldissera, A.F.; Freitas, D.B.; Ferreira, C.A. Electrochemical impedance spectroscopy investigation of chlorinated rubber-based coatings containing polyaniline as anticorrosion agent. Mater. Corros. 2010, 61, 790–801. [Google Scholar] [CrossRef]
- Deshpande, P.P.; Jadhav, N.G.; Gelling, V.J.; Sazou, D. Conducting polymers for corrosion protection: A review. J. Coat. Techn. Res. 2014, 11, 473–494. [Google Scholar] [CrossRef]
- Soganci, T.; Gumusay, O.; Soyleyici, H.C.; Ak, M. Synthesis of highly branched conducting polymer architecture for electrochromic applications. Polymer 2018, 134, 187–195. [Google Scholar] [CrossRef]
- Pagès, H.; Topart, P.; Lemordant, D. Wide band electrochromic displays based on thin conducting polymer films. Electrochim. Acta 2001, 46, 2137–2143. [Google Scholar] [CrossRef]
- Barnes, A.; Despotakis, A.; Wong, T.C.P.; Anderson, A.P.; Chambers, B.; Wright, P.V. Towards a ‘smart window’ for microwave applications. Smart Mater. Struct. 1998, 7, 752. [Google Scholar] [CrossRef]
- Barus, D.A.; Sebayang, K.; Ginting, J.; Ginting, R.T. Effect of Chemical Treatment on Conducting Polymer for Flexible Smart Window Application. J. Phys. Conf. Ser. 2018, 1116, 032006. [Google Scholar] [CrossRef]
- Wang, M.; Wang, X.; Moni, P.; Liu, A.; Kim, D.H.; Jo, W.J.; Sojoudi, H.; Gleason, K.K. CVD Polymers for Devices and Device Fabrication. Adv. Mater. 2017, 29, 1604606. [Google Scholar] [CrossRef]
- Park, C.S.; Kim, D.H.; Shin, B.J.; Tae, H.S. Synthesis and Characterization of Nanofibrous Polyaniline Thin Film Prepared by Novel Atmospheric Pressure Plasma Polymerization Technique. Materials 2016, 9, 39. [Google Scholar] [CrossRef]
- Liu, C.; Goeckner, M.; Walker, A.V. Plasma polymerization of poly (3,4-ethylenedioxyethene) films: The influence of plasma gas phase chemistry. J. Vac. Sci. Technol. A 2017, 35, 021302. [Google Scholar] [CrossRef]
- Loewe, R.S.; Ewbank, P.C.; Liu, J.; Zhai, L.; Mccullough, R.D. Regioregular, Head-to-Tail Coupled Poly(3-Alkylthiophenes) Made Easy by the GRIM Method: Investigation of the Reaction and the Origin of Regioselectivity. Macromolecules 2001, 34, 4324–4333. [Google Scholar] [CrossRef]
- Tamba, S.; Fuji, K.; Meguro, H.; Okamoto, S.; Tendo, T.; Komobuchi, R.; Sugie, A.; Nishino, T.; Mori, A. Synthesis of High-Molecular-Weight Head-to-Tail-Type Poly(3-Substituted-Thiophene)s by Cross-Coupling Polycondensation with [CpNiCl(NHC)] as a Catalyst. Chem. Lett. 2013, 42, 281–283. [Google Scholar] [CrossRef]
- Malinauskas, A. Chemical deposition of conducting polymers. Polymer 2001, 42, 3957–3972. [Google Scholar] [CrossRef]
- Erdem, E.; Karakisla, M.; Sacak, M. The chemical synthesis of conductive polyaniline doped with dicarboxylic acids. Eur. Polym. J. 2004, 40, 785–791. [Google Scholar] [CrossRef]
- Ramanavicius, A.; Kausaite, A.; Ramanaviciene, A. Polypyrrole-coated glucose oxidase nanoparticles for biosensor design. Sens. Actuators B 2005, 111, 532–539. [Google Scholar] [CrossRef]
- Jha, P.; Koiry, S.P.; Saxena, V.; Veerender, P.; Chauhan, A.K.; Aswal, D.K.; Gupta, S.K. Growth of Free-Standing Polypyrrole Nanosheets at Air/Liquid Interface Using J-Aggregate of Porphyrin Derivative as in-Situ Template. Macromolecules 2011, 44, 4583–4585. [Google Scholar] [CrossRef]
- Heinze, J.; Frontana-Uribe, B.A.; Ludwigs, S. Electrochemistry of Conducting Polymers—Persistent Models and New Concepts. Chem. Rev. 2010, 110, 4724–4771. [Google Scholar] [CrossRef]
- Park, Y.; Jung, J.; Chang, M. Research Progress on Conducting Polymer-Based Biomedical Applications. Appl. Sci. 2019, 9, 1070. [Google Scholar] [CrossRef]
- Nair, S.S.; Mishra, S.K.; Kumar, D. Recent progress in conductive polymeric materials for biomedical applications. Polym. Adv. Technol. 2019, 30, 2932–2953. [Google Scholar] [CrossRef]
- Geetha, S.; Rao, C.R.K.; Vijayan, M.; Trivedi, D.C. Biosensing and drug delivery by polypyrrole. Anal. Chim. Acta 2006, 568, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Boehler, C.; Oberueber, F.; Asplund, M. Tuning drug delivery from conducting polymer films for accurately controlled release of charged molecules. J. Control. Release 2019, 304, 173–180. [Google Scholar] [CrossRef]
- Krukiewicz, K.; Bednarczyk, B.; Turczyn, R.; Zak, J.K. EQCM verification of the concept of drug immobilization and release from conducting polymer matrix. Electrochim. Acta 2016, 212, 694–700. [Google Scholar] [CrossRef]
- Guo, B.; Ma, P.X. Conducting polymers for tissue engineering. Biomacromolecules 2018, 19, 1764–1782. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Ma, P.X.; Guo, B. Conductive biomaterials for muscle tissue engineering. Biomaterials 2020, 229, 119584. [Google Scholar] [CrossRef]
- Zarrintai, P.; Bakhshandeh, B.; Saeb, M.R.; Sefat, F.; Rezaeian, I.; Ganjali, M.R.; Ramakrishna, S.; Mozafari, M. Oligoaniline-based conductive biomaterials for tissue engineering. Acta Biomater. 2018, 72, 16–34. [Google Scholar] [CrossRef]
- Inal, S.; Hama, A.; Ferro, M.; Pitsalidis, C.; Oziat, J.; Iandolo, D.; Pappa, A.M.; Hadida, M.; Huerta, M.; Marchat, D.; et al. Conducting polymer scaffolds for hosting and monitoring 3D cell culture. Adv. Biosyst. 2017, 1, 1700052. [Google Scholar] [CrossRef]
- Lakard, S.; Morrand-Villeneuve, N.; Lesniewska, E.; Lakard, B.; Michel, G.; Herlem, G.; Gharbi, T.; Fahys, B. Synthesis of polymer materials for use as cell culture substrates. Electrochim. Acta 2007, 53, 1114–1126. [Google Scholar] [CrossRef]
- Ateh, D.D.; Navsaria, H.A.; Vadgama, P. Polypyrrole-based conducting polymers and interactions with biological tissues. J. R. Soc. Interface 2006, 3, 741–752. [Google Scholar] [CrossRef]
- He, H.; Zhang, L.; Guan, X.; Cheng, H.; Liu, X.; Yu, S.; Wei, J.; Ouyang, J. Biocompatible conductive polymers with high conductivity and high stretchability. ACS Appl. Mater. Interfaces 2019, 11, 26185–26193. [Google Scholar] [CrossRef]
- Humpolicek, P.; Kasparkova, V.; Pachernik, J.; Stejskal, J.; Bober, P.; Capakova, Z.; Radaszkiewicz, K.A.; Junkar, I.; Lehocky, M. The biocompatibility of polyaniline and polypyrrole: A comparative study of their cytotoxicity, embryotoxicity and impurity profile. Mat. Sci. Eng. C 2018, 91, 303–310. [Google Scholar] [CrossRef]
- Humpolicek, P.; Kasparkova, V.; Saha, P.; Stejskal, J. Biocompatibility of polyaniline. Synth. Met. 2012, 162, 722–727. [Google Scholar] [CrossRef]
- George, P.M.; Lyckman, A.W.; LaVan, D.A.; Hegde, A.; Leung, Y.; Avasare, R.; Testa, C.; Alexander, P.M.; Langer, R.; Sur, M. Fabrication and biocompatibility of polypyrrole implants suitable for neural prosthetics. Biomaterials 2005, 26, 3511–3519. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.M.; Cho, S.J.; Cho, C.H.; Kim, K.B.; Kim, M.Y.; Shim, Y.B. Disposable all-solid-state pH and glucose sensors based on conductivepolymer covered hierarchical AuZn oxide. Biosens. Bioelectron. 2016, 79, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Tuncagil, S.; Ozdemir, C.; Demirkol, D.O.; Timur, S.; Toppare, L. Gold nanoparticle modified conducting polymer of 4-(2,5-di(thiophen-2-yl)-1H-pyrrole-1-l) benzenamine for potential use as a biosensing material. Food Chem. 2011, 127, 1317–1322. [Google Scholar] [CrossRef]
- Kausaite-Minkstimiene, A.; Glumbokaite, L.; Ramanaviciene, A.; Dauskaite, E.; Ramanavicius, A. An Amperometric Glucose Biosensor Based on Poly (Pyrrole-2-Carboxylic Acid)/Glucose Oxidase Biocomposite. Electroanalysis 2018, 30, 1642–1652. [Google Scholar] [CrossRef]
- Gaikwad, P.; Shirale, D.; Gade, V.; Savale, P.; Kharat, H.; Kakde, K.; Shirsat, M. Immobilization of GOD on electrochemically synthesized PANI film by cross-linking via glutaraldehyde for determination of glucose. Int. J. Electrochem. Sci. 2006, 1, 425–434. [Google Scholar]
- Lakard, B.; Herlem, G.; Lakard, S.; Antoniou, A.; Fahys, B. Urea potentiometric biosensor based on modified electrodes with urease immobilized on polyethylenimine films. Biosens. Bioelectron. 2004, 19, 1641–1647. [Google Scholar] [CrossRef]
- Magnin, D.; Callegari, V.; Matefi-Tempfli, S.; Matefi-Tempfli, M.; Glinel, K.; Jonas, A.M.; Demoustier-Champagne, S. Functionalization of Magnetic Nanowires by Charged Biopolymers. Biomacromolecules 2008, 9, 2517–2522. [Google Scholar] [CrossRef]
- Xue, H.G.; Shen, Z.Q.; Li, Y.F. Polyaniline-polyisoprene composite film based glucose biosensor with high permselectivity. Synth. Met. 2001, 124, 345–349. [Google Scholar] [CrossRef]
- Molino, P.J.; Higgins, M.J.; Innis, P.C.; Kapsa, R.M.I.; Wallace, G.G. Fibronectin and bovine serum albumin adsorption and conformational dynamics on inherently conducting polymers: A QCM-D study. Langmuir 2012, 28, 8433–8445. [Google Scholar] [CrossRef] [PubMed]
- Lakard, B.; Magnin, D.; Deschaume, O.; Vanlancker, G.; Glinel, K.; Demoustier-Champagne, S.; Nysten, B.; Jonas, A.M.; Bertrand, P.; Yunus, S. Urea potentiometric enzymatic biosensor based on charged biopolymers and electrodeposited polyaniline. Biosens. Bioelectron. 2011, 26, 4139–4145. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Kampstra, K.L.; Abidian, M.R. High performance conducting polymer nanofiber biosensors for detection of biomolecules. Adv. Mater. 2014, 26, 4954–4960. [Google Scholar] [CrossRef] [PubMed]
- Soares, J.C.; Brisolari, A.; da Cruz Rodrigues, V.; Sanches, E.A.; Gonçalves, D. Amperometric urea biosensors based on the entrapment of urease in polypyrrole films. React. Funct. Polym. 2012, 72, 148–152. [Google Scholar] [CrossRef]
- Minett, A.I.; Barisci, J.N.; Wallace, G.G. Immobilisation of anti-Listeria in a polypyrrole film. React. Funct. Polym. 2002, 53, 217–227. [Google Scholar] [CrossRef]
- Mandli, J.; Amine, A. Impedimetric genosensor for miRNA-34a detection in cell lysates using polypyrrole. J. Solid State Electrochem. 2018, 22, 1007–1014. [Google Scholar] [CrossRef]
- Ramanavicius, A.; Ramanaviciene, A.; Malinauskas, A. Electrochemical sensors based on conducting polymer- polypyrrole. Electrochim. Acta 2006, 51, 6025–6037. [Google Scholar] [CrossRef]
- Adeloju, S.B.; Moline, A.N. Fabrication of ultra-thin polypyrrole–glucose oxidase film from supporting electrolyte-free monomer solution for potentiometric biosensing of glucose. Biosens. Bioelectron. 2001, 16, 133–139. [Google Scholar] [CrossRef]
- Leite, C.; Lakard, B.; Hihn, J.Y.; del Campo, F.J.; Lupu, S. Use of sinusoidal voltages with fixed frequency in the preparation of tyrosinase based electrochemical biosensors for dopamineelectroanalysis. Sens. Actuators B 2017, 240, 801–809. [Google Scholar] [CrossRef]
- Clark, L.C. Monitor and control of blood and tissue oxygen tensions. Trans. Am. Soc. Artif. Intern. Organs 1956, 2, 41–48. [Google Scholar]
- Forzani, E.S.; Zhang, H.; Nagahara, L.A.; Amlani, I.; Tsui, R.; Tao, N. A Conducting Polymer Nanojunction Sensor for Glucose Detection. Nano Lett. 2004, 4, 1785–1788. [Google Scholar] [CrossRef]
- Chauhan, N.; Chawla, S.; Pundir, C.S.; Jain, U. An electrochemical sensor for detection of neurotransmitter-acetylcholine using metal nanoparticles, 2D material and conducting polymer modified electrode. Biosens. Bioelectron. 2017, 89, 377–383. [Google Scholar] [CrossRef]
- Uwaya, G.E.; Fayemi, O.E. Electrochemical detection of serotonin in banana at green mediated PPy/Fe3O4 NPs nanocomposites modified electrodes. Sens. Bio Sens. Res. 2020, 28, 100338. [Google Scholar] [CrossRef]
- Hamid, H.H.; Harb, M.E.; Elshaer, A.M.; Erahim, S.; Soliman, M.M. Electrochemical Preparation and Electrical Characterization of Polyaniline as a Sensitive Biosensor. Microsyst. Technol. 2018, 24, 1775–1781. [Google Scholar] [CrossRef]
- Bahadir, E.B.; Sezgintürk, M.K. A review on impedimetric biosensors. Artif. Cells Nanomed Biotechn. 2016, 44, 248–262. [Google Scholar] [CrossRef]
- He, S.; Yuan, Y.; Nag, A.; Feng, S.; Afsarimanesh, N.; Han, T.; Mukhopadhyay, S.C.; Organ, D.R. A Review on the Use of Impedimetric Sensors for the Inspection of Food Quality. Int. J. Environ. Res. Public Health 2020, 17, 5220. [Google Scholar] [CrossRef] [PubMed]
- Leva-Bueno, J.; Peyman, S.A.; Millner, P.A. A review on impedimetric immunosensors for pathogen and biomarker detection. Med. Microbiol. Immunol. 2020, 209, 343–362. [Google Scholar] [CrossRef]
- Grant, S.; Davis, F.; Law, K.A.; Barton, A.C.; Collyer, S.D.; Higson, S.P.J.; Gibson, T.D. Label-free and reversible immunosensor based upon an ac impedance interrogation protocol. Anal. Chim. Acta 2005, 537, 163–168. [Google Scholar] [CrossRef]
- Aydin, E.B.; Aydin, M.; Sezgintürk, M.K. Highly sensitive electrochemical immunosensor based on polythiophene polymer with densely populated carboxyl groups as immobilization matrix for detection of interleukin 1β in human serum and saliva. Sens. Actuators B 2018, 270, 18–27. [Google Scholar] [CrossRef]
- Taleat, Z.; Ravalli, A.; Mazloum-Ardakami, M.; Marrazza, G. CA 125 Immunosensor Based on Poly-Anthranilic Acid Modified Screen-Printed Electrodes. Electroanalysis 2013, 25, 269–277. [Google Scholar] [CrossRef]
- Jugovic, B.; Grgur, B.; Antov, M.; Knezevic-Jugovic, Z.; Stevanovic, J.; Gvozdenovic, M. Polypyrrole-based Enzyme Electrode with Immobilized Glucose Oxidase for Electrochemical Determination of Glucose. Int. J. Electrochem. Sci. 2016, 11, 1152–1161. [Google Scholar]
- Gvozdenovic, M.M.; Jugovic, B.Z.; Bezbradica, D.I.; Antov, M.G.; Knezevic-Jugovic, Z.D.; Grgur, B.N. Electrochemical determination of glucose using polyaniline electrode modified by glucose oxidase. Food Chem. 2011, 124, 396–400. [Google Scholar] [CrossRef]
- Lai, J.; Yi, Y.; Zhu, P.; Shen, J.; Wu, K.; Zhang, L.; Liu, J. Polyaniline-based glucose biosensor: A review. J. Electroanal. Chem. 2016, 782, 138–153. [Google Scholar] [CrossRef]
- Singh, M.; Kathuroju, P.K.; Jampana, N. Polypyrrole based amperometric glucose biosensors. Sens. Actuators B 2009, 143, 430–443. [Google Scholar] [CrossRef]
- Lau, K.T.; de Fortescu, S.A.L.; Murphy, L.J.; Slater, J.M. Disposable glucose sensors for flow injection analysis using substituted 1,4-benzoquinonemediators. Electroanalysis 2003, 15, 975–981. [Google Scholar] [CrossRef]
- Qiu, J.D.; Zhou, W.M.; Guo, J.; Wang, R.; Liang, R.P. Amperometric sensor based on ferrocene-modified multiwalled carbon nanotube nanocomposites as electron mediator for the determination of glucose. Anal. Biochem. 2009, 385, 264–269. [Google Scholar] [CrossRef]
- Jian, L.; Shanhui, S.; Changchun, L.; Shoushui, W. An amperometric glucose biosensor based on a screen-printed electrode and Os-complex mediator for flow injection analysis. Measurement 2011, 44, 1878–1883. [Google Scholar]
- Shrestha, B.K.; Ahmad, R.; Mousa, H.M.; Kim, I.G.; Kim, J.I.; Neupane, M.P.; Park, C.H.; Kim, C.S. High-performance glucose biosensor based on chitosane-glucose oxidase immobilized polypyrrole/Nafion/functionalized multi-walled carbon nanotubes bio-nanohybrid film. J. Colloid Interf. Sci. 2016, 482, 39–47. [Google Scholar] [CrossRef]
- Shrestha, B.K.; Ahmad, R.; Shrestha, S.; Park, C.H.; Kim, C.S. Globular Shaped Polypyrrole Doped Well-Dispersed Functionalized Multiwall Carbon Nanotubes/Nafion Composite for Enzymatic Glucose Biosensor Application. Sci. Rep. 2017, 7, 16191. [Google Scholar] [CrossRef]
- Chen, X.; Chen, Z.; Tian, R.; Yan, W.; Yao, C. Glucose biosensor based on three dimensional ordered macroporous self-doped polyaniline/Prussian blue bicomponent film. Anal. Chim. Acta 2012, 723, 94–100. [Google Scholar] [CrossRef]
- Chowdhury, A.D.; Gangopadhyay, R.; De, A. Highly sensitive electrochemical biosensor for glucose, DNA and protein using gold-polyaniline nanocomposites as a common matrix. Sens. Actuators B 2014, 190, 348–356. [Google Scholar] [CrossRef]
- Mazeiko, V.; Kausaite-Minkstimiene, A.; Ramanaviciene, A.; Balevicius, Z.; Ramanavicius, A. Gold Nanoparticle and Conducting Polymer—Polyaniline—Based Nanocomposites for Glucose Biosensor Design. Sens. Actuators B 2013, 189, 187–193. [Google Scholar] [CrossRef]
- Zhai, D.; Liu, B.; Shi, Y.; Pan, L.; Wang, Y.; Li, Y.; Zhang, R.; Yu, G. Highly sensitive glucose sensor based on Pt nanoparticle/polyaniline hydrogel heterostructures. ACS Nano 2013, 7, 3540–3546. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Yuan, R.; Chai, Y.; Li, W.; Zhong, X.; Zhang, Y. In situ chemo-synthesized multi-wall carbon nanotube-conductive polyaniline nanocomposites: Characterization and application for a glucose amperometric biosensor. Talanta 2011, 85, 104–111. [Google Scholar] [CrossRef]
- Jimenez-Fierrez, F.; Gonzalez-Sanchez, M.I.; Jimenez-Perez, R.; Iniesta, J.; Valero, E. Glucose Biosensor Based on Disposable Activated Carbon Electrodes Modified with Platinum Nanoparticles Electrodeposited on Poly(Azure A). Sensors 2020, 20, 4489. [Google Scholar] [CrossRef]
- Djaalab, E.; El Hadi Samar, M.; Zougar, S.; Kherrat, R. Electrochemical Biosensor for the Determination of Amlodipine Besylate Based on Gelatin-Polyaniline Iron Oxide Biocomposite Film. Catalysts 2018, 8, 233. [Google Scholar] [CrossRef]
- Zhuang, X.; Tian, C.; Luan, F.; Wu, X.; Chen, L. One-step electrochemical fabrication of a nickel oxide nanoparticle/polyaniline nanowire/graphene oxide hybrid on a glassy carbon electrode for use as a non-enzymatic glucose biosensor. RSC Adv. 2016, 6, 92541–92546. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, S.; Wu, P.; Cai, C. Detection of Glucose Based on Direct Electron Transfer Reaction of Glucose Oxidase Immobilized on Highly Ordered Polyaniline Nanotubes. Anal. Chem. 2009, 81, 1638–1645. [Google Scholar] [CrossRef]
- Xu, G.; Adeloju, S.B.; Wu, Y.; Zhang, X. Modification of polypyrrole nanowires array with platinum nanoparticles and glucose oxidase for fabrication of a novel glucose biosensor. Anal. Chim. Acta 2012, 755, 100–107. [Google Scholar] [CrossRef]
- Komathi, S.; Gopalan, A.I.; Muthuchamy, N.; Lee, K.P. Polyaniline nanoflowers grafted onto nanodiamonds via a soft template-guided secondary nucleation process for high-performance glucose sensing. RSC Adv. 2017, 7, 15342–15351. [Google Scholar] [CrossRef]
- Ramanavicius, A.; Habermüller, K.; Csöregi, E.; Laurinavicius, V.; Schuhmann, W. Polypyrrole-Entrapped Quinohemoprotein Alcohol Dehydrogenase. Evidence for Direct Electron Transfer via Conducting-Polymer Chains. Anal. Chem. 1999, 71, 3581–3586. [Google Scholar] [CrossRef]
- Bollela, P.; Gorton, L.; Antiochia, R. Direct Electron Transfer of Dehydrogenases for Development of 3rd Generation Biosensors and Enzymatic Fuel Cells. Sensors 2018, 18, 1319. [Google Scholar] [CrossRef] [PubMed]
- Oztekin, Y.; Ramanaviciene, A.; Yazicigil, Z.; Solak, A.O.; Ramanavicius, A. Direct electron transfer from glucose oxidase immobilized on polyphenanthroline-modified glassy carbon electrode. Biosens. Bioelectron. 2011, 26, 2541–2546. [Google Scholar] [CrossRef] [PubMed]
- Bagdziunas, G.; Palinauskas, D. Poly (9H-carbazole) as a Organic Semiconductor for Enzymatic and Non-Enzymatic Glucose Sensors. Biosensors 2020, 10, 104. [Google Scholar] [CrossRef] [PubMed]
- Grennan, K.; Strachan, G.; Porter, A.J.; Killard, A.J.; Smyth, M.R. Atrazine analysis using an amperometric immunosensor based on single-chain antibody fragments and regeneration-free multi-calibrant measurement. Anal. Chim. Acta 2003, 500, 287–298. [Google Scholar] [CrossRef]
- Darain, F.; Park, D.S.; Park, J.S.; Shim, Y.B. Development of an immunosensor for the detection of vitellogenin using impedance spectroscopy. Biosens. Bioelectron. 2004, 19, 1245–1252. [Google Scholar] [CrossRef]
- Wang, H.; Ma, F. A cascade reaction signal-amplified amperometric immunosensor platform for ultrasensitive detection of tumour marker. Sens. Actuators B 2018, 254, 642–647. [Google Scholar] [CrossRef]
- Zhao, L.; Ma, Z. Facile Synthesis of Polyaniline-Polythionine Redox Hydrogel: Conductive, Antifouling and Enzyme-Linked Material for Ultrasensitive Label-Free Amperometric Immunosensor toward Carcinoma Antigen-125. Anal. Chim. Acta 2018, 997, 60–66. [Google Scholar] [CrossRef]
- Shaikh, M.O.; Srikanth, B.; Zhu, P.Y.; Chuang, C.H. Impedimetric Immunosensor Utilizing Polyaniline/Gold Nanocomposite-Modified Screen-Printed Electrodes for Early Detection of Chronic Kidney Disease. Sensors 2019, 19, 3990. [Google Scholar] [CrossRef]
- Liu, S.; Ma, Y.; Cui, M.; Luo, X. Enhanced Electrochemical Biosensing of Alpha-Fetoprotein Based on Three-Dimensional Macroporous Conducting Polymer Polyaniline. Sens. Actuators B 2018, 255, 2568–2574. [Google Scholar] [CrossRef]
- Dutta, S.; Chowdhury, A.D.; Biswas, S.; Park, E.Y.; Agnihotri, N.; De, A.; De, S. Development of an effective electrochemical platform for highly sensitive DNA detection using MoS2—polyaniline nanocomposites. Biochem. Eng. J. 2018, 140, 130–139. [Google Scholar] [CrossRef]
- Eguiluz, K.I.V.; Salazar-Banda, G.R.; Elizabeth, M.; Huacca, F.; Alberice, J.V.; Carrilho, E.; Machado, S.A.S.; Avaca, L.A. Sequence-specific electrochemical detection of Alicyclobacillus acidoterrestris DNA using electroconductive polymer-modified fluorine tin oxide electrodes. Analyst 2009, 134, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Tlili, C.; Jaffrezic-Renault, N.J.; Martelet, C.; Korri-Youssoufi, H. Direct electrochemical probing of DNA hybridization on oligonucleotide-functionalized polypyrrole. Mater. Sci. Eng. C 2008, 28, 848–854. [Google Scholar] [CrossRef]
- Wilson, J.; Radhakrishnan, S.; Sumathi, C.; Dharuman, V. Polypyrrole-polyaniline-Au (PPy-PANi-Au) nano composite films for label-free electrochemical DNA sensing. Sens. Actuators B 2012, 171, 216–222. [Google Scholar] [CrossRef]
- Radhakrishnan, S.; Sumathi, C.; Umar, A.; Kim, S.J.; Wilson, J.; Dharuman, V. Polypyrrole-poly (3,4-ethylenedioxythiophene)-Ag (PPy-PEDOT-Ag) nanocomposite films for label-free electrochemical DNA sensing. Biosens. Bioelectron. 2013, 47, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Ye, X.; Yang, L.; He, P.; Fang, Y. Impedance DNA biosensor using electropolymerized polypyrrole/multiwalled carbon nanotubes modified electrode. Electroanalysis 2006, 18, 1471–1478. [Google Scholar] [CrossRef]
- Chang, H.; Yuan, Y.; Shi, N.; Guan, Y. Electrochemical DNA biosensor based on conducting polyaniline nanotube Array. Anal. Chem. 2007, 79, 5111–5115. [Google Scholar] [CrossRef]
- Zhu, N.; Chang, Z.; He, P.; Fang, Y. Electrochemically fabricated polyaniline nanowire-modified electrode for voltammetric detection of DNA hybridization. Electrochim. Acta 2006, 51, 3758–3762. [Google Scholar] [CrossRef]
- Du, M.; Yang, T.; Li, X.; Jiao, K. Fabrication of DNA/graphene/polyaniline nanocomplex for label-free voltammetric detection of DNA hybridization. Talanta 2012, 88, 439–444. [Google Scholar] [CrossRef]
- Mohamad, F.S.; Zaid, M.H.M.; Abdullah, J.; Zawawi, R.M.; Lim, H.N.; Sulaiman, Y.; Rahman, N.A. Synthesis and Characterization of Polyaniline/Graphene Composite Nanofiber and Its Application as an Electrochemical DNA Biosensor for the Detection of Mycobacterium tuberculosis. Sensors 2017, 17, 2789. [Google Scholar] [CrossRef]
- Ding, L.; Du, D.; Zhang, X.; Ju, H. Trends in Cell-Based Electrochemical Biosensors. Curr. Med. Chem. 2008, 15, 3160–3170. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Mo, X.; Zhang, P.; Li, Y.; Liao, J.; Li, Y.; Zhang, J.; Ning, C.; Wang, S.; Deng, X.; et al. Directing Stem Cell Differentiation via Electrochemical Reversible Switching between Nanotubes and Nanotips of Polypyrrole Array. ACS Nano 2017, 11, 5915–5924. [Google Scholar] [CrossRef]
- Lakard, B.; Ploux, L.; Anselme, K.; Lallemand, F.; Lakard, S.; Nardin, M.; Hihn, J.Y. Effect of ultrasounds on the electrochemical synthesis of polypyrrole. Application to the adhesion and growth of biological cells. Bioelectrochemistry 2009, 75, 148–157. [Google Scholar] [CrossRef] [PubMed]
- El-Said, W.A.; Yea, C.H.; Choi, J.W.; Kwon, I.K. Ultrathin polyaniline film coated on an indium-tin oxide cell-based chip for study of anticancer effect. Thin Solid Film 2009, 518, 661–667. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lee, J.W.; Schmidt, C.E. Neuroactive conducting scaffolds: Nerve growth factor conjugation on active ester-functionalized polypyrrole. J. R. Soc. Interface 2009, 6, 801–810. [Google Scholar] [CrossRef]
- Nguyen-Vu, T.D.B.; Chen, H.; Cassell, A.M.; Andrews, R.; Meyyappan, M.; Li, J. Vertically Aligned Carbon Nanofiber Arrays: An Advance toward Electrical-Neural Interfaces. Small 2006, 2, 89–94. [Google Scholar] [CrossRef]
- El-Said, W.A.; Yea, C.H.; Jung, M.; Kim, H.C.; Choi, J.W. Analysis of effect of nanoporous alumina substrate coated with polypyrrole nanowire on cell morphology based on AFM topography. Ultramicroscopy 2010, 110, 676–681. [Google Scholar] [CrossRef]
- Apetrei, R.M.; Carac, G.; Bahrim, G.; Camurlu, P. Glucose biosensor based on whole cells of Aspergillus niger MIUG 34 coated with polypyrrole. J. Biotechnol. 2017, 256, S55–S56. [Google Scholar] [CrossRef]
- Cevik, E.; Cerit, A.; Tombuloglu, H.; Sabit, H.; Yildiz, H.B. Electrochemical Glucose Biosensors: Whole Cell Microbial and Enzymatic Determination based on 10-(4H-dithiyeno [3,2-b:2′,3′-d]pyroll-4-il) decan-1-amine Interfaces Glassy Carbon Electrodes. Anal. Lett. 2019, 52, 1138–1152. [Google Scholar] [CrossRef]
- Baskurt, E.; Ekiz, F.; Demirkol, D.O.; Timur, S.; Toppare, L. A conducting polymer with benzothiadiazole unit: Cell based biosensing applications and adhesion properties. Colloids Surf. B 2012, 97, 13–18. [Google Scholar] [CrossRef]
- Guler, E.; Soylevici, H.C.; Demirkol, D.O.; Ak, M.; Timur, S. A novel functional conducting polymer as an immobilization platform. Mat. Sci. Eng. C 2014, 40, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Tuncagil, S.; Odaci, D.; Yildiz, E.; Timur, S.; Toppare, L. Design of a microbial sensor using conducting polymer of 4-(2,5-di(thiophen-2-yl)-1H-pyrrole-1-l) benzenamine. Sens. Actuators B 2009, 137, 42–47. [Google Scholar] [CrossRef]
- Jha, S.K.; Kanungo, M.; Nath, A.; D’Souza, S.F.D. Entrapment of live microbial cells in electropolymerized polyaniline and their use as urea biosensor. Biosens. Bioelectron. 2009, 24, 2637–2642. [Google Scholar] [CrossRef] [PubMed]
- Ratautaite, V.; Plausinaitis, D.; Baleviciute, I.; Mikoliunaite, L.; Ramanaviciene, A.; Ramanavicius, A. Characterization of caffeine-imprinted polypyrrole by a quartz crystalmicrobalance and electrochemical impedance spectroscopy. Sens. Actuators B 2015, 212, 63–71. [Google Scholar] [CrossRef]
- Xing, X.; Liu, S.; Yu, J.; Lian, W.; Huan, J. Electrochemical sensor based on molecularly imprinted film at polypyrrole-sulfonated graphene/hyaluronic acid-multiwalled carbon nanotubes modified electrode for determination of tryptamine. Biosens. Bioelectron. 2012, 31, 277–283. [Google Scholar] [CrossRef]
- Zhou, H.; Xu, G.; Zhu, A.; Zhao, Z.; Ren, C.; Nie, L.; Kan, X. A multiporous electrochemical sensor for epinephrine recognition and detection based on molecularly imprinted polypyrrole. RSC Advances 2012, 2, 7803–7808. [Google Scholar] [CrossRef]
- Radi, A.E.; El-Naggar, A.E.; Nassef, H.M. Determination of coccidiostat clopidol on an electropolymerized-molecularly imprinted polypyrrole polymer modified screen printed carbon electrode. Anal. Methods 2014, 6, 7967–7972. [Google Scholar] [CrossRef]
- Lai, Y.X.; Zhang, C.X.; Deng, Y.; Yang, G.J.; Li, S.; Tang, C.L.; He, N.Y. A novel alpha-fetoprotein-MIP immunosensor based on AuNPs/PTh modified glass carbon electrode. Chin. Chem. Lett. 2019, 30, 160–162. [Google Scholar] [CrossRef]
- Karami, P.; Bagheri, H.; Johari-Ahar, M.; Khoshsafar, H.; Arduini, F.; Afkhami, A. Dual-modality impedimetric immunosensor for early detection of prostate-specific antigen and myoglobin markers based on antibody-molecularly imprinted polymer. Talanta 2019, 202, 111–122. [Google Scholar] [CrossRef]

 |  |  |
| (a) | (b) | (c) |
 |  |  |
| (d) | (e) | (f) |
 |  |  |
| (g) | (h) | (i) |
| Active Layer | Linear Range | Sensitivity | Detection Limit | Stability | Real Samples? | Ref. |
|---|---|---|---|---|---|---|
| Polypyrrole-CNT-chitosan | 1–4.7 mM | 2860 μA mM−1 cm−2 | 5.0 μM | 45 days | serum | [101] |
| Polypyrrole-CNT | 1–4.1 mM | 54.2 μA mM−1 cm−2 | 5.0 μM | 45 days | serum | [102] |
| Polyaniline-Prussian Blue | 2–1.6 MM | 99.4 μA mM−1 cm−2 | 0.4 μM. | 15 days | serum | [103] |
| Polyaniline-Au NP | 1–20 mM | 14.6 μA mM−1 cm−2 | 1.0 μM | --- | --- | [104] |
| Polyaniline-Pt NP | 0.01–8 mM | 96.1 μA mM−1 cm−2 | 0.7 μM | --- | --- | [106] |
| Polyaniline-CNT | 3–8.2 mM | 16.1 μA mM−1 cm−2 | 1.0 μM | 48 days | serum | [107] |
| Poly(Azure A)-Pt NP | 0.02–2.3 mM | 42.7μA mM−1 cm−2 | 7.6 μM | 3 month | fruit juice | [108] |
| Polyaniline-Graphene-NiO2 | 0.02–5.56 mM | 376.2 μA mM−1 cm−2 | 0.5 μM | --- | serum | [110] |
| Polyaniline | 0.01–5.5 mM | 97.2 μA mM−1 cm−2 | 0.3 μM | 15 days | urine | [111] |
| Polypyrrole-Pt NP | 0.1–9 mM | 34.7 μA mM−1 cm−2 | 27.7 μM | --- | --- | [112] |
| Polyaniline-nanodiamonds | 1–30 mM | 2.03 mA mM−1 cm−2 | 18.0 μM | 30 days | serum | [113] |
| Polycarbazole | 0.01–5 mM | 14.0 μA mM−1 cm−2 | 0.2 μM | --- | --- | [117] |
| Active Layer | Target | Detection Mode | Linear Range | Detection Limit | Ref. |
|---|---|---|---|---|---|
| Polyaniline-poly(vinylsulfonic acid) | atrazine | amperometry | 0.12–5 µM | 0.1 µg/L | [118] |
| Polythiophene derivative (with—COOH groups) | Carp vittelogenin | impedometry | 1–8 µg/L | 0.42 µg/L | [119] |
| Polypyrrole-polythionine | neuron-specific enolase | voltammetry | 0.001–100 pg/mL | 0.65 pg/mL | [120] |
| Polypyrrole-polythionine | carcinoma antigen-125 | 1–20 mM | 0.0001–1000 U/mL | 0.00125 U/mL | [121] |
| Polyaniline/Au nanocrystals | human serumalbumin | voltammetry + impedometry | 3–300 µg/mL | 3 µg/mL | [122] |
| Polyaniline-poly(sodium styrene sulfonate) | voltammetry | 0.01–1000 pg/L | 3.7 fg/mL | [123] |
| Active Layer | Detection Mode | Linear Range | Detection Limit | Ref. |
|---|---|---|---|---|
| Polyaniline–MoS2 | voltammetry | 10−15–10−6 M | 10−15 M | [124] |
| Polypyrrole–Au and Ag NPs | voltammetry | 7–150 nM | 7 nM | [125] |
| poly [3–acetic acid pyrrole,3–N–hydroxyphthalimide pyrrole)] | impedometry | 0.05–5.5 nM | 1 pM | [126] |
| Polypyrrole–Polyaniline– | impedometry | 10−13–10−6 M | 10−13 M | [127] |
| Polypyrrole–PEDOT–Ag NP | impedometry | 10−15–10−11 M | 5 × 10−15 M | [128] |
| Polypyrrole–CNT–COOH | impedometry | 10−12–10−7 M | 5 × 10−12 M | [129] |
| Polyaniline | voltammetry | 10−15–10−12 M | 10−15 M | [130] |
| Polyaniline–methylene blue | voltammetry | 10−12–10−10 M | 10−12 M | [131] |
| Polyaniline–graphene | voltammetry | 10−13–10−7 M | 3 × 10−14 M | [132] |
| Polyaniline–graphene | voltammetry | 10−9–10−6 M | 8 × 10−7 M | [133] |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lakard, B. Electrochemical Biosensors Based on Conducting Polymers: A Review. Appl. Sci. 2020, 10, 6614. https://doi.org/10.3390/app10186614
Lakard B. Electrochemical Biosensors Based on Conducting Polymers: A Review. Applied Sciences. 2020; 10(18):6614. https://doi.org/10.3390/app10186614
Chicago/Turabian StyleLakard, Boris. 2020. "Electrochemical Biosensors Based on Conducting Polymers: A Review" Applied Sciences 10, no. 18: 6614. https://doi.org/10.3390/app10186614
APA StyleLakard, B. (2020). Electrochemical Biosensors Based on Conducting Polymers: A Review. Applied Sciences, 10(18), 6614. https://doi.org/10.3390/app10186614





