Influence of Heterogeneous Catalysts and Reaction Parameters on the Acetylation of Glycerol to Acetin: A Review
Abstract
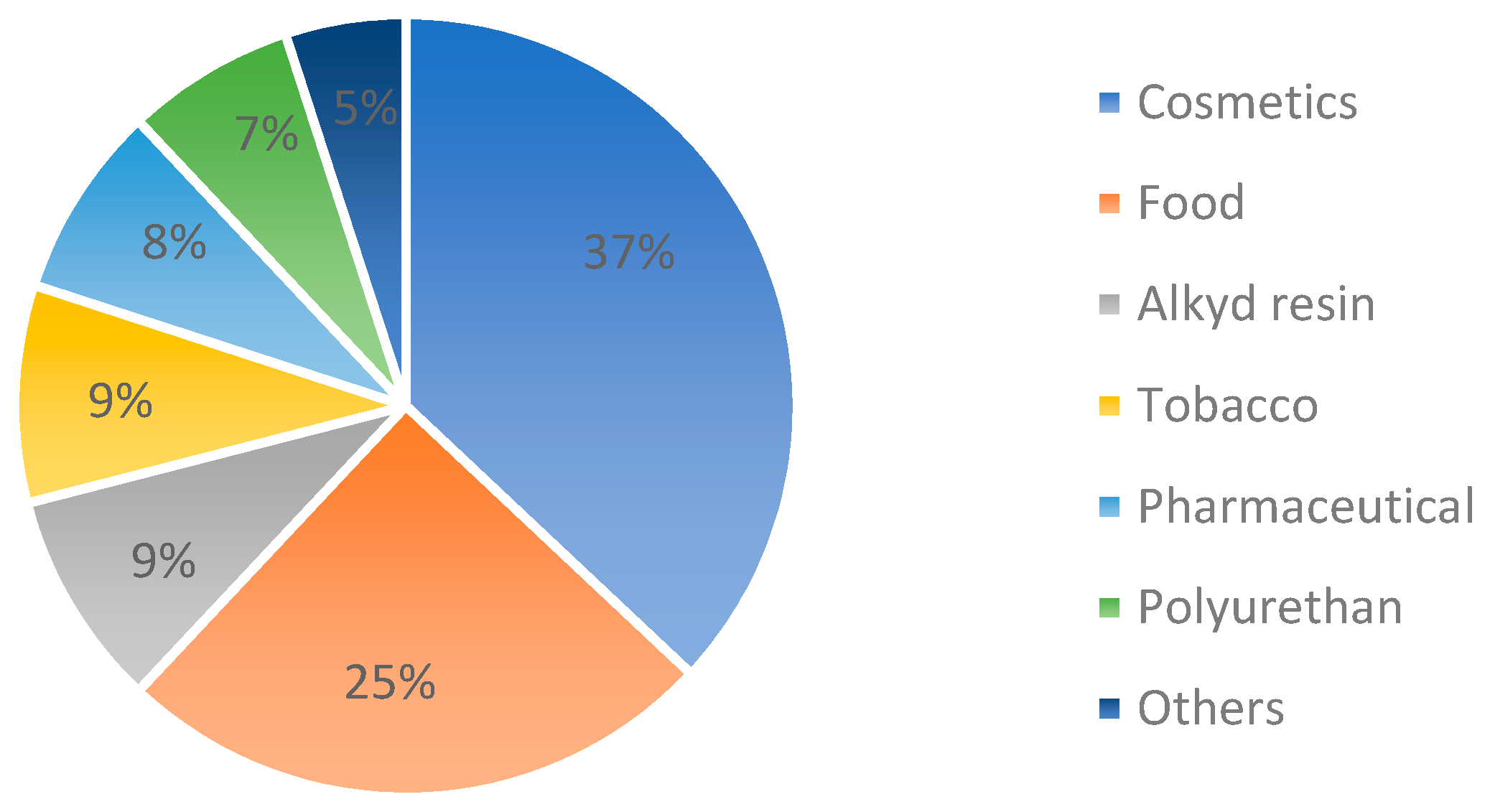
:1. Introduction
2. Acetin (Glycerol Esters or Acetyl Glycerol)
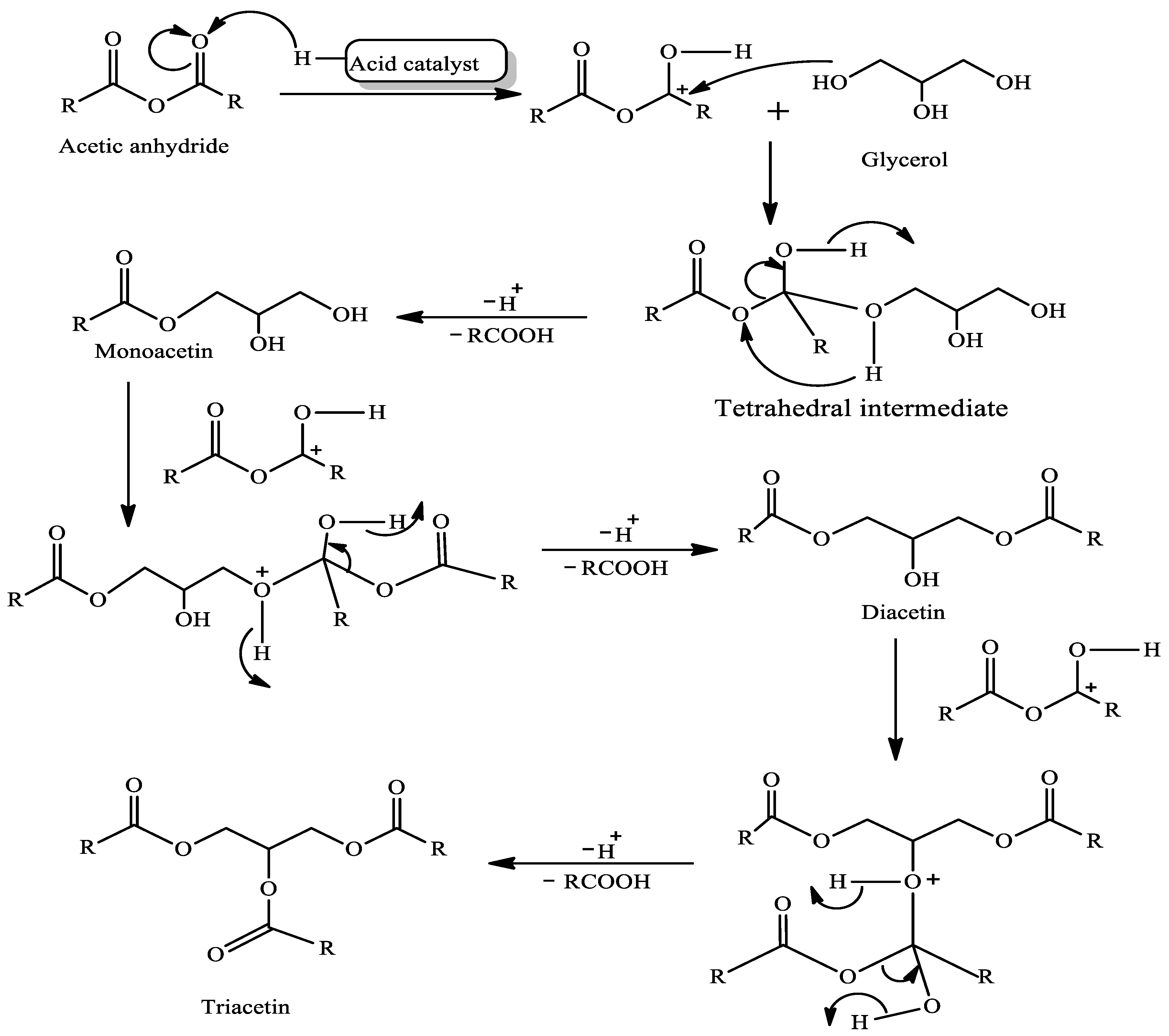
2.1. Mechanism of Acetylation of Glycerol
2.2. Influence of Reaction Parameters on the Acetylation of Glycerol
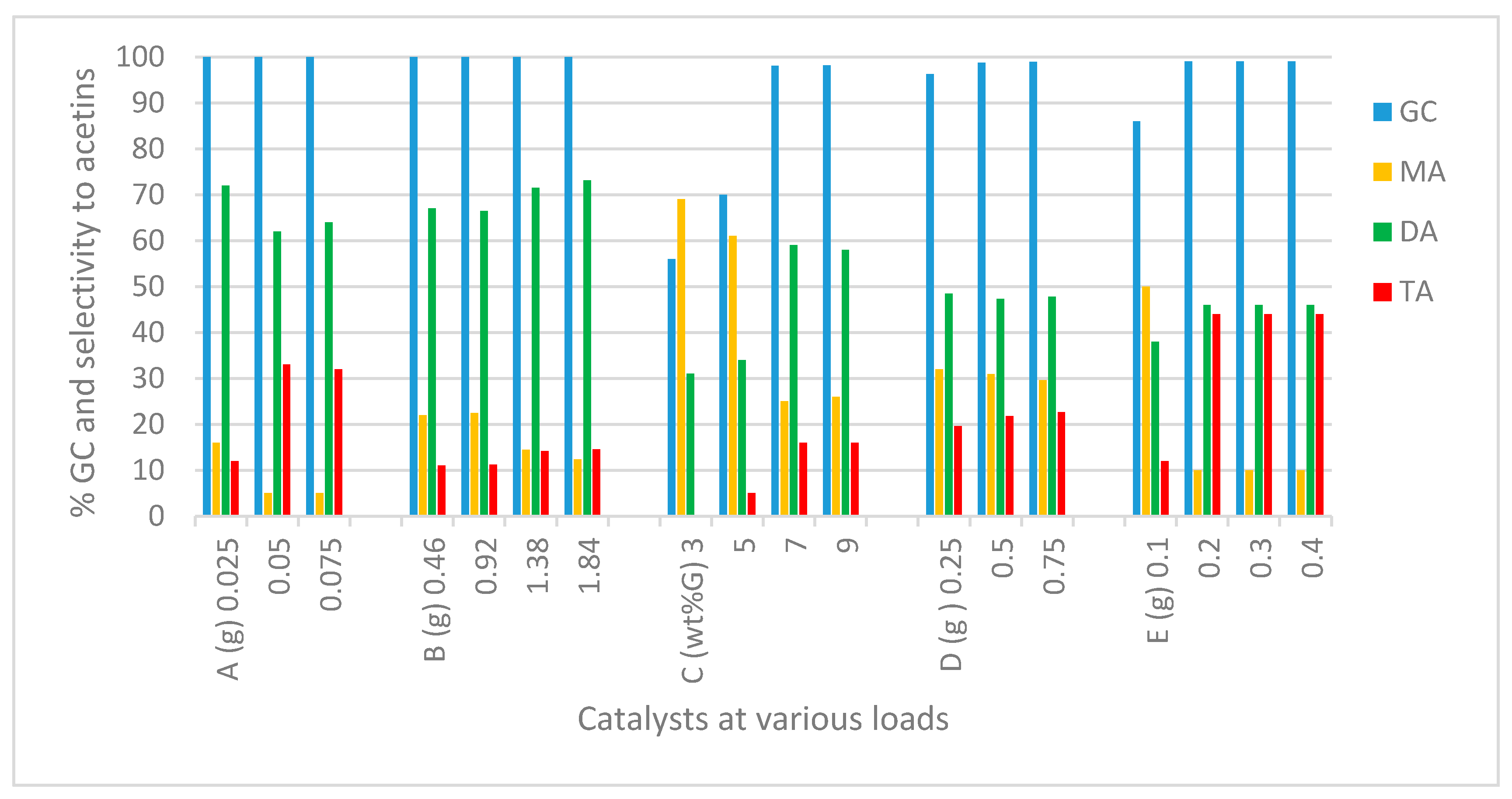
2.2.1. Catalyst and Catalyst Load
Montmorillonite (K-10 Clay) and Modified Montmorillonite
Metals and Metal Oxides
Mesoporous Silica and Functionalized Silica
Carbon and Functionalized Carbon-Based Materials
Ion-Exchange and Functionalized Resins
Heteropoly Acids and Supported Heteropoly Acids
Others
2.2.2. Temperature
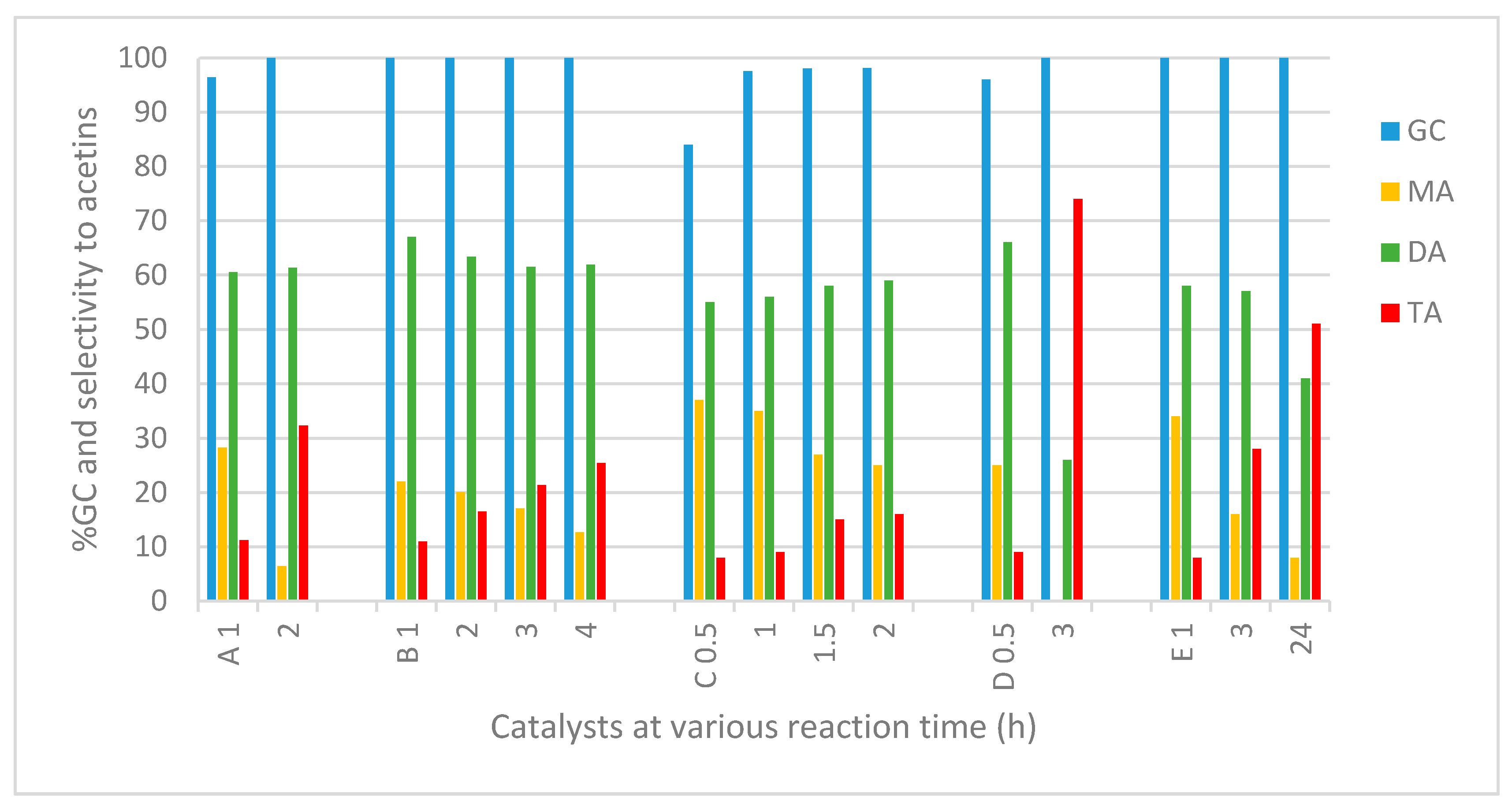
2.2.3. Reaction Time
2.2.4. The Reactants and Their Molar Ratios
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Johnson, D.T.; Taconi, K.A. The Glycerin Glut: Options for the Value-Added Conversion of Crude Glycerol Resulting from Biodiesel Production. Environ. Prog. 2009, 26, 338–348. [Google Scholar] [CrossRef]
- Ayoub, M.; Abdullah, A.Z. Critical review on the current scenario and significance of crude glycerol resulting from biodiesel industry towards more sustainable renewable energy industry. Renew. Sustain. Energy Rev. 2012, 16, 2671–2686. [Google Scholar] [CrossRef]
- Rahmat, N.; Abdullah, A.Z.; Mohamed, A.R. Recent progress on innovative and potential technologies for glycerol transformation into fuel additives: A critical review. Renew. Sustain. Energy Rev. 2010, 14, 987–1000. [Google Scholar] [CrossRef]
- Gupta, M.; Kumar, N. Scope and opportunities of using glycerol as an energy source. Renew. Sustain. Energy Rev. 2012, 16, 4551–4556. [Google Scholar] [CrossRef]
- Babajide, O. Sustaining Biodiesel Production via Value-Added Applications of Glycerol. J. Energy 2013, 2013, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Sakthivel, A.; Nakamura, R.; Komura, K.; Sugi, Y. Esterification of glycerol by lauric acid over aluminium and zirconium containing mesoporous molecular sieves in supercritical carbon dioxide medium. J. Supercrit. Fluids 2007, 42, 219–225. [Google Scholar] [CrossRef]
- Bagheri, S.; Julkapli, M.N.; Yehye, W.A. Catalytic conversion of biodiesel derived raw glycerol to value added products. Renew. Sustain. Energy Rev. 2015, 41, 113–127. [Google Scholar] [CrossRef]
- Konstantinović, S.S.; Danilović, B.R.; Ćirić, J.T.; Ilić, S.B.; Savić, D.S.; Veljković, V.B. Valorizacija sirovog glicerola iz proizvodnje biodizela. Chem. Ind. Chem. Eng. Q. 2016, 22, 461–489. [Google Scholar]
- Bagnato, G.; Iulianelli, A.; Sanna, A.; Basile, A. Glycerol production and transformation: A critical review with particular emphasis on glycerol reforming reaction for producing hydrogen in conventional and membrane reactors. Membranes 2017, 7, 17. [Google Scholar] [CrossRef]
- Werpy, T.; Petersen, G. Top Value Added Chemicals from Biomass Volume I—Results of Screening for Potential Candidates from Sugars and Synthesis Gas; BiblioGov: Columbus, OH, USA, 2004. [Google Scholar]
- He, Q.S.; Mcnutt, J.; Yang, J. Utilization of the residual glycerol from biodiesel production for renewable energy generation. Renew. Sustain. Energy Rev. 2017, 71, 63–76. [Google Scholar] [CrossRef]
- Quispe, C.A.G.; Coronado, C.J.R.; Carvalho, J.A. Glycerol: Production, consumption, prices, characterization and new trends in combustion. Renew. Sustain. Energy Rev. 2013, 27, 475–493. [Google Scholar] [CrossRef]
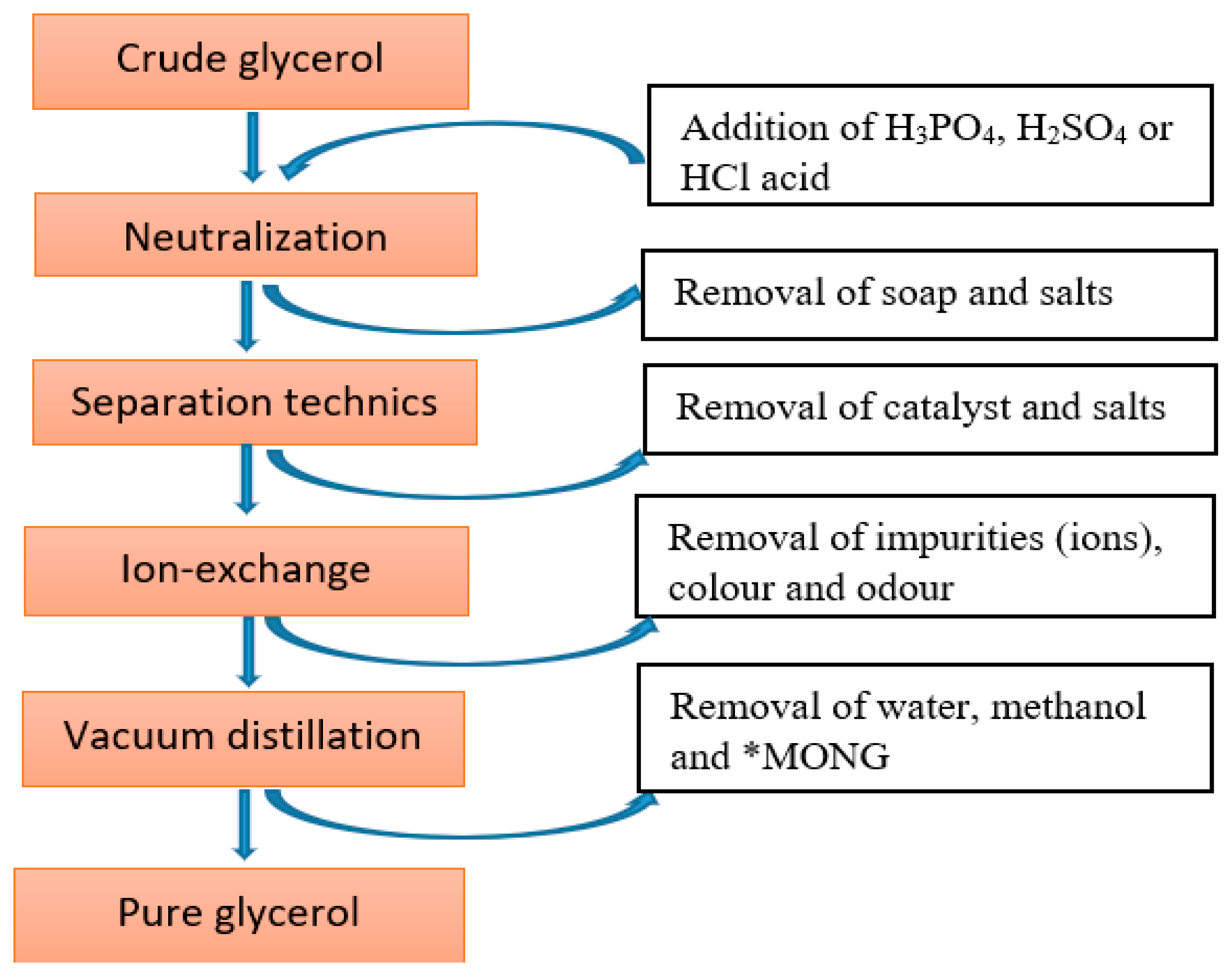
- Isahak, W.N.R.W.; Ramli, Z.A.C.; Ismail, M.; Jahim, J.M.; Yarmo, M.A. Recovery and Purification of Crude Glycerol from Vegetable Oil Transesterification Recovery and Purification of Crude Glycerol from Vegetable Oil Transesterification. Sep. Purif. Rev. 2015, 44, 250–267. [Google Scholar] [CrossRef]
- Christoph, R.; Schmidt, B.; Steinberner, U.; Dilla, W.; Karinen, R. Glycerol; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2012; ISBN 9780471238966. [Google Scholar]
- Marchetti, J.M.; Miguel, V.U.; Errazu, A.F. Possible methods for biodiesel production. Renew. Sustain. Energy Rev. 2007, 11, 1300–1311. [Google Scholar] [CrossRef]
- Knothe, G.; Razon, L.F. Biodiesel fuels. Prog. Energy Combust. Sci. 2017, 58, 36–59. [Google Scholar] [CrossRef]
- Bauer, F.; Hulteberg, C. Is there a future in glycerol as a feedstock in the production of biofuels and biochemicals? Biofuels Bioprod. Biorefining 2013, 7, 43–51. [Google Scholar] [CrossRef]
- Anuar, M.R.; Zuhairi, A. Challenges in biodiesel industry with regards to feedstock, environmental, social and sustainability issues: A critical review. Renew. Sustain. Energy Rev. 2016, 58, 208–223. [Google Scholar] [CrossRef]
- Nanda, M.R.; Yuan, Z.; Qin, W.; Ghaziaskar, H.S.; Poirier, M.; Xu, C.C. Thermodynamic and kinetic studies of a catalytic process to convert glycerol into solketal as an oxygenated fuel additive. Fuel 2014, 117, 470–477. [Google Scholar] [CrossRef]
- Naylor, R.L.; Higgins, M.M. The political economy of biodiesel in an era of low oil prices. Renew. Sustain. Energy Rev. 2017, 77, 695–705. [Google Scholar] [CrossRef]
- REN21. Renewables 2018-Global Status Report. Available online: https://cleanenergysolutions.org/sites/default/files/documents/180605_gsr_2018_presentation_webinar_rad.pdf (accessed on 5 June 2018).
- Trifoi, A.R.; Agachi, P.Ş.; Pap, T. Glycerol acetals and ketals as possible diesel additives. A review of their synthesis protocols. Renew. Sustain. Energy Rev. 2016, 62, 804–814. [Google Scholar] [CrossRef]
- Bajpai, D.; Tyagi, V.K. Biodiesel: Source, Production, Composition, Properties and Its Benefits. J. Oleo Sci. 2006, 55, 487–502. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.P.; Singh, D. Biodiesel production through the use of different sources and characterization of oils and their esters as the substitute of diesel: A review. Renew. Sustain. Energy Rev. 2010, 14, 200–216. [Google Scholar] [CrossRef]
- Takase, M.; Zhao, T.; Zhang, M.; Chen, Y.; Liu, H.; Yang, L.; Wu, X. An expatiate review of neem, jatropha, rubber and karanja as multipurpose non-edible biodiesel resources and comparison of their fuel, engine and emission properties. Renew. Sustain. Energy Rev. 2015, 43, 495–520. [Google Scholar] [CrossRef]
- Shah, M.; Dai, J.J.; Guo, Q.X.; Fu, Y. Products and production routes for the catalytic conversion of seed oil into fuel and chemicals: A comprehensive review. Sci. China Chem. 2015, 58, 1110–1121. [Google Scholar] [CrossRef]
- Pagliaro, M.; Ciriminna, R.; Kimura, H.; Rossi, M.; Pina, C. Della Review Article Recent advances in the conversion of bioglycerol into value-added products. Eur. J. Lipid Sci. Technol. 2009, 111, 788–799. [Google Scholar] [CrossRef]
- Coronado, C.R.; Carvalho, J.A.; Quispe, C.A.; Sotomonte, C.R. Ecological efficiency in glycerol combustion. Appl. Therm. Eng. 2014, 63, 97–104. [Google Scholar] [CrossRef]
- Deng, C.; Duan, X.; Zhou, J.; Chen, D.; Zhou, X.; Yuan, W. Size effects of Pt-Re bimetallic catalysts for glycerol hydrogenolysis. Catal. Today 2014, 234, 208–214. [Google Scholar] [CrossRef]
- Kant, A.; He, Y.; Jawad, A.; Li, X.; Rezaei, F.; Smith, J.D.; Rownaghi, A.A. Hydrogenolysis of glycerol over Ni, Cu, Zn, and Zr supported on H-beta. Chem. Eng. J. 2017, 317, 1–8. [Google Scholar] [CrossRef]
- Wang, C.; Dou, B.; Chen, H.; Song, Y.; Xu, Y.; Du, X.; Zhang, L.; Luo, T.; Tan, C. Renewable hydrogen production from steam reforming of glycerol by Ni-Cu-Al, Ni-Cu-Mg, Ni-Mg catalysts. Int. J. Hydrogen Energy 2013, 38, 3562–3571. [Google Scholar] [CrossRef]
- Bepari, S.; Pradhan, N.C.; Dalai, A.K. Selective production of hydrogen by steam reforming of glycerol over Ni/Fly ash catalyst. Catal. Today 2017, 291, 36–46. [Google Scholar] [CrossRef]
- Ma, T.; Ding, J.; Shao, R.; Xu, W.; Yun, Z. Dehydration of glycerol to acrolein over Wells–Dawson and Keggin type phosphotungstic acids supported on MCM-41 catalysts. Chem. Eng. J. 2017, 316, 797–806. [Google Scholar] [CrossRef]
- Zhou, L.; Al-zaini, E.; Adesina, A.A. Catalytic characteristics and parameters optimization of the glycerol acetylation over solid acid catalysts. Fuel 2013, 103, 617–625. [Google Scholar] [CrossRef]
- Indran, V.P.; Syuhada Zuhaimi, N.A.; Deraman, M.A.; Maniam, G.P.; Yusoff, M.M.; Yun Hin, T.-Y.; Rahim, M.H.A. An accelerated route of glycerol carbonate formation from glycerol using waste boiler ash as catalyst. RSC Adv. 2014, 4, 25257–25267. [Google Scholar] [CrossRef] [Green Version]
- Ishak, Z.I.; Sairi, N.A.; Alias, Y.; Aroua, M.K.T.; Yusoff, R. Production of glycerol carbonate from glycerol with aid of ionic liquid as catalyst. Chem. Eng. J. 2016, 297, 128–138. [Google Scholar] [CrossRef]
- Skrzynska, E.; Zaid, S.; Girardon, J.-S.; Capron, M.; Dumeignil, F. Catalytic behaviour of four different supported noble metals in the crude glycerol oxidation. Appl. Catal. A Gen. 2015, 499, 89–100. [Google Scholar] [CrossRef]
- Ning, X.; Li, Y.; Yu, H.; Peng, F.; Wang, H.; Yang, Y. Promoting role of bismuth and antimony on Pt catalysts for the selective oxidation of glycerol to dihydroxyacetone. J. Catal. 2016, 335, 95–104. [Google Scholar] [CrossRef]
- Bookong, P.; Ruchirawat, S.; Boonyarattanakalin, S. Optimization of microwave-assisted etherification of glycerol to polyglycerols by sodium carbonate as catalyst. Chem. Eng. J. 2015, 275, 253–261. [Google Scholar] [CrossRef]
- Galy, N.; Nguyen, R.; Blach, P.; Sambou, S.; Luart, D.; Len, C. Journal of Industrial and Engineering Chemistry Glycerol oligomerization in continuous fl ow reactor. J. Ind. Eng. Chem. 2017, 51, 312–318. [Google Scholar] [CrossRef]
- Kim, I.; Kim, J.; Lee, D. A comparative study on catalytic properties of solid acid catalysts for glycerol acetylation at low temperatures. Appl. Catal. B Environ. 2014, 148–149, 295–303. [Google Scholar] [CrossRef]
- Gao, X.; Zhu, S.; Li, Y. Graphene oxide as a facile solid acid catalyst for the production of bioadditives from glycerol esteri fi cation. Catal. Commun. 2015, 62, 48–51. [Google Scholar] [CrossRef]
- Ayoub, M.; Khayoon, M.S.; Abdullah, A.Z. Synthesis of oxygenated fuel additives via the solventless etherification of glycerol. Bioresour. Technol. 2012, 112, 308–312. [Google Scholar] [CrossRef]
- Pinto, B.P.; De Lyra, J.T.; Nascimento, J.A.C.; Mota, C.J.A. Ethers of glycerol and ethanol as bioadditives for biodiesel. Fuel 2016, 168, 76–80. [Google Scholar] [CrossRef]
- Gadamsetti, S.; Rajan, N.P.; Rao, G.S.; Chary, K.V.R. Acetalization of glycerol with acetone to bio fuel additives over supported molybdenum phosphate catalysts. J. Mol. Catal. A Chem. 2015, 410, 49–57. [Google Scholar] [CrossRef]
- Timofeeva, M.N.; Panchenko, V.N.; Krupskaya, V.V.; Gil, A.; Vicente, M.A. Effect of nitric acid modification of montmorillonite clay on synthesis of solketal from glycerol and acetone. Catal. Commun. 2017, 90, 65–69. [Google Scholar] [CrossRef]
- Valliyappan, T.; Bakhshi, N.N.; Dalai, A.K. Pyrolysis of glycerol for the production of hydrogen or syn gas. Bioresour. Technol. 2008, 99, 4476–4483. [Google Scholar] [CrossRef] [PubMed]
- Dianningrum, L.W.; Choi, H.; Kim, Y.; Jung, K.; Susanti, R.F.; Kim, J.; Sang, B. ScienceDirect Hydrothermal gasification of pure and crude glycerol in supercritical water: A comparative study. Int. J. Hydrogen Energy 2014, 39, 1262–1273. [Google Scholar] [CrossRef]
- Fan, X.; Burton, R.; Zhou, Y. Glycerol (Byproduct of Biodiesel Production) as a Source for Fuels and Chemicals—Mini Review. Open Fuels Energy Sci. J. 2010, 3, 17–22. [Google Scholar] [CrossRef] [Green Version]
- Leoneti, B.A.; Aragão-leoneti, V.; de Oliveira, S.V.W.B. Glycerol as a by-product of biodiesel production in Brazil: Alternatives for the use of unre fi ned glycerol. Renew. Energy 2012, 45, 138–145. [Google Scholar] [CrossRef]
- Len, C.; Luque, R. Continuous flow transformations of glycerol to valuable products: An overview. Sustain. Chem. Process. 2014, 2, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Luo, X.; Ge, X.; Cui, S.; Li, Y. Value-added processing of crude glycerol into chemicals and polymers. Bioresour. Technol. 2016, 215, 144–154. [Google Scholar] [CrossRef] [Green Version]
- Mota, C.J.A.; Silva, C.X.A.; Rosenbach, N.; Costa, J.; da Silva, F. Glycerin Derivatives as Fuel Additives: The Addition of Glycerol/Acetone Ketal (Solketal) in Gasolines. Energy Fuels 2010, 24, 2733–2736. [Google Scholar] [CrossRef]
- García, J.I.; García-Marín, H.; Pires, E. Glycerol based solvents: Synthesis, properties and applications. Green Chem. 2014, 16, 1007–1033. [Google Scholar] [CrossRef] [Green Version]
- Cornejo, A.; Barrio, I.; Campoy, M.; Lázaro, J.; Navarrete, B. Oxygenated fuel additives from glycerol valorization. Main production pathways and effects on fuel properties and engine performance: A critical review. Renew. Sustain. Energy Rev. 2017, 79, 1400–1413. [Google Scholar] [CrossRef]
- Farinha, J.; Caiado, M.; Castanheiro, J.E. Valorisation of glycerol into biofuel additives over heterogeneous catalysts. In Materials and Processes for Energy: Communicating Current Research and Technological Developments; Mendez-Vilas, A., Ed.; Formatex Research Center: Badajoz, Spain, 2013; pp. 422–429. [Google Scholar]
- Samoilov, V.O.; Ramazanov, D.N.; Nekhaev, A.I.; Maximov, A.L.; Bagdasarov, L.N. Heterogeneous catalytic conversion of glycerol to oxygenated fuel additives. Fuel 2016, 172, 310–319. [Google Scholar] [CrossRef]
- Mallesham, B.; Rao, B.G.; Reddy, B.M. Production of biofuel additives by esteri fi cation and acetalization of bioglycerol. Comptes Rendus Chim. 2016, 19, 1194–1202. [Google Scholar] [CrossRef]
- Kong, P.S.; Aroua, M.K.; Daud, W.M.A.W.; Lee, H.V.; Cognet, P.; Pérès, Y. Catalytic role of solid acid catalysts in glycerol acetylation for the production of bio-additives: A review. RSC Adv. 2016, 6, 68885–68905. [Google Scholar] [CrossRef]
- Okoye, P.U.; Hameed, B.H. Review on recent progress in catalytic carboxylation and acetylation of glycerol as a byproduct of biodiesel production. Renew. Sustain. Energy Rev. 2016, 53, 558–574. [Google Scholar] [CrossRef]
- Okoye, P.U.; Abdullah, A.Z.; Hameed, B.H. A review on recent developments and progress in the kinetics and deactivation of catalytic acetylation of glycerol—A byproduct of biodiesel. Renew. Sustain. Energy Rev. 2017, 74, 387–401. [Google Scholar] [CrossRef]
- Maximov, A.L.; Nekhaev, A.I.; Ramazanov, D.N. Ethers and Acetals, Promising Petrochemicals from Renewable Sources. Pet. Chem. 2015, 55, 3–24. [Google Scholar] [CrossRef]
- De Torres, M.; Jiménez-osés, G.; Mayoral, J.A.; Pires, E.; Santos, M.D.L. Glycerol ketals: Synthesis and profits in biodiesel blends. Fuel 2012, 94, 614–616. [Google Scholar] [CrossRef]
- Izquierdo, J.F.; Montiel, M.; Pales, I.; Outo, P.R.; Galan, M.; Jutglar, L.; Villarrubia, M.; Izquierdo, M.; Hermo, M.P.; Ariza, X. Fuel additives from glycerol etherification with light olefins: State of the art. Renew. Sustain. Energy Rev. 2012, 16, 6717–6724. [Google Scholar] [CrossRef]
- Agirre, I.; Garcia, I.; Requies, J.; Barrio, V.L.; Guemez, M.B.; Cambra, J.F.; Arias, P.L. Glycerol acetals, kinetic study of the reaction between glycerol and formaldehyde. Biomass Bioenergy 2011, 35, 3636–3642. [Google Scholar] [CrossRef]
- Di Serio, M.; Casale, L.; Tesser, R.; Santacesaria, E. New Process for the Production of Glycerol tert-Butyl Ethers. Energy Fuels 2010, 24, 4668–4672. [Google Scholar] [CrossRef]
- Garcı, E.; Laca, M.; Perez, E.; Garrido, A.; Bilbao, J. New Class of Acetal Derived from Glycerin as a Biodiesel Fuel Component. Energy Fuels 2008, 22, 4274–4280. [Google Scholar] [CrossRef]
- Deutsch, J.; Martin, A.; Lieske, H. Investigations on heterogeneously catalysed condensations of glycerol to cyclic acetals. J. Catal. 2007, 245, 428–435. [Google Scholar] [CrossRef]
- Nanda, M.R.; Zhang, Y.; Yuan, Z.; Qin, W.; Ghaziaskar, H.S.; Charles, C. Catalytic conversion of glycerol for sustainable production of solketal as a fuel additive: A review. Renew. Sustain. Energy Rev. 2016, 56, 1022–1031. [Google Scholar] [CrossRef]
- Moraes, D.S.; Angélica, R.S.; Costa, C.E.F.; Rocha-Filho, G.N.; Zamian, J.R. Bentonite functionalized with propyl sulfonic acid groups used as catalyst in esteri fi cation reactions. Appl. Clay Sci. 2011, 51, 209–213. [Google Scholar] [CrossRef] [Green Version]
- Khayoon, M.S.; Hameed, B.H. Synthesis of hybrid SBA-15 functionalized with molybdophosphoric acid as efficient catalyst for glycerol esterification to fuel additives. Appl. Catal. A Gen. 2012, 433–434, 152–161. [Google Scholar] [CrossRef]
- Kong, P.S.; Aroua, M.K.; Daud, W.M.A.W.; Wan Daud, W.M.A. Catalytic esterification of bioglycerol to value-added products. Rev. Chem. Eng. 2015, 31, 437–451. [Google Scholar] [CrossRef]
- Dalla Costa, B.O.; Decolatti, H.P.; Legnoverde, M.S.; Querini, C.A. Influence of acidic properties of different solid acid catalysts for glycerol acetylation. Catal. Today 2017, 289, 222–230. [Google Scholar] [CrossRef]
- Pinazo, A.; Lozano, N.; Perez, L.; Moran, M.C.; Infante, M.R.; Pons, R. Arginine diacyl-glycerolipid conjugates as multifunctional biocompatible surfactants. Comptes Rendus Chim. 2011, 14, 726–735. [Google Scholar] [CrossRef]
- Carvalho, W.A.; Galhardo, T.S.; Simone, N.; Goncalves, M.; Figueiredo, F.C.A.; Mandelli, D.; Gonçalves, M.; Figueiredo, F.C.A.; Mandelli, D.; Carvalho, W.A. Preparation of sulfonated carbons from rice husk and their application in catalytic conversion of glycerol. ACS Sustain. Chem. Eng. 2013, 1, 1381–1389. [Google Scholar]
- Zhou, C.H.; Beltramini, J.N.; Fan, Y.X.; Lu, G.Q. Chemoselective catalytic conversion of glycerol as a biorenewable source to valuable commodity chemicals. Chem. Soc. Rev. 2008, 37, 527–549. [Google Scholar] [CrossRef] [PubMed]
- Setyaningsih, L.; Siddiq, F.; Pramezy, A. Esterification of glycerol with acetic acid over Lewatit catalyst. MATEC Web Conf. 2018, 154, 2–5. [Google Scholar] [CrossRef]
- Goncalves, V.L.C.; Pinto, B.P.; Silva, J.C.; Mota, C.J.A. Acetylation of glycerol catalyzed by different solid acids. Catal. Today 2008, 135, 673–677. [Google Scholar] [CrossRef]
- Melero, J.A.; Vicente, G.; Morales, G.; Paniagua, M.; Moreno, J.M.; Roldan, R.; Ezquerro, A.; Perez, C. Acid-catalyzed etherification of bio-glycerol and isobutylene over sulfonic mesostructured silicas. Appl. Catal. A Gen. 2008, 346, 44–51. [Google Scholar] [CrossRef]
- Liao, X.; Zhu, Y.; Wang, S.; Li, Y. Producing triacetylglycerol with glycerol by two steps: Esterification and acetylation. Fuel Process. Technol. 2009, 90, 988–993. [Google Scholar] [CrossRef]
- Rashedul, H.K.; Masjuki, H.H.; Kalam, M.A.; Ashraful, A.M.; Rahman, S.M.A.; Shahir, S.A. The effect of additives on properties, performance and emission of biodiesel fuelled compression ignition engine. Energy Convers. Manag. 2014, 88, 348–364. [Google Scholar] [CrossRef]
- Khayoon, M.S.; Triwahyono, S.; Hameed, B.H.; Jalil, A.A. Improved production of fuel oxygenates via glycerol acetylation with acetic acid. Chem. Eng. J. 2014, 243, 473–484. [Google Scholar] [CrossRef]
- Zhou, L.; Nguyen, T.; Adesina, A.A. The acetylation of glycerol over amberlyst-15: Kinetic and product distribution. Fuel Process. Technol. 2012, 104, 310–318. [Google Scholar] [CrossRef]
- Zhu, S.; Gao, X.; Dong, F.; Zhu, Y.; Zheng, H.; Li, Y. Design of a highly active silver-exchanged phosphotungstic acid catalyst for glycerol esterification with acetic acid. J. Catal. 2013, 306, 155–163. [Google Scholar] [CrossRef]
- Sutter, M.; Da Silva, E.; Duguet, N.; Raoul, Y.; Métay, E.; Lemaire, M. Glycerol Ether Synthesis: A Bench Test for Green Chemistry Concepts and Technologies. Chem. Rev. 2015, 115, 8609–8651. [Google Scholar] [CrossRef] [PubMed]
- Alegría, A.; Cuellar, J. Esterification of oleic acid for biodiesel production catalyzed by 4-dodecylbenzenesulfonic acid. Appl. Catal. B Environ. 2015, 179, 530–541. [Google Scholar] [CrossRef]
- Venkatesha, N.J.; Bhat, Y.S.; Prakash, B.S.J. Volume accessibility of acid sites in modified montmorillonite and triacetin selectivity in acetylation of glycerol. RSC Adv. 2016, 6, 45819–45828. [Google Scholar] [CrossRef]
- Silva, L.N.; Gonçalves, V.L.C.; Mota, C.J.A. Catalytic acetylation of glycerol with acetic anhydride. Catal. Commun. 2010, 11, 1036–1039. [Google Scholar] [CrossRef]
- Sandesh, S.; Manjunathan, P.; Halgeri, A.B.; Shanbhag, G.V. Glycerol acetins: Fuel additive synthesis by acetylation and esterification of glycerol using cesium phosphotungstate catalyst. RSC Adv. 2015, 5, 104354–104362. [Google Scholar] [CrossRef]
- Ramalingam, R.J.; Radhika, T.; Adam, F.; Dolla, H.T. Acetylation of glycerol over bimetallic Ag-Cu doped rice husk silica based biomass catalyst for bio-fuel additives application. Int. J. Ind. Chem. 2016, 7, 187–194. [Google Scholar] [CrossRef] [Green Version]
- Kakasaheb, Y.N.; Prashant, S.N.; Vijay, V.B. Synthesis of Oxygenated Fuel Additives via Acetylation of Bio-Glycerol over H 2 SO 4 Modified Montmorillonite K10 Catalyst. Prog. Petrochem. Sci. 2018, 1, 1–5. [Google Scholar]
- Testa, M.L.; La Parola, V.; Liotta, L.F.; Venezia, A.M. Screening of different solid acid catalysts for glycerol acetylation. J. Mol. Catal. A Chem. 2013, 367, 69–76. [Google Scholar] [CrossRef]
- Gonçalves, C.E.; Laier, L.O.; Cardoso, A.L.; Silva, M.J. Da Bioadditive synthesis from H3PW12O40 -catalyzed glycerol esteri fi cation with HOAc under mild reaction conditions. Fuel Process. Technol. 2012, 102, 46–52. [Google Scholar] [CrossRef]
- Beejapur, H.A.; La Parola, V.; Liotta, L.F.; Testa, M.L. Glycerol Acetylation over Organic-Inorganic Sulfonic or Phosphonic Silica Catalysts. ChemistrySelect 2017, 2, 4934–4941. [Google Scholar] [CrossRef]
- Churipard, S.R.; Manjunathan, P.; Chandra, P.; Shanbhag, G.V.; Ravishankar, R.; Rao, P.V.C.; Sri Ganesh, G.; Halgeri, A.B.; Maradur, S.P. Remarkable catalytic activity of a sulfonated mesoporous polymer (MP-SO3H) for the synthesis of solketal at room temperature. New J. Chem. 2017, 41, 5745–5751. [Google Scholar] [CrossRef]
- Rao, B.G.; Sudarsanam, P.; Rangaswamy, A.; Reddy, B.M. Highly Efficient CeO2–MoO3/SiO2 Catalyst for Solvent-Free Oxidative Coupling of Benzylamines into N-Benzylbenzaldimines with O2 as the Oxidant. Catal. Lett. 2015, 145, 1436–1445. [Google Scholar]
- Fukumura, T.; Toda, T.; Seki, Y.; Kubo, M.; Shibasaki-kitakawa, N.; Yonemoto, T. Catalytic Synthesis of Glycerol Monoacetate Using a Continuous Expanded Bed Column Reactor Packed with Cation-Exchange Resin. Ind. Eng. Chem. Res. 2009, 48, 1816–1823. [Google Scholar] [CrossRef]
- Dosuna-Rodríguez, I.; Gaigneaux, E.M. Glycerol acetylation catalysed by ion exchange resins. Catal. Today 2012, 195, 14–21. [Google Scholar] [CrossRef]
- Lacerda, C.V.; Carvalho, M.J.S.; Ratton, A.R.; Soares, I.P.; Borges, L.E.P. Synthesis of Triacetin and Evaluation on Motor. J. Braz. Chem. Soc. 2015, 26, 1625–1631. [Google Scholar]
- Marwan, M.; Indarti, E.; Darmadi, D.; Rinaldi, W.; Hamzah, D.; Rinaldi, T. Production of triacetin by microwave assisted esterification of glycerol using activated natural zeolite. Bull. Chem. React. Eng. Catal. 2019, 14, 672–677. [Google Scholar] [CrossRef]
- Konwar, L.J.; Mäki-Arvela, P.; Kumar, N.; Mikkola, J.P.; Sarma, A.K.; Deka, D. Selective esterification of fatty acids with glycerol to monoglycerides over—SO3H functionalized carbon catalysts. React. Kinet. Mech. Catal. 2016, 119, 121–138. [Google Scholar] [CrossRef]
- Patel, A.; Singh, S. A green and sustainable approach for esterification of glycerol using 12-tungstophosphoric acid anchored to different supports: Kinetics and effect of support. Fuel 2014, 118, 358–364. [Google Scholar] [CrossRef]
- Ferreira, P.; Fonseca, I.M.; Ramos, A.M.; Vital, J.; Castanheiro, J.E. Acetylation of glycerol over heteropolyacids supported on activated carbon. Catal. Commun. 2011, 12, 573–576. [Google Scholar] [CrossRef]
- Magar, S.; Mohanraj, G.T.; Jana, S.K.; Rode, C.V. Synthesis and characterization of supported heteropoly acid: Efficient solid acid catalyst for glycerol esterification to produce biofuel additives. Inorg. Nano-Metal Chem. 2020, 50, 1157–1165. [Google Scholar] [CrossRef]
- Popova, M.; Szegedi, Á.; Ristic, A.; Tusar, N.N. Catalysis Science & Technology supported sulphated zirconia catalysts. Catal. Sci. Technol. Esterification 2014, 4, 3993–4000. [Google Scholar]
- Arun, P.; Pudi, S.M.; Biswas, P. Acetylation of Glycerol over Sulfated Alumina: Reaction Parameter Study and Optimization Using Response Surface Methodology. Energy Fuels 2016, 30, 584–593. [Google Scholar]
- Zhang, Z.; Huang, H.; Ma, X.; Li, G.; Wang, Y.; Sun, G.; Teng, Y.; Yan, R.; Zhang, N.; Li, A.J. Production of diacylglycerols by esterification of oleic acid with glycerol catalyzed by diatomite loaded SO42−/TiO2. J. Ind. Eng. Chem. 2017, 53, 307–316. [Google Scholar] [CrossRef]
- Kulkarni, R.M.; Britto, P.J.; Narula, A.; Saqline, S.; Anand, D.; Bhagyalakshmi, C.; Herle, R.N. Kinetic studies on the synthesis of fuel additives from glycerol using CeO2-ZrO2 metal oxide catalyst. Biofuel Res. J. 2020, 7, 1100–1108. [Google Scholar] [CrossRef] [Green Version]
- Mizugaki, T.; Arundhathi, R.; Mitsudome, T.; Jitsukawa, K.; Kaneda, K. Highly efficient and selective transformations of glycerol using reusable heterogeneous catalysts. ACS Sustain. Chem. Eng. 2014, 2, 574–578. [Google Scholar] [CrossRef]
- Ghoreishi, K.B.; Yarmo, M.A. Sol-gel Sulfated Silica as a Catalyst for Glycerol Acetylation with Acetic Acid. J. Sci. Technol. 2013, 1, 65–78. [Google Scholar]
- Gonzalez-Arellano, C.; De, S.; Luque, R. Selective glycerol transformations to high value-added products catalysed by aluminosilicate-supported iron oxide nanoparticles. Catal. Sci. Technol. 2014, 4, 4242–4249. [Google Scholar] [CrossRef]
- De La Calle, C.; Fraile, J.M.; García-bordejé, E.; Pires, E.; Roldán, L.; De Calle, C.; Fraile, J.M.; García-bordejé, E.; Pires, E.; Roldán, L. Biobased catalyst in biorefinery processes: Sulphonated hydrothermal carbon for glycerol esterification. Catal. Sci. Technol. 2015, 5, 2897–2903. [Google Scholar] [CrossRef] [Green Version]
- Tao, M.L.; Guan, H.Y.; Wang, X.H.; Liu, Y.C.; Louh, R.F. Fabrication of sulfonated carbon catalyst from biomass waste and its use for glycerol esterification. Fuel Process. Technol. 2015, 138, 355–360. [Google Scholar] [CrossRef]
- Okoye, P.U.; Abdullah, A.Z.; Hameed, B.H. Synthesis of oxygenated fuel additives via glycerol esterification with acetic acid over bio-derived carbon catalyst. Fuel 2017, 209, 538–544. [Google Scholar] [CrossRef]
- Konwar, L.J.; Mäki-Arvela, P.; Begum, P.; Kumar, N.; Jyoti, T.A.; Mikkola, J.; Deka, C.R.; Deka, D. Shape selectivity and acidity effects in glycerol acetylation with acetic anhydride: Selective synthesis of triacetin over Y-zeolite and sulfonated mesoporous carbons. J. Catal. 2015, 329, 237–247. [Google Scholar] [CrossRef]
- Goscianska, J.; Malaika, A. A facile post-synthetic modification of ordered mesoporous carbon to get efficient catalysts for the formation of acetins. Catal. Today 2019, 1–10. [Google Scholar] [CrossRef]
- Deutschman, O.; Knozinger, H.; Kochloefl, K. Heterogeneous Catalysis and Solid Catalysts. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/14356007.a05_313.pub2 (accessed on 15 April 2019).
- Mansir, N.; Taufiq-Yap, Y.H.; Rashid, U.; Lokman, I.M. Investigation of heterogeneous solid acid catalyst performance on low grade feedstocks for biodiesel production: A review. Energy Convers. Manag. 2017, 141, 171–182. [Google Scholar] [CrossRef]
- Mukasa-Tebandeke, I.Z.; Ssebuwufu, P.J.M.; Nyanzi, S.A.; Schumann, A.; Nyakairu, G.W.A.; Ntale, M.; Lugolobi, F. The Elemental, Mineralogical, IR, DTA and XRD Analyses Characterized Clays and Clay Minerals of Central and Eastern Uganda. Adv. Mater. Phys. Chem. 2015, 05, 67–86. [Google Scholar] [CrossRef] [Green Version]
- Ray, C.; Pal, T. Recent advances of metal-metal oxide nanocomposites and their tailored nanostructures in numerous catalytic applications. J. Mater. Chem. A 2017, 5, 9465–9487. [Google Scholar] [CrossRef]
- Veluturla, S.; Narula, A.; Rao, D.S.; Shetty, S.P. Kinetic study of synthesis of bio-fuel additives from glycerol using a hetropolyacid. Resour. Technol. 2017, 3, 337–341. [Google Scholar] [CrossRef]
- Rane, S.A.; Pudi, S.M.; Biswa, P. Esterification of Glycerol with Acetic Acid over Highly Active and Stable Alumina-based Catalysts: A Reaction Kinetics Study. Chem. Biochem. Eng. Q. J. 2016, 30, 33–45. [Google Scholar] [CrossRef]
- Hu, W.; Zhang, Y.; Huang, Y.; Wang, J.; Gao, J.; Xu, J. Selective esterification of glycerol with acetic acid to diacetin using antimony pentoxide as reusable catalyst. J. Energy Chem. 2015, 24, 632–636. [Google Scholar] [CrossRef]
- Sánchez, J.A.; Hernández, D.L.; Moreno, J.A.; Mondragón, F.; Fernández, J.J. Alternative carbon based acid catalyst for selective esterification of glycerol to acetylglycerols. Appl. Catal. A Gen. 2011, 405, 55–60. [Google Scholar] [CrossRef]
- Rafi, J.M.; Rajashekar, A.; Srinivas, M.; Rao, B.V.S.K.; Prasad, R.B.N.; Lingaiah, N. Esterification of glycerol over a solid acid biochar catalyst derived from waste biomass. RSC Adv. 2015, 5, 44550–44556. [Google Scholar] [CrossRef]
- Chandrakala, U.; Prasad, R.B.N.; Prabhavathi Devi, B.L.A. Glycerol valorization as biofuel additives by employing a carbon-based solid acid catalyst derived from glycerol. Ind. Eng. Chem. Res. 2014, 53, 16164–16169. [Google Scholar] [CrossRef]
- Prabhavathi Devi, B.L.A.; Vijaya Lakshmi, K.; Gangadhar, K.N.; Prasad, R.B.N.; Sai Prasad, P.S.; Jagannadh, B.; Kundu, P.P.; Kumari, G.; Narayana, C. Novel Heterogeneous SO3Na-Carbon Transesterification Catalyst for the Production of Biodiesel. ChemistrySelect 2017, 2, 1925–1931. [Google Scholar] [CrossRef]
- Tang, H.; Li, N.; Chen, F.; Li, G.; Wang, A.; Cong, Y.; Wang, X.; Zhang, T. Highly efficient synthesis of 5-hydroxymethylfurfural with carbohydrates over renewable cyclopentanone-based acidic resin. Green Chem. 2017, 19, 1855–1860. [Google Scholar] [CrossRef]
- Hara, M.; Nakajima, K.; Kamata, K. Recent progress in the development of solid catalysts for biomass conversion into high value-added chemicals. Sci. Technol. Adv. Mater. 2015, 16, 1–22. [Google Scholar] [CrossRef]
- Hoo, P.; Abdullah, A.Z. Direct synthesis of mesoporous 12-tungstophosphoric acid SBA-15 catalyst for selective esterification of glycerol and lauric acid to monolaurate. Chem. Eng. J. 2014, 250, 274–287. [Google Scholar] [CrossRef]
- Zhu, S.; Zhu, Y.; Gao, X.; Mo, T.; Zhu, Y.; Li, Y. Production of bioadditives from glycerol esterification over zirconia supported heteropolyacids. Bioresour. Technol. 2013, 130, 45–51. [Google Scholar] [CrossRef]
- Kotbagi, T.V.; Pandhare, S.L.; Dongare, M.K.; Umbarkar, S.B. In situ Formed Supported Silicomolybdic Heteropolyanions: Efficient Solid Catalyst for Acetylation of Glycerol. Environ. Anal. Chem. 2015, 2, 1–5. [Google Scholar]
- Khayoon, M.S.; Hameed, B.H. Acetylation of glycerol to biofuel additives over sulfated activated carbon catalyst. Bioresour. Technol. 2011, 102, 9229–9235. [Google Scholar] [CrossRef]
- Sun, J.; Tong, X.; Yu, L.; Wan, J. An efficient and sustainable production of triacetin from the acetylation of glycerol using magnetic solid acid catalysts under mild conditions. Catal. Today 2015, 264, 115–122. [Google Scholar] [CrossRef]
- Sandesh, S.; Kristachar, P.K.R.; Manjunathan, P.; Halgeri, A.B.; Shanbhag, G.V. Synthesis of biodiesel and acetins by transesterification reactions using novel CaSn(OH)6heterogeneous base catalyst. Appl. Catal. A Gen. 2016, 523, 1–11. [Google Scholar] [CrossRef]
- Li, L.; Yu, S.; Xie, C.; Liu, F.; Li, H. Synthesis of glycerol triacetate using functionalized ionic liquid as catalyst. J. Chem. Technol. Biotechnol. 2009, 84, 1649–1652. [Google Scholar] [CrossRef]
- Liu, X.; Ma, H.; Wu, Y.; Wang, C.; Yang, M.; Yan, P.; Welz-biermann, U. Esterification of glycerol with acetic acid using double SO 3 H-functionalized ionic liquids as recoverable catalysts. Green Chem. 2011, 13, 697–701. [Google Scholar] [CrossRef]
- Keogh, J.; Tiwari, M.S.; Manyar, H. Esterification of Glycerol with Acetic Acid Using Nitrogen-Based Brønsted-Acidic Ionic Liquids. Ind. Eng. Chem. Res. 2019, 58, 17235–17243. [Google Scholar] [CrossRef]
- Troncea, S.B.; Wuttke, S.; Kemnitz, E.; Coman, S.M.; Parvulescu, V.I. Hydroxylated magnesium fluorides as environmentally friendly catalysts for glycerol acetylation. Appl. Catal. B Environ. 2011, 107, 260–267. [Google Scholar] [CrossRef]
- Mufrodi, Z.; Rochmadi, R.; Sutijan, S.; Budiman, A. Synthesis Acetylation of Glycerol Using Batch Reactor and Continuous Reactive Distillation Column. Eng. J. 2014, 18, 29–40. [Google Scholar] [CrossRef]
- Goncalves, C.E.; Laier, L.O.; da Silva, M.J. Novel Esterification of Glycerol Catalysed by Tin Chloride (II): A Recyclable and Less Corrosive Process for Production of Bio-Additives. Catal. Lett. 2011, 14, 1111–1117. [Google Scholar] [CrossRef]
- Dosuna-Rodríguez, I.; Adriany, C.; Gaigneaux, E.M. Glycerol acetylation on sulphated zirconia in mild conditions. Catal. Today 2011, 167, 56–63. [Google Scholar] [CrossRef]
- Hamerski, F.; Corazza, M.L. General LDH-catalyzed esterification of lauric acid with glycerol in solvent-free system. Appl. Catal. A Gen. 2014, 475, 242–248. [Google Scholar] [CrossRef]
- Reddy, P.S.; Sudarsanam, P.; Raju, G.; Reddy, B.M. Selective acetylation of glycerol over CeO2-M and SO42-/CeO2-M (M = ZrO2 and Al2O3) catalysts for synthesis of bioadditives. J. Ind. Eng. Chem. 2012, 18, 648–654. [Google Scholar] [CrossRef]
- Reddy, P.S.; Sudarsanam, P.; Raju, G.; Reddy, B.M. Synthesis of bio-additives: Acetylation of glycerol over zirconia-based solid acid catalysts. Catal. Commun. 2010, 11, 1224–1228. [Google Scholar] [CrossRef]
- Da Silva, M.J.; Liberto, N.A.; De Andrade Leles, L.C.; Pereira, U.A. Fe4(SiW12O40)3-catalyzed glycerol acetylation: Synthesis of bioadditives by using highly active Lewis acid catalyst. J. Mol. Catal. A Chem. 2016, 422, 69–83. [Google Scholar] [CrossRef]
- Tangestanifard, M.; Ghaziaskar, H.S. Arenesulfonic Acid-Functionalized Bentonite as Catalyst in Glycerol Esterification with Acetic Acid. Catalysts 2017, 7, 211. [Google Scholar] [CrossRef] [Green Version]
- Sari, V.I.; Hambali, E.; Suryani, A.; Permadi, P. Esterification Reaction of Glycerol and Palm Oil Oleic Acid using Methyl Ester Sulfonate Acid Catalyst as Drilling Fluid Formation. In Proceedings of the Materials Science and Engineering, Purwokerto, Indonesia, 15–16 September 2016; IOP Publishing: Bristol, UK, 2017; Volume 172. [Google Scholar]
- Liu, J.; Wang, Z.; Sun, Y.; Jian, R.; Jian, P.; Wang, D. Selective synthesis of triacetin from glycerol catalyzed by HZSM-5/MCM-41 micro/mesoporous molecular sieve. Chin. J. Chem. Eng. 2019, 27, 1073–1078. [Google Scholar] [CrossRef]
- Rastegari, H.; Ghaziaskar, H.S.; Yalpani, M. Valorization of biodiesel derived glycerol to acetins by continuous esterification in acetic acid: Focusing on high selectivity to diacetin and triacetin with no byproducts. Ind. Eng. Chem. Res. 2015, 54, 3279–3284. [Google Scholar] [CrossRef]
- Kale, S.; Armbruster, U.; Umbarkar, S.; Dongare, M.; Martin, A. Esterification of glycerol with acetic acid for improved production of triacetin using toluene as an entrainer. In Proceedings of the 10th Green Chemistry Conference. An International Event, Barcelona, Spain, 15 November 2013; pp. 70–71. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |














| Feedstocks | Countries |
|---|---|
| Soybean oil (Glycine max) | America, Brazil, Argentina |
| Rapeseed oil (Brassica napus L.) | Germany, Australia, Argentina, France, Canada |
| Linseed oil (Linum usitatissimum) and Olive oil (Olea europaea) | Spain |
| Sunflower oil (Helianthus annuus) | France, Italy |
| Castor oil (Ricinus communis) | Brazil |
| Guang pi (Cornus wilsoniana) | China |
| Palm oil (Elaeis guineensis) and coconut oil (Cocus nucifera) | Indonesia, Malaysia, Colombia, Thailand |
| Animals fat/Beef tallow | Australia, Ireland, Canada |
| Jojoba oil (Simmondsia chinensis) | Mexico, Southwest and Central America |
| * Jatropha oil (J. curcas), * Karanja seed oil (Pongamia pinnata), * Neem oil (Azadirachta indica), * Rubber seed oil (Hevea brasiliensis), * Mahua oil (Madhuca indica), * Algae oil (Cyanobacteria) | Countries cut across Asia, Europe, and Africa |
| Catalyst | SA (m2/g) | Acidity (mmol/g) | Conditions for the Reaction | Performance | Reference | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylating Agent (AA) | T (°C) | MR (G:AA) | CL (g) | t (h) | Stirring (rpm) | GC (%) | MA (%) | DA (%) | TA (%) | ||||
| SO42−/CeO2-ZrO2 | 22.07 | NA | Acetic acid | 100 | 1:10 | 5 wt%G | 3 | Stirred | 99.12 | 21.46 | 57.28 | 21.26 | [108] |
| PVP–DTP | NA | NA | Acetic acid | 110 | 1:20 | 0.25 | 6 | NA | 100 | 13 | 54 | 34 | [104] |
| [H-NEP][HSO4] Ionic liquid | NA | 0.40g NaOH/g IL | Acetic acid | 100 | 1:6 | 2 mol% | 0.30 | 800 | 99 | 23.4 | 55.2 | 21.3 | [138] |
| HCl-activated zeolite | NA | NA | Acetic acid | 90 | 1:9 | 3% AA | 130 | 400 | 95 | 43.0 | 48.6 | 8.3 | [100] |
| HZSM-5/MCM-41 micro/mesoporous molecular sieve | 501 | NA | Acetic acid | 125 | 1:8 | 1.5 | 24 | Stirred | 100 | 0.20 | 17.9 | 81.9 | [149] |
| Sucrose–SBA-15–BDS | 547 | 0.96 | Acetic acid | 110 | 1:9 | 0.7 | 6 | Stirred | 95 | 19 | 56 | 22 | [116] |
| 20%SO4/K10 | 187 | 0.19 | Acetic acid | 120 | 1:12 | 0.4 | 5 | Stirred | 99 | 23 | 59 | 15 | [91] |
| Arenesulfonic acid functionalized bentonite | 5.4 | 1.7 | Acetic acid/ * Toluene | 100 | 1:7:1.4 | 1 | 3 | na | 100 | 0 | 26 | 74 | [147] |
| Propyl-SO3H SBA-15 | 366 | 1.80 | Acetic acid | 120 | 1:6 | 4 wt%G | 2.5 | na | 96 | 13 | 55 | 32 | [73] |
| SBA-Pr NH–SO3H | na | 1.02 | Acetic acid | 105 | 1:3 | 0.05 | 3 | na | 100 | 16 | 57 | 28 | [94] |
| Diatomite-loaded SO42−/TiO2 | na | na | Oleic acid | 210 | 1:2 | 0.1 wt% | 6 | 200 | 95.1 | 20.2 | 59.6 | 15.3 | [107] |
| Sulfonated glycerol-based carbon | na | 3.6 | Acetic acid | 110 | 1:3 | 0.2 | 3 | stirred | 99 | 12 | 45 | 43 | [114] |
| Sulfonated activated carbon (ACSO3H) | 469 | 5.41 | Oleic acid | 125 | 1:1 | 0.5 | 24 | 650 | 90 | 77 | na | na | [101] |
| ACSO3H | 469 | 5.41 | Lauric acid | 100 | 1:1 | 0.5 | 24 | 650 | 90 | 70 | na | na | [101] |
| Fe–Sn–Ti(SO42−)-400 | 18.88 | na | Acetic anhydride | 80 | 1:6 | 0.05 | 0.5 | Stirred | 100 | 0 | 1 | 99 | [134] |
| CaSn(OH)6 | 3.95 | na | Methyl acetate | 30 | 1:10 | 7 wt%G | 2 | Stirred | 78.2 | 67.3 | 32.6 | na | [135] |
| 2M SO42−/γ-Al2O3 | 8.4 | 2.51 | Acetic acid | 110 | 1:9 | 0.25 | 2 | 700 | 97 | 27 | 49.9 | 23.1 | [122] |
| 1Ag–10Cu-doped rice husk silica | 108 | na | Acetic acid | 110 | 1:10 | 0.08 | 5 | 700 | 83.4 | 68.3 | 18.5 | 13.2 | [90] |
| Fe4(SiW12O40)3 | na | na | Acetic acid | 60 | 1:3 | 0.06 mmol | 8 | Stirred | 100 | 24 | 69 | 7 | [146] |
| PDSA-treated montmorillonite | 276 | 0.258 | Acetic acid | 120 | 1:3 | 1 | 1 | 500 | 96 | 14 | 26 | 56 | [87] |
| MSA-treated montmorillonite | 204 | 0.254 | Acetic acid | 120 | 1:3 | 1 | 1 | 500 | 94 | 22 | 31 | 41 | [87] |
| p-TSA-treated montmorillonite | 141 | 0.256 | Acetic acid | 120 | 1:3 | 1 | 1 | 500 | 94 | 30 | 35 | 29 | [87] |
| 2M SO42−/γ-Al2O3 | 8.4 | 2.51 | Acetic acid | 95 | 1:12 | 0.75 | 5 | na | 99 | 22.6 | 48.2 | 29.2 | [106] |
| 20 mol% MnO3/SiO2 | 217 | 0.94 | Acetic acid | 100 | 1:10 | 10 wt%G | 8 | na | 100 | 17 | 33 | 50 | [132] |
| Amberlyst-15 | na | na | Acetic anhydride | 60 | 1:3 | 5% per mol G | 2 | na | 100 | 0.7 | 1.3 | 98.1 | [99] |
| Amberlyst-15 | na | na | Acetic acid | 120 | 1:6 | 5% per mol G | 2 | na | 100 | 3.5 | 8.7 | 87.8 | [99] |
| Sulphated willow catkins-based carbon | na | 5.14 | Acetic acid | 120 | 1:5 | 5 wt%G | 2 | stirred | 98.4 | 32.8 | 54.5 | 12.7 | [113] |
| Biochar-400 | 14 | 6.328 | Acetic acid | 120 | 1:5 | 0.2 | 1 | na | 88.5 | 56 | 40 | 4 | [125] |
| Graphene oxide | na | na | Acetic acid | 120 | 1:10 | 0.1 | 1 | Stirred | 98.5 | 15.5 | 60 | 24.5 | [42] |
| CsPWA | 110 | 1.87 | Acetic acid | 85 | 1:8 | 7 wt%R | 2 | Stirred | 98.1 | 25 | 59 | 16 | [89] |
| CsPWA | 110 | 1.87 | Acetic anhydride | 30 | 1:3 | 4 wt%R | 2 | Stirred | 100 | 1.2 | 21.5 | 77.3 | [89] |
| Sb2O5 | 53 | 0.094 | Acetic acid | 120 | 1:6 | 0.1 | 1 | Stirred | 96.8 | 33.2 | 54.2 | 12.6 | [123] |
| Amberlyst-36 | na | na | Acetic acid | 100 | 1:7 | 3 | 2 | na | 100 | 43 | 44 | 13 | [150] |
| SO42−/12ZrKIL-2 | 306 | na | Acetic acid | 100 | 1:10 | 0.1 | 2 | Stirred | 100 | 2.7 | 77.1 | 20.1 | [105] |
| SO42−/12ZrKIL-2 | 306 | na | Acetic acid | 100 | 1:10 Crude G | 0.1 | 2 | Stirred | 91.1 | 63.7 | 26.4 | 20.5 | [105] |
| Fe-SBA-15 | 688 | 0.104 | Levulinic acid | 140 | 1:4 | 0.05 | 8 | 1200 | >99 | 0 | 80 | 20 | [111] |
| 3%Y-SBA-15 | 1568 | 1.342 | Acetic acid | 110 | 1:4 | 0.2 | 3 | 350 | 100 | 11 | 34 | 55 | [82] |
| Sulphated glycerol-based carbon | na | na | Acetic acid | 115 | 1:10 | 0.46 | 1 | Stirred | 100 | 8.4 | 71.8 | 19.8 | [126] |
| PrSO3H SBA-15 | 701 | 1.2 | Acetic acid | 80 | 1:6 | 5 wt%G | 8 | Stirred | 100 | 15.8 | 64.6 | 19.6 | [41] |
| Amberlyst-15 | 53 | 4.9 | Acetic acid | 80 | 1:6 | 5 wt%G | 8 | Stirred | 100 | 21.1 | 63.8 | 15.1 | [41] |
| SO3H-SBA15 | 515 | 0.8 | Acetic acid | 80 | 1:6 | 5 wt%G | 8 | Stirred | 100 | 11.1 | 61.9 | 27.0 | [41] |
| La3+-montmorillonite | na | na | Acetic acid/ * Toluene | 120 | 1:2 | 0.02 mmol | 24 | na | 98 | 0 | >99 | 0 | [109] |
| 30%TPA/MCM-41 | 360 | 0.855 | Acetic acid | 100 | 1:6 | 0.15 | 6 | Stirred | 87 | na | 60 | 15 | [102] |
| Layer double hydroxide–hydrotalcite | 106 | na | Lauric acid | 180 | 1:3 | 2 wt%G | 1 | 500 | >99 | ≈58 | 30 | ≈10 | [143] |
| Amberlyst-15 (1.6 wt) | 37.6 | 1.41/** 4.7 | Acetic acid | 110 | 1:9 | 2.645 | 4.5 | 1100 | 97.1 | <20 | 47.7 | 44.5 | [34] |
| H4SiW12O40/ZrO2 | 48.7 | 0.69 | Acetic acid | 120 | 1:10 | 0.3 | 4 | 250 | 100 | 6.4 | 61.3 | 32.3 | [131] |
| Silver-exchanged phosphotungstic acid (Ag1PW) | na | 1.92 | Acetic acid | 120 | 1:10 | 1 wt%G | 0.25 | Stirred | 96.8 | 48.4 | 46.4 | 5.2 | [84] |
| Ag1PW | na | 1.92 | Propanoic acid | 120 | 1:10 | 1 wt%G | 0.25 | na | 70.9 | 55.0 | 43.1 | 1.9 | [84] |
| Ag1PW | na | 1.92 | 1-Butanoic acid | 120 | 1:10 | 1 wt%G | 0.25 | na | 64.3 | 53.5 | 46.4 | 0.1 | [84] |
| 20% sulphated silica | na | 479 | Acetic acid | 50 | 1:6 | 0.2 | 2 | Stirred | 96.88 | 51.90 | 45.27 | 2.11 | [110] |
| PrSO3H SAS | 640 | 1.7 | Acetic acid | 105 | 1:3 | 0.05 | 3 | Stirred | 100 | 0 | 51 | 49 | [92] |
| PrSO3H SBA-15 | 489 | 1.2 | Acetic acid | 105 | 1:3 | 0.05 | 3 | Stirred | 100 | 5 | 62 | 33 | [92] |
| Sulfonated carbonized rice husk | <80 | 5.8 | Acetic acid | 150 | 1:4 | 5 wt%G | 5 | Stirred | 90 | 11 | 52 | 37 | [75] |
| Amberlyst-70 | 36 | 2.55 | Acetic acid/ * Toluene | 105 | 1:6 | 5 wt%G | 10 | na | 100 | 0 | 7.5 | 87.6 | [151] |
| Amberlyst-15 | 53 | 4.7 | Acetic acid/ * Toluene | 105 | 1:6 | 5 wt%G | 10 | na | 100 | 0 | 12.3 | 83.9 | [151] |
| H3PW12O40 | na | na | Acetic acid | 60 | 1:3 | 0.03 mmol | 8 | Stirred | 96 | 66 | 34 | 0 | [93] |
| Amberlyst-15 | na | ** 4.7 | Acetic acid | 110 | 1:9 | 2.645 | 5 | 1100 | 97.13 | 7.59 | 46.29 | 43.23 | [83] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nda-Umar, U.I.; Ramli, I.B.; Muhamad, E.N.; Azri, N.; Amadi, U.F.; Taufiq-Yap, Y.H. Influence of Heterogeneous Catalysts and Reaction Parameters on the Acetylation of Glycerol to Acetin: A Review. Appl. Sci. 2020, 10, 7155. https://doi.org/10.3390/app10207155
Nda-Umar UI, Ramli IB, Muhamad EN, Azri N, Amadi UF, Taufiq-Yap YH. Influence of Heterogeneous Catalysts and Reaction Parameters on the Acetylation of Glycerol to Acetin: A Review. Applied Sciences. 2020; 10(20):7155. https://doi.org/10.3390/app10207155
Chicago/Turabian StyleNda-Umar, Usman Idris, Irmawati Binti Ramli, Ernee Noryana Muhamad, Norsahida Azri, Uchenna Fidelis Amadi, and Yun Hin Taufiq-Yap. 2020. "Influence of Heterogeneous Catalysts and Reaction Parameters on the Acetylation of Glycerol to Acetin: A Review" Applied Sciences 10, no. 20: 7155. https://doi.org/10.3390/app10207155
APA StyleNda-Umar, U. I., Ramli, I. B., Muhamad, E. N., Azri, N., Amadi, U. F., & Taufiq-Yap, Y. H. (2020). Influence of Heterogeneous Catalysts and Reaction Parameters on the Acetylation of Glycerol to Acetin: A Review. Applied Sciences, 10(20), 7155. https://doi.org/10.3390/app10207155






