A Comprehensive Physical Profile for Aqueous Dispersions of Carbon Derivatives as Solar Working Fluids
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
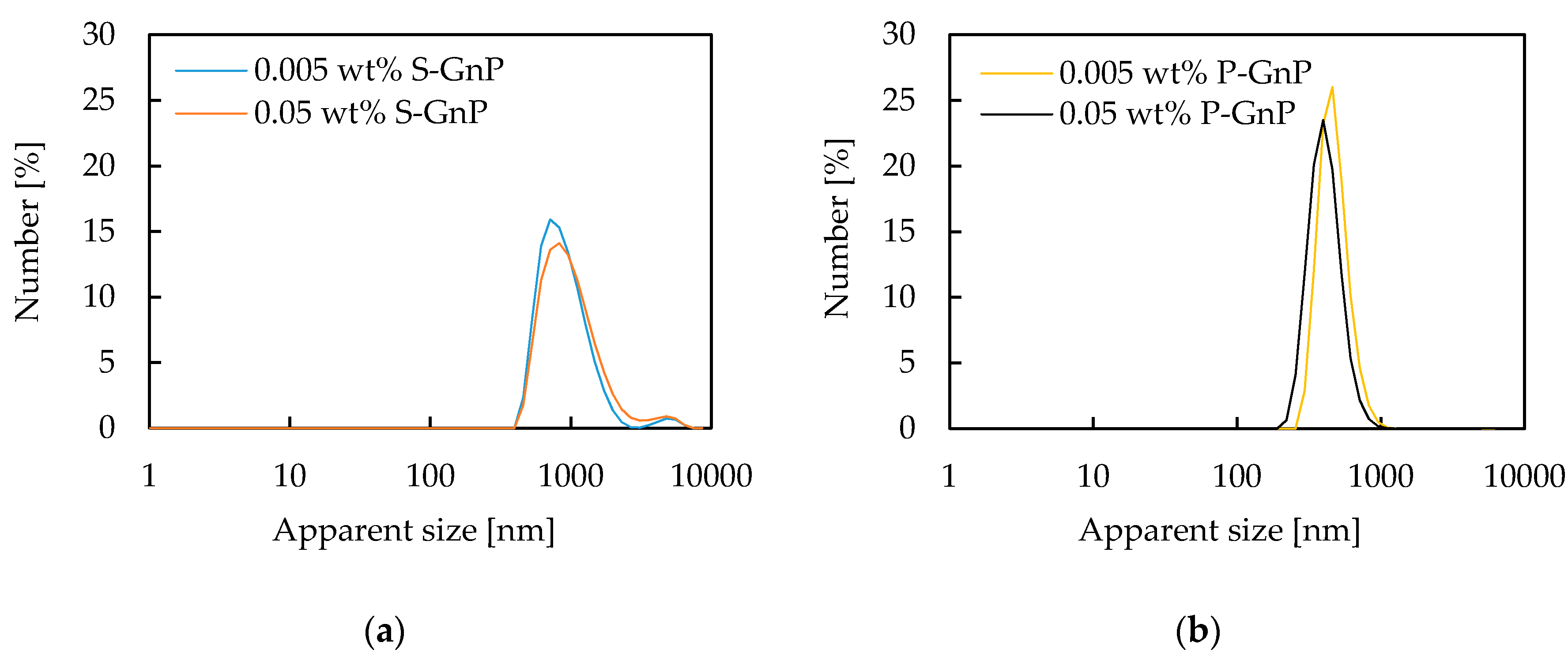
3.1. Nanopowder Chemical Composition and Nanofluids’ Stability
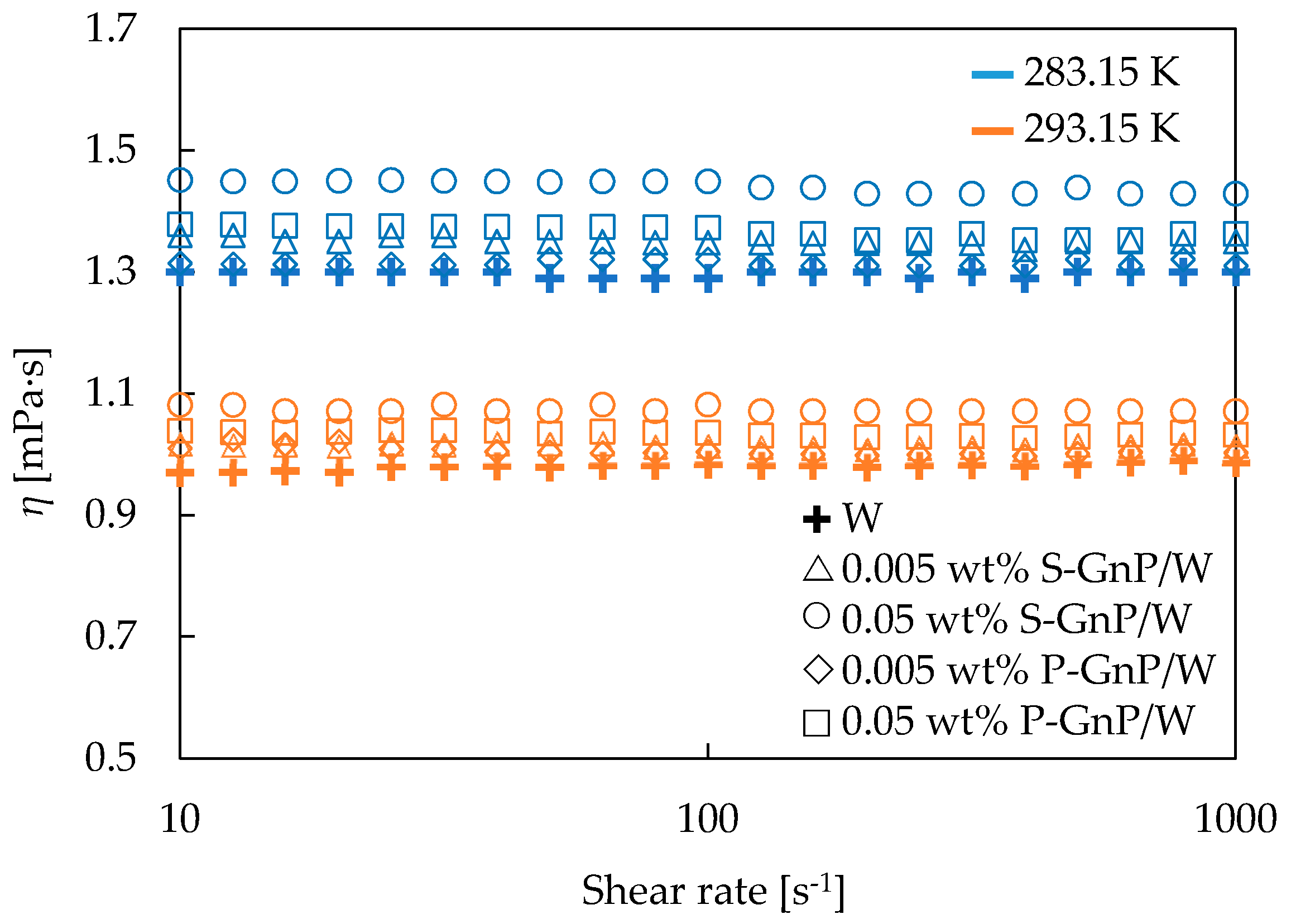
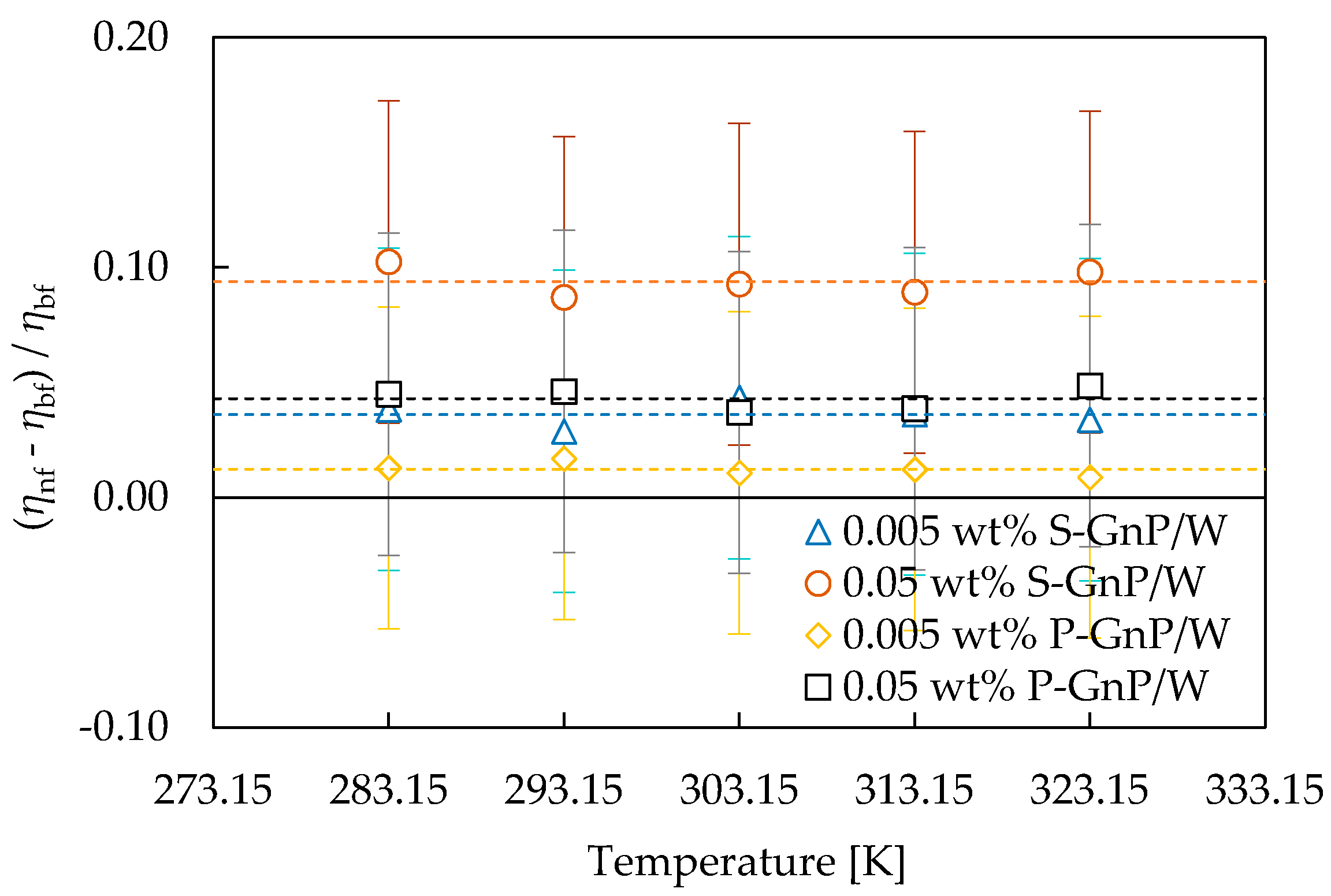
3.2. Rheological and Thermophysical Properties
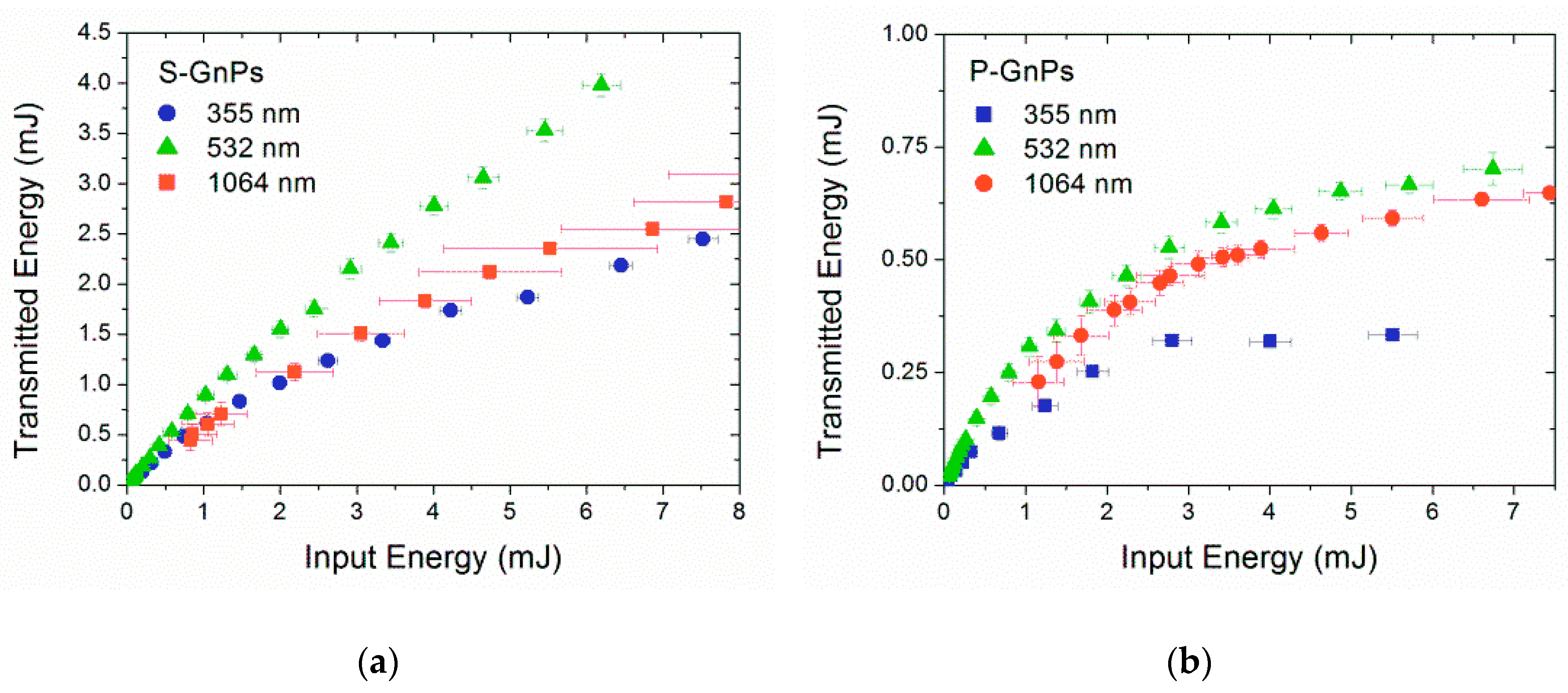
3.3. Optical Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Choi, S.U.S.; Eastman, J.A. Enhancing Thermal Conductivity of Fluids with Nanoparticles. In Proceedings of the 1995 International Mechanical Engineering Congress and Exhibition, San Francisco, CA, USA, 12–17 November 1995. [Google Scholar]
- Patil, M.S.; Seo, J.H.; Kang, S.J.; Lee, M.Y. Review on synthesis, thermo-physical property, and heat transfer mechanism of nanofluids. Energies 2016, 9, 840. [Google Scholar] [CrossRef] [Green Version]
- Bellos, E.; Tzivanidis, C. Optimization of a solar-driven trigeneration system with nanofluid-based parabolic trough collectors. Energies 2017, 10, 848. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Hwang, S.G.; Lee, G.H. Efficiency Improvement of a Photovoltaic Thermal (PVT) System Using Nanofluids. Energies 2019, 12, 3063. [Google Scholar] [CrossRef] [Green Version]
- Minardi, J.E.; Chuang, H.N. Performance of a “black” liquid flat-plate solar collector. Sol. Energy 1975, 17, 179–183. [Google Scholar] [CrossRef]
- Bortolato, M.; Dugaria, S.; Agresti, F.; Barison, S.; Fedele, L.; Sani, E.; Del Col, D. Investigation of a single wall carbon nanohorn-based nanofluid in a full-scale direct absorption parabolic trough solar collector. Energy Convers. Manag. 2017, 150, 693–703. [Google Scholar] [CrossRef]
- Lin, M.; Reinhold, J.; Monnerie, N.; Haussener, S. Modeling and design guidelines for direct steam generation solar receivers. Appl. Energy 2018, 216, 761–776. [Google Scholar] [CrossRef]
- Bouvier, J.-L.; Michaux, G.; Salagnac, P.; Nepveu, F.; Rochier, D.; Kientz, T. Experimental characterisation of a solar parabolic trough collector used in a micro-CHP (micro-cogeneration) system with direct steam generation. Energy 2015, 83, 474–485. [Google Scholar] [CrossRef]
- Taylor, R.A.; Phelan, P.E.; Adrian, R.J.; Gunawan, A.; Otanicar, T.P. Characterization of light-induced, volumetric steam generation in nanofluids. Int. J. Therm. Sci. 2012, 56, 1–11. [Google Scholar] [CrossRef]
- Sani, E.; Papi, N.; Mercatelli, L.; Żyła, G. Graphite/diamond ethylene glycol-nanofluids for solar energy applications. Renew. Energy 2018, 126, 692–698. [Google Scholar] [CrossRef]
- Wang, X.; He, Y.; Liu, X.; Shi, L.; Zhu, J. Investigation of photothermal heating enabled by plasmonic nanofluids for direct solar steam generation. Sol. Energy 2017, 157, 35–46. [Google Scholar] [CrossRef]
- Wang, X.; He, Y.; Cheng, G.; Shi, L.; Liu, X.; Zhu, J. Direct vapor generation through localized solar heating via carbon-nanotube nanofluid. Energy Convers. Manag. 2016, 130, 176–183. [Google Scholar] [CrossRef]
- Ni, G.; Miljkovic, N.; Ghasemi, H.; Huang, X.; Boriskina, S.V.; Lin, C.T.; Wang, J.; Xu, Y.; Rahman, M.M.; Zhang, T.J.; et al. Volumetric solar heating of nanofluids for direct vapor generation. Nano Energy 2015, 17, 290–301. [Google Scholar] [CrossRef] [Green Version]
- Gorji, T.B.; Ranjbar, A.A. Thermal and exergy optimization of a nanofluid-based direct absorption solar collector. Renew. Energy 2017, 106, 274–287. [Google Scholar] [CrossRef]
- Hordy, N.; Rabilloud, D.; Meunier, J.-L.; Coulombe, S. High temperature and long-term stability of carbon nanotube nanofluids for direct absorption solar thermal collectors. Sol. Energy 2014, 105, 82–90. [Google Scholar] [CrossRef]
- Karami, M.; Akhavan Bahabadi, M.A.; Delfani, S.; Ghozatloo, A. A new application of carbon nanotubes nanofluid as working fluid of low-temperature direct absorption solar collector. Sol. Energy Mater. Sol. Cells 2014, 121, 114–118. [Google Scholar] [CrossRef]
- Delfani, S.; Karami, M.; Akhavan-Behabadi, M.A. Performance characteristics of a residential-type direct absorption solar collector using MWCNT nanofluid. Renew. Energy 2016, 87, 754–764. [Google Scholar] [CrossRef]
- Boldoo, T.; Ham, J.; Cho, H. Comparison Study on Photo-Thermal Energy Conversion Performance of Functionalized and Non-Functionalized MWCNT Nanofluid. Energies 2019, 12, 3763. [Google Scholar] [CrossRef] [Green Version]
- Moradi, A.; Sani, E.; Simonetti, M.; Francini, F.; Chiavazzo, E.; Asinari, P. Carbon-Nanohorn Based Nanofluids for a Direct Absorption Solar Collector for Civil Application. J. Nanosci. Nanotechnol. 2015, 15, 3488–3495. [Google Scholar] [CrossRef] [Green Version]
- Dugaria, S.; Bortolato, M.; Del Col, D. Modelling of a direct absorption solar receiver using carbon based nanofluids under concentrated solar radiation. Renew. Energy 2018, 128, 495–508. [Google Scholar] [CrossRef]
- Vallejo, J.P.; Mercatelli, L.; Martina, M.R.; Di Rosa, D.; Dell’Oro, A.; Lugo, L.; Sani, E. Comparative study of different functionalized graphene-nanoplatelet aqueous nanofluids for solar energy applications. Renew. Energy 2019, 141, 791–801. [Google Scholar] [CrossRef]
- Sani, E.; Vallejo, J.P.; Cabaleiro, D.; Lugo, L. Functionalized graphene nanoplatelet-nanofluids for solar thermal collectors. Sol. Energy Mater. Sol. Cells 2018, 185, 205–209. [Google Scholar] [CrossRef]
- Xiao, X.; Zhang, G.; Ding, Y.; Wen, D. Rheological Characteristics of Molten Salt Seeded with Al2O3 Nanopowder and Graphene for Concentrated Solar Power. Energies 2019, 12, 467. [Google Scholar] [CrossRef] [Green Version]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Si, Y.; Samulski, E.T. Synthesis of water soluble graphene. Nano Lett. 2008, 8, 1679–1682. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; An, J.; Piner, R.D.; Jung, I.; Yang, D.; Velamakanni, A.; Nguyen, S.T.; Ruoff, R.S. Aqueous suspension and characterization of chemically modified graphene sheets. Chem. Mater. 2008, 20, 6592–6594. [Google Scholar] [CrossRef]
- Vallejo, J.P.; Pérez-Tavernier, J.; Cabaleiro, D.; Fernández-Seara, J.; Lugo, L. Potential heat transfer enhancement of functionalized graphene nanoplatelet dispersions in a propylene glycol-water mixture. Thermophysical profile. J. Chem. Thermodyn. 2018, 123, 174–184. [Google Scholar] [CrossRef]
- Cabaleiro, D.; Colla, L.; Barison, S.; Lugo, L.; Fedele, L.; Bobbo, S. Heat Transfer Capability of (Ethylene Glycol + Water)-Based Nanofluids Containing Graphene Nanoplatelets: Design and Thermophysical Profile. Nanoscale Res. Lett. 2017, 12, 53. [Google Scholar] [CrossRef] [Green Version]
- Agromayor, R.; Cabaleiro, D.; Pardinas, A.A.; Vallejo, J.P.; Fernandez-Seara, J.; Lugo, L. Heat Transfer Performance of Functionalized Graphene Nanoplatelet Aqueous Nanofluids. Materials 2016, 9, 455. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Xu, C.; Liu, J.; Fang, X.; Zhang, Z. Optical absorption property and photo-thermal conversion performance of graphene oxide/water nanofluids with excellent dispersion stability. Sol. Energy 2017, 148, 17–24. [Google Scholar] [CrossRef]
- Chen, L.; Liu, J.; Fang, X.; Zhang, Z. Reduced graphene oxide dispersed nanofluids with improved photo-thermal conversion performance for direct absorption solar collectors. Sol. Energy Mater. Sol. Cells 2017, 163, 125–133. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, L.; Liu, J.; Fang, X.; Zhang, Z. Effect of morphology of carbon nanomaterials on thermo-physical characteristics, optical properties and photo-thermal conversion performance of nanofluids. Renew. Energy 2016, 99, 888–897. [Google Scholar] [CrossRef]
- Neumann, O.; Feronti, C.; Neumann, A.D.; Dong, A.; Schell, K.; Lu, B.; Kim, E.; Quinn, M.; Thompson, S.; Grady, N.; et al. Compact solar autoclave based on steam generation using broadband light-harvesting nanoparticles. Proc. Natl. Acad. Sci. USA 2013, 110, 11677–11681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishii, S.; Sugavaneshwar, R.P.; Chen, K.; Dao, T.D.; Nagao, T. Solar water heating and vaporization with silicon nanoparticles at mie resonances. Opt. Mater. Express 2016, 6, 640–648. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Blau, W.J. Optical Limiting Properties of Single-Walled Carbon Nanotube Dispersions in Amide Solvents. In Proceedings of the SPIE 6988, Nanophotonics II, Strasbourg, France, 23 April 2008; p. 69881F. [Google Scholar]
- Ghasemi, H.; Ni, G.; Marconnet, A.M.; Loomis, J.; Yerci, S.; Miljkovic, N.; Chen, G. Solar steam generation by heat localization. Nat. Commun. 2014, 5, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Vallejo, J.P.; Álvarez-Regueiro, E.; Cabaleiro, D.; Fernández-Seara, J.; Fernández, J.; Lugo, L. Functionalized graphene nanoplatelet nanofluids based on a commercial industrial antifreeze for the thermal performance enhancement of wind turbines. Appl. Therm. Eng. 2019, 152, 113–125. [Google Scholar] [CrossRef]
- Żyła, G.; Vallejo, J.P.; Fal, J.; Lugo, L. Nanodiamonds—Ethylene Glycol nanofluids: Experimental investigation of fundamental physical properties. Int. J. Heat Mass Transf. 2018, 121, 1201–1213. [Google Scholar] [CrossRef]
- Żyła, G.; Vallejo, J.P.; Lugo, L. Isobaric heat capacity and density of ethylene glycol based nanofluids containing various nitride nanoparticle types: An experimental study. J. Mol. Liq. 2018, 261, 530–539. [Google Scholar] [CrossRef]
- Sani, E.; Dell’Oro, A. Spectral optical constants of ethanol and isopropanol from ultraviolet to far infrared. Opt. Mater. 2016, 60, 137–141. [Google Scholar] [CrossRef]
- Sani, E.; Dell’Oro, A. Optical Constants of Ethylene Glycol over an Extremely Wide Spectral Range. Opt. Mater. 2014, 37, 36–41. [Google Scholar] [CrossRef]
- Vallejo, J.P.; Gómez-Barreiro, S.; Cabaleiro, D.; Gracia-Fernández, C.; Fernández-Seara, J.; Lugo, L. Flow behaviour of suspensions of functionalized graphene nanoplatelets in propylene glycol–water mixtures. Int. Commun. Heat Mass Transf. 2018, 91, 150–157. [Google Scholar] [CrossRef]
- Vallejo, J.P.; Żyła, G.; Fernández-Seara, J.; Lugo, L. Rheological behaviour of functionalized graphene nanoplatelet nanofluids based on water and propylene glycol: Water mixtures. Int. Commun. Heat Mass Transf. 2018, 99, 43–53. [Google Scholar] [CrossRef]
- Nidhin, M.; Indumathy, R.; Sreeram, K.J.; Nair, B.U. Synthesis of iron oxide nanoparticles of narrow size distribution on polysaccharide templates. Bull. Mater. Sci. 2008, 31, 93–96. [Google Scholar] [CrossRef] [Green Version]
- Das, P.K.; Mallik, A.K.; Ganguly, R.; Santra, A.K. Synthesis and characterization of TiO2–water nanofluids with different surfactants. Int. Commun. Heat Mass 2016, 75, 341–348. [Google Scholar] [CrossRef]
- Ghadimi, A.; Saidur, R.; Metselaar, H.S.C. A review of nanofluid stability properties and characterization in stationary conditions. Int. J. Heat Mass Transf. 2011, 54, 4051–4068. [Google Scholar] [CrossRef]
- Huang, J.; Wang, X.; Long, Q.; Wen, X.; Zhou, Y.; Li, L. Influence of pH on the Stability Characteristics of Nanofluids. In Proceedings of the 2009 Symposium on Photonics and Optoelectronics, Wuhan, China, 14–16 August 2009. [Google Scholar]
- Babita; Sharma, S.K.; Gupta, S.M. Preparation and evaluation of stable nanofluids for heat transfer application: A review. Exp. Therm. Fluid Sci. 2016, 79, 202–212. [Google Scholar] [CrossRef]
- Hodgman, C.D. Handbook of Chemistry and Physics; Chemical Rubber Company: Boca Raton, FL, USA, 1976. [Google Scholar]
- Deckwer, W.D. Density, Viscosity, Vapor Pressure, and Hydrogen Solubility of Aqueous Manganese (II) Sulfate Solutions. J. Chem. Eng. Data 1980, 25, 75–76. [Google Scholar] [CrossRef]
- James, C.J.; Mulcahy, D.E.; Steel, B.J. Viscometer calibration standards: Viscosities of water between 0 and 60 degrees C and of selected aqueous sucrose solutions at 25 degrees C from measurements with a flared capillary viscometer. J. Phys. D Appl. Phys. 1984, 17, 225–230. [Google Scholar] [CrossRef]
- Hartono, A.; Svendsen, H.F. Density, viscosity, and excess properties of aqueous solution of diethylenetriamine (DETA). J. Chem. Thermodyn. 2009, 41, 973–979. [Google Scholar] [CrossRef]
- Lemmon, E.W.; Huber, M.L.; McLinden, M.O. Reference Fluid Thermodynamic and Transport Properties (REFPROP), National Institute of Standards and Technology. In Nist Standard Reference Database 23; NIST: Gaithersburg, MD, USA, 2010. [Google Scholar]
- Vallejo, J.P.; Żyła, G.; Fernández-Seara, J.; Lugo, L. Influence of Six Carbon-Based Nanomaterials on the Rheological Properties of Nanofluids. Nanomaterials 2019, 9, 146. [Google Scholar] [CrossRef] [Green Version]
- Douglas, J.F.; Gasiorek, J.M.; Swaffield, J.A.; Jack, L.B. Fluid Mechanics; Prentice Hall: Upper Saddle River, NJ, USA, 2005. [Google Scholar]
- Dembicki, H., Jr. Interpreting crude oil and natural gas data. In Practical Petroleum Geochemistry for Exploration and Production; Elsevier: Amsterdam, The Netherlands, 2017; pp. 135–188. [Google Scholar]
- ASTM G173-03(2012), Standard Tables for Reference Solar Spectral Irradiances: Direct Normal and Hemispherical on 37° Tilted Surface; ASTM International: West Conshohocken, PA, USA, 2012.
- Sani, E.; Mercatelli, L.; Barison, S.; Pagura, C.; Agresti, F.; Colla, L.; Sansoni, P. Potential of carbon nanohorn-based suspensions for solar thermal collectors. Sol. Energy Mater. Sol. Cells 2011, 95, 2994–3000. [Google Scholar] [CrossRef]
- Sani, E.; Barison, S.; Pagura, C.; Mercatelli, L.; Sansoni, P.; Fontani, D.; Jafrancesco, D.; Francini, F. Carbon nanohorns-based nanofluids as direct sunlight absorbers. Opt. Express 2010, 18, 5179–5187. [Google Scholar] [CrossRef] [PubMed]
- Sani, E.; Papi, N.; Mercatelli, L.; Barison, S.; Agresti, F.; Rossi, S.; Dell’Oro, A. Optical Limiting of Carbon Nanohorn-Based Aqueous Nanofluids: A Systematic Study. Nanomaterials 2020. submitted. [Google Scholar]
- Hagan, D.J.; Xia, T.J.; Said, A.A.; Van Stryland, E.W. Tandem Limiter Optimization. In Proceedings of the SPIE 2229, Nonlinear Optical Materials for Switching and Limiting, Orlando, FL, USA, 6 July 1994. [Google Scholar]
- Vincent, D.; Cruickshank, J. Optical limiting with C60 and other fullerenes. Appl. Opt. 1997, 36, 7794–7798. [Google Scholar] [CrossRef] [PubMed]
- McEwan, K.J.; Milsom, P.K.; James, D.B. Nonlinear Optical Effects in Carbon Suspension. In Proceedings of the SPIE 3472, Nonlinear Optical Liquids for Power Limiting and Imaging, San Diego, CA, USA, 12 October 1998. [Google Scholar]

| Element | S-GnP | P-GnP |
|---|---|---|
| C [%] | 69.1 | 52.4 |
| H [%] | 3.1 | 2.2 |
| N [%] | 3.2 | 0 |
| S [%] | 5.4 | 0 |
| O [%] | 12.6 | 22.4 |
| Total [%] | 93.4 | 77.0 |
| Sample | pH |
|---|---|
| W | 6.2 |
| 0.005 wt% S-GnP/W | 4.3 |
| 0.05 wt% S-GnP/W | 3.3 |
| 0.005 wt% P-GnP/W | 5.2 |
| 0.05 wt% P-GnP/W | 5.0 |
| η [mPa·s] | |||||
|---|---|---|---|---|---|
| Temperature [K] | W | 0.005 wt% S-GnP/W | 0.05 wt% S-GnP/W | 0.005 wt% P-GnP/W | 0.05 wt% P-GnP/W |
| 283.15 | 1.298 | 1.350 | 1.429 | 1.315 | 1.356 |
| 293.15 | 0.986 | 1.014 | 1.071 | 1.002 | 1.031 |
| 303.15 | 0.787 | 0.821 | 0.860 | 0.796 | 0.816 |
| 313.15 | 0.639 | 0.662 | 0.696 | 0.647 | 0.664 |
| 323.15 | 0.535 | 0.553 | 0.587 | 0.539 | 0.561 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sani, E.; Vallejo, J.P.; Mercatelli, L.; Martina, M.R.; Di Rosa, D.; Dell’Oro, A.; Lugo, L. A Comprehensive Physical Profile for Aqueous Dispersions of Carbon Derivatives as Solar Working Fluids. Appl. Sci. 2020, 10, 528. https://doi.org/10.3390/app10020528
Sani E, Vallejo JP, Mercatelli L, Martina MR, Di Rosa D, Dell’Oro A, Lugo L. A Comprehensive Physical Profile for Aqueous Dispersions of Carbon Derivatives as Solar Working Fluids. Applied Sciences. 2020; 10(2):528. https://doi.org/10.3390/app10020528
Chicago/Turabian StyleSani, Elisa, Javier P. Vallejo, Luca Mercatelli, Maria Raffaella Martina, Daniele Di Rosa, Aldo Dell’Oro, and Luis Lugo. 2020. "A Comprehensive Physical Profile for Aqueous Dispersions of Carbon Derivatives as Solar Working Fluids" Applied Sciences 10, no. 2: 528. https://doi.org/10.3390/app10020528







