Comparison of Different Biofilter Media During Biological Bed Maturation Using Common Carp as a Biogen Donor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Conditions
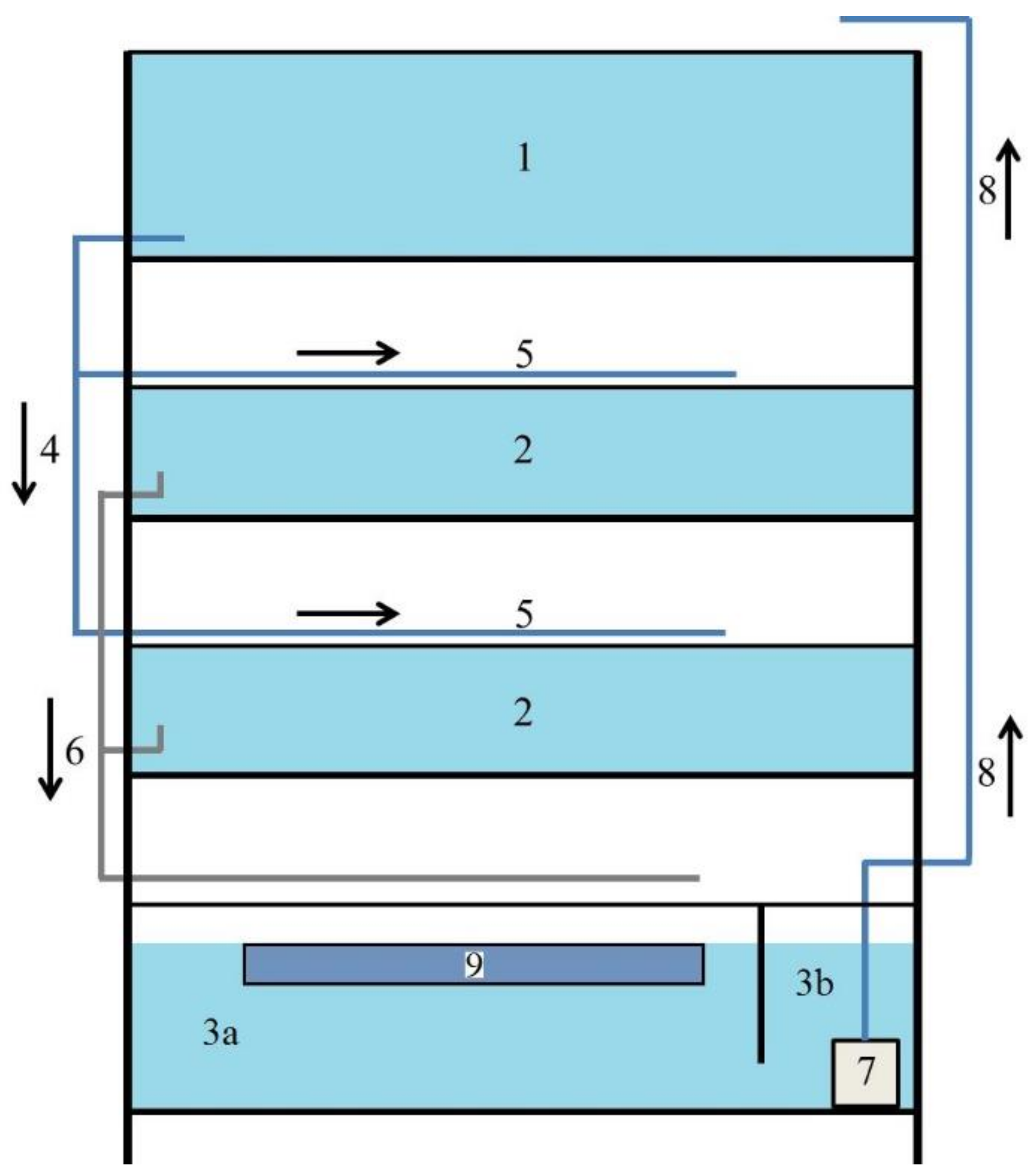
2.1.1. RAS Systems
- -
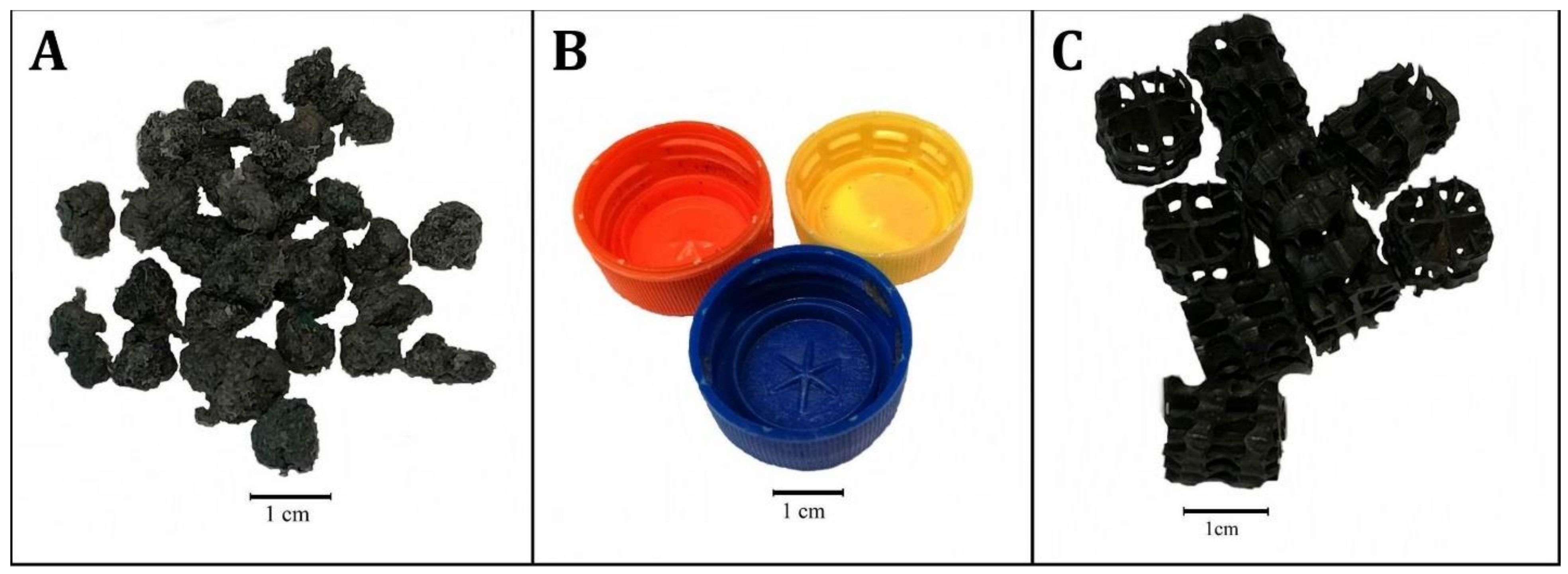
- Polypropylene aggregate (PPA) (active surface area 300–500 m2/m3 —manufacturer’s data) (Figure 1A).
- -
- Polyethylene screw caps for PET bottles (PSC) (active surface area ca. 300 m2/m3) (Figure 1B).
- -
- Commercial fittings HXF12KLL (CF) (active surface area 859 m2/m3, Stöhr GmhH & Co. KG, Marktrodach, Germany) (Figure 1C).
2.1.2. Fish
2.2. Water Quality Measurements
- Ammonium—cuvette test LCK 304
- Nitrite—cuvette test LCK 341
- Nitrate—cuvette test LCK 340
- -
- pH—using a portable pH-meter manufactured by Oxyguard,
- -
- Oxygen concentration (mg O2/dm3, saturation)—using an oxygen probe, a portable Polaris 2 manufactured by Oxyguard,
- -
- Temperature—using an oxygen probe, a portable Polaris 2 manufactured by Oxyguard,
- -
- Temperature—a probe combined with a thermostat controlling heater work,
- -
- pH—using a portable pH-meter manufactured by Oxyguard,
- -
- Oxygen concentration (mg O2/dm3, saturation)—using an oxygen probe, connected with the Total Commander system manufactured by Oxyguard.
2.3. Fish Measurements
2.4. Statistical Analysis
3. Results
3.1. Variant I—Use of Submerged Biological Filter
3.1.1. Ammonium Nitrogen
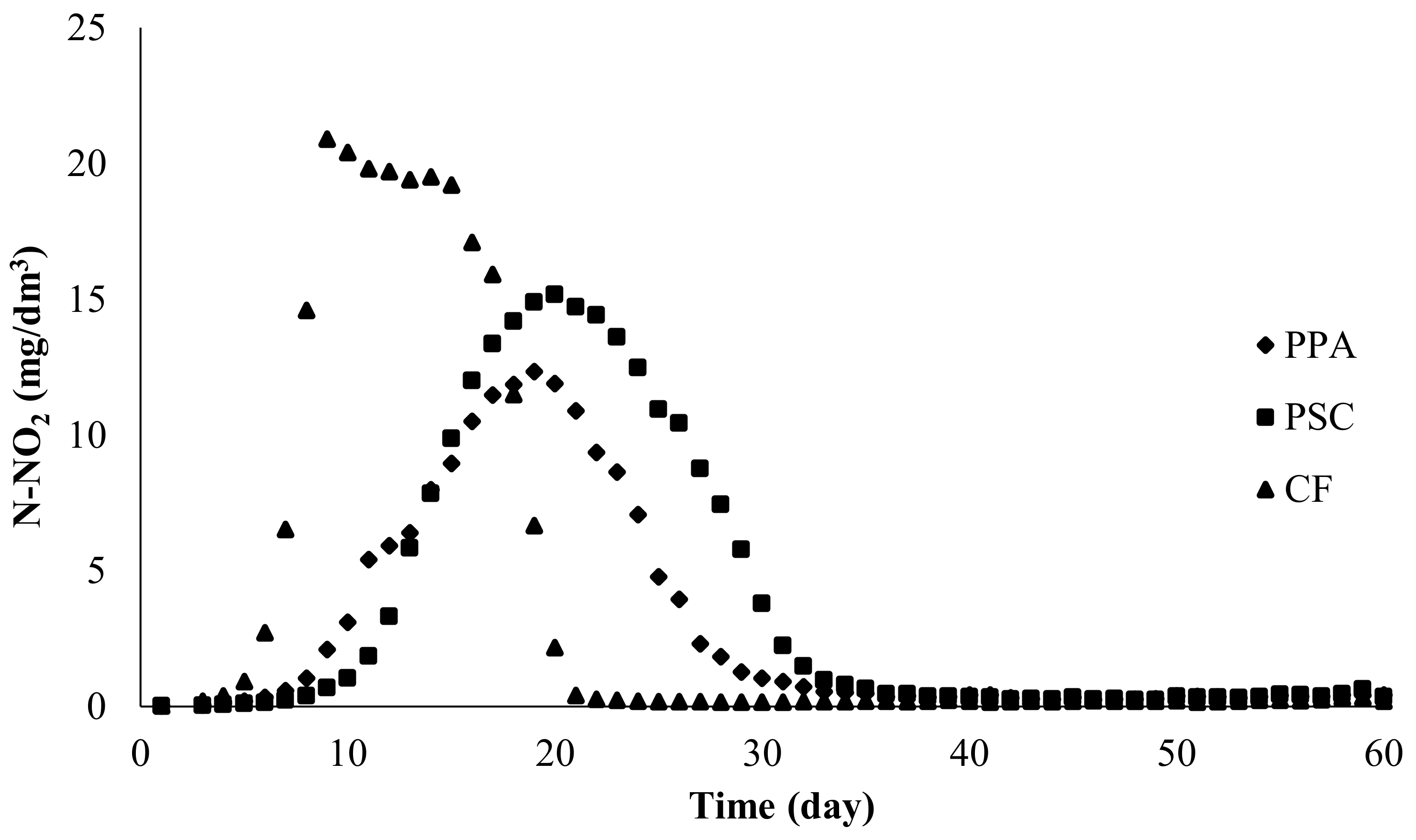
3.1.2. Nitrite Nitrogen
3.1.3. Nitrate Nitrogen
3.1.4. Other Water Parameters
3.2. Variant II—Use of Dripping Filters
3.2.1. Ammonium Nitrogen
3.2.2. Nitrite Nitrogen
3.2.3. Nitrate Nitrogen
3.2.4. Other Water Parameters
3.3. Statistical Analysis of Results
3.4. Fish
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- FAO. HLEF2050 Global Agriculture. 2016. Available online: http://www.fao.org/fileadmin/templates/wsfs/docs/Issues_papers/HLEF2050_Global_Agriculture.pdf (accessed on 10 March 2016).
- FAO. The State of World Fisheries and Aquaculture, Contributing to Food Security and Nutrition for All; Food and Agriculture Organization of the United Nations: Rome, Italy, 2016. [Google Scholar]
- Timmons, N.; Timmons, M.B.; Ebeling, J.M. Recirculating Aquaculture System (RAS) Technologies. Aquac. Mag. 2006, 32, 32–39. [Google Scholar]
- Kristensen, T.; Åtland, Å.; Rosten, T.; Urke, H.A.; Rosseland, B.O. Important influent-water quality parameters at freshwater production sites in two salmon producing countries. Aquac. Eng. 2009, 41, 53–59. [Google Scholar] [CrossRef]
- Greiner, A.D.; Timmons, M.B. Evaluation of the nitrification rates of microbead and trickling filters in an intensive recirculating tilapia production facility. Aquac. Eng. 1998, 18, 189–200. [Google Scholar] [CrossRef]
- Chen, S.; Ling, J.; Blancheton, J.P. Nitrification kinetics of biofilm as affected by water quality factors. Aquac. Eng. 2006, 34, 179–197. [Google Scholar] [CrossRef]
- Emparanza, E.J.M. Problems affecting nitrification in commercial RAS with fixed-bed biofilters for salmonids in Chile. Aquac. Eng. 2009, 41, 91–96. [Google Scholar] [CrossRef]
- Eding, E.H.; Kamstra, A.; Verreth, J.A.J.; Huisman, E.A.; Klapwijk, A. Design and operation of nitrifying trickling filters in recirculating aquaculture: A review. Aquac. Eng. 2006, 34, 234–260. [Google Scholar] [CrossRef]
- Wicks, B.J.; Joensen, R.; Tang, Q.; Randall, D.J. Swimming and ammonia toxicity in salmonids: The effect of sub lethal ammonia exposure on the swimming performance of coho salmon and the acute toxicity of ammonia in swimming and resting rainbow trout. Aquat. Toxicol. 2002, 59, 55–69. [Google Scholar] [CrossRef]
- Randall, D.J.; Tsui, T.K.N. Ammonia toxicity in fish. Marine Pollut. Bull. 2002, 45, 17–23. [Google Scholar] [CrossRef]
- Thurston, R.V.; Russo, R.C. Acute toxicity of ammonia to rainbow trout. Trans. Am. Fish. Soc. 1983, 112, 696–704. [Google Scholar] [CrossRef]
- Thurston, R.V.; Russo, R.J.; Luedtke, R.J.; Smith, C.E.; Meyn, E.L.; Chakoumalos, C.; Wang, K.C.; Brown, C.J.D. Chronic toxicity of ammonia to rainbow trout. Trans. Am. Fish. Soc. 1984, 113, 56–73. [Google Scholar] [CrossRef]
- Wood, C.M. Toxic responses of the gill. In Target Organ Toxicity in Marine and Freshwater Teleosts; Schlenk, D., Benson, W.H., Eds.; Taylor & Francis: London, UK, 2001; Volume 1, pp. 1–89. [Google Scholar]
- Cheng, W.; Hsiao, I.S.; Chen, J.C. Effect of nitrite on immune response of Taiwan abalone Haliotis diversicolor supertexta and its susceptibility to Vibrio parahaemolyticus. Dis. Aquat. Org. 2004, 60, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Miron, D.S.; Moraes, B.; Becker, A.G.; Crestani, M.; Spanevello, R.; Loro, V.L.; Baldisserotto, B. Ammonia and pH effects on some metabolic parameters and gill histology of silver catfish, Rhamdiaquelen (Heptapteridae). Aquaculture 2008, 277, 192–196. [Google Scholar] [CrossRef]
- Benli, A.C.K.; Köksal, G.; Özkul, A. Sublethal ammonia exposure of Nile tilapia (Oreochromis niloticus L.): Effects on gill, liver and kidney histology. Chemosphere 2008, 72, 1355–1358. [Google Scholar] [CrossRef] [PubMed]
- Hurtado, C.F.; Cancino-Madariaga, B. Ammonia retention capacity of nanofiltration and reverse osmosis membranes in a non-steady state system, to be use in recirculation aquaculture systems (RAS). Aquac. Eng. 2014, 58, 29–34. [Google Scholar] [CrossRef]
- Sikora, M.; Nowosad, J.; Biegaj, M.; Kucharczyk, D.; Dębowski, M. The possibility of application of agglomerate elastomers (EPP) as media for biological bed in aquaculture. Aquac. Res. 2018, 49, 2988–2994. [Google Scholar] [CrossRef]
- Zhu, S.; Chen, S. The impact of temperature on nitrification rate in fixed film biofilters. Aquac. Eng. 2002, 26, 221–237. [Google Scholar] [CrossRef]
- Suhr, K.I.; Pedersen, P.B. Nitrification in moving bed and fixed bed biofilters treating effluent water from a large commercial outdoor rainbow trout RAS. Aquac. Eng. 2010, 42, 31–37. [Google Scholar] [CrossRef]
- Hamlin, H.J. Nitrate toxicity in Siberian sturgeon (Acipenser baeri). Aquaculture 2006, 253, 688–693. [Google Scholar] [CrossRef]
- Malone, R.F.; Pfeiffer, T.J. Rating fixed-film nitrifying biofilters used in recirculating aquaculture systems. Aquac. Eng. 2006, 34, 389–402. [Google Scholar] [CrossRef]
- Camargo, J.A.; Alonso, A.; Salamanca, A. Nitrate toxicity to aquatic animals: A review with new data for freshwater invertebrates. Chemosphere 2005, 58, 1255–1267. [Google Scholar] [CrossRef]
- Badiola, M.; Mendiola, D.; Bostock, J. Recirculating Aquaculture Systems (RAS) analysis: Main issues on management and future challenges. Aquac. Eng. 2012, 51, 26–35. [Google Scholar] [CrossRef] [Green Version]
- Ling, J.; Chen, S. Impact of organic carbon on nitrification performance of different biofilters. Aquac. Eng. 2005, 33, 150–162. [Google Scholar] [CrossRef]
- Guedart, T.C.; Losordo, T.M.; Classen, J.J.; Osborne, J.A.; DeLong, D.P. An evaluation of commercially available biological filters for recirculating aquaculture systems. Aquac. Eng. 2010, 42, 38–49. [Google Scholar] [CrossRef]
- Guedart, T.C.; Losordo, T.M.; Classen, J.J.; Osborne, J.A.; De Long, D.P. Evaluating the effects of organic carbon on biological filtration performance in a large scale recirculating aquaculture system. Aquac. Eng. 2011, 44, 10–18. [Google Scholar] [CrossRef]
- Kucharczyk, D.; Targońska, K.; Hliwa, P.; Gomułka, P.; Kwiatkowski, M.; Krejszeff, S.; Perkowski, J. Reproductive parameters of common carp (Cyprinus carpio L.) spawners during natural season and out-of-season spawning. Reprod. Biol. 2008, 8, 285–289. [Google Scholar] [CrossRef]
- Kucharczyk, D.; Nowosad, J.; Łuczyński, M.J.; Targońska, K. New technique for fertilizing eggs of burbot, asp and ide under hatchery conditions. Anim. Reprod. Sci. 2016, 172, 143–147. [Google Scholar] [CrossRef]
- Nowosad, J.; Kucharczyk, D.; Biłas, M.; Palińska-Żarska, K.; Krejszeff, S. Optimization of feeding rate of juvenile common carp, (Cyprinus carpio L.), during short intensive rearing under controlled conditions. Experiment 2013, 15, 1056–1063. [Google Scholar]
- Nowosad, J.; Żarski, D.; Biłas, M.; Dryl, K.; Krejszeff, S.; Kucharczyk, D. Dynamics of ammonia excretion in juvenile common tench, Tincatinca (L.), during intensive rearing under controlled conditions. Aquac. Int. 2013, 21, 629–637. [Google Scholar] [CrossRef]
- Svobodová, Z.; Máchová, J.; Poleszczuk, G.; Hòda, J.; Hamáâková, J.; Kroupová, H. Nitrite poisoning of fish in aquaculture facilities with water-recirculating systems. Acta Vet. Brno 2005, 74, 129–137. [Google Scholar] [CrossRef] [Green Version]
- Kuhn, D.D.; Smith, S.A.; Boardman, G.D.; Angier, M.W.; Marsh, L.; Flick, G.J., Jr. Chronic toxicity of nitrate to Pacific white shrimp. Litopenaeus vannamei: Impacts on survival, growth, antennae length, and pathology. Aquaculture 2010, 309, 109–114. [Google Scholar] [CrossRef]
- Tanikawa, D.; Nakamura, Y.; Tokuzawa, H.; Hirakata, Y.; Hatamoto, M.; Yamaguchi, T. Effluent treatment in an aquaponics-based closed aquaculture system with single-stage nitrification–denitrification using a down-flow hanging sponge reactor. Int. Biodeterior. Biodegrad. 2018, 132, 268–273. [Google Scholar] [CrossRef]
- Losordo, T.M.; Hobbs, A.O. Using computer spreadsheets for water flow and biofilter sizing in recirculating aquaculture production systems. Aquac. Eng. 2000, 23, 95–102. [Google Scholar] [CrossRef]
- Crab, R.; Avnimelech, Y.; Defoirdt, T.; Bossier, P.; Verstraete, W. Nitrogen removal techniques in aquaculture for sustainable production. Aquaculture 2007, 270, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Hargrove, L.L.; Westerman, P.W.; Losordo, T.M. Nitrification in three-stage and single-stage floating bead biofilters in a laboratory-scale recirculating aquaculture system. Aquac. Eng. 1996, 15, 67–80. [Google Scholar] [CrossRef]
- van Rijn, J. The potential for integrated biological treatment systems in recirculating fish culture. Rev. Aquac. 1996, 139, 181–201. [Google Scholar] [CrossRef]
- Ridha, M.T.; Cruz, E.M. Effect of biofilter media on water quality and biological performance of the Nile tilapia Oreochromis niloticus L. reared in a simple recirculating system. Aquac. Eng. 2001, 24, 157–166. [Google Scholar] [CrossRef]
- Zhu, S.; Chen, S. An experimental study on nitrification biofilm performances using a series reactor system. Aquac. Eng. 1999, 20, 245–259. [Google Scholar] [CrossRef]
- Żarski, D.; Kucharczyk, D.; Targoñska, K.; Chyła, B.; Dobrołowicz, A. Dynamics of changes in nitrogen and phosphorus compounds during intensive rearing of ide, leuciscusidus (L.), in a recirculating system. Arch. Pol. Fish. 2008, 16, 459–467. [Google Scholar] [CrossRef]
- Żarski, D.; Kucharczyk, D.; Targońska, K.; Krejszeff, S.; Czarkowski, T.; Babiarz, E.; Nowosielska, D.B. Dynamics of nitrogen and phosphorus in closed and semi-closed recirculating aquaculture systems during the intensive culture of goldfish, Carassius auratus auratus (L.), juveniles. Arch. Pol. Fish. 2010, 18, 187–193. [Google Scholar] [CrossRef]
- Ebeling, J. Biofiltration. In Presentation Notebook of the Aquacultural Engineering Society Workshop; Intensive Fin-fish Systems and Technologies: Orlando, FL, USA, 2001; pp. 47–56. [Google Scholar]
- Itoi, S.; Ebihara, N.; Washio, S.; Sugita, H. Nitrite-oxidizing bacteria, nitrospira, distribution in the outer layer of the biofilm from filter materials of a recirculating water system for the goldfish Carassius auratus. Aquaculture 2007, 264, 297–308. [Google Scholar] [CrossRef]
- Kuhn, D.D.; Drahos, D.D.; Marsh, L.; Flick, G.J., Jr. Evaluation of nitrifying bacteria product to improve nitrification efficacy in recirculating aquaculture systems. Aquac. Eng. 2010, 43, 78–82. [Google Scholar] [CrossRef]
- Pulkkinen, J.T.; Eriksson-Kallio, A.M.; Aalto, S.L.; Tiirola, M.; Koskela, J.; Kiuru, T.; Vielma, J. The effects of different combinations of fixed and moving bed bioreactors on rainbow trout (Oncorhynchus mykiss) growth and health, water quality and nitrification in recirculating aquaculture systems. Aquac. Eng. 2019, 85, 98–105. [Google Scholar] [CrossRef]
- Davidson, J.; Good, C.; Williams, C.; Summerfelt, S.T. Evaluating the chronic effects of nitrate on the health and performance of post-smolt Atlantic salmon Salmo salar in freshwater recirculation aquaculture systems. Aquac. Eng. 2017, 79, 1–8. [Google Scholar] [CrossRef]
- Hamlin, H.J.; Michaels, J.T.; Beaulaton, C.M.; Graham, W.F.; Dutt, W.; Steinbach, P.; Losordo, T.M.; Schrader, K.K.; Main, K.L. Comparing denitrification rates and carbon sources in commercial scale upflow denitrification biological filters in aquaculture. Aquac. Eng. 2008, 38, 79–92. [Google Scholar] [CrossRef]
- delos Reyes, A.A., Jr.; Lawson, T.B. Combination of a bead filter and rotating biological contactor in a recirculating fish culture system. Aquac. Eng. 1996, 15, 27–39. [Google Scholar] [CrossRef]
- Barak, Y.; van Rijn, J. Biological phosphate removal in a prototype recirculating aquaculture treatment system. Aquac. Eng. 2000, 22, 121–136. [Google Scholar] [CrossRef]
- Seo, J.-K.; Jung, I.-H.; Kim, M.-R.; Kim, B.J.; Nam, S.-W.; Kim, S.-K. Nitrification performance of nitrifiers immobilized in PVA (polyvinyl alcohol) for a marine recirculating aquarium system. Aquac. Eng. 2001, 24, 181–194. [Google Scholar] [CrossRef]
- Hu, Z.; Lee, J.W.; Chandran, K.; Kim, S.; Brotto, A.C.; Khanal, S.K. Effect of plant species on nitrogen recovery in aquaponics. Bioresour. Technol. 2015, 188, 92–98. [Google Scholar] [CrossRef]
- Karpiński, A.; Szkudlarek, M.; Zakęś, Z. Nitrification in recirculation systems. Practical remarks about maturation of the biologically active filter. Komunikaty Rybackie 1999, 3, 11–14. (In Polish) [Google Scholar]
- Sandu, S.I.; Boardman, G.D.; Watten, B.J.; Brazil, B.L. Factors influencing the nitrification efficiency of fluidized bed filter with a plastic bead medium. Aquac. Eng. 2002, 26, 41–59. [Google Scholar] [CrossRef]
- Kolman, R. Effectiveness of a biological shelf filter used to treat water in a recirculation system during trout rearing. Arch. Pol. Fish. 1992, 1 (Suppl. 1), 1–37. (In Polish) [Google Scholar]






| Filling | Stage I | Break Down | Stage II | |
|---|---|---|---|---|
| PPA | Average ± SD (N-NH4/dm3) | 2.971 ± 0.059 | 0.960 | 0.100 ± 0.022 |
| Duration (d) | 3–10 | 11 | 12–60 | |
| PSC | Average ± SD (N-NH4/dm3) | 2.996 ± 0.056 | 0.860 | 0.133 ± 0.031 |
| Duration (d) | 3–11 | 12 | 13–60 | |
| CF | Average ± SD (N-NH4/dm3) | 2.906 ± 0.029 | 0,675 | 0.118 ± 0.040 |
| Duration (d) | 3–6 | 7 | 8–60 |
| Filling | Stage I | Stage II | |||
|---|---|---|---|---|---|
| Increase (d) | Maximum Concentration (N-NO2/dm3) | Decrease (d) | Average ± SD (N-NO2/dm3) | Duration (d) | |
| PPA | 1–19 | 12.340 | 19–31 | 0.404 ± 0.137 | 14 |
| PSC | 1–20 | 15.180 | 20–33 | 0.412 ± 0.169 | 12 |
| CF | 1–9 | 20.900 | 9–21 | 0.211 ± 0.049 | 24 |
| Filling | Stage I | Break Down | Stage II | |
|---|---|---|---|---|
| PPA | Average ± SD (N-NH4/dm3) | 2.996 ± 0.033 | 0.799 | 0.162 ± 0.048 |
| Duration | 3–9 | 10 | 11–45 | |
| PSC | Average ± SD (N-NH4/dm3) | 3.010 ± 0.055 | 0.313 | 0.209 ± 0.033 |
| Duration | 3–10 | 11 | 12–45 | |
| CFsf | Average ± SD (N-NH4/dm3) | 2.986 ± 0.033 | 0.884 | 0.120 ± 0.019 |
| Duration | 3–9 | 10 | 11–45 |
| Filling | Stage I | Stage II | |||
|---|---|---|---|---|---|
| Increase (d) | Maximum Concentration (N-NO2/dm3) | Decrease (d) | Average ± SD (N-NO2/dm3) | Duration (d) | |
| PPA | 1–17 | 12.500 | 17–27 | 0.480 ± 0.139 | 18 |
| PSC | 1–19 | 12.900 | 19–30 | 0.438 ± 0.137 | 15 |
| CF | 1–8 | 5.780 | 8–15 | 0.385 ± 0.126 | 30 |
| CFsf | 1–17 | 11.700 | 17–26 | 0.261 ± 0.178 | 19 |
| Submerged Filter | Dripping Filter | ||||||
|---|---|---|---|---|---|---|---|
| PPA | PSC | CF | PPA | PSC | CF | CFsf | |
| Ammonium | a | b | a | bc | c | bc | ab |
| Nitrite | bcd | bcd | a | cd | d | abc | ab |
| Nitrate | bc | bc | a | b | c | ab | bc |
| (a) | |||||
| Type of Filter, Filling | Stage | ||||
| I | II | III | IV | ||
| Submerged, PPA | Reg. | y = 0.0137e0.5439x | y = −0.1324x2 + 4.8955x − 33.778 | y = 6E + 10x−7.218 | y = 0.0007x2 − 0.0651x + 1.8372 |
| (R) | 0.9988 | 0.9538 | 0.9916 | 0.6644 | |
| Duration (d) | 1–11 | 11–24 | 24–36 | 36–60 | |
| Submerged, PSC | Reg. | y = 0.0117e0.4586x | y = −0.16x2 + 6.5599x − 52.287 | y = 5E + 16x−10.96 | y = 0.0013x2 - 0.1243x + 3.1995 |
| (R) | 0.9894 | 0.9909 | 0.9931 | 0.7628 | |
| Duration (d) | 1–13 | 13–27 | 27–36 | 36–60 | |
| Submerged, CF | Reg. | y = 0.0115e0.8991x | y = −0.24x2 + 5.8161x − 14.642 | y = 7E + 26x−20.47 | y = 0.0002x2 − 0.0119x + 0.4015 |
| (R) | 0.9991 | 0.9031 | 0.9817 | 0.6522 | |
| Duration (d) | 1–8 | 8–18 | 18–22 | 22–60 | |
| Dripping, PPA | Reg. | y = 0.0109e0.6266x | y = −0.1126x2 + 3.5649x – 16.232 | y = 6E + 09x−6.821 | y = −0.0002x2 − 0.0057 + 0.4272 |
| (R) | 0.9953 | 0.9216 | 0.9914 | 0.4423 | |
| Duration (d) | 1–11 | 11–19 | 19–31 | 31–45 | |
| Dripping, PSC | Reg | y = 0.0151e0.5446x | y = −0.0698x2 + 2.5974x – 11.5 | y = 5E + 14x−9.983 | y = 0.002x2 − 0.1489x + 3.1431 |
| (R) | 0.9979 | 0.9807 | 0.9913 | 0.9381 | |
| Duration (d) | 1–12 | 12–22 | 22–33 | 33–45 | |
| Submerged, CFsf | Reg. | y = 0.0127e0.6536x | y = −0.0698x2 + 2.1765x – 5.6077 | y = 2E + 12x−8.719 | y = 0.0013x2 − 0.1001x + 2.1323 |
| (R) | 0.9995 | 0.9390 | 0.9914 | 0.9199 | |
| Duration (d) | 1–10 | 10–19 | 19–29 | 29–45 | |
| (b) | |||||
| Type of Filter, Filling | Stage | ||||
| I | II | III | |||
| Dripping, CF | Reg. | y = −0.0351x3 + 0.469x2 – 0.8346x + 0.4202 | y = 18770x−3.647 | y = 0.001x2 – 0.067x + 1.4065 | |
| (R) | 0.9998 | 0.9917 | 0.8223 | ||
| Duration (d) | 1–9 | 9–21 | 21–45 | ||
| Type of Filter, Filling | Regress Equation | Correlation Coefficients (R) |
|---|---|---|
| Submerged, PPA | y = −0.0005x3 + 0.0392x2 − 0.1142x + 0.135 | 0.9844 |
| Submerged, PSC | y = −0.0005x3 + 0.0425x2 − 0.3631x + 1.5747 | 0.9946 |
| Submerged, CF | y = −0.0006x3 + 0.0367x2 + 0.5992x − 2.1153 | 0.9797 |
| Dripping, PPA | y = −0.0017x3 + 0.1118x2 − 1.1623x + 3.931 | 0.9884 |
| Dripping, PSC | y = −0.0015x3 + 0.1082x2 − 1.3589x + 4.808 | 0.9882 |
| Submerged, CFsf | y = −0.0005x3 + 0.0174x2 + 0.9072x − 1.355 | 0.9968 |
| Dripping, CF | y = −0.0015x3 + 0.1052x2 − 1.1256x + 3.7311 | 0.9926 |
| Temperature (°C) | Time (d) | °D | Source of Biogens | ||
|---|---|---|---|---|---|
| Sandu, et al., 2002 | 25.0 | 53 | 1325 | No data | |
| Greiner and Timmons, 1998 | 26.4 | 56 | 1478.4 | Fish | |
| Kolman, 2002 | 18.0 | 40–60 | 720–1080 | No data | |
| Sikora et al. 2018 | 23.0 | 35 | 805 | Fish | |
| This paper | Submerged, PPA | 25.0 | 35 | 875 | Fish |
| Submerged, PSC | 25.0 | 36 | 900 | Fish | |
| Submerged, CF | 25.0 | 21 | 525 | Fish | |
| Dripping PPA | 25.0 | 37 | 924 | Fish | |
| Dripping PSC | 25.0 | 32 | 800 | Fish | |
| Submerged CFsf | 25.0 | 18 | 700 | Fish | |
| Dripping CF | 25.0 | 18 | 450 | Fish | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sikora, M.; Nowosad, J.; Kucharczyk, D. Comparison of Different Biofilter Media During Biological Bed Maturation Using Common Carp as a Biogen Donor. Appl. Sci. 2020, 10, 626. https://doi.org/10.3390/app10020626
Sikora M, Nowosad J, Kucharczyk D. Comparison of Different Biofilter Media During Biological Bed Maturation Using Common Carp as a Biogen Donor. Applied Sciences. 2020; 10(2):626. https://doi.org/10.3390/app10020626
Chicago/Turabian StyleSikora, Mateusz, Joanna Nowosad, and Dariusz Kucharczyk. 2020. "Comparison of Different Biofilter Media During Biological Bed Maturation Using Common Carp as a Biogen Donor" Applied Sciences 10, no. 2: 626. https://doi.org/10.3390/app10020626
APA StyleSikora, M., Nowosad, J., & Kucharczyk, D. (2020). Comparison of Different Biofilter Media During Biological Bed Maturation Using Common Carp as a Biogen Donor. Applied Sciences, 10(2), 626. https://doi.org/10.3390/app10020626





