Edge-of-Field Technologies for Phosphorus Retention from Agricultural Drainage Discharge
Featured Application
Abstract
1. Introduction
2. Edge-of-Field Technologies
2.1. Constructed Wetlands
2.2. Restored Wetlands
2.3. Vegetated Buffer Strips
2.4. Filter Materials
3. Conclusions and Perspectives
Funding
Conflicts of Interest
References
- Ulén, B.; Bechmann, M.; Fölster, J.; Jarvie, H.P.; Tunney, H. Agriculture as a phosphorus source for eutrophication in the north-west European countries, Norway, Sweden, United Kingdom and Ireland: A review. Soil Use Manag. 2007, 23, 5–15. [Google Scholar] [CrossRef]
- Withers, P.J.A.; Haygarth, P.M. Agriculture, phosphorus and eutrophication: A European perspective. Soil Use Manag. 2007, 23, 1–4. [Google Scholar] [CrossRef]
- Bechmann, M.; Stålnacke, P. Effect of policy-induced measures on suspended sediments and total phosphorus concentrations from three Norwegian agricultural catchments. Sci. Total Environ. 2005, 304, 238–250. [Google Scholar] [CrossRef]
- Sharpley, A.N.; McDowell, R.W.; Kleinman, P.J.A. Phosphorus loss from land to water: Integrating agricultural and environmental management. Plant Soil 2001, 237, 287–307. [Google Scholar] [CrossRef]
- Sundareshwar, P.V.; Morris, J.T.; Koepfler, E.K.; Fornwalt, B. Phosphorus limitation of coastal ecosystem processes. Science 2003, 299, 563–565. [Google Scholar] [CrossRef]
- Foy, R.H. The return of the phosphorus paradigm: Agricultural phosphorus and eutrophication. In Phosphorus: Agriculture and the Environment; Sims, J.T., Sharpley, A.N., Eds.; American Society of Agronomy Monograph No. 46: Madison, WI, USA, 2005; pp. 911–939. [Google Scholar]
- Correll, D.L. The Role of Phosphorus in the Eutrophication of Receiving Waters: A Review. J. Environ. Qual. 1998, 27, 261–266. [Google Scholar] [CrossRef]
- Schindler, D.W. Evolution of phosphorus limitation in lakes. Science 1977, 195, 260–262. [Google Scholar] [CrossRef]
- Strock, J.S.; Kleinman, P.J.A.; King, K.W.; Delgado, J.A. Drainage water management for water quality protection. J. Soil Water Conserv. 2010, 65, 131A–136A. [Google Scholar] [CrossRef]
- Sims, J.T.; Simard, R.R.; Joern, B.C. Phosphorus Loss in Agricultural Drainage: Historical Perspective and Current Research. J. Environ. Qual. 1998, 27, 277–293. [Google Scholar] [CrossRef]
- Sharpley, A.N.; Withers, P.J.A. The environmentally-sound management of agricultural phosphorus. Fertil. Res. 1994, 39, 133–146. [Google Scholar] [CrossRef]
- Blicher-Mathiesen, G.; Rasmussen, A.; Rolighed, J.; Andersen, H.E.; Carstensen, M.V.; Jensen, P.G.; Wienke, J.; Hansen, B.; Thorling, L. Landovervågningsoplande 2015; NOVANA; Videnskabelig rapport fra DCE—Nationalt Center for Miljø og Energi nr. 205; Aarhus Universitet, DCE—Nationalt Center for Miljø og Energi: Roskilde, Denmark, 2016; 167p. [Google Scholar]
- Gelbrecht, J.; Lengsfeld, H.; Pöthig, R.; Opitz, D. Temporal and spatial variation of phosphorus input, retention and loss in a small catchment of NE Germany. J. Hydrol. 2005, 304, 151–165. [Google Scholar] [CrossRef]
- Ruark, M.; Madison, A.; Cooley, E.; Stuntebeck, T.; Komiskey, M. Phosphorus loss from tile drains: Should we be concerned? In Proceedings of the Wisconsin Crop Management Conference, University of Wisconsin, Madison, WI, USA, 10–12 January 2012.
- King, K.W.; Williams, M.R.; Macrae, M.L.; Fausey, N.R.; Frankenberger, J.; Smith, D.R.; Kleinman, P.J.A.; Brown, L.C. Phosphorus Transport in Agricultural Subsurface Drainage: A Review. J. Environ. Qual. 2015, 44, 467–485. [Google Scholar] [CrossRef] [PubMed]
- Johansson, G.; Gustafson, A. Observation Fields on Arable Land; Technical Report; Division of Water Quality Management: Trenton, NJ, USA, 2005. [Google Scholar]
- Takeno, N. Atlas of Eh-pH Diagrams: Intercomparison of Thermodynamic Databases; Geological Survey of Japan Open File Report No. 419; National Institute of Advanced Industrial Science and Technology, Research Center for Deep Geological Environments: Tsukuba, Tokyo, 2005. [Google Scholar]
- Scalenghe, R.; Edwards, A.C.; Marsan, F.A.; Barberis, E. The effect of reducing conditions on the solubility of phosphorus in a diverse range of European agricultural soils. Eur. J. Soil Sci. 2002, 53, 439–447. [Google Scholar] [CrossRef]
- Grant, R.; Laubel, A.; Kronvang, B.; Andersen, H.E.; Svendsen, L.M.; Fuglsang, A. Loss of dissolved and particulate phosphorus from arable catchments by subsurface drainage. Water Res. 1996, 30, 2633–2642. [Google Scholar] [CrossRef]
- Mcdowell, R.W.; Sharpley, A.N. Approximating Phosphorus Release from Soils to Surface Runoff and Subsurface Drainage. J. Environ. Qual. 2001, 30, 508–520. [Google Scholar] [CrossRef]
- Heckrath, G.; Brookes, P.C.; Poulton, P.R.; Goulding, K.W.T. Phosphorus Leaching from Soils Containing Different Phosphorus Concentrations in the Broadbalk Experiment. J. Environ. Qual. 1995, 24, 904. [Google Scholar] [CrossRef]
- McDowell, R.W.; Sharpley, A.N.; Brookes, P.A.; Poulton, P. Relationship between soil test phosphorus and phosphorus release to solution. Soil Sci. 2001, 166, 137–149. [Google Scholar] [CrossRef]
- Heiberg, L.; Pedersen, T.V.; Jensen, H.S.; Kjaergaard, C.; Hansen, H.C.B. A Comparative Study of Phosphate Sorption in Lowland Soils under Oxic and Anoxic Conditions. J. Environ. Qual. 2010, 39, 734–743. [Google Scholar] [CrossRef]
- Ulén, B. Size and settling velocities of phosphorus-containing particles in water from agricultural drains. Water Air Soil Pollut. 2004, 157, 331–343. [Google Scholar] [CrossRef]
- Gentry, L.E.; David, M.B.; Royer, T.V.; Mitchell, C.A.; Starks, K.M. Phosphorus Transport Pathways to Streams in Tile-Drained Agricultural Watersheds. J. Environ. Qual. 2007, 36, 408–415. [Google Scholar] [CrossRef]
- Kronvang, B.; Laubel, A.; Grant, R. Suspended sediment and particulate phosphorus transport and delivery pathways in an arable catchment, Gelbaek stream, Denmark. Hydrol. Process. 1997, 11, 627–642. [Google Scholar] [CrossRef]
- Steegen, A.; Govers, G.; Takken, I.; Nachtergaele, J.; Poesen, J.; Merckx, R. Factors Controlling Sediment and Phosphorus Export from Two Belgian Agricultural Catchments. J. Environ. Qual. 2001, 30, 1249–1258. [Google Scholar] [CrossRef] [PubMed]
- Stone, M.; Mudroch, A. The effect of particle size, chemistry and mineralogy of river sediments on phosphate adsorption. Environ. Technol. Lett. 1989, 10, 501–510. [Google Scholar] [CrossRef]
- Eastman, M.; Gollamudi, A.; Stampfli, N.; Madramootoo, C.A.; Sarangi, A. Comparative evaluation of phosphorus losses from subsurface and naturally drained agricultural fields in the Pike River watershed of Quebec, Canada. Agric. Water Manag. 2010, 97, 596–604. [Google Scholar] [CrossRef]
- Beauchemin, S.; Simard, R.R.; Cluis, D. Forms and Concentration of Phosphorus in Drainage Water of Twenty-Seven Tile-Drained Soils. J. Environ. Qual. 1998, 27, 721–728. [Google Scholar] [CrossRef]
- Syers, J.K.; Johnston, A.E.; Curtin, D. Efficiency of Soil and Fertilizer Phosphorus Use. Reconciling Changing Concepts of Soil Phosphorous Behaviour with Agronomic Information; FAO: Rome, Italy, 2008. [Google Scholar]
- Bergström, L.; Kirchmann, H.; Djodjic, F.; Kyllmar, K.; Ulén, B.; Liu, J.; Andersson, H.; Aronsson, H.; Börjesson, G.; Kynkäänniemi, P.; et al. Turnover and Losses of Phosphorus in Swedish Agricultural Soils: Long-Term Changes, Leaching Trends, and Mitigation Measures. J. Environ. Qual. 2015, 44, 512–523. [Google Scholar] [CrossRef]
- Andreini, M.S.; Steenhuis, T.S. Preferential paths of flow under conventional and conservation tillage. Geoderma 1990, 46, 85–102. [Google Scholar] [CrossRef]
- Gaynor, J.D.; Findlay, W.I. Soil and Phosphorus Loss from Conservation and Conventional Tillage in Corn Production. J. Environ. Qual. 1995, 24, 734–741. [Google Scholar] [CrossRef]
- Kleinman, P.J.; Sharpley, A.N.; Saporito, L.S.; Buda, A.R.; Bryant, R.B. Application of manure to no-till soils: Phosphorus losses by sub-surface and surface pathways. Nutr. Cycl. Agroecosyst. 2009, 84, 215–227. [Google Scholar] [CrossRef]
- Lemunyon, J. SERA-17: Innovative Solutions to Minimize Phosphorus Losses from Agriculture. Cover Crops. Available online: https://sera17.org/ (accessed on 12 May 2019).
- Kleinman, P.J.; Smith, D.R.; Bolster, C.H.; Easton, Z. M Phosphorus Fate, Management, and Modeling in Artificially Drained Systems. J. Environ. Qual. 2015, 44, 460–466. [Google Scholar] [CrossRef]
- Schoumans, O.F.; Van den Berg, R.; Beusen, A.H.W.; Van den Born, G.J.; Renaud, L.; Roelsma, J.; Gronendijk, P. Quick Scan of the Impact of Proposed Application Standards for Animal Manure and Chemical Fertilisers on Nutrient Losses to Groundwater and Surface Waters; Rep. 730.6; Alterra: Wageningen, The Netherlands, 2004. (In Dutch) [Google Scholar]
- Djodjic, F.; Bergström, L.; Ulén, B. Phosphorus losses from a structured clay soil in relation to tillage practices. Soil Use Manag. 2002, 18, 79–83. [Google Scholar] [CrossRef]
- Peron, H.; Hueckel, T.; Laloui, L.; Hu, L.B. Fundamentals of desiccation cracking of finegrained soils: Experimental characterisation and mechanisms identification. Can. Geotech. J. 2009, 46, 1177–1201. [Google Scholar] [CrossRef]
- Liu, J.; Ulén, B.; Bergkvist, G.; Aronsson, H. Freezing–thawing effects on phosphorus leaching from catch crops. Nutr. Cycl. Agroecosyst. 2014, 99, 17–30. [Google Scholar] [CrossRef]
- Liu, J.; Khalaf, R.; Ulén, B.; Bergkvist, G. Potential phosphorus release from catch crop shoots and roots after freezing-thawing. Plant Soil 2013, 371, 543–557. [Google Scholar] [CrossRef]
- Ulén, B. Episodic precipitation and discharge events and their influence on losses of phosphorus and nitrogen from tile-drained arable fields. Swed. J. Agric. Res. 1995, 25, 25–31. [Google Scholar]
- Svanbäck, A.; Ulén, B.; Etana, A.; Bergström, L.; Kleinman, P.J.A.; Mattsson, L. Influence of soil phosphorus and manure on phosphorus leaching in Swedish topsoils. Nutr. Cycl. Agroecosyst. 2013, 96, 133–147. [Google Scholar] [CrossRef]
- Haygarth, P.M.; Chapman, P.J.; Jarvis, S.C.; Smith, R.V. Phosphorus budgets for two contrasting grassland farming systems in the UK. Soil Use Manag. 1998, 14, 160–167. [Google Scholar] [CrossRef]
- Valero, C.S.; Madramootoo, C.A.; Stämpfli, N. Water table management impacts on phosphorus loads in tile drainage. Agric. Water Manag. 2007, 89, 71–80. [Google Scholar] [CrossRef]
- Bryant, R.B.; Buda, A.R.; Kleinman, P.J.A.; Church, C.D.; Saporito, L.S.; Folmar, G.J.; Bose, S.; Allen, A.L. Using Flue Gas Desulfurization Gypsum to Remove Dissolved Phosphorus from Agricultural Drainage Waters. J. Environ. Qual. 2012, 41, 664–671. [Google Scholar] [CrossRef]
- Schoumans, O.F.; Kruijne, R.; Van der Molen, D.T. Methods to reduce phosphorus leaching from phosphate saturated soils. Landschap 1995, 12, 63–73. (In Dutch) [Google Scholar]
- McDowell, R.W.; Sharpley, A.N.; Bourke, W. Treatment of Drainage Water with Industrial By-Products to Prevent Phosphorus Loss from Tile-Drained Land. J. Environ. Qual. 2008, 37, 1575–1582. [Google Scholar] [CrossRef] [PubMed]
- Gburek, W.J.; Sharpley, A.N. Hydrologic Controls on Phosphorus Loss from Upland Agricultural Watersheds. J. Environ. Qual. 1998, 27, 267–277. [Google Scholar] [CrossRef]
- Sharpley, A.N.; Bergström, L.; Aronsson, H.; Bechmann, M.; Bolster, C.H.; Börling, K.; Djodjic, F.; Jarvie, H.P.; Schoumans, O.F.; Stamm, C.; et al. Future agriculture with minimized phosphorus losses to waters: Research needs and direction. Ambio 2015, 44, 163–179. [Google Scholar] [CrossRef] [PubMed]
- Tanner, C.C.; Nguyen, M.L.; Sukias, J.P.S. Nutrient removal by a constructed wetland treating subsurface drainage from grazed dairy pasture. Agric. Ecosyst. Environ. 2005, 105, 145–162. [Google Scholar] [CrossRef]
- Thiere, G.; Milenkovski, S.; Lindgren, P.-E.; Sahlén, G.; Berglund, O.; Weisner, S.E.B. Wetland creation in agricultural landscapes: Biodiversity benefits on local and regional scales. Biol. Conserv. 2009, 142, 964–973. [Google Scholar] [CrossRef]
- Kovacic, D.A.; David, M.B.; Gentry, L.E.; Starks, K.M.; Cooke, R.A. Effectiveness of Constructed Wetlands in Reducing Nitrogen and Phosphorus Export from Agricultural Tile Drainage. J. Environ. Qual. 2000, 29, 1262–1274. [Google Scholar] [CrossRef]
- De Laney, T.A. Benefits to downstream flood attenuation and water quality as a result of constructed wetlands in agricultural landscapes. J. Soil Water Conserv. 1995, 50, 620–626. [Google Scholar]
- Verhoeven, J.T.A.; Arheimer, B.; Yin, C.; Hefting, M.M. Regional and global concerns over wetlands and water quality. Trends Ecol. Evol. 2006, 21, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Keddy, P.A. Wetland Ecology: Principles and Conservation, 2nd ed.; Cambridge University Press: New York, NY, USA, 2010. [Google Scholar]
- Reddy, K.R.; Kadlec, R.H.; Flaig, E.; Gale, P.M. Phosphorus Retention in Streams and Wetlands: A Review. Crit. Rev. Environ. Sci. Technol. 1999, 29, 83–146. [Google Scholar] [CrossRef]
- Décamps, H.; Naiman, R.J.; McClain, M.E. Riparian Zones. In River Ecosystem Ecology: A Global Perspective; Likens, G.E., Ed.; Academic Press: San Diego, CA, USA, 2009; pp. 182–189. [Google Scholar]
- Blann, K.L.; Anderson, J.L.; Sands, G.R.; Vondracek, B. Effects of Agricultural Drainage on Aquatic Ecosystems: A Review. Crit. Rev. Environ. Sci. Technol. 2009, 39, 909–1001. [Google Scholar] [CrossRef]
- McCorvie, M.R.; Lant, C.L. Drainage district formation and the loss of Midwestern wetlands, 1850–1930. Agric. Hist. 1993, 67, 13–39. [Google Scholar]
- Stromberg, J.C. Restoration of riparian vegetation in the south-western United States: Importance of flow regimes and fluvial dynamism. J. Arid. Environ. 2001, 49, 17–34. [Google Scholar] [CrossRef]
- Nair, V.D.; Graetz, D.A. Phosphorus Saturation in Spodosols Impacted by Manure. J. Environ. Qual. 2002, 31, 1279–1285. [Google Scholar] [CrossRef] [PubMed]
- Allen, B.L.; Mallarino, A.P. Relationships between Extractable Soil Phosphorus and Phosphorus Saturation after Long-Term Fertilizer or Manure Application. Soil Sci. Soc. Am. J. 2006, 70, 454–463. [Google Scholar] [CrossRef]
- Börling, K.; Otabbonga, E.; Barberis, E. Soil Variables for Predicting Potential Phosphorus Release in Swedish Noncalcareous Soils. J. Environ. Qual. 2004, 33, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhang, J.; Ngo, H.H.; Guo, W.; Hub, Z.; Liang, S.; Fan, J.; Liu, H. A review on the sustainability of constructed wetlands for wastewater treatment: Design and operation. Bioresour. Technol. 2015, 175, 594–601. [Google Scholar] [CrossRef]
- Kadlec, R.H.; Wallace, S.D. Treatment Wetlands, 2nd ed.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2008. [Google Scholar]
- Braskerud, B.C. Factors affecting phosphorus retention in small constructed wetlands treating agricultural non-point source pollution. Ecol. Eng. 2002, 19, 41–61. [Google Scholar] [CrossRef]
- Johannesson, K.M.; Andersson, J.L.; Tonderski, K.S. Efficiency of a constructed wetland for retention of sediment-associated phosphorus. Hydrobiologia 2011, 674, 179–190. [Google Scholar] [CrossRef][Green Version]
- Johannesson, K.M.; Kynkäänniemi, P.; Ulén, B.; Weisner, S.E.B.; Tonderski, K.S. Phosphorus and particle retention in constructed wetlands—A catchment comparison. Ecol. Eng. 2015, 80, 20–31. [Google Scholar] [CrossRef]
- Kynkäänniemi, P.; Ulén, B.; Torstensson, G.; Tonderski, K.S. Phosphorus Retention in a Newly Constructed Wetland Receiving Agricultural Tile Drainage Water. J. Environ. Qual. 2013, 42, 596–605. [Google Scholar] [CrossRef]
- Maynard, J.J.; Geen, A.T.O.; Dahlgren, R.A.; Davis, C. Bioavailability and Fate of Phosphorus in Constructed Wetlands Receiving Agricultural Runoff in the San Joaquin Valley, California. J. Environ. Qual. 2009, 38, 360–372. [Google Scholar] [CrossRef]
- Mendes, L.R.D.; Tonderski, K.; Iversen, B.V.; Kjaergaard, C. Phosphorus retention in surface-flow constructed wetlands targeting agricultural drainage water. Ecol. Eng. 2018, 120, 94–103. [Google Scholar] [CrossRef]
- Kroeger, A.C.; Madramootoo, C.A.; Enright, P.; Laflamme, C. Efficiency of a small constructed wetland in southern Québec for treatment of agricultural runoff waters. In Proceedings of the Wastewater Biosolids Sustainability: Technical, Managerial, and Public Synergy, Moncton, NB, Canada, 24–27 June 2007; pp. 1057–1062. [Google Scholar]
- Tanner, C.C.; Sukias, J.P.S. Multiyear nutrient removal performance of three constructed wetlands intercepting tile drain flows from grazed pastures. J. Environ. Qual. 2011, 40, 620–633. [Google Scholar] [CrossRef]
- Reinhardt, M.; Gächter, R.; Wehrli, B.; Müller, B. Phosphorus retention in small constructed wetlands treating agricultural drainage water. J. Environ. Qual. 2005, 34, 1251–1259. [Google Scholar] [CrossRef] [PubMed]
- Crumpton, W.G.; Kovacic, D.A.; Hey, D.L.; Kostel, J.A. Potential of Restored and Constructed Wetlands to Reduce Nutrient Export from Agricultural Watersheds in the Corn Belt. In Final Report: Gulf Hypoxia and Local Water Quality Concerns Workshop; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2008; pp. 29–42. [Google Scholar]
- DeBusk, T.A.; Grace, K.A.; Dierberg, F.E. Treatment wetlands for removing phosphorus from agricultural drainage water. In Nutrient Management in Agricultural Watersheds: A Wetlands Solution; Dunne, E.J., Reddy, K.R., Carton, O.T., Eds.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2005; pp. 167–178. [Google Scholar]
- O’Geen, A.T.; Budd, R.; Gan, J.; Maynard, J.J.; Parikh, S.J.; Dahlgren, R.A. Mitigating Nonpoint Source Pollution in Agriculture with Constructed and Restored Wetlands. Adv. Agron. 2010, 108, 1–76. [Google Scholar]
- Mendes, L.R.D.; Tonderski, K.; Kjaergaard, C. Phosphorus accumulation and stability in sediments of surface-flow constructed wetlands. Geoderma 2018, 331, 109–120. [Google Scholar] [CrossRef]
- Hoagland, C.R.; Gentry, L.E.; David, M.B.; Kovacic, D.A. Plant Nutrient Uptake and Biomass Accumulation in a Constructed Wetland. J. Freshw. Ecol. 2001, 16, 527–540. [Google Scholar] [CrossRef]
- Gu, B.; Dreschel, T. Effects of plant community and phosphorus loading rate on constructed wetland performance in Florida, USA. Wetlands 2008, 28, 81–91. [Google Scholar] [CrossRef]
- Kao, J.T.; Titus, J.E.; Zhu, W.-X. Differential nitrogen and phosphorus retention by five wetland plant species. Wetlands 2003, 23, 979–987. [Google Scholar] [CrossRef]
- Vymazal, J. Emergent plants used in free water surface constructed wetlands: A review. Ecol. Eng. 2013, 61, 582–592. [Google Scholar] [CrossRef]
- Pant, H.K.; Reddy, K.R.; Dierberg, F.E. Bioavailability of Organic Phosphorus in a Submerged Aquatic Vegetation-Dominated Treatment Wetland. J. Environ. Qual. 2002, 31, 1748–1756. [Google Scholar] [CrossRef]
- Pant, H.K.; Reddy, K.R. Hydrologic influence on stability of organic phosphorus in wetland detritus. J. Environ. Qual. 2001, 30, 668–674. [Google Scholar] [CrossRef]
- Dunne, E.J.; Culleton, N.; O’Donovan, G.; Harrington, R.; Daly, K. Phosphorus retention and sorption by constructed wetland soils in Southeast Ireland. Water Res. 2005, 39, 4355–4362. [Google Scholar] [CrossRef] [PubMed]
- Lai, D.Y.F. Phosphorus fractions and fluxes in the soils of a free surface flow constructed wetland in Hong Kong. Ecol. Eng. 2014, 73, 73–79. [Google Scholar] [CrossRef]
- Braskerud, B.C.; Tonderski, K.S.; Wedding, B.; Bakke, R.; Blankenberg, A.G.; Ulen, B.; Koskiaho, J. Can constructed wetlands reduce the diffuse phosphorus loads to eutrophic water in cold temperate regions? J. Environ. Qual. 2005, 34, 2145–2155. [Google Scholar] [CrossRef]
- Braskerud, B.C. The influence of vegetation on sedimentation and resuspension of soil particles in small constructed wetlands. J. Environ. Qual. 2001, 30, 1447–1457. [Google Scholar] [CrossRef] [PubMed]
- Braskerud, B.C. Clay particle retention in small constructed wetlands. Water Res. 2003, 37, 3793–3802. [Google Scholar] [CrossRef]
- Lavrnić, S.; Braschi, I.; Anconelli, S.; Blasioli, S.; Solimando, D.; Mannini, P.; Toscano, A. Long-Term Monitoring of a Surface Flow Constructed Wetland Treating Agricultural Drainage Water in Northern Italy. Water 2018, 10, 644. [Google Scholar] [CrossRef]
- Johannesson, K.M.; Tonderski, K.S.; Ehde, P.M.; Weisner, S.E.B. Temporal phosphorus dynamics affecting retention estimates inagricultural constructed wetlands. Ecol. Eng. 2017, 103, 436–445. [Google Scholar] [CrossRef]
- Land, M.; Granéli, W.; Grimvall, A.; Hoffmann, C.C.; Mitsch, W.J.; Tonderski, K.S.; Verhoeven, J.T. How effective are created or restored freshwater wetlands for nitrogen and phosphorus removal? A systematic review. Environ. Evid. 2016, 5, 9. [Google Scholar] [CrossRef]
- Geranmayeh, P.; Johannesson, K.M.; Ulén, B.; Tonderski, K.S. Particle deposition, resuspension and phosphorus accumulation in small constructed wetlands. Ambio 2018, 47, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Persson, J.; Somes, N.L.G.; Wong, T.H.F. Hydraulic efficiency of constructed wetlands and ponds. Water Sci. Technol. 1999, 40, 291–300. [Google Scholar] [CrossRef]
- Dierberg, F.E.; Juston, J.J.; DeBusk, T.A.; Pietro, K.; Gu, B. Relationship between hydraulic efficiency and phosphorus removal in a submerged aquatic vegetation-dominated treatment wetland. Ecol. Eng. 2005, 25, 9–23. [Google Scholar] [CrossRef]
- Maynard, J.J.; O’Geen, A.T.; Dahlgren, R.A. Spatial Relationships of Phosphorus Sorption in a Seasonally Saturated Constructed Wetland Soil. Soil Sci. Soc. Am. J. 2009, 73, 1741–1753. [Google Scholar] [CrossRef]
- Su, T.-M.; Yang, S.-C.; Shih, S.-S.; Lee, H.-Y. Optimal design for hydraulic efficiency performance of free-water-surface constructed wetlands. Ecol. Eng. 2009, 35, 1200–1207. [Google Scholar] [CrossRef]
- Guo, C.; Cui, Y.; Dong, B.; Luo, Y.; Liu, F.; Zhao, S.; Wu, H. Test study of the optimal design for hydraulic performance and treatment performance of free water surface flow constructed wetland. Bioresour. Technol. 2017, 238, 461–471. [Google Scholar] [CrossRef]
- Braskerud, B.C. Design considerations for increased sedimentation in small wetlands treating agricultural runoff. Water Sci Technol. 2002, 45, 77–85. [Google Scholar] [CrossRef]
- Dierberg, F.E.; DeBusk, T.A. Particulate phosphorus transformations in south Florida stormwater treatment areas used for Everglades protection. Ecol. Eng. 2008, 34, 100–115. [Google Scholar] [CrossRef]
- Van der Zee, S.E.A.T.M.; Van Riemsdijk, W.H. Sorption kinetics and transport of phosphate in sandy soil. Geoderma 1986, 38, 293–309. [Google Scholar] [CrossRef]
- Zou, Y.; Zhang, L.; Wang, L.; Zhang, S.; Yu, X. Effects of Aeration, Vegetation, and Iron Input on Total P Removal in a Lacustrine Wetland Receiving Agricultural Drainage. Water. 2018, 10, 61. [Google Scholar] [CrossRef]
- Flessa, H. Plant-induced changes in the redox potential of the rhizospheres of the submerged vascular macrophytes Myriophyllum verticillatum L. and Ranunculus circinatus L. Aquat. Bot. 1994, 47, 119–129. [Google Scholar] [CrossRef]
- Armstrong, J.; Armstrong, W. Light-enhanced convective throughflow increases oxygenation in rhizomes and rhizosphere of Phragmites australis (Cav.) Trin. ex Steud. New Phytol. 1990, 114, 121–128. [Google Scholar] [CrossRef]
- Moore, B.C.; Lafer, J.E.; Funk, W.H. Influence of aquatic macrophytes on phosphorus and sediment porewater chemistry in a freshwater wetland. Aquat. Bot. 1994, 49, 137–148. [Google Scholar] [CrossRef]
- White, J.R.; Reddy, K.R.; Moustafa, M.Z. Influence of hydrologic regime and vegetation on phosphorus retention in Everglades stormwater treatment area wetlands. Hydrol. Process. 2004, 18, 343–355. [Google Scholar] [CrossRef]
- Olila, O.G.; Reddy, K.R.; Stites, D.L. Influence of draining on soil phosphorus forms and distribution in a constructed wetland. Ecol. Eng. 1997, 9, 157–169. [Google Scholar] [CrossRef]
- Ann, Y.; Reddy, K.R.; Delfino, J.J. Influence of chemical amendments on phosphorus immobilization in soils from a constructed wetland. Ecol. Eng. 1999, 14, 157–167. [Google Scholar] [CrossRef]
- Ballantine, D.J.; Tanner, C.C. Substrate and filter materials to enhance phosphorus removal in constructed wetlands treating diffuse farm runoff: A review. N. Z. J. Agric. Res. 2010, 53, 71–95. [Google Scholar] [CrossRef]
- Li, Y.; Wang, S.; Li, Y.; Kong, F.; Xi, H.; Liu, Y. Corn Straw as a Solid Carbon Source for the Treatment of Agricultural Drainage Water in Horizontal Subsurface Flow Constructed Wetlands. Water 2018, 10, 511. [Google Scholar] [CrossRef]
- Vymazal, J. Constructed Wetlands for Wastewater Treatment. Water 2010, 2, 530–549. [Google Scholar] [CrossRef]
- Gachango, F.G.; Pedersen, S.M.; Kjaergaard, C. Cost-Effectiveness Analysis of Surface Flow Constructed Wetlands (SFCW) for Nutrient Reduction in Drainage Discharge from Agricultural Fields in Denmark. Environ. Manag. 2015, 56, 1478–1486. [Google Scholar] [CrossRef]
- Schoumans, O.F.; Chardon, W.J.; Bechmann, M.E.; Gascuel-Odoux, C.; Hofman, G.; Kronvang, B.; Ulén, B.; Dorioz, J.-M. Mitigation options to reduce phosphorus losses from the agricultural sector and improve surface water quality: A review. Sci. Total Environ. 2014, 468–469, 1255–1266. [Google Scholar] [CrossRef] [PubMed]
- Kronvang, B.; Bechmann, M.; Lundekvam, H.; Behrendt, H.; Rubæk, G.H.; Schoumans, O.F.; Syversen, N.; Andersen, H.E.; Hoffmann, C.C. Phosphorus Losses from Agricultural Areas in River Basins: Effects and Uncertainties of Targeted Mitigation Measures. J. Environ. Qual. 2005, 34, 2129–2144. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, C.C.; Kjaergaard, C.; Uusi-Kämppä, J.; Hansen, H.C.B.; Kronvang, B. Phosphorus Retention in Riparian Buffers: Review of Their Efficiency. J. Environ. Qual. 2009, 38, 1942–1955. [Google Scholar] [CrossRef]
- Shenker, M.; Seitelbach, S.; Brand, S.; Haim, A.; Litaor, M.I. Redox reactions and phosphorus release in re-flooded soils of an altered wetland. Eur. J. Soil Sci. 2005, 56, 515–525. [Google Scholar] [CrossRef]
- Hoffmann, C.C.; Baattrup-Pedersen, A.; Amsinck, S.L.; Clausen, P. Overvågning af Vandmiljøplan II Vådområder 2005; Faglig rapport fra DMU nr. 576; Danmarks Miljøundersøgelser: Silkeborg, Denmark, 2006. (In Danish) [Google Scholar]
- Hoffmann, C.C.; Heiberg, L.; Audet, J.; Schønfeldt, B.; Fuglsang, A.; Kronvang, B.; Ovesen, N.B.; Kjaergaard, C.; Hansen, H.C.B.; Jensen, H.S.; et al. Low phosphorus release but high nitrogen removal in two restored riparian wetlands inundated with agricultural drainage water. Ecol. Eng. 2012, 46, 75–87. [Google Scholar] [CrossRef]
- Hogan, D.M.; Jordan, T.E.; Walbridge, M.R. Phosphorus retention and soil organic carbon in restored and natural freshwater wetlands. Wetlands 2004, 24, 573–585. [Google Scholar] [CrossRef]
- Woltemade, C.J. Ability of restored wetlands to reduce nitrogen and phosphorus concentrations in agricultural drainage water. J. Soil Water Conserv. 2000, 55, 303–309. [Google Scholar]
- Liikanen, A.; Puustinen, M.; Koskiaho, J.; Väisänen, T.; Martikainen, P.; Hartikainen, H. Phosphorus Removal in a Wetland Constructed on Former Arable Land. J. Environ. Qual. 2004, 33, 1124–1132. [Google Scholar] [CrossRef]
- Zedler, J.B. Wetlands at your service: Reducing impacts of agriculture at the watershed scale. Front. Ecol. Environ. 2003, 1, 65–72. [Google Scholar] [CrossRef]
- Jordan, T.E.; Whigham, D.F.; Hofmockel, K.H.; Pittek, M.A. Nutrient and Sediment Removal by a Restored Wetland Receiving Agricultural Runoff. J. Environ. Qual. 2003, 32, 1534–1547. [Google Scholar] [CrossRef]
- Hickey, M.B.C.; Doran, B. A Review of the Efficiency of Buffer Strips for the Maintenance and Enhancement of Riparian Ecosystems. Water Qual. Res. J. Can. 2004, 39, 311–317. [Google Scholar] [CrossRef]
- Muscutt, A.D.; Harris, G.L.; Bailey, S.W.; Davies, D.B. Buffer zones to improve water quality: A review of their potential use in UK agriculture. Agric. Ecosyst. Environ. 1993, 45, 59–77. [Google Scholar] [CrossRef]
- Parkyn, S. Review of Riparian Buffer Zone Effectiveness; Technical Paper No 2004/05; Ministry of Agriculture and Forestry: Wellington, New Zealand, 2004. [Google Scholar]
- Osborne, L.L.; Kovacic, D.A. Riparian vegetated buffer strips in water-quality restoration and stream management. Freshw. Biol. 1993, 29, 243–258. [Google Scholar] [CrossRef]
- McDowell, R.W.; Biggs, B.J.F.; Sharpley, A.N.; Nguyen, L. Connecting phosphorus loss from agricultural landscapes to surface water quality. Chem. Ecol. 2004, 20, 1–40. [Google Scholar] [CrossRef]
- Wenger, S. A Review of the Scientific Literature on Riparian Buffer Width, Extent and Vegetation; Office of Public Service and Outreach, Institute of Ecology, University of Georgia: Athens, GA, USA, 1999. [Google Scholar]
- Roberts, W.M.; Stutter, M.I.; Haygarth, P.M. Phosphorus Retention and Remobilization in Vegetated Buffer Strips: A Review. J. Environ. Qual. 2012, 41, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Uusi-Kämppä, J.; Yläranta, T. Effect of buffer strips on controlling soil erosion and nutrient losses in southern Finland. In Wetlands: Environmental Gradients, Boundaries, and Buffers; Mulamoottil, G., Warner, B.G., McBean, E.A., Eds.; CRC Press, Lewis Publishers: Boca Raton, FL, USA, 1996; pp. 221–235. [Google Scholar]
- Helmers, M.J.; Isenhart, T.M.; Dosskey, M.G.; Dabney, S.M.; Strock, J.S. Buffers and Vegetative Filter Strips. In UMRSHNC (Upper Mississippi River Sub-basin Hypoxia Nutrient Committee) Final Report: Gulf Hypoxia and Local Water Quality Concerns Workshop; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2008; pp. 43–58. [Google Scholar]
- Bhattarai, R.; Kalita, P.K.; Patel, M.K. Nutrient transport through a Vegetative Filter Strip with subsurface drainage. J. Environ. Manag. 2009, 90, 1868–1876. [Google Scholar] [CrossRef]
- Zak, D.; Kronvang, B.; Carstensen, M.V.; Hoffmann, C.C.; Kjeldgaard, A.; Larsen, S.E.; Audet, J.; Egemose, S.; Jorgensen, C.A.; Feuerbach, P.; et al. Nitrogen and Phosphorus Removal from Agricultural Runoff in Integrated Buffer Zones. Environ. Sci. Technol. 2018, 52, 6508–6517. [Google Scholar] [CrossRef]
- Penn, C.; McGrath, J.; Bowen, J.; Wilson, S. Phosphorus removal structures: A management option for legacy phosphorus. J. Soil Water Conserv. 2014, 69, 51A–56A. [Google Scholar] [CrossRef]
- Penn, C.J.; Bryant, R.B.; Kleinman, P.J.A.; Allen, A.L. Removing dissolved phosphorus from drainage ditch water with phosphorus sorbing materials. J. Soil Water Conserv. 2007, 62, 269–276. [Google Scholar]
- Lyngsie, G.; Borggaard, O.K.; Hansen, H.C.B. A three-step test of phosphate sorption efficiency of potential agricultural drainage filter materials. Water Res. 2014, 51, 256–265. [Google Scholar] [CrossRef]
- Canga, E.; Heckrath, G.J.; Kjaergaard, C. Agricultural Drainage Filters. II. Phosphorus Retention and Release at Different Flow Rates. Water Air Soil Pollut. 2016, 227, 276–288. [Google Scholar] [CrossRef]
- Lyngsie, G.; Penn, C.J.; Hansen, H.C.B.; Borggaard, O.K. Phosphate sorption by three potential filter materials as assessed by isothermal titration calorimetry. J. Environ. Manag. 2014, 143, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Allred, B.J.; Racharaks, R. Laboratory Comparison of Four Iron-Based Filter Materials for Drainage Water Phosphate Treatment. Water Environ. Res. 2014, 86, 852–862. [Google Scholar] [CrossRef] [PubMed]
- Allred, B.J. Laboratory evaluation of porous iron composite for agricultural drainage water filter treatment. Trans. ASABE 2012, 55, 1683–1697. [Google Scholar] [CrossRef]
- Allred, B.J. Laboratory batch test evaluation of five filter materials for removal of nutrients and pesticides from drainage waters. Trans ASABE 2010, 53, 39–54. [Google Scholar] [CrossRef]
- King, K.W.; McDonald, J.; Moore, J.F.; Agrawal, S.G.; Fischer, E.N.; Balogh, J.C. Nutrient and pesticide removal from laboratory-simulated tile drainage discharge. Trans. ASABE 2010, 53, 769–777. [Google Scholar] [CrossRef]
- Kirkkala, T.; Ventelä, A.-M.; Tarvainen, M. Fosfilt filters in an agricultural catchment: A long-term field-scale experiment. Agric. Food Sci. 2012, 21, 237–246. [Google Scholar] [CrossRef]
- Gottschall, N.; Edwards, M.; Craiovan, E.; Frey, S.K.; Sunohara, M.; Ball, B.; Zoski, E.; Topp, E.; Khana, I.; Clark, I.D.; et al. Amending woodchip bioreactors with water treatment plant residuals to treat nitrogen, phosphorus, and veterinary antibiotic compounds in tile drainage. Ecol. Eng. 2016, 95, 852–864. [Google Scholar] [CrossRef]
- Carstensen, M.V.; Larsen, S.E.; Kjærgaard, C.; Hoffmann, C.C. Reducing adverse side effects by seasonally lowering nitrate removal in subsurface flow constructed wetlands. J. Environ. Manag. 2019, 240, 190–197. [Google Scholar] [CrossRef]
- Christianson, L.E.; Lepine, C.; Sibrell, P.L.; Penn, C.; Summerfelt, S.T. Denitrifying woodchip bioreactor and phosphorus filter pairing to minimize pollution swapping. Water Res. 2017, 121, 129–139. [Google Scholar] [CrossRef]
- Goodwin, G.E.; Bhattarai, R.; Cooke, R. Synergism in nitrate and orthophosphate removal in subsurface bioreactors. Ecol. Eng. 2015, 84, 559–568. [Google Scholar] [CrossRef]
- Hua, G.; Salo, M.W.; Schmit, C.G.; Hay, C.H. Nitrate and phosphate removal from agricultural subsurface drainage using laboratory woodchip bioreactors and recycled steel byproduct filters. Water Res. 2016, 102, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Thapa, U. Evaluation of Woodchip Bioreactors and Phosphorus Adsorption Media for Nutrient Removal from Subsurface Drainage Water; South Dakota State University: Brookings, SD, USA, 2017. [Google Scholar]
- Stoner, D.; Penn, C.; McGrath, J.; Warren, J. Phosphorus Removal with By-Products in a Flow-Through Setting. J. Environ. Qual. 2012, 41, 654–663. [Google Scholar] [CrossRef] [PubMed]
- Penn, C.; Chagas, I.; Klimeski, A.; Lyngsie, G. A Review of Phosphorus Removal Structures: How to Assess and Compare Their Performance. Water 2017, 9, 583. [Google Scholar] [CrossRef]
- Buda, A.R.; Koopmans, G.F.; Bryant, R.B.; Chardon, W.J. Emerging Technologies for Removing Nonpoint Phosphorus from Surface Water and Groundwater: Introduction. J. Environ. Qual. 2012, 41, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Lyngsie, G.; Penn, C.J.; Pedersen, H.L.; Borggaard, O.K.; Hansen, H.C.B. Modelling of phosphate retention by Ca- and Fe-rich filter materials under flow-through conditions. Ecol. Eng. 2015, 75, 93–102. [Google Scholar] [CrossRef]

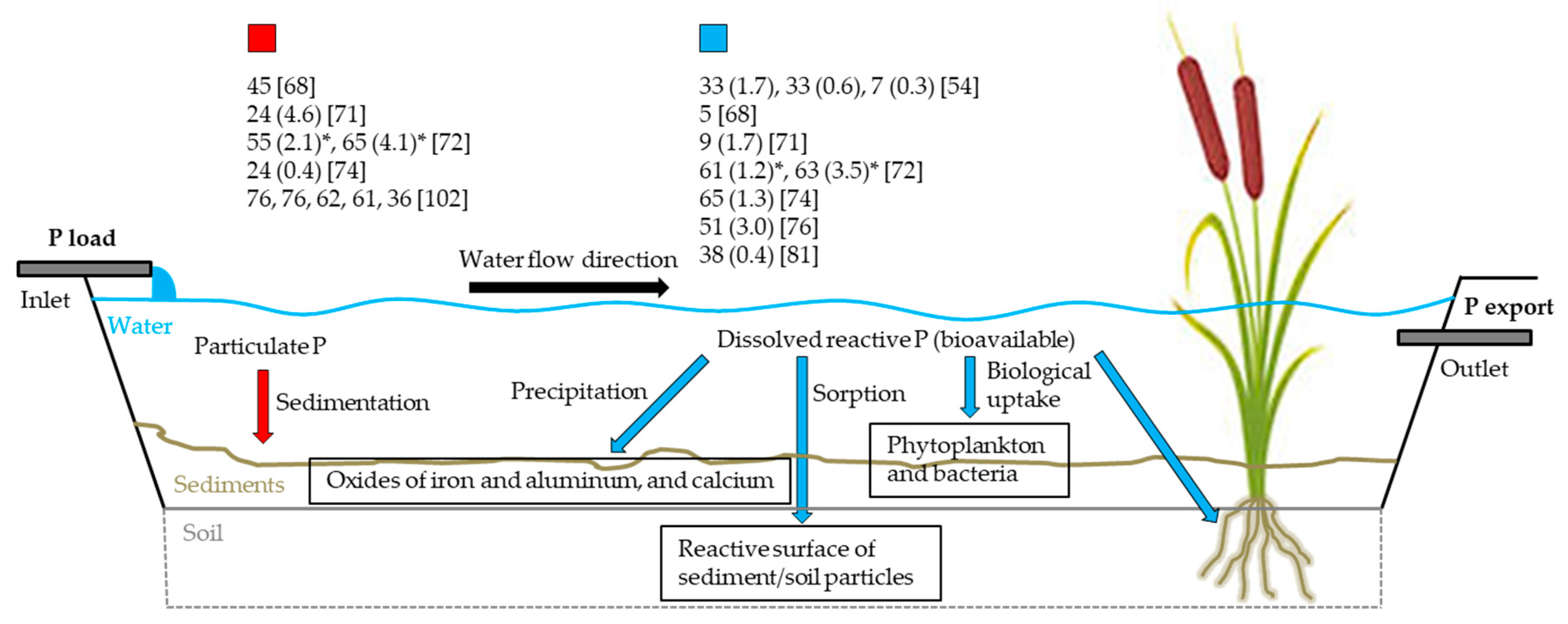
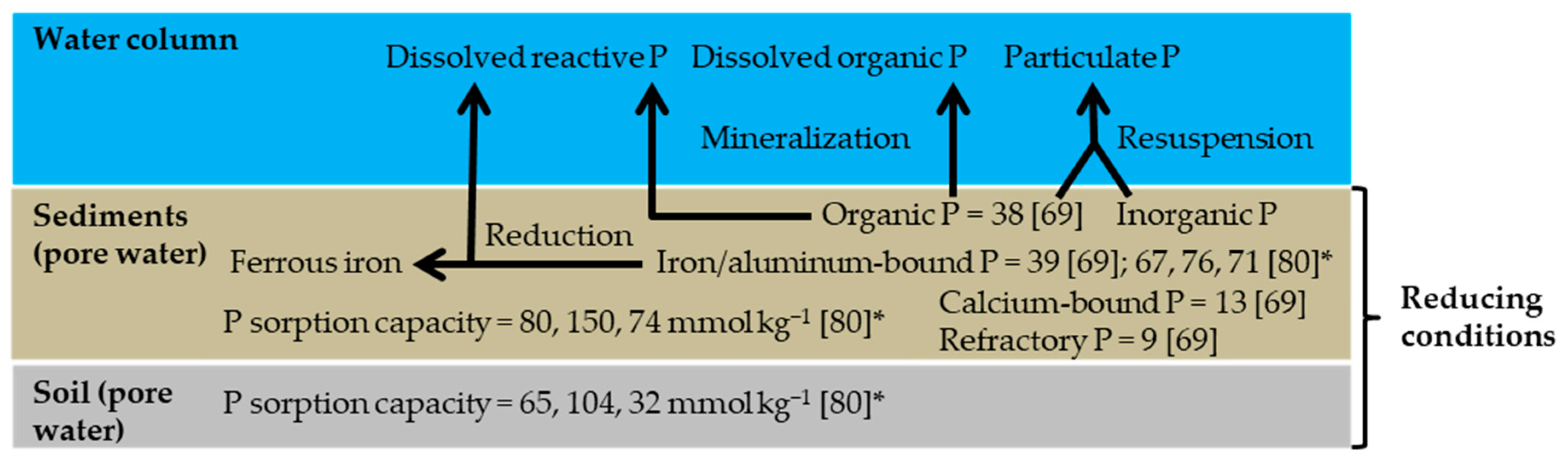
| Country | Name | ASFCW | Ratio of ASFCW to AAC | Monitoring Time | Hydraulic Load | P Concentration | P Load | DP/PP Fraction | Hydraulic Residence Time | P Retention | Study | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| m2 | % | yr | m yr−1 | mg L−1 | g m−2 yr−1 | % | d | g m−2 yr−1 | % | |||
| Norway | Berg | 900 | 0.06 | 7 | 595 | 0.17 | 97 | - | - | 40 | 41 | [68] |
| Norway | Kinn | 345 | 0.07 | 7 | 661 | 0.25 | 178 | 12/88 | - | 57 | 32 | [68] |
| Norway | Flatabekken | 870 | 0.08 | 4 | 588 | 0.22 | 124 | 49/51 | - | 26 | 21 | [68] |
| Norway | Grautholen 1 | 460 | 0.21 | 3 | 445 | 0.43 | 191 | - | - | 71 | 37 | [68] |
| Norway | Grautholen 2 | 840 | 0.38 | 3 | 241 | 0.43 | 106 | - | - | 47 | 44 | [68] |
| Sweden | Södra Stene | 21,000 | 2.2 | 4 | 6.7 | 0.13 a | 1.62 | 31/69 | - | 0.27 | 16 | [69] |
| Sweden | Bergaholm | 800 | 0.3 | 2 | 60 | 0.30 | 19 | 33/67 | 7 b | 6.9 | 36 | [71] |
| Denmark | Rodstenseje | 8950 | 1.1 | 3 | 17 | 0.18 c | 2.80 | 60/40 d | 20 | 1.2 | 42 | [73] |
| Denmark | Ryaa 1 | 21,190 | 0.9 | 3 | 60 | 0.21 c | 14 | 46/54 d | 4 | 6.7 | 51 | [73] |
| Denmark | Ryaa 3 | 8480 | 1.1 | 3 | 32 | 0.22 c | 11 | 46/50 d | 9 | 4.3 | 41 | [73] |
| Canada | Walbridge | 1215 | 0.004 e | 4 | 107 | 0.08 | 5.02 | 65/35 | - | 1.7 | 34 | [74] |
| New Zealand | Toenepi | 260 | 1 | 2 | 25 | 0.1–0.2 f | 3.17 | 92 g | 4 h | −2.4 | −76 | [52] |
| USA | Wetland A | 6000 | 4 | 3 | 8.0 | 0.21 | 1.67 | 100/0 | 41 | 0.39 | 23 | [54] |
| USA | Wetland B | 3000 | 6 | 3 | 5.3 | 0.13 | 0.67 | 100/0 | 28 | 0.18 | 27 | [54] |
| USA | Wetland D | 8000 | 3.2 | 3 | 6.3 | 0.18 | 1.15 | 100/0 | 38 | −0.30 | −26 | [54] |
| New Zealand | Titoki | 898 | 1.6 | 3 | 53 | 0.26 i | 14 | 15–24 j | - | −1.7 | −12 | [75] |
| New Zealand | Toenepi | 293 | 1.1 | 5 | 25 | 0.07 i | 1.60 | 70–93 j | - | −1.7 | −107 | [75] |
| New Zealand | Bog Burn | 113 | 0.66 | 4 | 41 | 0.30 i | 12 | 36–60 j | - | −7.4 | −64 | [75] |
| Switzerland | Sonnhof | 2350 | 1.2 | 2 | 34 | 0.01–1.3 k | 4.67 | 75/25 | 0.9–50 k | 1.1 | 23 | [76] |
| Italy | - | 3750 | 3 | 2 | 3.7 | 0.10 | 0.35 | - | 40 | −0.03 | −8 | [92] |
| Country | Name | ARW | Ratio of ARW to AAC | Monitoring Time | Hydraulic Load | P Concentration | P Load | P Retention | Study | |
|---|---|---|---|---|---|---|---|---|---|---|
| m2 | % | yr | m yr−1 | mg L−1 | g m−2 yr−1 | % | ||||
| Denmark | Ulleruplund | 130,000 | 21.7 | 1 | 0.4 | 0.03 | 0.01 | −0.04 | −88 | [119] |
| Denmark | Snaremose | 340,000 | 6.6 | 2 | - | - | 1.43 | 0.26 | 18 | [119] |
| Denmark | Lindkær | 840,000 | 9.2 | 1.4 | - | 0.07–0.93 a | 0.45 | −0.05 | −11 | [119] |
| Denmark | Geddebækken | 410,000 | 16.7 | 1.8 | - | 0.08–0.51 a | 0.25 | 0.05 | 21 | [119] |
| Denmark | Egeskov | 6200 | 13.7 | 2 | 2.7 | 0.04 b | 0.09 | −0.004 | −4 | [120] |
| Denmark | Stor Å | 5870 | 2.4 | 2 | 4.8 | 0.02 b | 0.11 | −0.06 | −56 | [120] |
| USA | Embarras River | 6000–8000 | 3.3–4.0 | - | - | - | - | - | 20 | [122] |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dantas Mendes, L.R. Edge-of-Field Technologies for Phosphorus Retention from Agricultural Drainage Discharge. Appl. Sci. 2020, 10, 634. https://doi.org/10.3390/app10020634
Dantas Mendes LR. Edge-of-Field Technologies for Phosphorus Retention from Agricultural Drainage Discharge. Applied Sciences. 2020; 10(2):634. https://doi.org/10.3390/app10020634
Chicago/Turabian StyleDantas Mendes, Lipe Renato. 2020. "Edge-of-Field Technologies for Phosphorus Retention from Agricultural Drainage Discharge" Applied Sciences 10, no. 2: 634. https://doi.org/10.3390/app10020634
APA StyleDantas Mendes, L. R. (2020). Edge-of-Field Technologies for Phosphorus Retention from Agricultural Drainage Discharge. Applied Sciences, 10(2), 634. https://doi.org/10.3390/app10020634





