Sediment Characteristics of Beachrock: A Baseline Investigation Based on Microbial Induced Carbonate Precipitation at Krakal-Sadranan Beach, Yogyakarta, Indonesia
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Investigation
2.2. Laboratory Analysis
2.2.1. Petrographic Studies
2.2.2. Inductively Coupled Plasma-Atomic Emission Spectroscopy and Mass Spectrometry (ICP-AES and MS)
2.2.3. Algae Taxonomy
2.2.4. Isolation and Identification of a Suitable Urease Active Bacterium
2.2.5. Cultivation of Bacteria and Assessing the Potential for Microbial Induced Carbonate Precipitation
2.2.6. Syringe Biocementation Test
3. Results
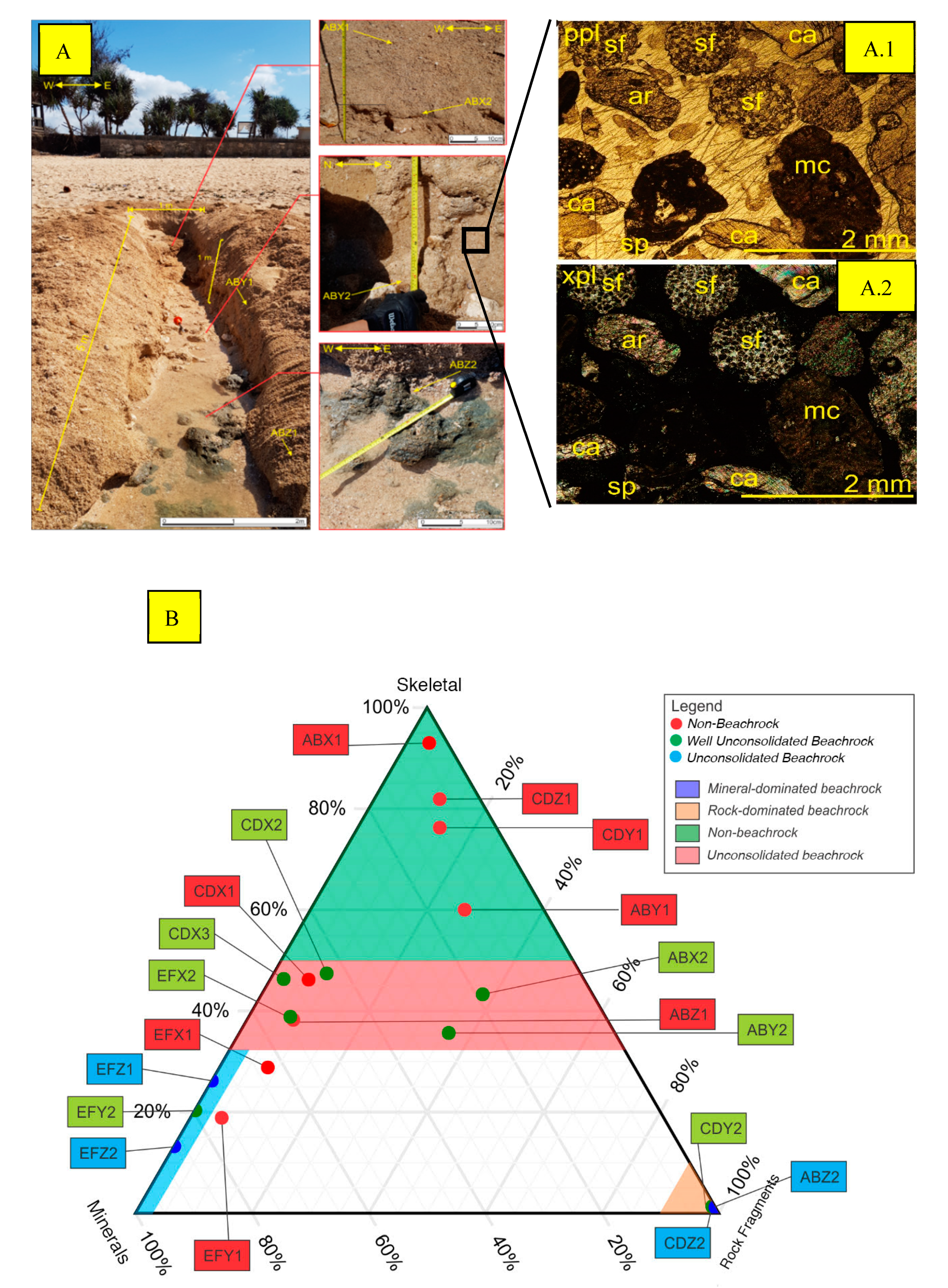
3.1. Stratigraphy of Krakal-Sadranan Beachrock
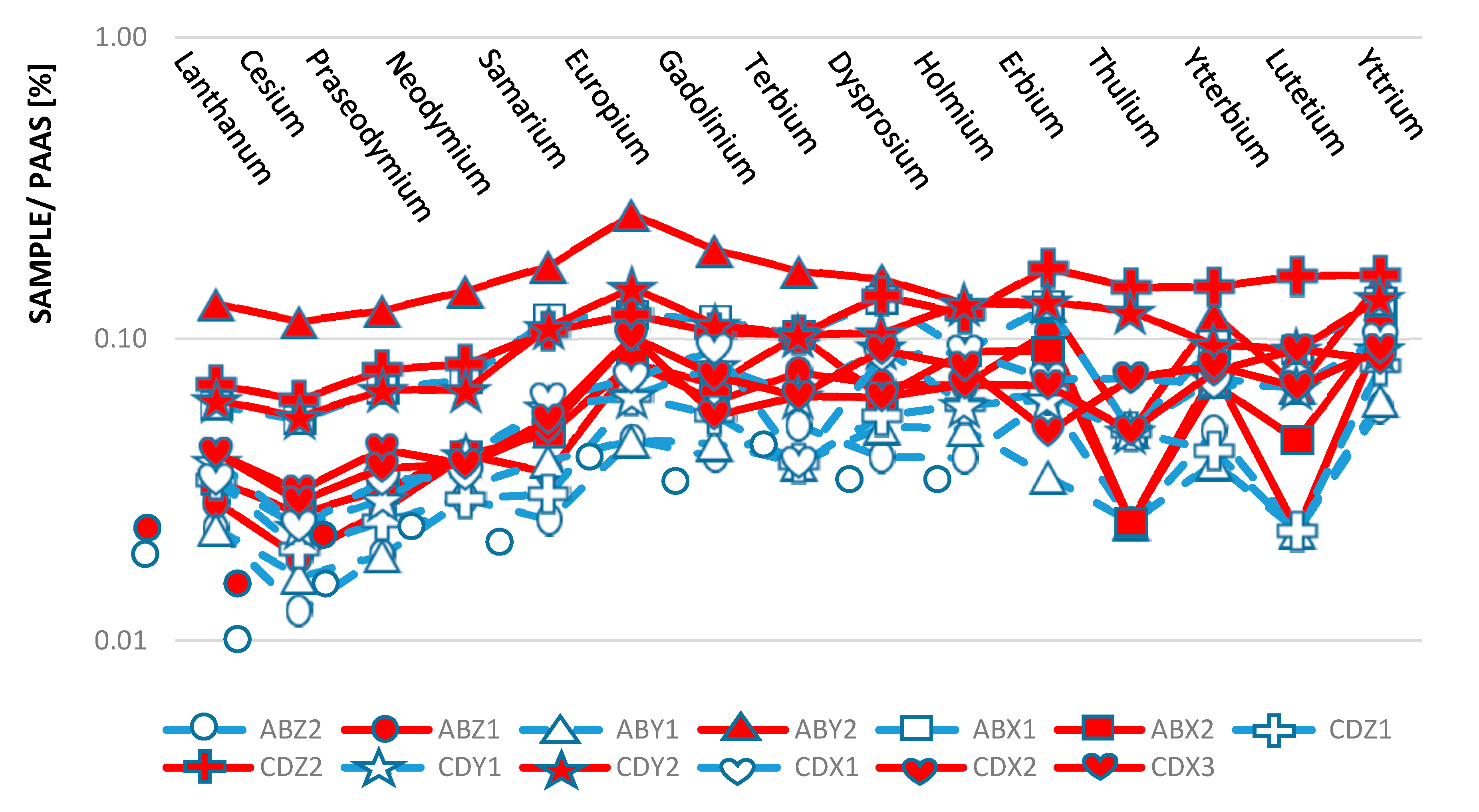
3.2. ICP-AES and ICP-MS Analysis
3.3. Biodiversity Observations
3.3.1. Algae Taxonomy
3.3.2. Isolation of Indigenous Ureolytic Bacteria
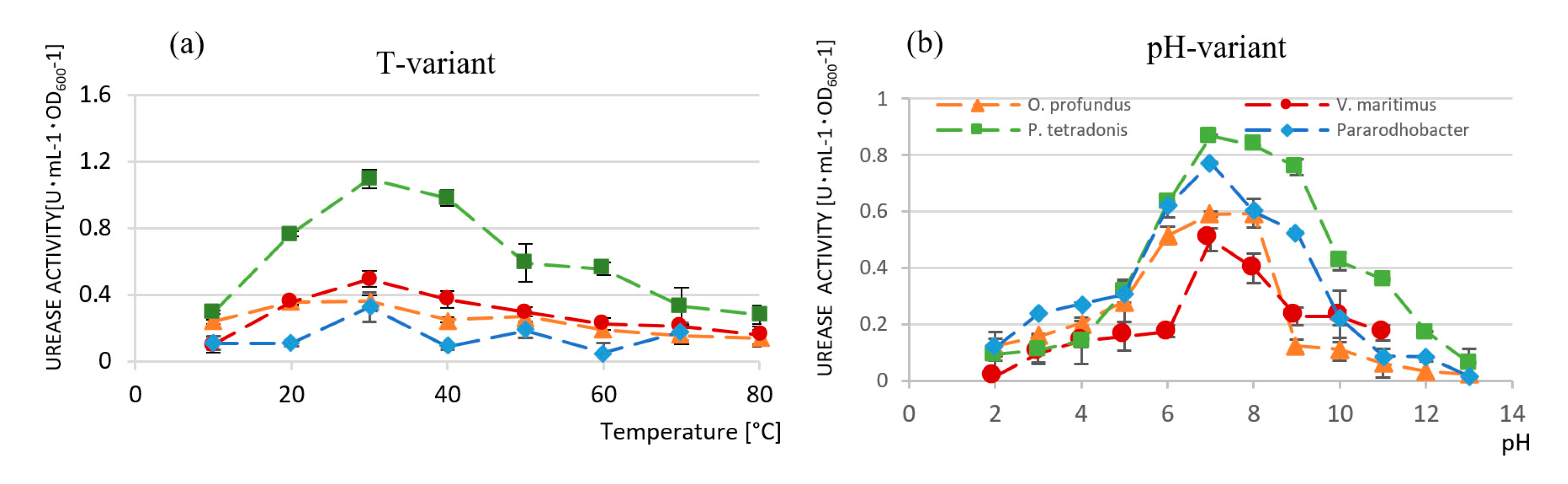
3.3.3. Microbial Population Count and Urease Activity Test
3.4. Indigenous Ureolytic Bacteria Ability for Beach Sand Solidification by MICP Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vousdoukas, M.I.; Velegrakis, A.F.; Plomaritis, T.A. Beachrock occurrence, characteristics, formation mechanisms and impacts. Earth Sci. Rev. 2007, 85, 23–46. [Google Scholar] [CrossRef]
- Russell, R.J.; McIntire, W.G. Beach cusps. Geol. Soc. Am. Bull. 1965, 76, 307–320. [Google Scholar] [CrossRef]
- Woodroffe, C.D.; Stoddart, D.R.; Spencer, T.; Scoffin, T.P.; Tudhope, A.W. Holocene emergence in the Cook Islands, South Pacific. Coral Reefs 1990, 9, 31–39. [Google Scholar] [CrossRef]
- Pirazzoli, P.A.; Costa, S.; Dornbusch, U. Flood threat anomaly for the low coastal areas of the English Channel based on analysis of recent characteristic flood occurrences. Ocean Dyn. 2007, 57, 501–510. [Google Scholar] [CrossRef]
- McLean, R. Beach Rockin the Encyclopedia of Modern Coral Reefs: Structure, Form and Process; Springer: Dordrecht, The Netherland, 2011; p. 1236. [Google Scholar]
- Avcioğlu, M.; Yiğitbaş, E.; Erginal, A.E. Beachrock formation on the coast of Gökçeada Island and its relation to the active tectonics of the region, northern Aegean Sea, Turkey. Quat. Int. 2016, 401, 141–152. [Google Scholar] [CrossRef]
- Ginsburg, R.N. Beachrock in south Florida. J. Sediment. Res. 1953, 23, 85–92. [Google Scholar] [CrossRef]
- Kelletat, D. Beachrock as sea-level indicator? Remarks from a geomorphological point of view. J. Coast. Res. 2006, 22, 1558–1564. [Google Scholar] [CrossRef]
- Moore, W.S. The subterranean estuary: A reaction zone of ground water and sea water. Mar. Chem. 1999, 65, 111–125. [Google Scholar] [CrossRef]
- Neumeier, U. Le Rôle de L’activité Microbienne dans la Cimentation Précoce des Beachrock (Sédiments Intertidaux)—The Role of Microbial Activity in Early Cementation of Beachrock. Ph.D. Thesis, University of Geneva, Geneva, Switzerland, 1998. [Google Scholar]
- Zhu, X.; Linham, M.M.; Nicholls, R.J. Technologies for Climate Change Adaptation—Coastal Erosion and Flooding; TNA Guidebook Series; Danmarks Tekniske Universitet, Risø Nationallaboratoriet for Bæredygtig Energi: Roskilde, Denmark; University of Southampton: Southampton, UK, 2010; p. 167. [Google Scholar]
- Jones, A.; Phillips, M. Disappearing Destinations: Climate Change and the Future Challenges for Coastal Tourism; Cabi: London, UK, 2011; p. 296. [Google Scholar]
- Pilkey, O.H.; Cooper, A. The Last Beach; Duke University Press: Durham, UK, 2014; p. 256. [Google Scholar]
- Barragan, J.M.; Andreis, M. Analysis and trends of the world’s coastal cities and agglomerations. Ocean Coast Manag. 2015, 114, 11–20. [Google Scholar] [CrossRef]
- Shi, P.; Kasperson, R. World Atlas of Natural Disaster Risk; Springer: Berlin/Heidelberg, Germany, 2015; p. 368. [Google Scholar] [CrossRef]
- Van Bemmelen, R.W. General Geology of Indonesia and Adjacent Archipelagoes; Government Printing House: The Hague, The Netherlands, 1949; p. 732. [Google Scholar]
- Surono, B.T.; Sudarno, I. Geological Map of the Surakarta-Giritontro Quadrangles, Jawa, 1408-3 and 1407-6 Quadrangles, Scale 1:100.000; Research Center and Geology Development, Department of Mining and Energy: Jakarta, Indonesia, 1992. [Google Scholar]
- Toha, B.; Purtyasti, R.D.; Srijono Soetoto Rahardjo, W.; Pramumijoyo, S. Geology of Southern Mountain Area: A Contribution (in Indonesian). In Geology and Geotechnic Java Island, Since Late Mesozoic to Quarternary; Indonesian Journal ON Geoscience: Jakarta, Indonesia, 1994; pp. 19–36. [Google Scholar]
- Sudarno, I. Tectonic Control toward Structure Occurrences in Paleogene and Neogene Rock of Java Southern Mountain, Yogyakarta, Indonesia. Master’s Thesis, Bandung Institute of Technology, Bandung, Indonesia, 1997. [Google Scholar]
- Verstappen, H.T. Outline of the Geomorphology of Indonesia; ITC Enschede: Enschede, The Netherlands, 2000; Volume 79, p. 212. [Google Scholar]
- Nichols, G. Sedimentology and Stratigraphy; John Wiley & Sons: West Sussex, UK, 2009; p. 420. [Google Scholar]
- Martin, T.D.; Brockhoff, J.T.; Creed, J.T. Method 200.7 Determination of Metals and Trace Elements in Water and Wastes by Inductively Coupled Plasmaeatomic Emission Spectrometry Revision 4.4 EMMC Version, 200.7 e1, 58; U. S. EPA: Washington, DC, USA, 1994.
- Condie, K.C.; Wilks, M.; Rosen, D.M.; Zlobin, V.L. Geochemistry of metasediments from the Precambrian Hapschan series, eastern Anabar Shield, Siberia. Precambrian Res. 1991, 50, 37–47. [Google Scholar] [CrossRef]
- Guimaraes, J.T.F.; Cohen, M.C.L.; Franca, M.C.; Silva, A.K.T.D.; Rodrigues, S.F.S. Mineralogical and Geochemical Influences on Sediment Color from Amazon Wetlands Analyzed by Visible Spectrophotometry. Acta Amaz. 2013, 43, 331–342. [Google Scholar] [CrossRef]
- Rollinson, H.R. Using Geochemical Data: Evaluation, Presentation, Interpretation; Routledge: London, UK, 2014; p. 73. [Google Scholar]
- Folk, R.L. Spectral Subdivision of Limestone Types. In Classification of Carbonate Rocks; Ham, W.E., Ed.; American Association of Petroleum Geologists: Tulsa, OK, USA, 1962; pp. 62–84. [Google Scholar] [CrossRef]
- Pratama, W.; Dewi, S.C.; Sari, I.Z.R.; Hardiyati, A.; Wajong, A.E. Distribution and Abundance of Macroalgae in Intertidal Zone of Drini Beach, Gunung Kidul, DIY. KnE Life Sci. 2015, 2, 514–515. [Google Scholar] [CrossRef] [Green Version]
- Romimohtarto, K.; Juwana, S. Biologi Laut: Ilmu Pengetahuan Tentang Biota Laut; Djambatan: Jakarta, Indonesia, 2009. [Google Scholar]
- Trono, G.C., Jr. Seaweed. In The Living Marine Resources of the Western Central Pacific. Volume 1. Seaweed, Corals, Bivalves, and Gastropods; Carpenter, K.E., Niem, V.H., Eds.; Food and Agriculture Organization of the United Nations: Rome, Italy, 1998; pp. 19–96. [Google Scholar]
- Baldrian, P. The known and the unknown in soil microbial ecology. FEMS Microbiol. Ecol. 2019, 95. [Google Scholar] [CrossRef] [PubMed]
- Karkani, A.; Evelpidou, N.; Maroukian, H.; Kawasaki, S. Study of Beachrock in East Attica. Bull. Geol. Soc. Greece 2016, 50, 434–440. [Google Scholar] [CrossRef] [Green Version]
- Porta, G.D. Carbonate Build-Ups in Lacustrine, Hydrothermal and Fluvial Settings: Comparing Depositional Geometry, Fabric Types and Geochemical Signature; Special Publication; Geological Society: London, UK, 2015; Volume 418, pp. 17–68. [Google Scholar] [CrossRef]
- Alshuaibi, A.A.; Khalaf, F.I.; Al-Zamel, A. Calcareous thrombolitic crust on Late Quaternary beachrock in Kuwait, Arabian Gulf. Arab. J. Geosci. 2015, 8, 9721–9732. [Google Scholar] [CrossRef]
- Atmoko, D.D.; Titisari, A.D.; Idrus, A. Geochemical Characteristics of Limestone of Wonosari-Punung Formation, Gunungkidul Regency, Yogyakarta, Indonesia. Indones. J. Geosci. 2018, 5, 179–197. [Google Scholar] [CrossRef]
- Bolhar, R.; Kamber, B.S.; Moorbath, S.; Fedo, C.M.; Whitehouse, M.J. Characterisation of Early Archaean Chemical Sediments by Trace Element Signatures. Earth Planet. Sci. Lett. 2004, 222, 43–60. [Google Scholar] [CrossRef]
- Janetzki, N.; Fairweather, P.G.; Benkendorff, K. Inventory of Rock Types, Habitats, and Biodiversity on Rocky Seashores in South Australia’s Two South-East Marine Parks: Pilot Study; A Report to the South Australian Department of Environment, Water, and Natural Resources; Flinders University: Adelaide, Australia, 2015; p. 61. [Google Scholar]
- Mauz, B.; Vacchi, M.; Green, A.; Hoffmann, G.; Cooper, A. Beachrock: A tool for reconstructing relative sea level in the far-field. Mar. Geol. 2015, 362, 1–16. [Google Scholar] [CrossRef]
- Tostevin, R.; Shields, G.A.; Tarbuck, G.M. Effective Use of Cerium Anomalies as a Redox Proxy in Carbonate-dominated Marine Settings. Chem. Geol. 2016, 438, 146–162. [Google Scholar] [CrossRef] [Green Version]
- Fujita, M.; Nakashima, K.; Achal, V.; Kawasaki, S. Whole-cell Evaluation of Urease Activity of Pararhodobacter sp., Isolated from Peripheral Beachrock. Biochem. Eng. J. 2017, 124, 1–5. [Google Scholar] [CrossRef]
- Oliver, J.D.; Pruzzo, C.; Vezzulli, L.; Kaper, J.B. Vibrio species. In Food Microbiology; American Society of Microbiology: Washington, DC, USA, 2013; pp. 401–439. [Google Scholar]
- Elhadi, N.; Radu, S.; Chen, C.H.; Nishibuchi, M. Prevalence of potentially pathogenic Vibrio species in the seafood marketed in Malaysia. J. Food Prot. 2004, 67, 1469–1475. [Google Scholar] [CrossRef] [PubMed]
- Danjo, T.; Kawasaki, S. Formation Mechanism of Beachrock in Okinawa and Ishikawa, Japan, with a Focus on Cements. Mater. Trans. 2014, 55, 493–500. [Google Scholar] [CrossRef] [Green Version]
- Taylor, J.C.; Martin, H.C.; Lise, S.; Broxholme, J.; Cazier, J.B.; Rimmer, A.; Kanapin, A.; Lunter, G.; Fiddy, S.; Allan, C.; et al. Factors influencing success of clinical genome sequencing across a broad spectrum of disorders. Nat. Genet. 2015, 47, 717. [Google Scholar] [CrossRef]
- Daryono, L.R.; Titisari, A.D.; Warmada, I.W.; Kawasaki, S. Comparative characteristics of cement materials in natural and artificial beachrock using a petrographic method. Bull. Eng. Geol. Environ. 2018, 78, 3943–3958. [Google Scholar] [CrossRef]
- Kandianis, M.T.; Fouke, B.W.; Johnson, R.W.; Veysey, J.; Inskeep, W.P. Microbial biomass: A catalyst for CaCO3 precipitation in advection-dominated transport regimes. Geol. Soc. Am. Bull. 2008, 120, 442–450. [Google Scholar] [CrossRef]
- Van Paassen, L.A.; Pieron, M.; Mulder, A.; Van der Linden, T.J.M.; Van Loosdrecht, M.C.M.; Ngan-Tillard, D.J.M. Strength and deformation of biologically cemented sandstone. In Proceedings of the ISRM Regional Conference EUROCK, Cavtat, Croatia, 29–31 October 2009; pp. 405–410, ISBN 978-0-415-80481-3. [Google Scholar]
- Ng, W.S.; Lee, L.M.; Khun, T.C.; Ling, H.S. Factors Affecting Improvement in Engineering Properties of. Residual Soil through Microbial-Induced Calcite Precipitation. J. Geotech. Geoenviron. Eng 2014. [Google Scholar] [CrossRef]
- Okwadha, G.D.O.; Li, J. Optimum conditions for microbial carbonate precipitation. Chemosphere 2010, 81, 1143–1148. [Google Scholar] [CrossRef]
- Zhu, T.; Dittrich, M. Carbonate Precipitation through Microbial Activities in Natural Environment, and Their Potential in Biotechnology: A Review. Front. Bioeng. Biotechnol. 2016, 4. [Google Scholar] [CrossRef] [Green Version]
- Almajed, A.; Khodadadi Tirkolaei, H.; Kavazanjian, E., Jr. Baseline Investigation on Enzyme Induced Calcium Carbonate Precipitation. J. Geotech. Geoenviron. Eng. 2018, 144. [Google Scholar] [CrossRef]
- Bathurst, R.G.C. Boring algae, micrite envelopes and lithification of molluscan biosparites. Geol. J. 1966, 5, 15–32. [Google Scholar] [CrossRef]
- Mwandira, W.; Nakashima, K.; Kawasaki, S.; Ito, M.; Sato, T.; Igarashi, T.; Chirwa, M.; Banda, K.; Nyambe, I.; Nakayama, S.; et al. Solidification of sand by Pb (II)-tolerant bacteria for capping mine waste to control metallic dust: Case of the abandoned Kabwe Mine, Zambia. Chemosphere 2019, 228, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.; Barnhart, A.; Butcher, A.; Freimuth, C.; Khaw, F.; Lafromboise, E.; Landeros, M.; Morrish, S.; Olson, E.; Ritzinger, B.; et al. Beachrock horizons of the Nicoya Peninsula, Costa Rica: Geomorphology, petrology, and neotectonic significance. In Proceedings of the Coastal Sediments, San Diego, CA, USA, 11–15 May 2015. [Google Scholar] [CrossRef]
- Arrieta, N.; Iturregui, A.; Martínez-Arkarazo, I.; Murelaga, X.; Baceta, J.I.; de Diego, A.; Olazabal, M.Á.; Madariaga, J.M. Characterization of ferruginous cements related with weathering of slag in a temperate anthropogenic beachrock. Sci. Total Environ. 2017, 581, 49–65. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.L. Carbonate Facies in Geologic History; Springer: New York, NY, USA, 1975; p. 471. [Google Scholar]
- James, N.P.; Bourque, P.A. Reefs and mounds. In Facies Models; Walker, R.G., James, N.P., Eds.; Geological Association of Canada: St. Johns, NL, Canada, 1992; pp. 323–345. [Google Scholar]
- Schlager, W. Carbonate Sedimentology and Sequence Stratigraphy (No. 8); SEPM Society for Sedimentary Geology: Tulsa, OK, USA, 2005. [Google Scholar]
- Wolf, K.H. Gradational sedimentary products of calcareous algae. Sedimentology 1965, 5, 1–37. [Google Scholar] [CrossRef]




| Reagent | (g/20 L) |
|---|---|
| MgCl2·6H2O | 222.23 |
| CaCl2·2H2O | 30.7 |
| SrCl2·6H2O | 0.85 |
| SrCl2·6H2O | 13.89 |
| KCl | 4.02 |
| NaHCO3 | 2.01 |
| KBr | 0.54 |
| H3BO3 | 0.06 |
| NaF | 490.68 |
| NaCl | 81.88 |
| Na2SO4 | 0.06 |
| Reagent | Content (g/L) |
|---|---|
| Nutrient broth | 3.0 |
| NH4Cl | 10.0 |
| NaHCO3 | 2.12 |
| Urea, Co(NH2)2 | 18.02 |
| CaCl2 | 33.3 |
| No. | Location 07°08′42,55″–110°26′11,31″ | |||||
|---|---|---|---|---|---|---|
| Division | Class | Ordo | Familia | Genus | Species Name | |
| 1. | Rhodophyta | Florideophyceae | Ceramiales | Rhodomelaceae | Laurencia | Laurencia pappilosa (C. Agardh) Greville |
| 2. | Gelidiales | Gelidiaceae | Gelidium | Gelidium corneum (Hudson) Lam. | ||
| 3. | Rhodophyceae | Gigartinales | Gigartinaceae | Gigartina | Gigartina sp | |
| 4. | Rhodymeniales | Rhodymeniceae | Rhodymenia | Rhodymenia pseudopalmata (Lam.) P.Silva | ||
| 5. | Phaeophyta | Phaeophyceae | Fucales | Sargassaceae | Sargassum | Sargassum duplicatum Bory |
| 6. | Chlorophyta | Chlorophyceae | Ulotrichales | Ulvaceae | Ulva | Ulva Lactuca L. |
| Condition | Limestone Sands | Beachrock | Interpretation |
|---|---|---|---|
| Sr concentration | Raised (~3830 ppm) | Depleted (~3417 ppm) | Beachrock postdepositional diagentic alteration |
| Tm Anomaly | Negative | Negative | Higher temperature (37 °C–50 °C) and intensive evaporation |
| REE concentration | Raised (∑REE = 12.52 ppm) | Raised (∑REE = 24.03 ppm) | Oxidative Environment |
| Mn* | Mn* positive | Mn * positive | Oxidative Environment |
| Ce/Ce* value and Ce anomaly | Less 1 | Less 1 | Precipitation from seawater under oxidative conditions |
| Eu/Eu* value and Eu anomaly | Positive anomaly with value > 1 | Positive anomaly with value > 1 | Terigenus material contamination |
| Y/Ho ratio | High ratio > 26 | High ratio > 26 | Terigenus material contamination |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daryono, L.R.; Nakashima, K.; Kawasaki, S.; Titisari, A.D.; Barianto, D.H. Sediment Characteristics of Beachrock: A Baseline Investigation Based on Microbial Induced Carbonate Precipitation at Krakal-Sadranan Beach, Yogyakarta, Indonesia. Appl. Sci. 2020, 10, 520. https://doi.org/10.3390/app10020520
Daryono LR, Nakashima K, Kawasaki S, Titisari AD, Barianto DH. Sediment Characteristics of Beachrock: A Baseline Investigation Based on Microbial Induced Carbonate Precipitation at Krakal-Sadranan Beach, Yogyakarta, Indonesia. Applied Sciences. 2020; 10(2):520. https://doi.org/10.3390/app10020520
Chicago/Turabian StyleDaryono, Lutfian Rusdi, Kazunori Nakashima, Satoru Kawasaki, Anastasia Dewi Titisari, and Didit Hadi Barianto. 2020. "Sediment Characteristics of Beachrock: A Baseline Investigation Based on Microbial Induced Carbonate Precipitation at Krakal-Sadranan Beach, Yogyakarta, Indonesia" Applied Sciences 10, no. 2: 520. https://doi.org/10.3390/app10020520





