Research on Corrosion Damage Evolution of Aluminum Alloy for Aviation
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
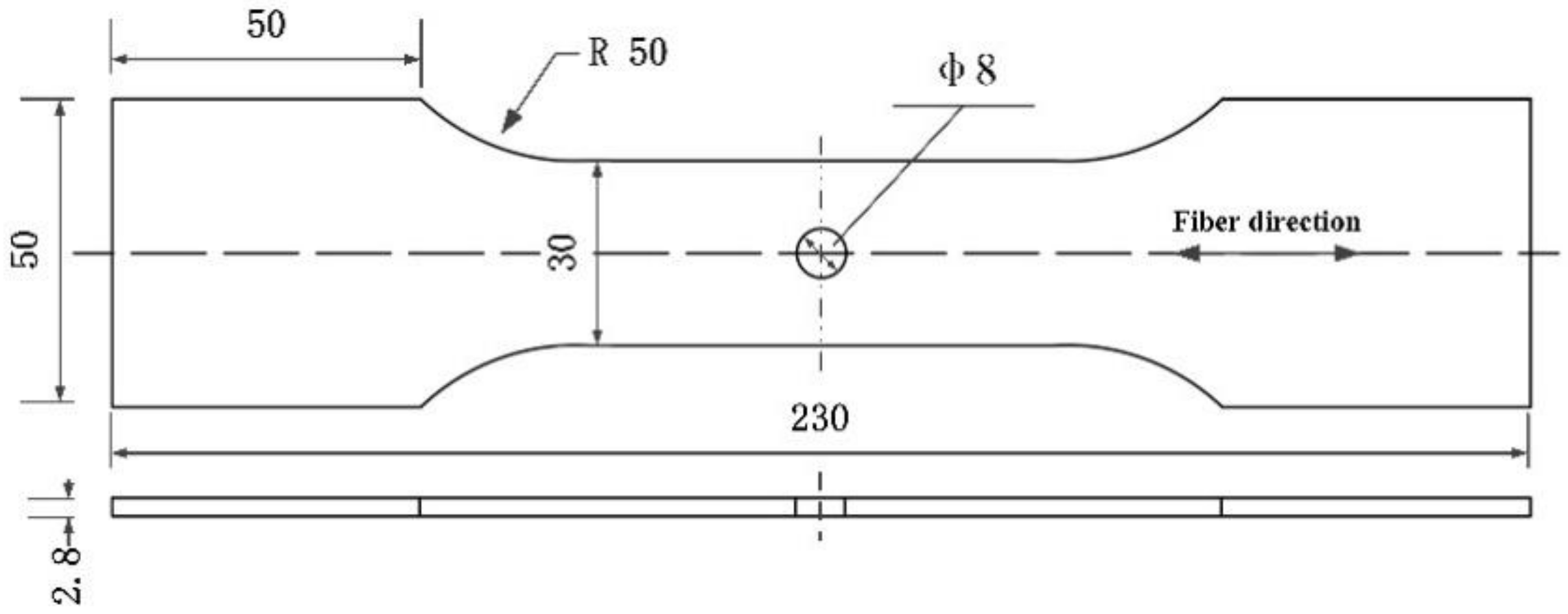
2.1. Materials
2.2. Methods
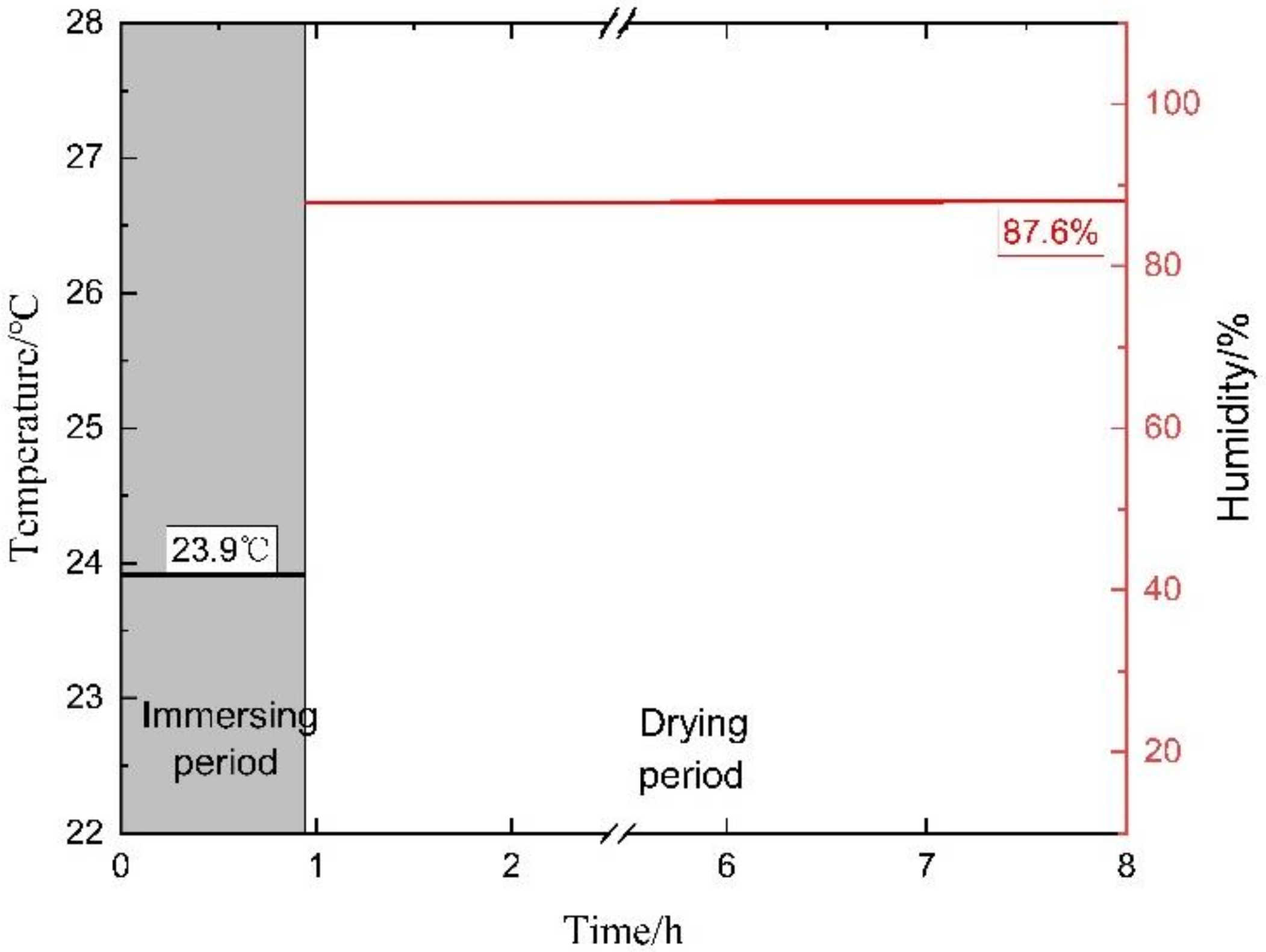
2.2.1. Determination of Corrosion Solution
2.2.2. A Method of Alternate Immersion Corrosion for Simulating the Environment of the Internal Structure of the Aircraft
3. Results and Discussion
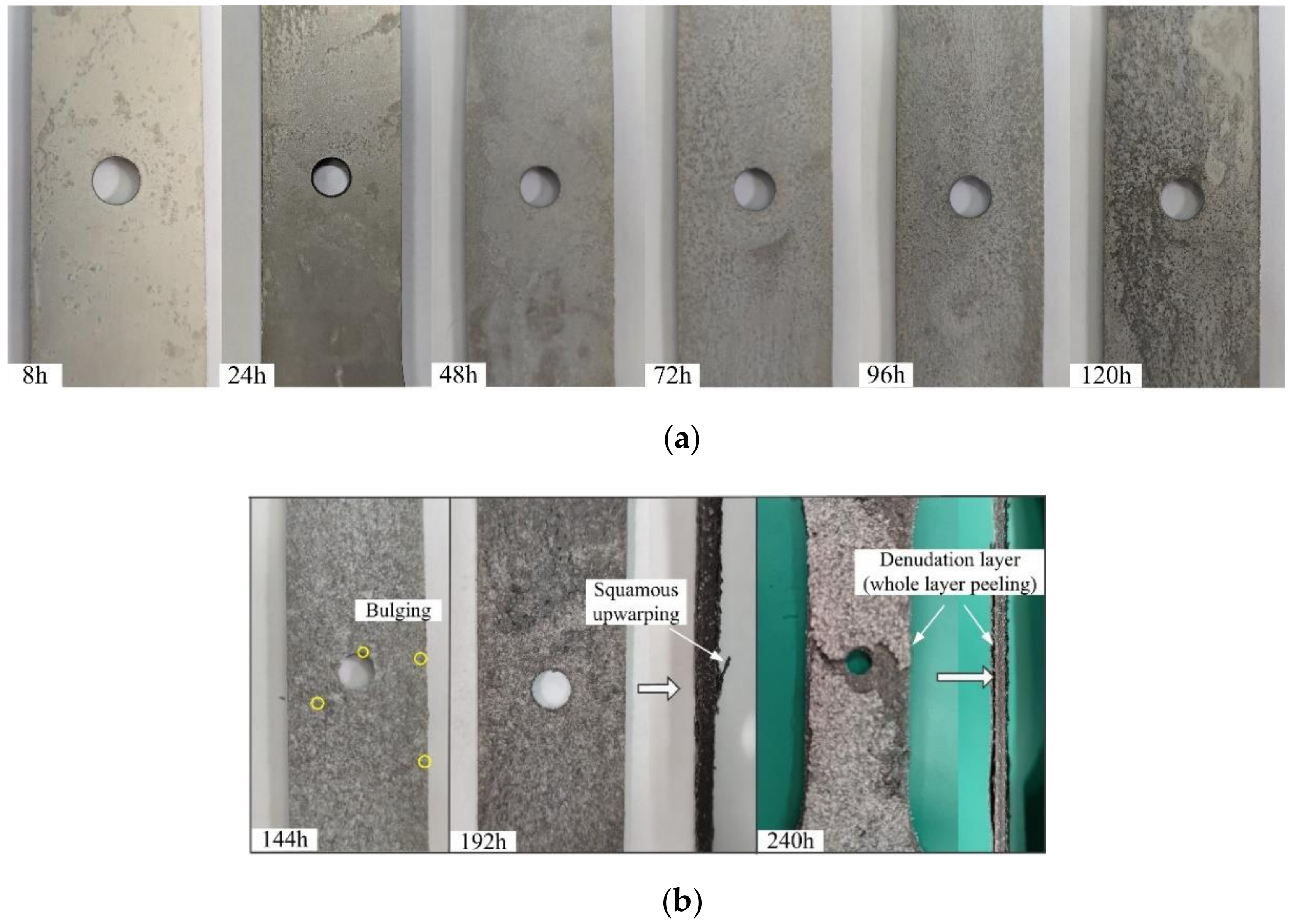
3.1. Corrosion Evaluation
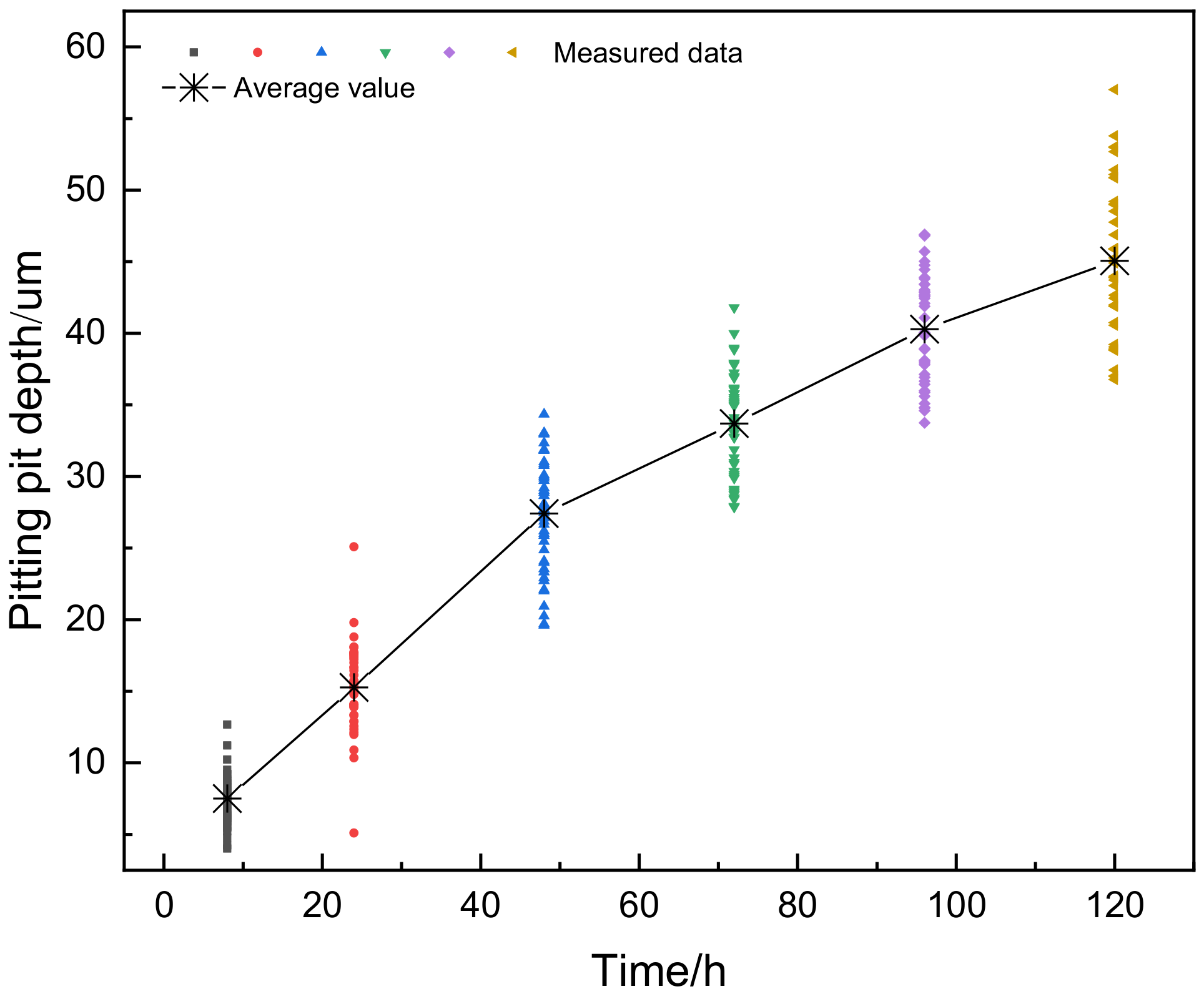
3.2. Depth of Corrosion Pits
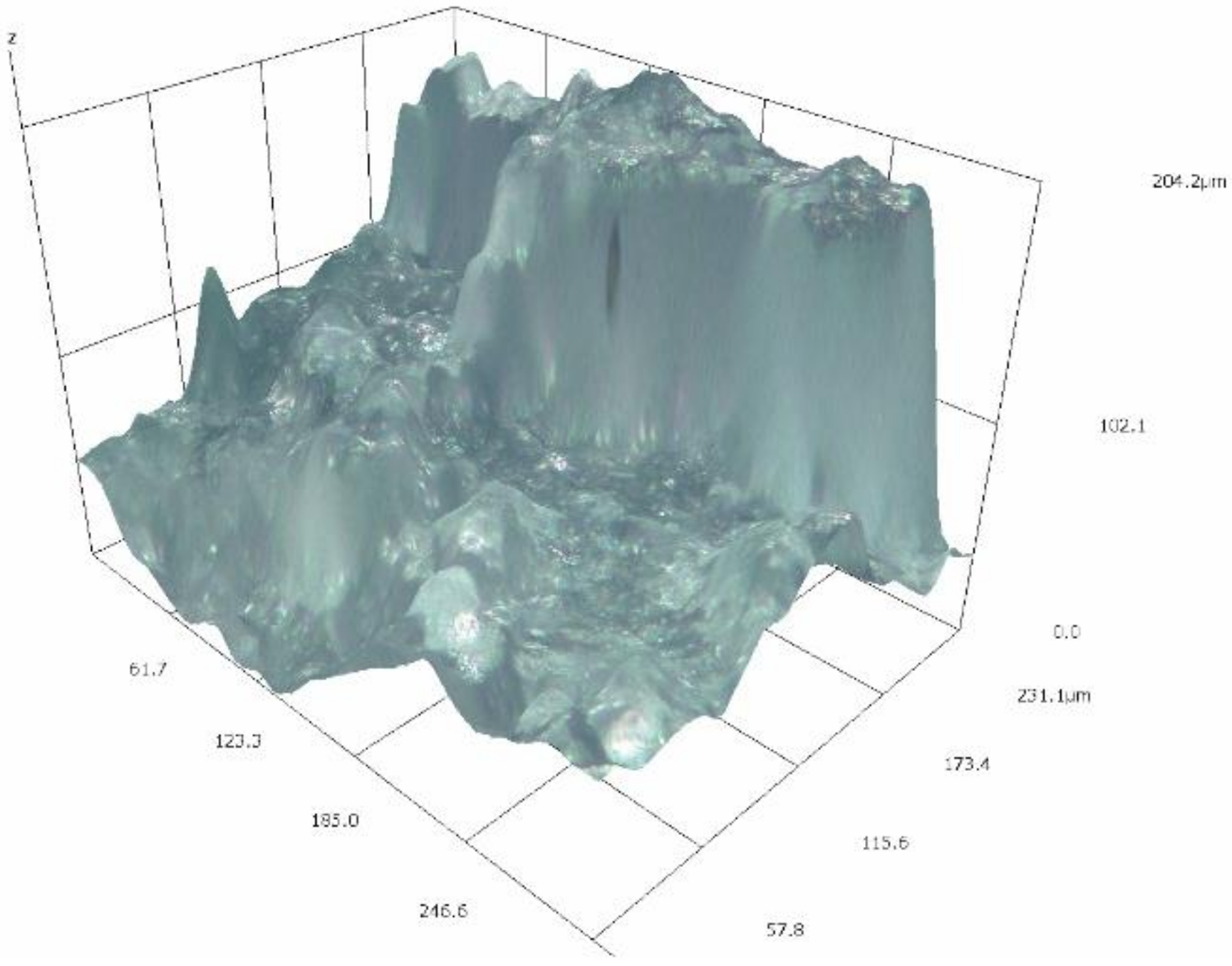
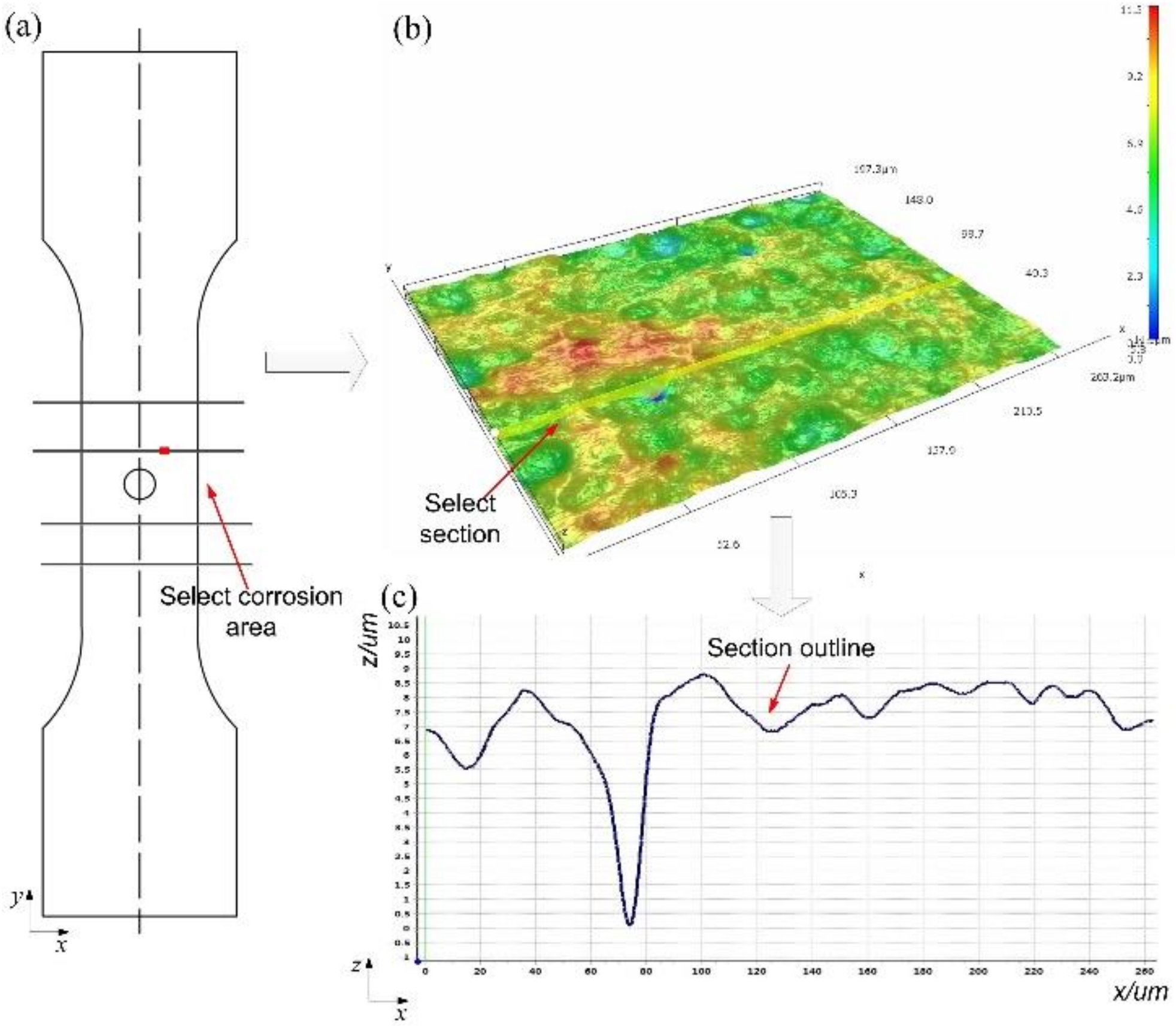
3.2.1. Measurement Method
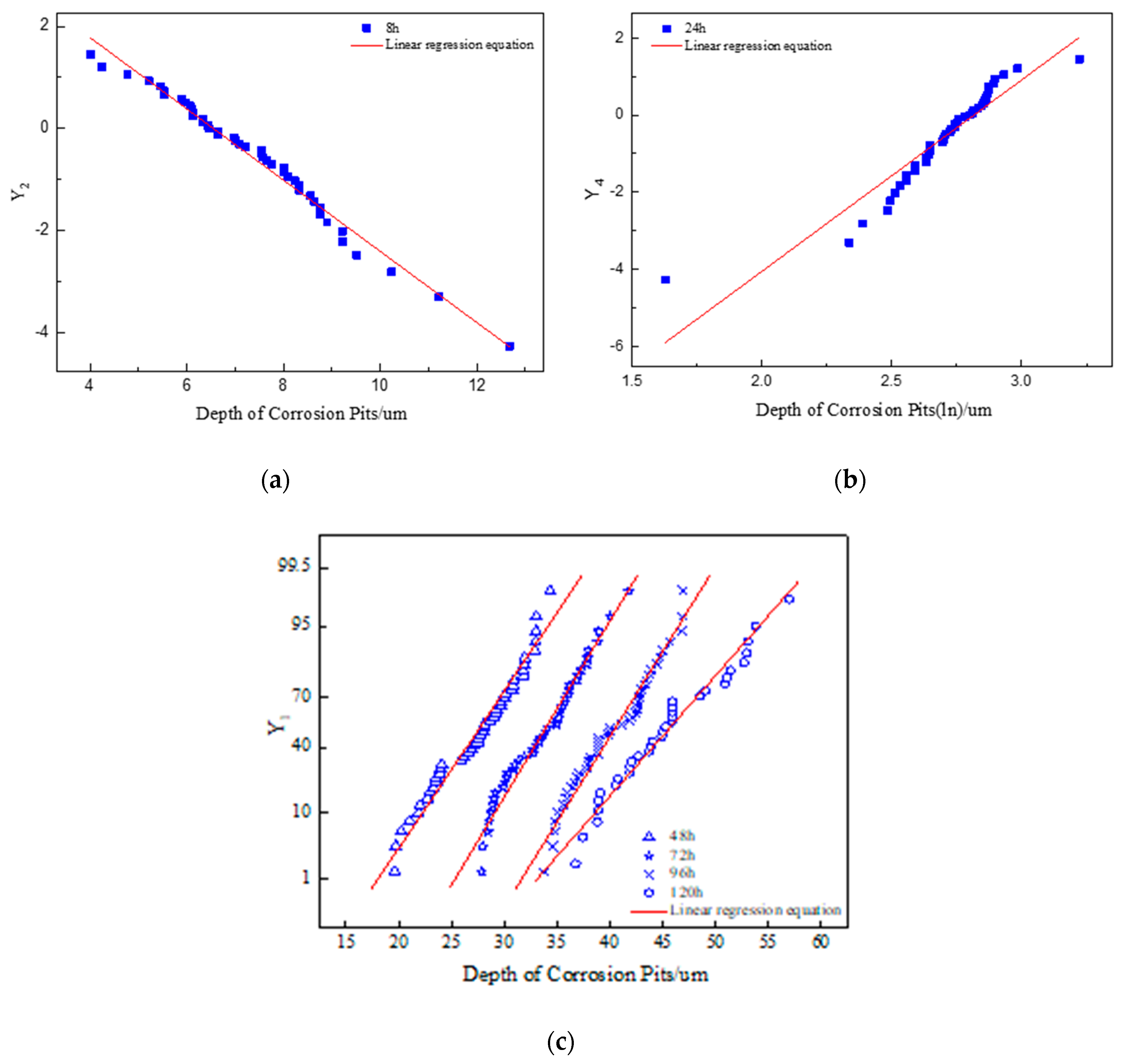
3.2.2. Distribution Law
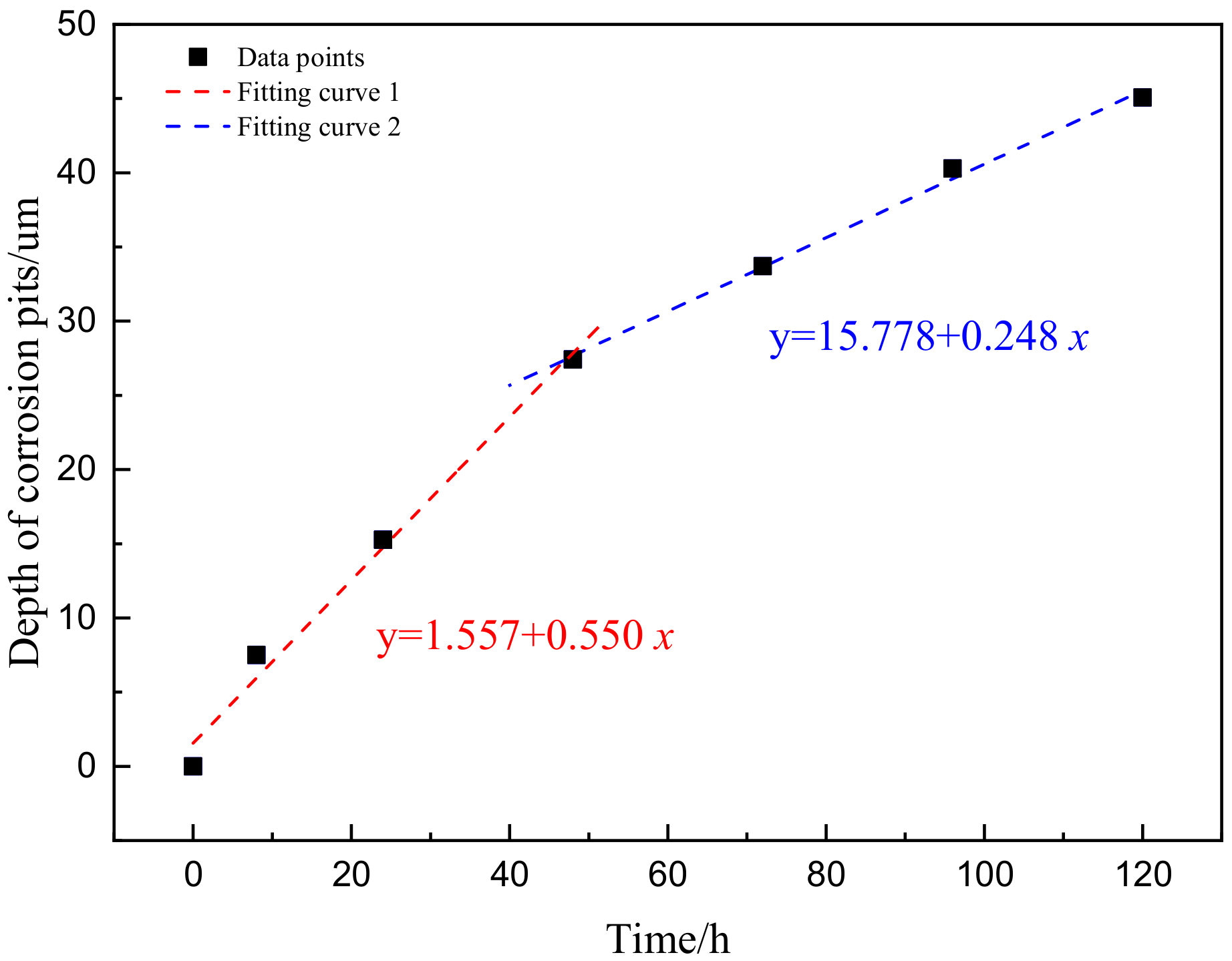
3.2.3. Dynamic Evolution Model
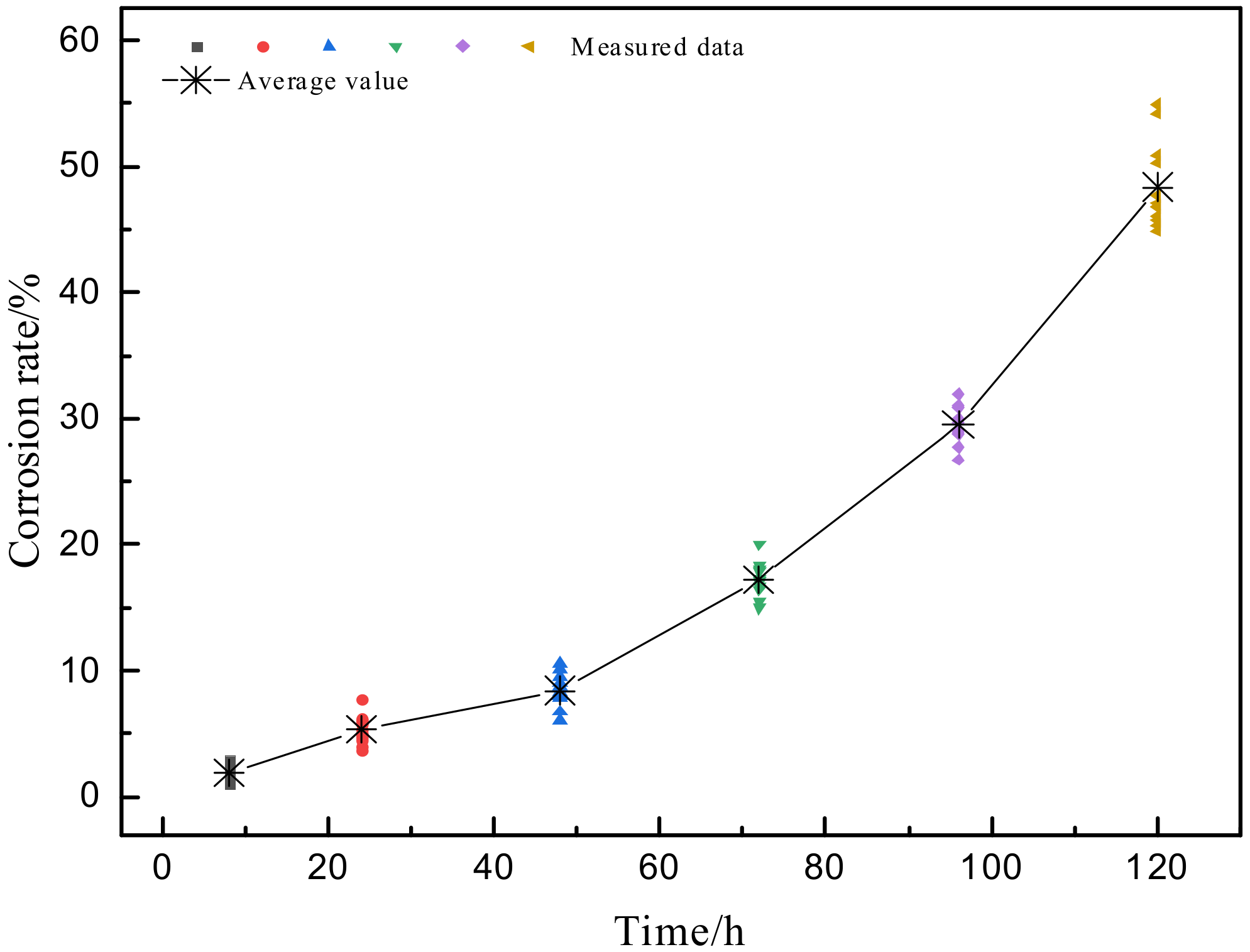
3.3. Corrosion Rate
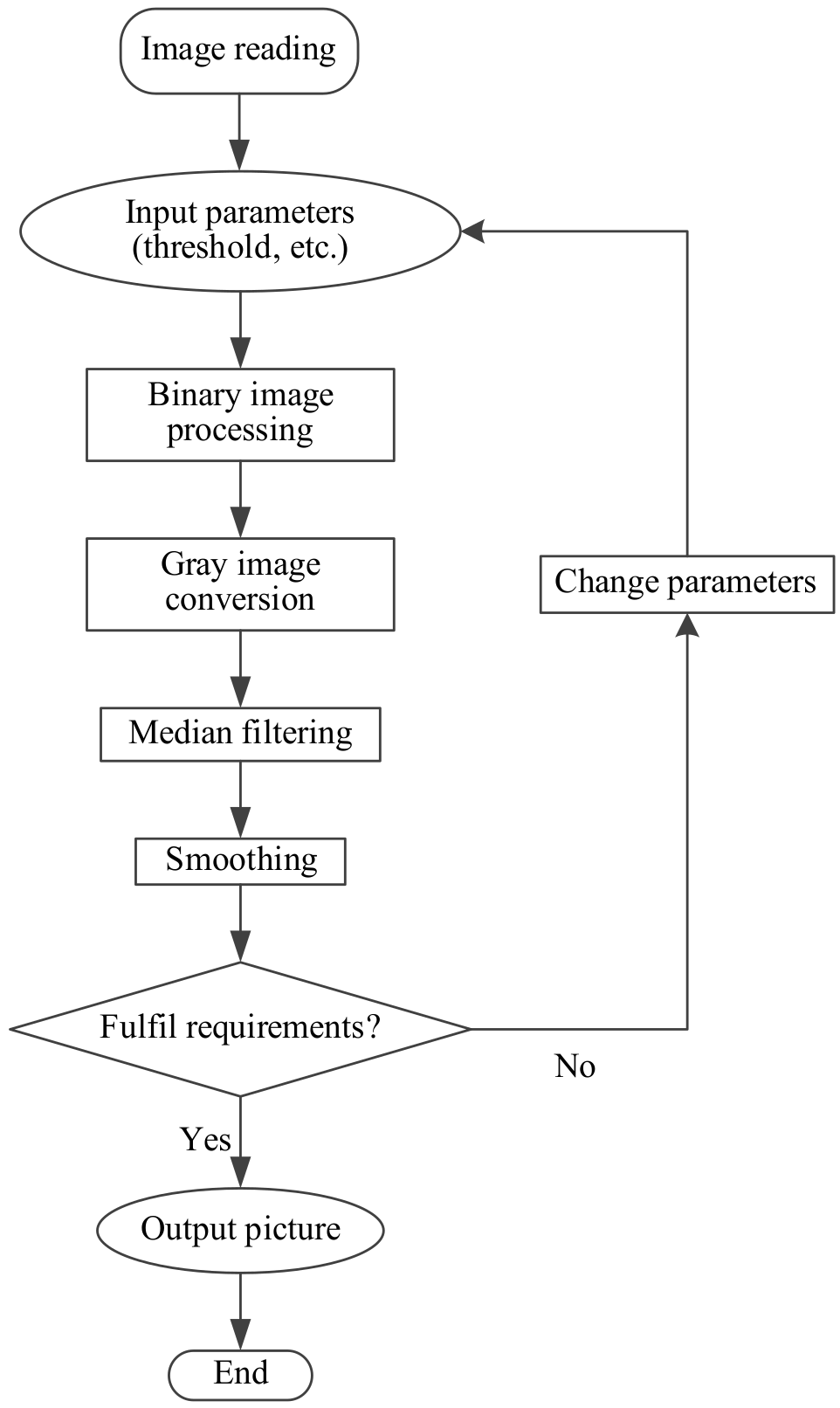
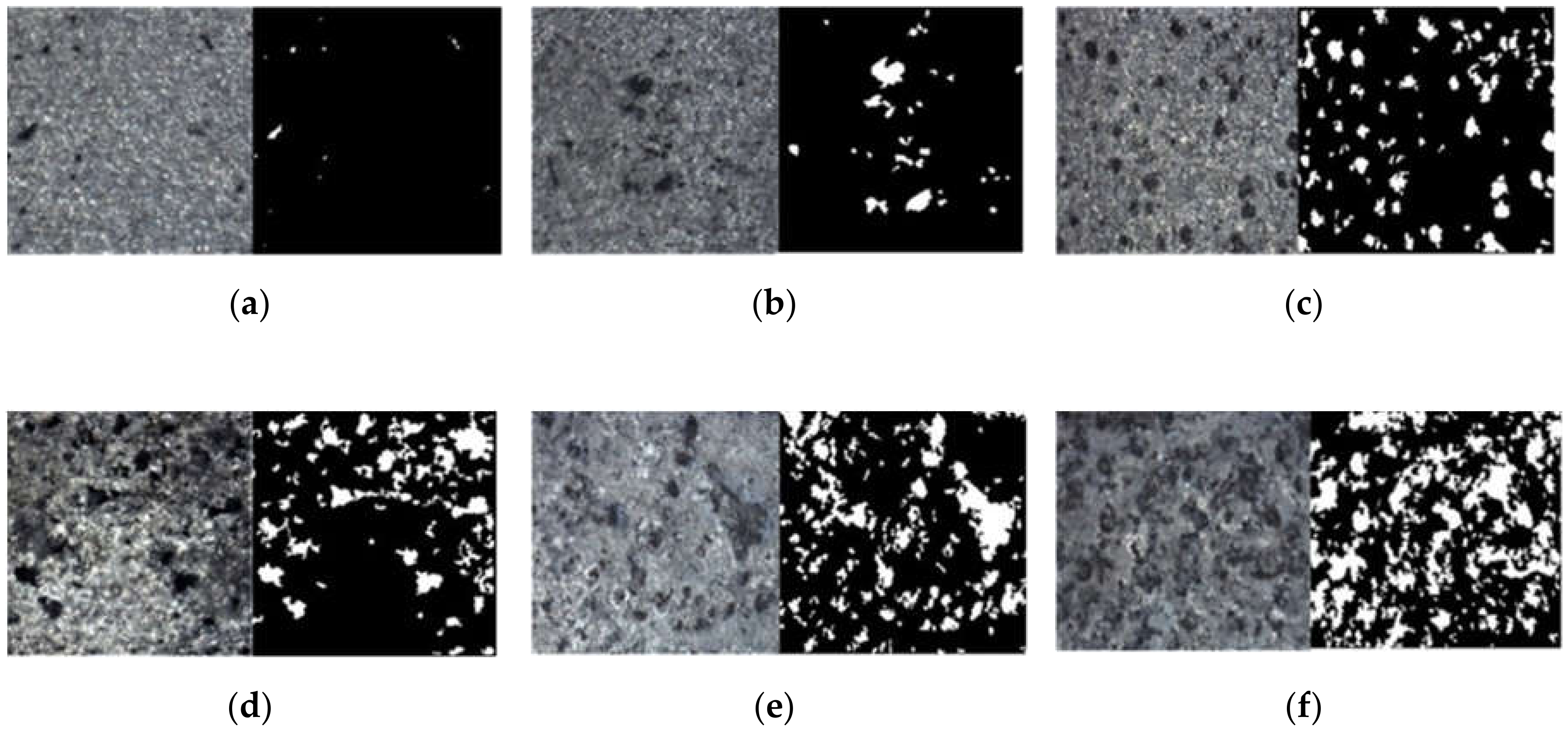
3.3.1. Image Binarization
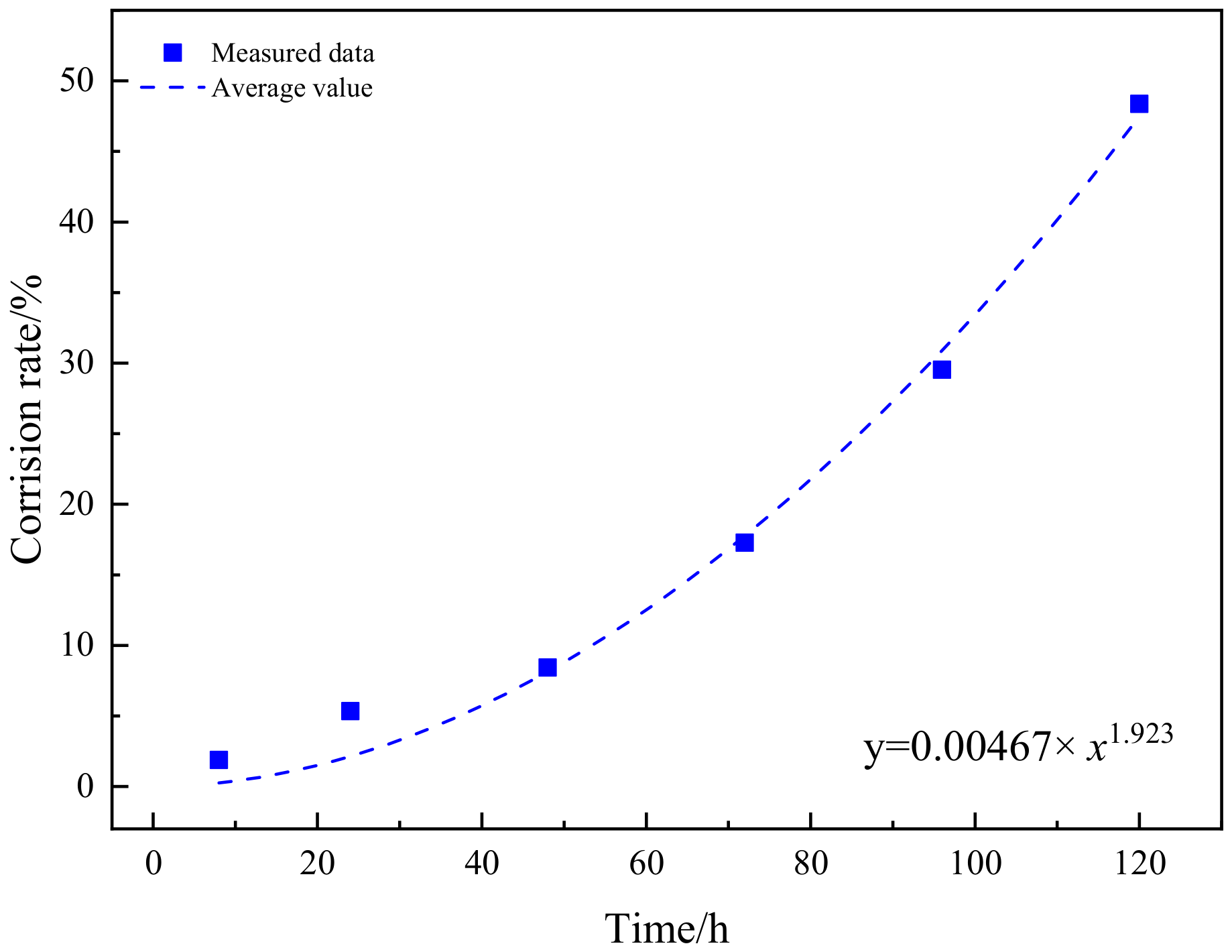
3.3.2. Dynamic Evolution Model
4. Conclusions
- (1)
- According to the characteristics of the atmospheric environment in Wanning, Hainan, a corrosion solution was designed and the traditional alternate immersion corrosion method of using the alternate immersion corrosion test box was improved to simulate the environment of the internal structure of the aircraft.
- (2)
- According to the results of statistical analysis, the depth of corrosion pits can well obey Gumbel distribution, normal distribution, lognormal distribution, and two-parameter Weibull distribution. When the accelerated corrosion time was 8 h and 24 h, the optimal distribution model was Gumbel distribution and Weibull distribution, respectively. When the accelerated corrosion time was 48 h, 72 h, 96 h, and 120 h, the optimal distribution model was normal distribution.
- (3)
- The proposed image binarization method and 3D profile recognition method can accurately and conveniently reflect the morphology characteristics of the corrosion pits and quantitatively calculate the depth of corrosion pits and corrosion rate. Compared with the traditional estimation methods, the proposed method had great improvement and had strong engineering value.
- (4)
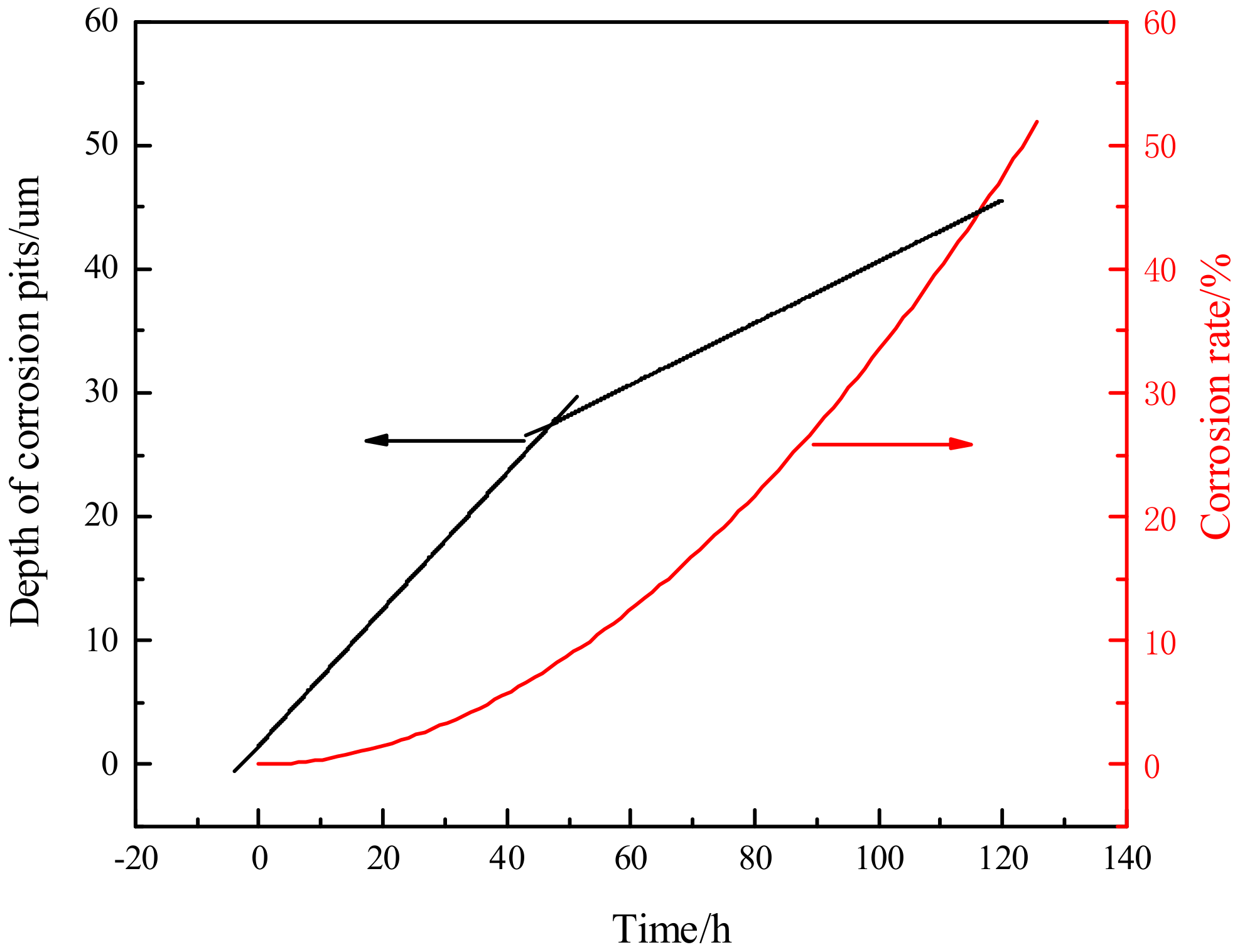
- The dynamic model of the depth of corrosion pits and corrosion rate evolution was obtained. Through comparison, it was found that the corrosion damage was mainly reflected in the increase of the depth of corrosion pits in the early stage of corrosion, and the growth rate of the pitting corrosion rate was relatively slow. After 48 h, the growth rate of the depth of corrosion pits began to slow down, while the growth rate of pitting corrosion rate accelerated.
Author Contributions
Funding
Conflicts of Interest
References
- Chantzis, D.; Van-Der-Veen, S.; Zettler, J. An industrial workflow to minimise part distortion for machining of large monolithic components in aerospace industry. Procedia CIRP 2013, 8, 281–286. [Google Scholar] [CrossRef]
- Chen, Z.; Zheng, Y.; Zhu, C. Effect of pre-stretching on geometric accuracy and mechanical properties of 7075 aluminum alloy plats. Nonferrous Met. Sci. Eng. 2019, 10, 40–47. [Google Scholar]
- Gurgen, S.; Sackesen, I.; Kushan, M.C. Fatigue and corrosion behavior of in-service AA7075 aircraft component after thermo-mechanical and retrogression and re-aging treatments. Proc. Inst. Mech. Eng. 2019, 233, 1764–1772. [Google Scholar] [CrossRef]
- Zhao, P.F.; Su, X.Q.; Wu, J.S. Accelerated corrosion test spectrum of typical reef atmospheric environment. Equip. Environ. Eng. 2019, 16, 14–21. [Google Scholar]
- Cui, T.F. Effect of Stress and Egalvanic Factors on the Durability of Aluminum Alloy; Northwestern Polytechnical University: Xi’an, China, 2017; pp. 41–49. [Google Scholar]
- Brooks, C.L.; Simpson, D. Integrating real time age degradation into the structural integrity process. In Proceedings of the RTO Meeting Proceedings, Corfu, Greece, 7–9 October 1998; pp. 195–207. [Google Scholar]
- Chlistovsky, R.M.; Heffernan, P.J.; Duquesnay, D.L. Corrosion-fatigue behaviour of 7075-T651 aluminum alloy subjected to periodic overloads. Int. J. Fatigue 2007, 29, 1941–1949. [Google Scholar] [CrossRef]
- Li, X.; Mu, Z.; Jia, M. Effect of Loading Frequency on Corrosion Fatigue Crack Growth Rate of Aerospace Aluminum Alloy. Mater. Mech. Eng. 2014, 38, 50–52, 98. [Google Scholar] [CrossRef]
- Ye, Z.Y.; Liu, D.X.; Zhang, X.H. Corrosion Fatigue Behavior of 7A85 Aluminum Alloy Thick Plate in NaCl Solution. Acta Metall. Sin. Engl. Lett. 2015, 28, 1047–1054. [Google Scholar] [CrossRef]
- Dan, Z.; Takigawa, S.; Muto, I. Applicability of constant dew point corrosion tests for evaluating atmospheric corrosion of aluminium alloys. Corros. Sci. 2011, 53, 2006–2014. [Google Scholar] [CrossRef]
- Pao, P.S.; Gill, S.J.; Feng, C.R. On fatigue crack initiation from corrosion pits in 7075-T7351 aluminum alloy. Scr. Mater. 2000, 43, 391–396. [Google Scholar] [CrossRef]
- Miedlar, P.C.; Berens, A.P.; Gunderson, A. USAF Damage Tolerant Design Handbook: Guidelines for the Analysis and of Damage Tolerant Aircraft Structure; AFRL-VA-WP-TR-2003-3002; University of Dayton Research Institute: Dayton, OH, USA, 2002. [Google Scholar]
- Zhang, Y.H. The Corrosion Damage and Its Effect e on Life of Aircraft Structure; Northwestern Polytechnical University: Xi’an, China, 2007. [Google Scholar]
- Sun, L. The Study on Corrosion Morphology and Residual Fatigue Life of Corroded Aluminium Alloy; Nanjing University of Aeronautics and Astronautics: Nanjing, China, 2013. [Google Scholar]
- Ren, K.L.; Lv, G.Z.; Zhang, Y.H. The correlation between corrosion pit with equivalent initial surface crack. Struct. Environ. Eng. 2006, 3, 50–57. [Google Scholar]
- DuQuesnay, D.L.; Underhill, P.R.; Britt, H.J. Fatigue crack growth from corrosion damage in 7075-T6511 aluminum alloy under aircraft loading. Int. J. Fatigue 2003, 25, 371–377. [Google Scholar] [CrossRef]
- Yu, M.F. Finite Element Simulation of the Influence of 20CrMnTi Pitting Corrosion on Stress Concentration; Guizhou University: Guizhou, China, 2018. [Google Scholar]
- Walde, K.; Hillberry, B.M. Characterization of pitting damage and prediction of remaining fatigue life. Int. J. Fatigue 2008, 30, 106–118. [Google Scholar]
- Murakami, Y. Metal Fatigue: Effects of Small Defects and Nonmetallic Inclusions; Elsevier: Amsterdam, The Netherlands, 2002. [Google Scholar]
- Adjel, S.; Merakeb, N.; Benchouia, S. Effect of corrosion pit density on the fatigue life of aluminum 1050A. Int. J. Adv. Manuf. Technol. 2018, 97, 3163–3177. [Google Scholar] [CrossRef]
- Ye, B.Y.; Zhu, F.; Xu, H.B. Probability Model Study on Corrosion Damage of Aircraft Structure. Aviat. Maint. Eng. 2019, 7, 36–37. [Google Scholar]
- Silva, J.W.J.; Bustamante, A.G.; Codaro, E.N. Morphological analysis of pits formed on Al 2024-T3 in chloride aqueous solution. Appl. Surf. Sci. 2004, 236, 356–365. [Google Scholar] [CrossRef]
- Wang, J. The Role of Liquid Film Morphology in Atmospheric Corrosion; Chemical Industry Press: Beijing, China, 2016; pp. 49–56. [Google Scholar]
- Zhang, Z. Electrochemical Detection of Atmospheric Corrosion of Aluminum Alloy for Aircraft; Tianjin University: Tianjin, China, 2007; pp. 20–35. [Google Scholar]
- Zhang, T.; He, Y.T.; Gao, C. Damage rule of 2A12-T4aluminum alloy with long-term atmospheric corrosion. Acta Aeronaut. Astronaut. Sin. 2015, 36, 661–671. [Google Scholar]
- Hao, G.Z.; Xing, L.Y.; Liang, Q.W. Humidity Fixed Point of Saturated Salt Water Solution (2)-Data Source and Salt Solution Selection. Sens. World 1999, 12, 10–14. [Google Scholar]
- Sun, S.; Zheng, Q.; Li, D.; Hu, S.; Wen, J. Exfoliation corrosion of extruded 2024-T4 in the coastal environments in China. Corros. Sci. 2011, 53, 2527–2538. [Google Scholar] [CrossRef]
- Chen, Q.Z.; Cui, C.J.; Sun, Z.D.; Wang, Y.Y.; Xi, H.Z. Probability Distribution and Variations of Corrosion Damage of LY12CZ Aluminum Alloys. Equip. Environ. Eng. 2005, 3, 1–6. [Google Scholar]
- Chen, Y.; Yang, X.; Qin, H. Study on corrosion damage distribution law of aircraft structure. Mater. Sci. Eng. 2002, 20, 378–380. [Google Scholar]
- Deng, Z.; Meng-Si, L.I. Dynamic Law of Corrosion Damage Distribution of High Strength Aluminum Alloy Based on Two-parameter Weibull Distribution. Equip. Environ. Eng. 2019, 16, 27–31. [Google Scholar]
- Xing, S.B.; Li, X.G.; Li, L. Corrosion Behavior of 7A04 Aluminium Alloy in Xisha Marine Atmosphere. Corros. Prot. 2013, 34, 796. [Google Scholar]


| Si | Fe | Cu | Mn | Mg | Cr | Zn | Ti | Al |
|---|---|---|---|---|---|---|---|---|
| 0.40 | 0.50 | 1.2~2 | 0.30 | 2.1~2.9 | 0.18~0.28 | 5.1~6.1 | 0.20 | allowance |
| Environment Parameters | Relative Humidity/(%) | Temperature/(°C) | Wind Speed/(m/s) | NO2/(mg/m3) | SO2/(mg/m3) | Cl− Deposition Rate/(mg/100 cm2·d) |
|---|---|---|---|---|---|---|
| Annual Average | 87.6 | 23.9 | 2.43 | 3.03 × 10−3 | 0.045 × 10−3 | 14.59 |
| Corrosive Medium | Distilled Water | H2SO4/(mol/L) | HNO3/(mol/L) | NaCl/(%) |
|---|---|---|---|---|
| Concentration | — | 4.06 × 10−3 | 9.19 × 10−2 | 3.5 |
| Distribution Type | Parameter | 8 h | 24 h | 48 h | 72 h | 96 h | 120 h |
|---|---|---|---|---|---|---|---|
| Normal distribution (Y1) | μ | 7.352 | 15.272 | 27.414 | 33.698 | 40.291 | 45.064 |
| σ | 1.749 | 3.043 | 4.053 | 3.623 | 3.741 | 5.120 | |
| r | 0.957 | 0.909 | 0.990 | 0.991 | 0.994 | 0.983 | |
| Gumbel distribution (Y2) | μ | 6.892 | 13.991 | 26.072 | 31.891 | 38.021 | 44.123 |
| σ | 2.312 | 3.221 | 4.121 | 3.458 | 3.889 | 4.451 | |
| r | −0.991 | −0.935 | −0.939 | −0.967 | −0.959 | −0.971 | |
| Lognormal distribution (Y3) | μ | 1.967 | 2.703 | 3.222 | 3.512 | 3.692 | 3.846 |
| σ | 0.238 | 0.233 | 0.167 | 0.108 | 0.0933 | 0.108 | |
| r | 0.968 | 0.837 | 0.978 | 0.990 | 0.989 | 0.981 | |
| Weibull distribution (Y4) | β | 4.362 | 5.261 | 7.556 | 10.302 | 12.120 | 9.812 |
| σ | 8.036 | 16.438 | 27.105 | 35.320 | 41.990 | 49.377 | |
| r | 0.980 | 0.939 | 0.982 | 0.960 | 0.961 | 0.959 |
| Corrosion Time/h | Distribution Form | Linear Regression Equation |
|---|---|---|
| 8 | Gumbel distribution | Y2 = −4.569 − 0.698d |
| 24 | Weibull distribution | Y4 = −13.987 + 4.966lnd |
| 48 | Normal distribution | Y1 = −144.350 + 7.089d |
| 72 | Normal distribution | Y1 = −217.591 + 7.940d |
| 96 | Normal distribution | Y1 = −260.837 + 7.715d |
| 120 | Normal distribution | Y1 = −194.385 + 5.590d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Z.; He, Y.; Zhang, S.; Zhang, T.; Yang, F. Research on Corrosion Damage Evolution of Aluminum Alloy for Aviation. Appl. Sci. 2020, 10, 7184. https://doi.org/10.3390/app10207184
Gao Z, He Y, Zhang S, Zhang T, Yang F. Research on Corrosion Damage Evolution of Aluminum Alloy for Aviation. Applied Sciences. 2020; 10(20):7184. https://doi.org/10.3390/app10207184
Chicago/Turabian StyleGao, Zhigang, Yuting He, Sheng Zhang, Tianyu Zhang, and Fei Yang. 2020. "Research on Corrosion Damage Evolution of Aluminum Alloy for Aviation" Applied Sciences 10, no. 20: 7184. https://doi.org/10.3390/app10207184
APA StyleGao, Z., He, Y., Zhang, S., Zhang, T., & Yang, F. (2020). Research on Corrosion Damage Evolution of Aluminum Alloy for Aviation. Applied Sciences, 10(20), 7184. https://doi.org/10.3390/app10207184





