Wax Formation Mechanisms, Wax Chemical Inhibitors and Factors Affecting Chemical Inhibition
Abstract
:1. Introduction
2. Wax Deposition Mechanism
3. Wax Deposition in Multiphase Flow
4. Oil−Water Wax Deposition
5. Wax Chemical Inhibitors
5.1. Wax Dispersant
5.2. Pour Point Depressant (PPD)
5.3. Wax Crystal Modifier
6. Factors Affecting Wax Inhibition Performance
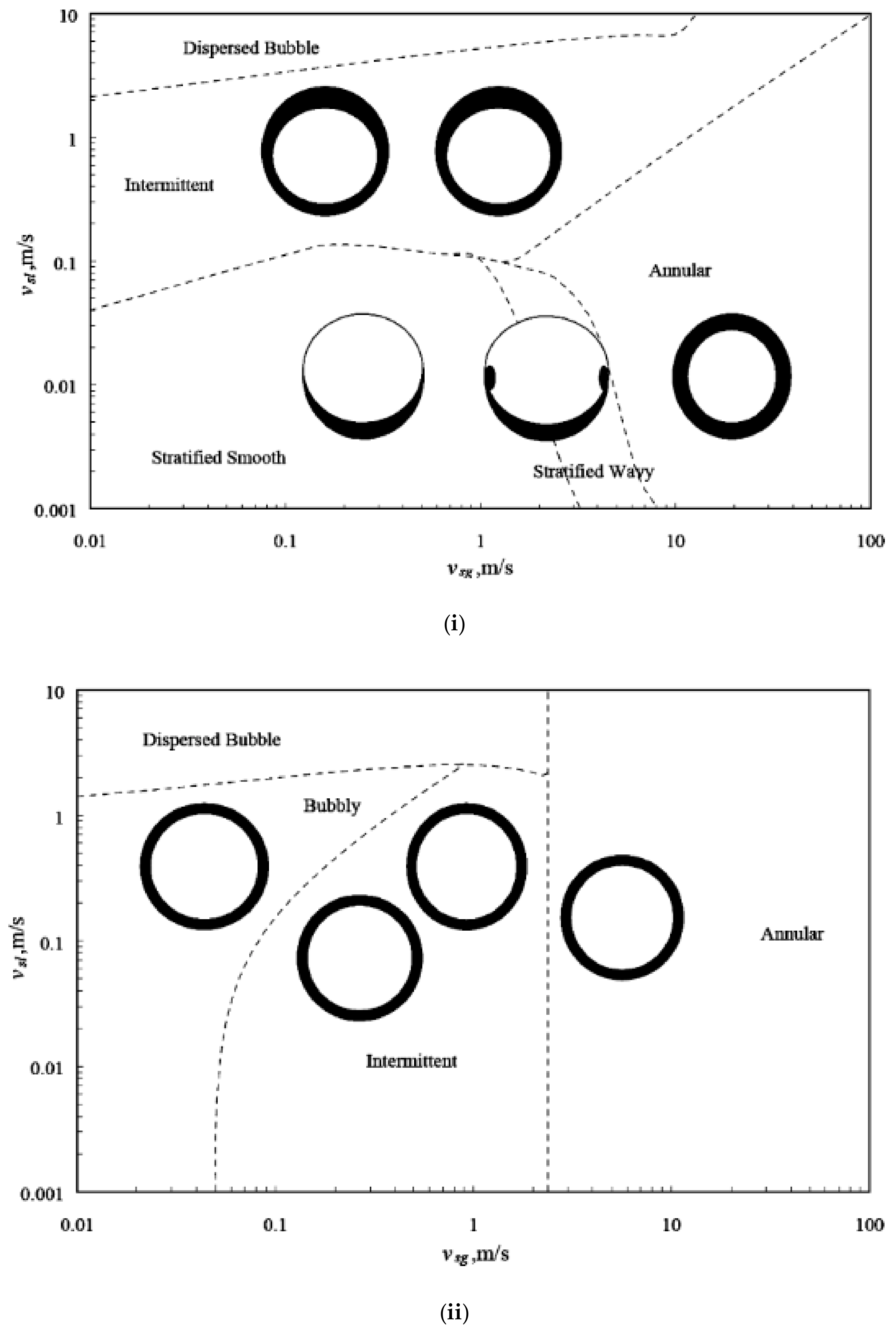
6.1. Flow Regimes
6.2. Temperature
6.3. Wax Content
6.4. Chemical Inhibitor’s Molecular Structure
6.5. Effect of Solvent and Dilution
6.6. Polar Crude Fractions
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| DEA | Diethanolamine |
| EVA | Ethylene vinyl acetate |
| MAC | Olefin-maleic anhydride copolymer |
| MMA | Methyl methacrylate |
| PA | Poly-acrylate |
| PEB | Polyethylene butene |
| PE-PEP | Polyethylene–polyethylene propylene |
| PMA | Poly-methacrylate |
| PPDs | Pour point depressants |
| TEX | Trichloroethylene-xylene |
| VA | Vinyl acetate |
| WAT | Wax appearance temperature |
| HTGC | High temperature gas chromatography |
| Superficial gas velocity | |
| Superficial liquid velocity |
References
- Arnold, K.; Stewart, M. Surface Production Operations; Gulf Professional Publishing: Oxford, UK, 2008. [Google Scholar]
- Pedersen, K.S.; Fredenslund, A.; Thomassen, P. Properties of Oils and Natural Gases 5; Gulf Pub Co: Pittsburgh, PA, USA, 1989. [Google Scholar]
- Srivastava, S.; Handoo, J.; Agrawal, K.; Joshi, G. Phase-transition studies in n-alkanes and petroleum-related waxes—A review. J. Phys. Chem. Solids 1993, 54, 639–670. [Google Scholar] [CrossRef]
- Ferworn, K.A.; Fluid, D.B.R.; Hammami, A.; Robinson, D.B.; Ellis, H. Control of Wax Deposition: An Experimental Investigation of Crystal Morphology and an Evaluation of Various Chemical Solvents. In Proceedings of the SPE International Symposium on Oilfield Chemistry, Houston, TX, USA, 18–21 February 1997; pp. 1–20. [Google Scholar]
- Aiyejina, A.; Chakrabarti, D.P.; Pilgrim, A.; Sastry, M. Wax formation in oil pipelines: A critical review. Int. J. Multiph. Flow 2011, 37, 671–694. [Google Scholar] [CrossRef]
- Behbahani, T.J.; Beigi, A.A.M.; Taheri, Z.; Ghanbari, B. Investigation of wax precipitation in crude oil: Experimental and modeling. Petroleum 2015, 1, 223–230. [Google Scholar] [CrossRef] [Green Version]
- Lira-Galeana, C.; Hammami, A. Chapter 21 Wax precipitation from petroleum fluids: A Review. Dev. Pet. Sci. 2000, 40, 557–608. [Google Scholar]
- Weingarten, J.S.; Euchner, J.A. Methods for predicting wax precipitation and deposition. SPE Prod. Eng. 1988, 3, 121–126. [Google Scholar] [CrossRef]
- Struchkov, I.A.; Rogachev, M.K. Wax precipitation in multicomponent hydrocarbon system. J. Pet. Explor. Prod. Technol. 2017, 7, 543–553. [Google Scholar] [CrossRef] [Green Version]
- Garcia, M.D.C.; Urbina, A. Effect of crude oil composition and blending on flowing properties. Pet. Sci. Technol. 2003, 21, 863–878. [Google Scholar] [CrossRef]
- García, M.D.C.; Orea, M.; Carbognani, L.; Urbina, A. The effect of paraffinic fractions on crude oil wax crystallization. Pet. Sci. Technol. 2001, 19, 189–196. [Google Scholar] [CrossRef]
- Hammami, A.; Ratulowski, J.; Coutinho, J.A.P. Cloud points: Can we measure or model them? SPE Repr. Ser. 2004, 21, 144–154. [Google Scholar] [CrossRef]
- Daraboina, N.; Soedarmo, A.; Sarica, C. Microscopic Study of Wax Inhibition Mechanism. In Proceedings of the Offshore Technology Conference, Society of Petroleum Engineers, Houston, TX, USA, 2–5 May 2016; pp. 1–10. [Google Scholar]
- Chi, Y.; Daraboina, N.; Sarica, C. Investigation of inhibitors efficacy in wax deposition mitigation using a laboratory scale flow loop. AIChE J. 2016, 62, 4131–4139. [Google Scholar] [CrossRef]
- Perez, P.; Boden, E.; Chichak, K.; Gurnon, A.K.; Hu, L.; Lee, J.; McDermott, J.; Osaheni, J.; Peng, W.; Richards, W.; et al. Evaluation of Paraffin Wax Inhibitors: An Experimental Comparison of Bench-Top Test Results and Small-Scale Deposition Rigs for Model Waxy Oils. In Proceedings of the Offshore Technology Conference, Houston, TX, USA, 4–7 May 2015; pp. 4–7. [Google Scholar]
- Singh, P.; Venkatesan, R.; Fogler, H.S.; Nagarajan, N. Formation and aging of incipient thin film wax-oil gels. AIChE J. 2000, 46, 1059–1074. [Google Scholar] [CrossRef] [Green Version]
- Soedarmo, A.A.; Daraboina, N.; Sarica, C. Validation of wax deposition models with recent laboratory scale flow loop experimental data. J. Pet. Sci. Eng. 2017, 149, 351–366. [Google Scholar] [CrossRef]
- Akinyemi, O.; Udonne, J.; Oyedeko, K. Study of effects of blend of plant seed oils on wax deposition tendencies of Nigerian waxy crude oil. J. Pet. Sci. Eng. 2018, 161, 551–558. [Google Scholar] [CrossRef]
- Correra, S.; Fasano, A.; Fusi, L.; Primicerio, M. Modelling wax diffusion in crude oils: The cold finger device. Appl. Math. Model. 2007, 31, 2286–2298. [Google Scholar] [CrossRef] [Green Version]
- Deshmukh, S.; Bharambe, D.P. Wax dispersant additives for improving the low temperature flow behavior of waxy crude oil. Energy Sources Part A Recover. Util. Environ. Eff. 2012, 34, 1121–1129. [Google Scholar] [CrossRef]
- Santos, A.D.; Fernandes, A.; Giulietti, M. Study of the paraffin deposit formation using the cold finger metholodogy for Brazilian crude oils. J. Pet. Sci. Eng. 2004, 45, 47–60. [Google Scholar] [CrossRef]
- Fan, K.; Huang, Q.; Li, S.; Zhao, D. Wax Deposition Study in a Cold-finger System with Model Oil. In Proceedings of the SPE/IATMI Asia Pacific Oil & Gas Conference and Exhibition, Bali, Indonesia, 20–22 October 2015; pp. 20–22. [Google Scholar]
- Hunt, E.B., Jr. Laboratory study of paraffin deposition. J. Pet. Technol. 1962, 14, 1259–1269. [Google Scholar] [CrossRef]
- Ridzuan, N.; Adam, F.; Yaacob, Z. Effects of shear rate and inhibitors on wax deposition of Malaysian crude oil. Orient. J. Chem. 2015, 31, 1999–2004. [Google Scholar] [CrossRef]
- Wei, F.; Acosta, E.; Gawas, K.; Krishnamurthy, P. Targeting High Molecular Weight Wax. In Proceedings of the SPE International Symposium on Oilfield Chemistry, The Woodlands, TX, USA, 13–15 April 2015; pp. 13–15. [Google Scholar]
- Zhang, Y.; Gong, J.; Wu, H. An experimental study on wax deposition of water in waxy crude oil emulsions. Pet. Sci. Technol. 2010, 28, 1653–1664. [Google Scholar] [CrossRef]
- Akinyemi, O.P.; Udonne, J.D.; Efeovbokhan, V.E.; Ayoola, A.A. A study on the use of plant seed oils, triethanolamine and xylene as flow improvers of Nigerian waxy crude oil. J. Appl. Res. Technol. 2016, 14, 195–205. [Google Scholar] [CrossRef] [Green Version]
- Patel, M.R.; Chitte, P.S.; Bharambe, D. Oleic acid based polymeric flow improvers for Langhnaj (North Gujarat, India) crude oil. Egypt. J. Pet. 2017, 26, 895–903. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, R.; Amundsen, L. Single-phase wax deposition experiments. Energy Fuels 2010, 24, 1069–1080. [Google Scholar] [CrossRef]
- Leporini, M.; Terenzi, A.; Marchetti, B.; Giacchetta, G.; Corvaro, F. Experiences in numerical simulation of wax deposition in oil and multiphase pipelines: Theory versus reality. J. Pet. Sci. Eng. 2019, 174, 997–1008. [Google Scholar] [CrossRef]
- Rosvold, K. Wax Deposition Models; Department of Petroleum Engineering, NTNU: Taipei, Taiwan, 2008. [Google Scholar]
- Coutinho, J.A.P.; Edmonds, B.; Moorwood, T.; Szczepanski, R.; Zhang, X. Reliable wax predictions for flow assurance. Energy Fuels 2006, 20, 1081–1088. [Google Scholar] [CrossRef]
- Huang, Z.; Zheng, S.; Fogler, H.S. Wax Deposition: Experimental Characterizations, Theoretical Modeling, and Field Practices, 1st ed.; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Chi, Y.; Yang, J.; Sarica, C.; Daraboina, N. A critical review of controlling paraffin deposition in production lines using chemicals. Energy Fuels 2019, 33, 2797–2809. [Google Scholar] [CrossRef]
- Harun, A.; Ab Lah, N.K.I.N.; Husin, H.; Hassan, Z. An overview of wax crystallization, deposition mechanism and effect of temperature & shear. In Proceedings of the ICIMSA 2016 3rd International Conference on Industrial Engineering, Management Science and Applications, Jeju, Korea, 23–26 May 2016; pp. 1–5. [Google Scholar]
- Leiroz, A.; Azevedo, L. Studies on the Mechanisms Of Wax Deposition In Pipelines. In Proceedings of the Offshore Technology Conference, Houston, TX, USA, 2–5 May 2005. [Google Scholar]
- Li, M.; Su, J.; Wu, Z.; Yang, Y.; Ji, S. Study of the mechanisms of wax prevention in a pipeline with glass inner layer. Colloids Surf. A Phys. Eng. Asp. 1997, 123, 635–649. [Google Scholar] [CrossRef]
- Burger, E.; Perkins, T.; Striegler, J. Studies of Wax Deposition in the Trans Alaska Pipeline. J. Pet. Technol. 1981, 33, 1075–1086. [Google Scholar] [CrossRef]
- Wilke, C.R.; Chang, P. Correlation of diffusion coefficients in dilute solutions. AIChE J. 1955, 1, 264–270. [Google Scholar] [CrossRef]
- Ekweribe, C.K.; Civan, F.; Lee, H.S.; Singh, P. Interim report on pressure effect on waxy-crude pipeline-restart conditions investigated by a model system. SPE Proj. Facil. Constr. 2009, 4, 61–74. [Google Scholar] [CrossRef]
- Merino-Garcia, D.; Correra, S. Cold Flow: A review of a technology to avoid wax deposition. Pet. Sci. Technol. 2008, 26, 446–459. [Google Scholar] [CrossRef]
- Banki, R.; Hoteit, H.; Firoozabadi, A. Mathematical formulation and numerical modeling of wax deposition in pipelines from enthalpy–porosity approach and irreversible thermodynamics. Int. J. Heat Mass Transf. 2008, 51, 3387–3398. [Google Scholar] [CrossRef]
- Majeed, A.; Bringeal, B.; Overa, S. Model calculates wax deposition for North sea. J. Oil Gas 1990, 88, 63–69. [Google Scholar]
- Azevedo, L.F.A.; Teixeira, A.M. A critical review of the modeling of wax deposition mechanisms. Pet. Sci. Technol. 2003, 21, 393–408. [Google Scholar] [CrossRef]
- Fasano, A.; Fusi, L.; Correra, S. Mathematical models for waxy crude oils. Meccanica 2004, 39, 441–482. [Google Scholar] [CrossRef]
- Hao, L.Z.; Al-Salim, H.S.; Ridzuan, N. A review of the mechanism and role of wax inhibitors in the wax deposition and precipitation. Pertanika J. Sci. Technol. 2019, 27, 499–526. [Google Scholar]
- Hernandez, O.; Hensley, H.; Sarica, C.; Brill, J.; Volk, M.; Dellecase, E. Improvements in Single-Phase Paraffin Deposition Modeling. In Proceedings of the SPE Annual Technical Conference and Exhibition, Society of Petroleum Engineers, Denver, CO, USA, 5–8 October 2003. [Google Scholar]
- Gong, J.; Zhang, Y.; Liao, L.; Duan, J.; Wang, P.; Zhou, J. Wax deposition in the oil/gas two-phase flow for a horizontal pipe. Energy Fuels 2011, 25, 1624–1632. [Google Scholar] [CrossRef]
- Matzain, A.; Apte, M.S.; Zhang, H.-Q.; Volk, M.; Brill, J.P.; Creek, J.L. Investigation of Paraffin Deposition During Multiphase Flow in Pipelines and Wellbores—Part 1: Experiments. J. Energy Resour. Technol. 2002, 124, 180–186. [Google Scholar] [CrossRef]
- Sultan, R.; Leulmi, H. CFD Simulation Investigation of Natural Gas Components through a Drilling Pipe Faculty of Engineering and Applied Sciences; University of Newfoundland: St. John’s, NL, Canada, 2016. [Google Scholar]
- Matzain, A. Multiphase Flow Paraffin Deposition Modeling. 1999. Available online: https://www.osti.gov/servlets/purl/834175 (accessed on 22 September 2019).
- Taitel, Y.; Dukler, A. A model for predicting flow regime transitions in horizontal and near horizontal gas-liquid ow. AIChE J. 1976, 22, 47–55. [Google Scholar] [CrossRef]
- Kilincer, N. Multiphase Paraffin Deposition Behavior of a Garden Banks Condensate, M.E. Project. 2003. Available online: https://www.osti.gov/servlets/purl/834175 (accessed on 21 September 2019).
- Rittirong, A.; Panacharoensawad, E.; Sarica, C. Experimental Study of Paraffin Deposition under Two-Phase Gas/Oil Slug Flow in Horizontal Pipes. SPE Prod. Oper. 2016, 32, 99–117. [Google Scholar]
- Anosike, C.F. Effect of Flow Patterns on Oil−Water Flow Paraffin Deposition in Horizontal Pipes; University of Tulsa: Tulsa, OK, USA, 2007. [Google Scholar]
- Bruno, A. Paraffin Deposition of Crude Oil and Water Dispersions under Flowing Conditions; Tulsa University: Tulsa, OK, USA, 2006. [Google Scholar]
- Couto, G.H.; Chen, H.; Delle-Case, E.; Sarica, C.; Volk, M. An Investigation of Two-Phase Oil/Water Paraffin Deposition. SPE Prod. Oper. 2008, 23, 49–55. [Google Scholar] [CrossRef]
- Vuong, D.H.; Zhang, H.-Q.; Sarica, C.; Li, M. Experimental Study on High Viscosity Oil/Water Flow in Horizontal and Vertical Pipes. In Proceedings of the SPE Annual Technical Conference and Exhibition, New Orleans, Louisiana, USA, 4–7 October 2009; pp. 1–10. [Google Scholar]
- Huang, Z.; Lu, Y.; Hoffmann, R.; Amundsen, L.; Fogler, H.S. The effect of operating temperatures on wax deposition. Energy Fuels 2011, 25, 5180–5188. [Google Scholar] [CrossRef]
- Robustillo, M.D.; Coto, B.; Martos, C.; Espada, J.J. Assessment of different methods to determine the total wax content of crude oils. Energy Fuels 2012, 26, 6352–6357. [Google Scholar] [CrossRef]
- Phan, T.; Faust, M.; Balsamo, V.; Champion, N. A More Effective Solution to Treat Paraffinic Crude Oil Wells. In Proceedings of the SPE Production & Operations, Galveston, TX, USA, 8–9 April 2019; pp. 8–9. [Google Scholar]
- Kasumu, A.S.; Mehrotra, A.K. Solids deposition from two-phase wax–solvent–water “Waxy” mixtures under turbulent flow. Energy Fuels 2013, 27, 1914–1925. [Google Scholar] [CrossRef]
- Anisuzzaman, S.M.; Abang, S.; Bono, A.; Krishnaiah, D.; Karali, R.; Safuan, M.K. Wax inhibitor based on ethylene vinyl acetate with methyl methacrylate and diethanolamine for crude oil pipeline. IOP Conf. Ser. Mater. Sci. Eng. 2017, 206, 12074. [Google Scholar] [CrossRef] [Green Version]
- Patton, C.C. Paraffin deposition from refined wax-solvent systems. Soc. Petrol. Eng. 1970, 10, 17–24. [Google Scholar] [CrossRef]
- Subramanie, P.A.; Padhi, A.; Ridzuan, N.; Adam, F. Experimental study on the effect of wax inhibitor and nanoparticles on rheology of Malaysian crude oil. J. King Saud Univ.-Eng. Sci. 2019, in press. [Google Scholar] [CrossRef]
- Kumar, R.; Banerjee, S.; Mandal, A.; Naiya, T.K. Flow improvement of heavy crude oil through pipelines using surfactant extracted from soapnuts. J. Pet. Sci. Eng. 2017, 152, 353–360. [Google Scholar] [CrossRef]
- Atta, A.M.; El-Ghazawy, R.A.; Morsy, F.A.; Hebishy, A.M.S.; Elmorsy, A. Adsorption of polymeric additives based on Vinyl Acetate copolymers as wax dispersant and its relevance to polymer crystallization mechanisms. J. Chem. 2015, 2015, 1–8. [Google Scholar] [CrossRef]
- Yang, F.; Paso, K.; Norrman, J.; Li, C.; Oschmann, H.; Sjöblom, J. Hydrophilic nanoparticles facilitate wax inhibition. Energy Fuels 2015, 29, 1368–1374. [Google Scholar] [CrossRef]
- Tung, N.P.; Phong, N.T.P.; Long, B.Q.K.; Thuc, P.D.; Son, T.C. Studying the Mechanisms of Crude Oil Pour Point and Viscosity Reductions When Developing Chemical Additives With the Use of Advanced Analytical Tools. In Proceedings of the Society of Petroleum Engineers, Houston, TX, USA, 13–16 February 2001; pp. 1–12. [Google Scholar]
- Ruwoldt, J.; Sørland, G.H.; Simon, S.; Oschmann, H.-J.; Sjöblom, J. Inhibitor-wax interactions and PPD effect on wax crystallization: New approaches for GC/MS and NMR, and comparison with DSC, CPM, and rheometry. J. Pet. Sci. Eng. 2019, 177, 53–68. [Google Scholar] [CrossRef]
- Adeyanju, O.A.; Oyekunle, L.O. Experimental study of water-in-oil emulsion flow on wax deposition in subsea pipelines. J. Pet. Sci. Eng. 2019, 182, 106294. [Google Scholar] [CrossRef]
- El-Sheshtawy, H.S.; Khidr, T.T. Some biosurfactants used as pour point depressant for waxy egyptian crude oil. Pet. Sci. Technol. 2016, 34, 1475–1482. [Google Scholar] [CrossRef]
- Al-Sabagh, A.M.; El-Din, M.R.N.; Morsi, R.E.; Elsabee, M.Z.; El-Din, M.R.N. Styrene-Maleic Anhydride copolymer esters as flow improvers of waxy crude oil. J. Dispers. Sci. Technol. 2009, 30, 420–426. [Google Scholar] [CrossRef]
- Hafiz, A.; Khidr, T. Hexa-triethanolamine oleate esters as pour point depressant for waxy crude oils. J. Pet. Sci. Eng. 2007, 56, 296–302. [Google Scholar] [CrossRef]
- Al-Sabagh, A.M.; El-Kafrawy, A.F.; Khidr, T.T.; El-Ghazawy, R.A.; Mishrif, M.R. Synthesis and evaluation of some novel polymeric surfactants ased on aromatic amines used as wax dispersant for waxy gas oil. J. Dispers. Sci. Technol. 2007, 28, 976–983. [Google Scholar] [CrossRef]
- Machado, A.L.C.; Lucas, E.F.; González, G. Poly(ethylene-co-vinyl acetate) (EVA) as wax inhibitor of a Brazilian crude oil: Oil viscosity, pour point and phase behavior of organic solutions. J. Pet. Sci. Eng. 2001, 32, 159–165. [Google Scholar] [CrossRef]
- Lim, Z.H.; Al Salim, H.S.; Ridzuan, N.; Nguele, R.; Sasaki, K. Effect of surfactants and their blend with silica nanoparticles on wax deposition in a Malaysian crude oil. Pet. Sci. 2018, 15, 577–590. [Google Scholar] [CrossRef] [Green Version]
- He, C.; Ding, Y.; Chen, J.; Wang, F.; Gao, C.; Zhang, S.; Yang, M. Influence of the nano-hybrid pour point depressant on flow properties of waxy crude oil. Fuel 2016, 167, 40–48. [Google Scholar] [CrossRef]
- Farag, R.K. Poly (Cinnamoyloxy Ethyl Methacrylate-Co-Octadecyl Acrylate) as flow improver for Egyptian waxy crude oils. Int. J. Polym. Mater. 2008, 57, 189–202. [Google Scholar] [CrossRef]
- Taraneh, J.B.; Rahmatollah, G.; Hassan, A.; Alireza, D. Effect of wax inhibitors on pour point and rheological properties of Iranian waxy crude oil. Fuel Process. Technol. 2008, 89, 973–977. [Google Scholar] [CrossRef]
- Ansaroudi, H.R.J.; Vafaie-Sefti, M.; Masoudi, S.; Behbahani, T.J.; Jafari, H. Study of the morphology of wax crystals in the presence of Ethylene-co-vinyl Acetate copolymer. Pet. Sci. Technol. 2013, 31, 643–651. [Google Scholar] [CrossRef]
- Soni, H.P.; Kiranbala, K.S.; Agrawal, A.; Bharambe, D.P. Designing maleic anhydride-α-olifin copolymeric combs as wax crystal growth nucleators. Fuel Process. Technol. 2010, 91, 997–1004. [Google Scholar] [CrossRef]
- Al-Yaari, M. Paraffin Wax Deposition: Mitigation and Removal Techniques. In Proceedings of the SPE Saudi Arabia, Dhahran, Saudi Arabia, 14–16 March 2011. [Google Scholar]
- Zhang, C.; Gao, C.; Gao, F.; Wang, J.; Zhang, D.; Wang, Y.; Xu, D. Synthesis of comb bipolymers and their pour point depressing properties. Pet. Sci. 2014, 11, 155–160. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, S.M.; Khidr, T.T.; Ismail, D.A. Effect of gemini surfactant additives on pour point depressant of crude oil. J. Dispers. Sci. Technol. 2018, 39, 1160–1164. [Google Scholar] [CrossRef]
- Maithufi, M.N.; Joubert, D.J.; Klumperman, B. Application of Gemini Surfactants as diesel fuel wax dispersants. Energy Fuels 2011, 25, 162–171. [Google Scholar] [CrossRef]
- Sahai, M.; Singh, R.K.; Kukrety, A.; Kumar, A.; Ray, S.S.; Chhibber, V.K.; Kumar, S. Application of Triazine-Based Gemini surfactants as viscosity reducing agents of Tar Sand derived bituminous crude. Energy Fuels 2018, 32, 3031–3038. [Google Scholar] [CrossRef]
- Coto, B.; Martos, C.; Espada, J.J.; Robustillo, M.D.; Peña, J.L. Experimental study of the effect of inhibitors in wax precipitation by different techniques. Energy Sci. Eng. 2014, 2, 196–203. [Google Scholar] [CrossRef]
- Sivakumar, P.; Sircar, A.; Deka, B.; Anumegalai, A.S.; Moorthi, P.S.; Yasvanthrajan, N. Flow improvers for assured flow of crude oil in midstream pipeline—A review. J. Pet. Sci. Eng. 2018, 164, 24–30. [Google Scholar] [CrossRef]
- Taiwo, E.; Otolorin, J.; Afolabi, T. Crude Oil Transportation: Nigerian Niger Delta Waxy Crude, Crude Oil Exploration in the World 2010. 2012. Available online: https://www.intechopen.com/books/crude-oil-exploration-in-the-world/crude-oil-transportation-nigerian-niger-delta-waxy-crude-oil (accessed on 15 September 2019).
- Ridzuan, N.; Adam, F.; Yaacob, Z.; Ump, P. Molecular Recognition of Wax Inhibitor through Pour Point Depressant. Spetember 2014, pp. 1–9. Available online: http://www.pertanika.upm.edu.my/Pertanika%20PAPERS/JST%20Vol.%2027%20(1)%20Jan.%202019/29%20JST-1110-2018.pdf (accessed on 15 September 2019).
- Xu, J.; Zhang, X.; Sun, J.; Li, L.; Guo, X. How comb-type poly (maleic acid alkylamide-co-a-olefin) assemble in waxy oils and improve flowing ability. Asia-Pac. J. Chem. Eng. 2018, 58, 2254–2261. [Google Scholar] [CrossRef]
- Li, L.; Xu, J.; Tinsley, J.; Adamson, D.; Pethica, B.; Huang, J.; Prud’homme, R.; Guo, X. Improvement of oil flowability by assembly of comb-type copolymers with paraffin and asphaltene. AIChE J. 2012, 58, 2254–2261. [Google Scholar] [CrossRef]
- Borthakur, A.; Chanda, D.; Choudhury, S.R.D.; Rao, K.V.; Subrahmanyam, B. Alkyl Fumarate−Vinyl Acetate copolymer as flow improver for high waxy Indian crude oils. Energy Fuels 1996, 10, 844–848. [Google Scholar] [CrossRef]
- Yang, F.; Li, C.; Lin, M.; Li, Z.; Yu, T. Depressive effect of polyacrylate (PA) pour point depressant on waxy crude oils. J. Petrochem. Univ. 2009, 22, 20–25. [Google Scholar]
- Wang, F.; Zhang, D.; Ding, Y.; Zhang, L.; Yang, M.; Jiang, B.; Huo, L. The effect of nanohybrid materials on the pour-point and viscosity depressing of waxy crude oil. Chin. Sci. Bull. 2011, 56, 14–17. [Google Scholar] [CrossRef] [Green Version]
- Yang, F.; Yao, B.; Li, C.; Shi, X.; Sun, G.; Ma, X. Performance improvement of the ethylene-vinyl acetate copolymer (EVA) pour point depressant by small dosages of the polymethylsilsesquioxane (PMSQ) microsphere: An experimental study. Fuel 2017, 207, 204–213. [Google Scholar] [CrossRef]
- Gateau, P.; Henaut, I.; Barre, L.; Argillier, J. Heavy oil dilution. Oil Gas Sci. Technol. 2004, 59, 503–509. [Google Scholar] [CrossRef]
- Bello, O.; Fasesan, S.; Teodoriu, C.; Reinicke, K. An Evaluation of the performance of selected wax inhibitors on paraffin deposition of Nigerian crude oils. Pet. Sci. Technol. 2006, 24, 195–206. [Google Scholar] [CrossRef]
- Straub, T.; Autry, S.; King, G. An Investigation into Practical Removal of Downhole Paraffin by Thermal Methods and Chemical Solvents. In Proceedings of the SPE Production & Operations, Olahoma City, OK, USA, 13–14 March 1989. [Google Scholar]
- Woo, G.; Garbis, S.; Gray, T. Long-Term Control of Paraffin Deposition. In Proceedings of the SPE Annual Technical Conference and Exhibition, Houston, TX, USA, 16–19 September 1984. [Google Scholar]
- Ahmed, N.S.; Nassar, A.M.; Zaki, N.N.; Gharieb, H.K. Stability and rheology of heavy crude oil-in-water emulsion stabilized by an anionic- nonionic surfactant mixture. Pet. Sci. Technol. 1999, 17, 553–576. [Google Scholar] [CrossRef]
- Khidr, T.T.; Doheim, M.M.; El-Shamy, O.A.A. Pour point depressant of fuel oil using non-ionic surfactants. Pet. Sci. Technol. 2015, 33, 1619–1626. [Google Scholar] [CrossRef]
- Okoliegbe, L.; Agarry, O. Application of microbial surfactant (A review). Sch. J. Biotechnol. 2012, 1, 15–23. [Google Scholar]
- Oguntimein, G.; Erdmann, H.; Schmid, R. Lipase catalysed synthesis of sugar eser in organic solvents. Biotechnol. Lett. 1993, 15, 175–180. [Google Scholar] [CrossRef]
- Chen, G.; Bai, Y.; Zhang, J.; Yuan, W.; Song, H.; Jeje, A. Synthesis of new flow improvers from canola oil and application to waxy crude oil. Pet. Sci. Technol. 2016, 34, 1285–1290. [Google Scholar] [CrossRef]
- Marie, E.; Chevalier, Y.; Eydoux, F.; Germanaud, L.; Flores, P. Control of n-alkanes crystallization by ethylene–vinyl acetate copolymers. J. Colloid Interface Sci. 2005, 290, 406–418. [Google Scholar] [CrossRef] [PubMed]
- Naiya, T.K.; Banerjee, S.; Kumar, R.; Mandal, A. Heavy Crude Oil Rheology Improvement Using Naturally Extracted Surfactant. In Proceedings of the SPE Oil & Gas India Conference and Exhibition, Society of Petroleum Engineers, Mumbai, India, 24–26 November 2015. [Google Scholar]
- Wei, B. Recent advances on mitigating wax problem using polymeric wax crystal modifier. J. Pet. Explor. Prod. Technol. 2015, 5, 391–401. [Google Scholar] [CrossRef] [Green Version]
- Ashbaugh, H.S.; Fetters, L.J.; Adamson, D.; Prud’homme, R.K. Flow improvement of waxy oils mediated by self-aggregating partially cystallizable diblock copolymers. J. Rheol. 2002, 46, 763–776. [Google Scholar] [CrossRef]
- Leube, W.; Monkenbusch, M.; Schneiders, D.; Richter, D.; Adamson, D.; Fetters, L.; Dounis, P.; Lovegrove, R. Wax-Crystal modification for fuel oils by self-aggregating partially crystallizable hydrocarbon block copolymers. Energy Fuels 2000, 14, 419–430. [Google Scholar] [CrossRef] [Green Version]
- Chi, Y.; Daraboina, N.; Sarica, C. Effect of the Flow Field on the Wax Deposition and Performance of Wax Inhibitors: Cold Finger and Flow Loop Testing. Energy Fuels 2017, 31, 4915–4924. [Google Scholar] [CrossRef]
- Dubey, A.; Chi, Y.; Daraboina, N. Investigating the Performance of Paraffin Inhibitors under Different Operating Conditions. In Proceedings of the SPE Annual Technical Conference and Exhibitionn, Society of Petroleum Engineers, San Antonio, Texas, USA, 9–11 October 2017; pp. 1–20. [Google Scholar]
- Quan, Q.; Gong, J.; Wang, W.; Gao, G. Study on the aging and critical carbon number of wax deposition with temperature for crude oils. J. Pet. Sci. Eng. 2015, 130, 1–5. [Google Scholar] [CrossRef]
- Jennings, D.W.; Breitigam, J. Paraffin inhibitor formulations for different application environments: From heated injection in the desert to extreme cold arctic temperatures. Energy Fuels 2010, 24, 2337–2349. [Google Scholar] [CrossRef]
- Kelland, M. Production Chemicals for the Oil and Gas Industry. CRC Press. 2014. Available online: https://books.google.com.my/books/about/Production_Chemicals_for_the_Oil_and_Gas.html?id=Aw6xjq2cBaQC&redir_esc=y (accessed on 1 October 2019).
- Beiny, D.H.M.; Mullins, J.W.; Lewtas, K. Crystallization of N-Dotriacontaine from hydrocarbon solution with polymeric additives. J. Cryst. Growth 1990, 102, 801–806. [Google Scholar] [CrossRef]
- Manka, J.S.; Ziegler, K.L. Factors affecting the performance of crude oil wax-control additives. Soc. Pet. Eng. SPE 2001, 67326, 1–7. [Google Scholar]
- Qian, J.W.; Qi, G.R.; Xu, Y.L.; Yang, S.L. Solvent effect on the action of ethylene-vinyl acetate copolymer pour point depressant in waxy solutions. J. Appl. Polym. Sci. 1996, 60, 1575–1578. [Google Scholar] [CrossRef]
- Xiong, C.X. The structure and activity of polyalphaolefins as pour point depressants. Lub. Eng. 1993, 49, 196–200. [Google Scholar]
- Venkatesan, R.; Östlund, J.-A.; Chawla, H.; Wattana, P.; Nydén, M.; Fogler, H.S. The effect of asphaltenes on the gelation of waxy oils. Energy Fuels 2003, 17, 1630–1640. [Google Scholar] [CrossRef]


| Model | Software | Description | References |
|---|---|---|---|
| Rygg, Rydahl and R∅nningsen | OLGATM | Multiphase flow wax deposition model which predicts wax deposition in wells as well as pipelines. | [31] |
| Matzain | OLGATM | Semi empirical model which takes into account shear stripping, molecular diffusion and shear dispersion to predict wax deposition. | [5] |
| Heat Analogy | OLGATM | Computes the mass transfer rate of wax utilizing the heat transfer analogy. | [30] |
| University of Michigan Model | LedaFlow | Models the wax crystallization and the wax deposition on pipe wall. | [30] |
| FloWax | FloWax | A complete compositional wax deposition model that considers the thermodynamics wax precipitation, wax diffusion based on heat and mass transfer analogy and the shearing effect. | [32] |
| Type of Crude Oil | Sample Used as Wax Dispersant | Year/References |
|---|---|---|
| Malaysia | Poly (ethylene-co-vinyl acetate)/poly (maleic anhydride-alt-1-octadecene)/nano particle sodium cloisite | 2019/[65] |
| Indian | Sapindus mukorossi sp. | 2017/[66] |
| Egyptian | Poly (octadecyl acrylate) | 2015/[67] |
| Laboratory sample | Poly (octadecyl acrylate)/nanosilica hybrid particles | 2015/[68] |
| Nada | Poly (n-alkyl recinoleate-co-N-hexadecyl maleimide) | 2012/[20] |
| Dragon RPI | Non-ionic surfactant | 2001/[69] |
| Type of Crude Oil | Sample Used as Pour Point Depressant | Year/References |
|---|---|---|
| Norwegian | Polycarboxilate/poly acrylate/poly vinyl acetate | 2019/[70] |
| Nigerian | Poly acrylate ester copolymer | 2019/[71] |
| Nigerian | Jatropha seed oil | 2018/[18] |
| Laboratory sample | Ethylene vinyl acetate-co-diethanolamine | 2017/[63] |
| Langhnaj | Poly (hexyl oleate-co-hexadecyl maleamide-co-n-alkyl oleate) | 2017/[28] |
| Egyptian | Halomonas xianhensis sp. | 2016/[72] |
| Changqing | Poly (octadecylarylate)/Clay nano composite | 2009/[73] |
| Umbaraka | Monohexatriethanolamine | 2007/[74] |
| Egyptian | Polyster R 1000 (1,3-dicarboxymethoxy benzene with polyethylene glycol with molecular weight of 1000). | 2007/[75] |
| White Tiger CTP2 | Poly (ethylene-co-vinyl acetate) | 2001/[69] |
| Albacor | Poly (ethylene-co-vinyl acetate) | 2001/[76] |
| Badejo | Poly (ethylene-co-vinyl acetate) | 2001/[76] |
| Type of Crude Oil | Sample Used as Wax Crystal Modifier | Year/References |
|---|---|---|
| Malaysia | Silane-based surfactants with SiO2 particles | 2018/[77] |
| China (Xuzhou) | Nano composite of montmorillonite | 2016/[78] |
| Karama | Poly (cinamoyloxy ethyl methacrylate-co-octadecyl arylate) | 2008/[79] |
| Iranian | Ethylene vinyl acetate copolymer | 2008/[80] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ragunathan, T.; Husin, H.; Wood, C.D. Wax Formation Mechanisms, Wax Chemical Inhibitors and Factors Affecting Chemical Inhibition. Appl. Sci. 2020, 10, 479. https://doi.org/10.3390/app10020479
Ragunathan T, Husin H, Wood CD. Wax Formation Mechanisms, Wax Chemical Inhibitors and Factors Affecting Chemical Inhibition. Applied Sciences. 2020; 10(2):479. https://doi.org/10.3390/app10020479
Chicago/Turabian StyleRagunathan, Thevaruban, Hazlina Husin, and Colin D. Wood. 2020. "Wax Formation Mechanisms, Wax Chemical Inhibitors and Factors Affecting Chemical Inhibition" Applied Sciences 10, no. 2: 479. https://doi.org/10.3390/app10020479
APA StyleRagunathan, T., Husin, H., & Wood, C. D. (2020). Wax Formation Mechanisms, Wax Chemical Inhibitors and Factors Affecting Chemical Inhibition. Applied Sciences, 10(2), 479. https://doi.org/10.3390/app10020479






