Methanol Production from Pyrolysis Oil Gasification—Model Development and Impacts of Operating Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Pyrolysis Oil Characteristics
2.2. Modeling Methodology
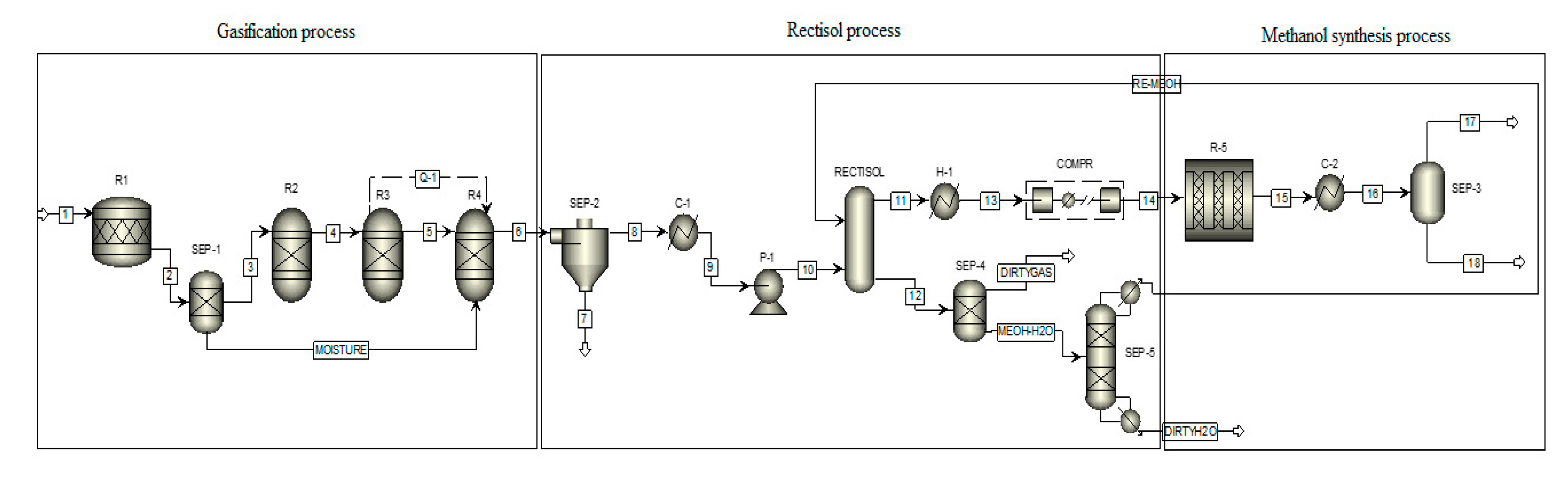
2.2.1. Gasification Process
- (1)
- Pyrolysis oil feed rate is 1000 kg/h.
- (2)
- Gasification is assumed to be steady state, isothermal, and simulated using a kinetic-free model [32].
- (3)
- Pyrolysis oil devolatilization occurs instantaneously and the volatile products mainly include H2, CO, CO2, CH4, H2S, NH3, and H2O.
- (4)
- All gases are ideal gases and uniformly distributed in the gas phase.
- (5)
- All reactions take place at a chemical equilibrium state and the pressure loss was not considered.
2.2.2. Rectisol Unit
2.2.3. Methanol Synthesis and Water Gas Shift Reaction Process
- (1)
- Pressure loss in the methanol synthesis reactor is not considered.
- (2)
- Syngas is preheated sufficiently and the temperature distribution in the methanol synthesis reactor is assumed to be uniform.
- Ri = reaction rate [mol/kgcat*s]
- ki = kinetic factor [kmol/kgcat*s*bar] or [kmol/kgcat*s*bar2]
- pi = partial pressure [bar]
- KEi = equilibrium constant [-] or [bar−2]
- k1/2/3 = adsorption constants [barn]
3. Results and Discussion
3.1. Model Validation
3.1.1. Gasification
3.1.2. Rectisol
3.1.3. Methanol Synthesis
3.2. Effect of Operating Conditions
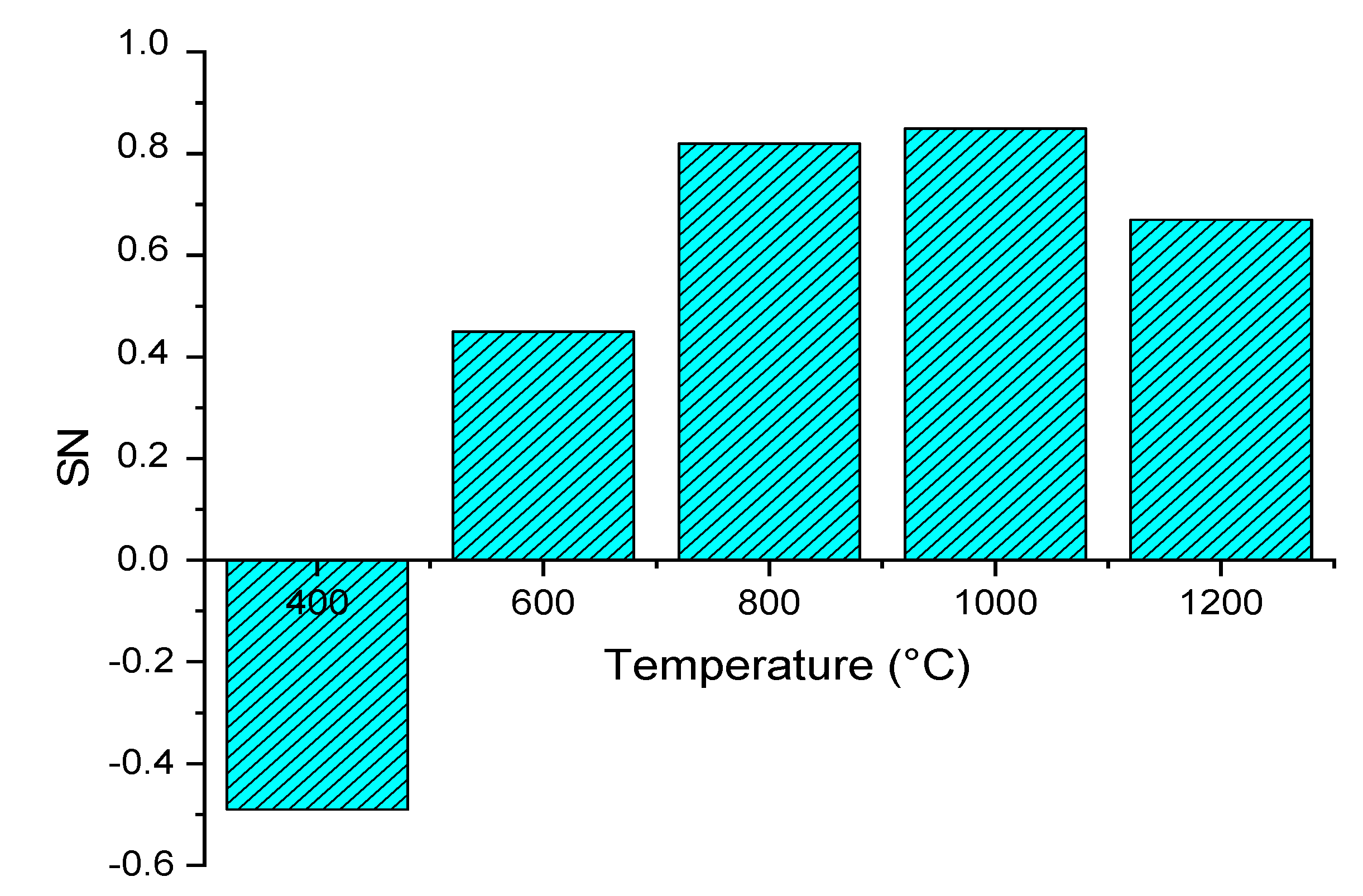
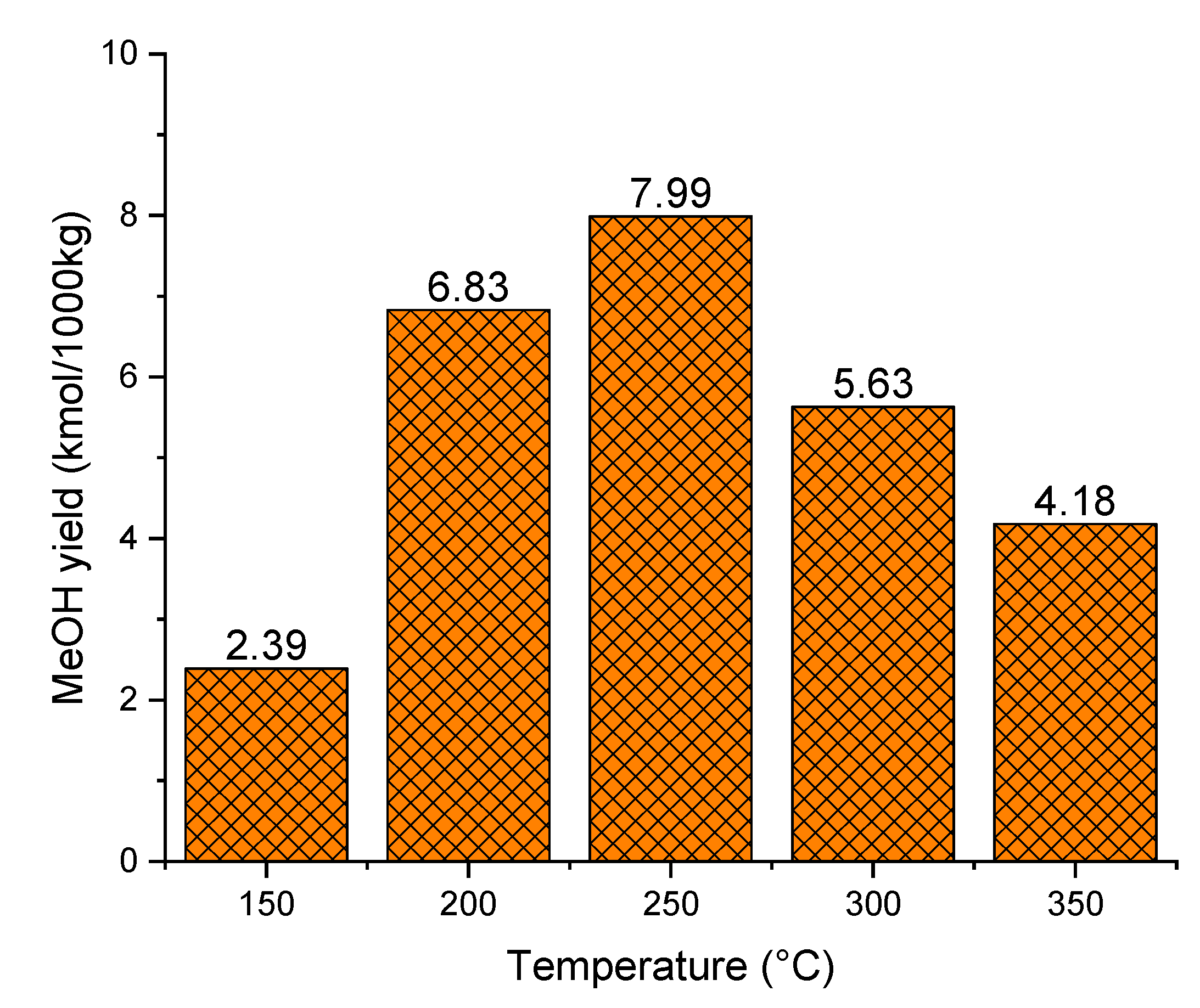
3.2.1. Effect of Gasifier Temperature
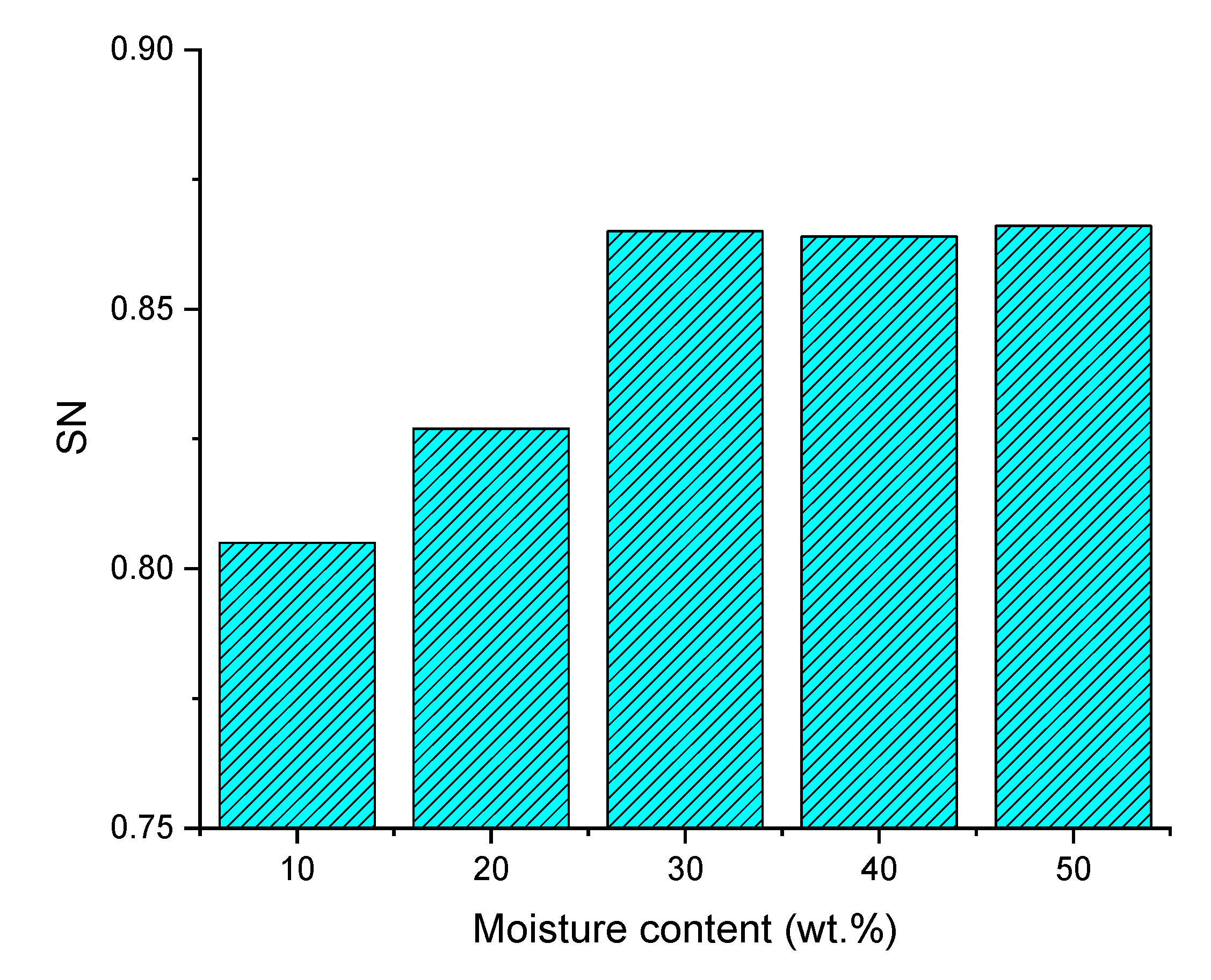
3.2.2. Effect of Pyrolysis Oil Moisture Content
3.2.3. Effect of Rectisol Operating Temperature
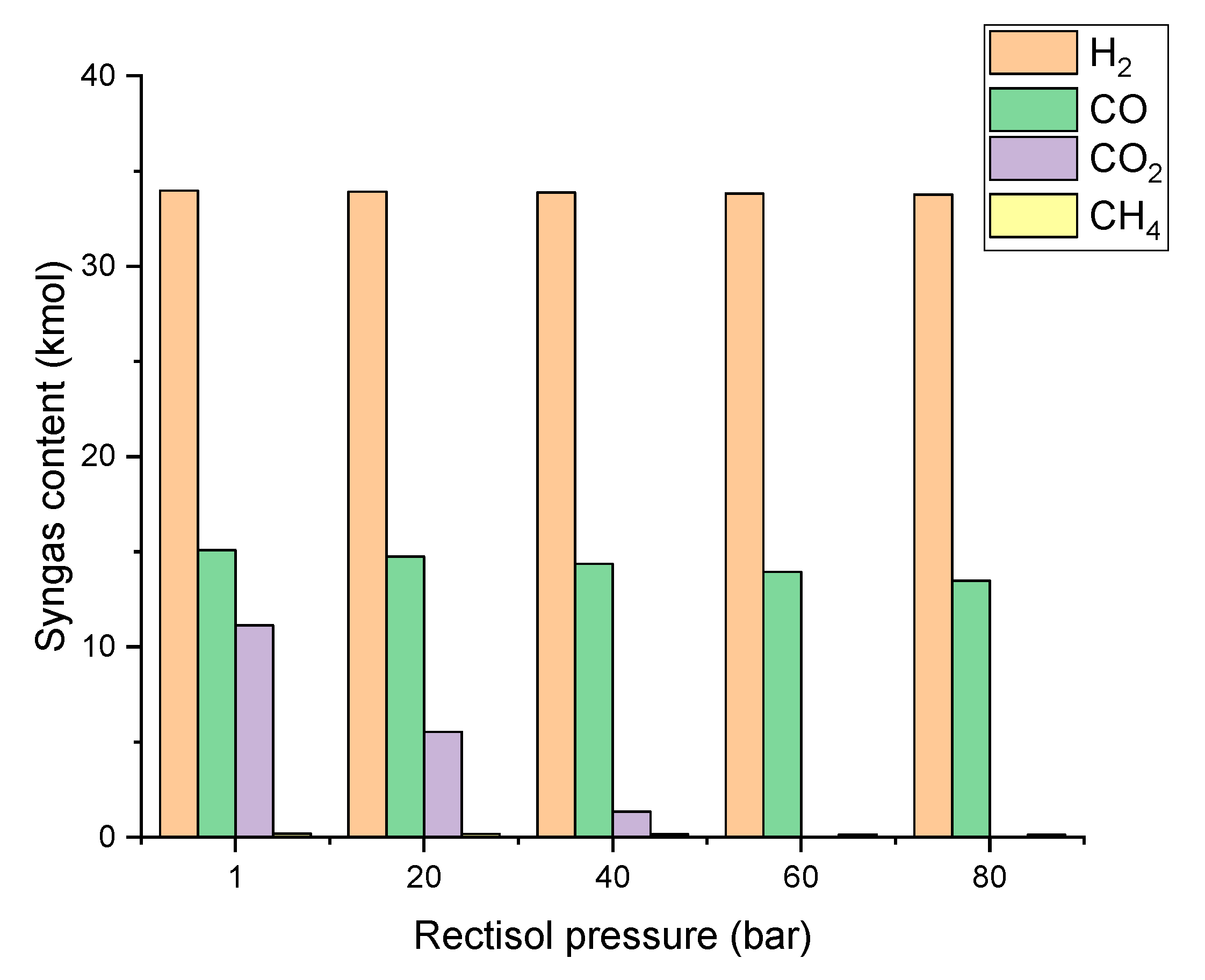
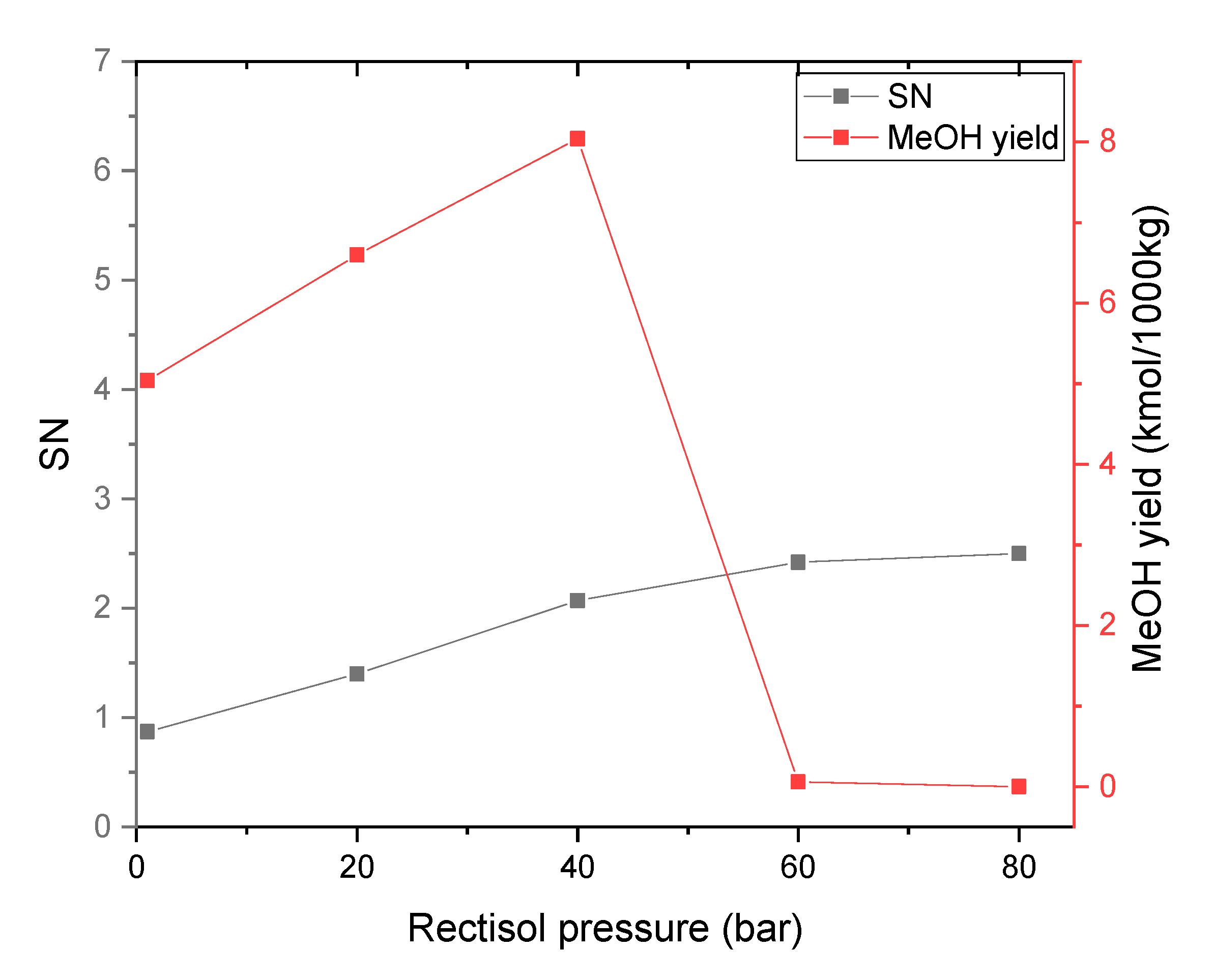
3.2.4. Effect of Rectisol Operating Pressure
3.2.5. Effect of Methanol Synthesis Operating Temperature
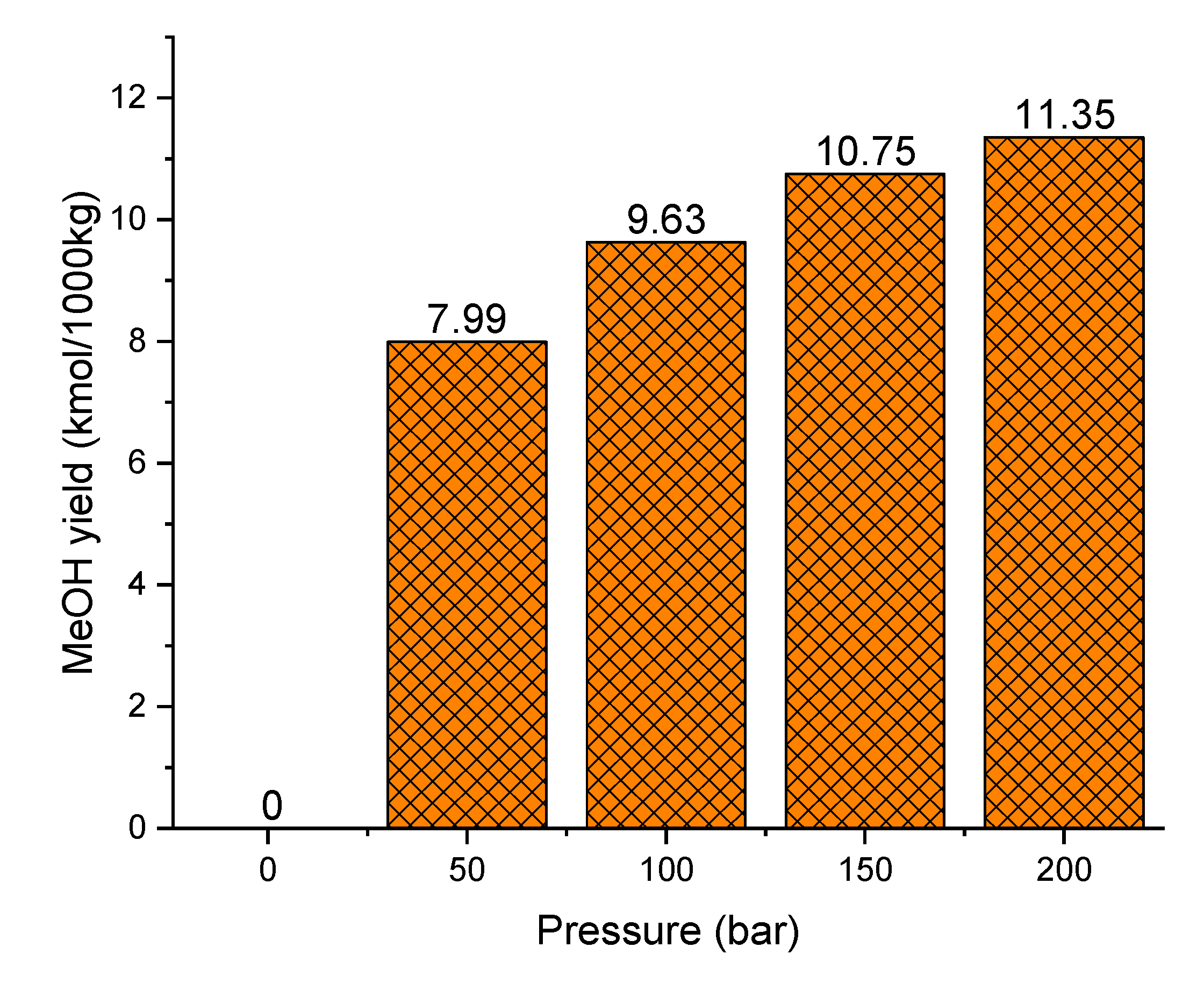
3.2.6. Effect of Methanol Synthesis Operating Pressure
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Attribute ID: | |||
|---|---|---|---|
| PROXANAL | SULFANAL | ||
| MOISTURE | 32.5 | PYRITIC | 0.001 |
| FC | SULFATE | ||
| VM | ORGANIC | ||
| ASH | |||
Appendix B
| Component Mass Flow (kg/hr) | 1 | 2 | 3 | MOISTURE | 4 | 5 | 6 |
|---|---|---|---|---|---|---|---|
| Temp (°C) | 50 | 100 | 100 | 100 | 500 | 800 | 800 |
| Pressure (bar) | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Pyrolysis oil | 1000 | ||||||
| Oil | 675 | 675 | |||||
| H2O | 325 | 325 | |||||
| -H2 | 4.15 | ||||||
| -O2 | 32.82 | ||||||
| -C | 30.40 | ||||||
| -N2 | 0.27 | ||||||
| -S | 0.001 | ||||||
| H2O | trace | 42.7 | |||||
| H2 | 40.9 | 64.2 | |||||
| CO | 570.1 | 386.0 | |||||
| CO2 | trace | 500.4 | |||||
| CH4 | 2.8 | 2.8 | |||||
| C | 59.7 | ||||||
| H2S | 0.01 | trace | |||||
| H3N | 3.8 | 3.3 | |||||
| C2H6 | trace | trace |
Appendix C
| Component Mass Flow (kg/hr) | 6 | 7 | 8 | 9 | 10 | RE-MEOH | 11 | 12 | DIRTY GAS | MEOH- H2O | DIRTY H2O | 13 | 14 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Temp (℃) | 800 | 800 | 800 | 80 | 80 | −20 | −20 | −20 | −20 | −20 | 250 | 250 | |
| Pressure (bar) | 1 | 1 | 1 | 1 | 40 | 40 | 40 | 1 | 1 | 1 | 40 | 50 | |
| H2O | 42.7 | 42.7 | 42.7 | 42.7 | 42.7 | 42.7 | 42.7 | ||||||
| H2 | 64.2 | 64.2 | 64.2 | 64.2 | 64.2 | 64.2 | 64.2 | ||||||
| CO | 386.0 | 386.0 | 386.0 | 386.0 | 386.0 | 386.0 | 386.0 | ||||||
| CO2 | 500.4 | 500.4 | 500.4 | 500.4 | 50.2 | 450.2 | 450.2 | 50.2 | 50.2 | ||||
| CH4 | 2.8 | 2.8 | 2.8 | 2.8 | 2.8 | 2.8 | 2.8 | ||||||
| H2S | trace | trace | trace | trace | trace | trace | |||||||
| H3N | 3.3 | 3.3 | 3.3 | 3.3 | 3.3 | 3.3 | |||||||
| ASH | 0 | 0 | |||||||||||
| MeOH | 640 | 640 | 640 |
Appendix D
| Component Mass Flow (kg/hr) | 14 | 15 | 16 | 17 | 18 |
|---|---|---|---|---|---|
| Temp (°C) | 250 | 250 | 50 | 50 | 50 |
| Pressure (bar) | 50 | 50 | 1 | 1 | 1 |
| H2 | 64.2 | 31.0 | 31.0 | 31.0 | |
| CO | 386.0 | 175.6 | 175.6 | 175.6 | |
| CO2 | 50.2 | 26.8 | 26.8 | 26.8 | |
| CH4 | 2.8 | 2.8 | 2.8 | 2.8 | |
| H2O | 9.5 | 9.5 | 9.5 | ||
| MeOH | 257.3 | 257.3 | 257.3 |
References
- Taba, L.E.; Irfan, M.F.; Daud, W.A.M.W.; Chakrabarti, M.H. The effect of temperature on various parameters in coal, biomass and CO-gasification: A review. Renew. Sustain. Energy Rev. 2012, 16, 5584–5596. [Google Scholar] [CrossRef]
- Zickfeld, K.; Solomon, S.; Gilford, D.M. Centuries of thermal sea-level rise due to anthropogenic emissions of short-lived greenhouse gases. Proc. Natl. Acad. Sci. USA 2017, 114, 657–662. [Google Scholar] [CrossRef]
- McKendry, P. Energy production from biomass (part 1): Overview of biomass. Bioresour. Technol 2002, 83, 37–46. [Google Scholar] [CrossRef]
- Ruiz, J.A.; Juárez, M.; Morales, M.; Muñoz, P.; Mendívil, M. Biomass gasification for electricity generation: Review of current technology barriers. Renew. Sustain. Energy Rev. 2013, 18, 174–183. [Google Scholar] [CrossRef]
- Rauch, R.; Hrbek, J.; Hofbauer, H. Biomass gasification for synthesis gas production and applications of the syngas. Wiley Interdiscip. Rev. Energy Environ. 2014, 3, 343–362. [Google Scholar] [CrossRef]
- Abdoulmoumine, N.; Adhikari, S.; Kulkarni, A.; Chattanathan, S. A review on biomass gasification syngas cleanup. Appl. Energy 2015, 155, 294–307. [Google Scholar] [CrossRef]
- Moioli, S.; Pellegrini, L.A.; Gamba, S. Simulation of CO2 capture by MEA scrubbing with a rate-based model. Proc. Eng. 2012, 42, 1651–1661. [Google Scholar] [CrossRef]
- Ortiz, F.G.; Serrera, A.; Galera, S.; Ollero, P. Methanol synthesis from syngas obtained by supercritical water reforming of glycerol. Fuel 2013, 105, 739–751. [Google Scholar] [CrossRef]
- Gupta, M.; Coyle, I.; Thambimuthu, K. CO2 Capture Technologies and Opportunities in Canada. In Proceedings of the 1st Canadian CC&S Technology Roadmap Workshop, Calgary, AB, Canada, 18–19 September 2003; p. 19. [Google Scholar]
- Chihara, H. Kagaku Binran–Kiso Handbook of Basic Chemistry, 3rd ed.; Maruzen: Tokyo, Japan, 1984; Volume 2, p. 158. [Google Scholar]
- Hochgesand, G. Rectisol and purisol. Ind. Eng. Chem. Res. 1970, 62, 37–43. [Google Scholar] [CrossRef]
- Gatti, M.; Martelli, E.; Marechal, F.; Consonni, S. Review, modeling, Heat Integration, and improved schemes of Rectisol®-based processes for CO2 capture. Appl. Therm. Eng. 2014, 70, 1123–1140. [Google Scholar] [CrossRef]
- Vancoillie, J.; Demuynck, J.; Sileghem, L.; Van De Ginste, M.; Verhelst, S.; Brabant, L.; Van Hoorebeke, L. The potential of methanol as a fuel for flex-fuel and dedicated spark-ignition engines. Appl. Energy 2013, 102, 140–149. [Google Scholar] [CrossRef]
- Breeze, P. Chapter 8-direct methanol fuel cell. In Fuel Cells; Breeze, P., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 75–82. [Google Scholar] [CrossRef]
- Larson, E.D.; Katofsky, R.E. Production of hydrogen and methanol via biomass gasification. In Advances in Thermochemical Biomass Conversion; Springer: Berlin/Heidelberg, Germany, 1993; pp. 495–510. [Google Scholar] [CrossRef]
- Chmielniak, T.; Sciazko, M. Co-gasification of biomass and coal for methanol synthesis. Appl. Energy 2003, 74, 393–403. [Google Scholar] [CrossRef]
- Alvarez, J.; Lopez, G.; Amutio, M.; Bilbao, J.; Olazar, M. Bio-oil production from rice husk fast pyrolysis in a conical spouted bed reactor. Fuel 2014, 128, 162–169. [Google Scholar] [CrossRef]
- Staš, M.; Kubička, D.; Chudoba, J.; Pospíšil, M. Overview of analytical methods used for chemical characterization of pyrolysis bio-oil. Energy Fuels 2014, 28, 385–402. [Google Scholar] [CrossRef]
- Bridgwater, A.; Cottam, M. Opportunities for biomass pyrolysis liquids production and upgrading. Energy Fuels 1992, 6, 113–120. [Google Scholar] [CrossRef]
- Radlein, D. The production of chemicals from fast pyrolysis bio-oils. In Fast Pyrolysis of Biomass: A Handbook; CLC Press: Newnury, UK, 1999; p. 164. [Google Scholar]
- Van Rossum, G.; Kersten, S.R.; van Swaaij, W.P. Catalytic and noncatalytic gasification of pyrolysis oil. Ind. Eng. Chem. Res. 2007, 46, 3959–3967. [Google Scholar] [CrossRef]
- Huber, G.W.; Iborra, S.; Corma, A. Synthesis of transportation fuels from biomass: Chemistry, catalysts, and engineering. Chem. Rev. 2006, 106, 4044–4098. [Google Scholar] [CrossRef]
- Demirbas, A. The influence of temperature on the yields of compounds existing in bio-oils obtained from biomass samples via pyrolysis. Fuel Process. Technol. 2007, 88, 591–597. [Google Scholar] [CrossRef]
- Brown, R.C.; Radlein, D.; Piskorz, J. Pretreatment processes to increase pyrolytic yield of levoglucosan from herbaceous feedstocks. In Chemicals and Materials from Renewable Resources, Proceedings of the ACS Symposium Series, Washington, DC, USA, 2001; American Chemical Society: Washington, DC, USA, 1999; pp. 123–132. [Google Scholar] [CrossRef]
- Özbay, G.; Özçifçi, A.; Aysal, S. Pyrolysis of beech wood catalysed by FeCl3: Production and characterisation of bio-oil. In Proceedings of the 9th Wood Science and Engineering in the Third Millennium–ICWSE, Brasov, Romania, 7–9 September 2013; Volume 1, pp. 75–81. [Google Scholar]
- Rossum, G. Steam Reforming and Gasification of Pyrolysis Oil; University of Twente: Enschede, The Netherlands, 2009. [Google Scholar]
- Oasmaa, A.; Meier, D. Norms and standards for fast pyrolysis liquids: 1. Round robin test. J. Anal. Appl. Pyrolysis 2005, 73, 323–334. [Google Scholar] [CrossRef]
- Krutof, A.; Hawboldt, K. Blends of pyrolysis oil, petroleum, and other bio-based fuels: A review. Renew. Sustain. Energy Rev. 2016, 59, 406–419. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, J.; Shen, L. Simulation of methanol production from biomass gasification in interconnected fluidized beds. Ind. Eng. Chem. Res 2009, 48, 5351–5359. [Google Scholar] [CrossRef]
- Yin, X.; Leung, D.Y.; Chang, J.; Wang, J.; Fu, Y.; Wu, C. Characteristics of the synthesis of methanol using biomass-derived syngas. Energy Fuels 2005, 19, 305–310. [Google Scholar] [CrossRef]
- Begum, S.; Rasul, M.; Akbar, D. A numerical investigation of municipal solid waste gasification using aspen plus. Proc. Eng. 2014, 90, 710–717. [Google Scholar] [CrossRef]
- Ramzan, N.; Ashraf, A.; Naveed, S.; Malik, A. Simulation of hybrid biomass gasification using Aspen plus: A comparative performance analysis for food, municipal solid and poultry waste. Biomass Bioenergy 2011, 35, 3962–3969. [Google Scholar] [CrossRef]
- Bussche, K.V.; Froment, G. A Steady-state kinetic model for methanol synthesis and the water gas shift reaction on a commercial Cu/ZnO/Al2O3 catalyst. J. Catal 1996, 161, 1–10. [Google Scholar] [CrossRef]
- Zhang, C.; Jun, K.-W.; Kwak, G.; Lee, Y.-J.; Park, H.-G. Efficient utilization of carbon dioxide in a gas-to-methanol process composed of CO2/steam–mixed reforming and methanol synthesis. J. CO2 Util. 2016, 16, 1–7. [Google Scholar] [CrossRef]
- Luyben, W.L. Design and control of a methanol reactor/column process. Ind. Eng. Chem. Res. 2010, 49, 6150–6163. [Google Scholar] [CrossRef]
- Bell, D.A.; Towler, B.F.; Fan, M. Coal Gasification and Its Applications; William Andrew: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Chen, L.; Jiang, Q.; Song, Z.; Posarac, D. Optimization of methanol yield from a Lurgi reactor. Chem. Eng. Technol. 2011, 34, 817–822. [Google Scholar] [CrossRef]
- Atkin, P. The Elements of Physical Chemistry: With Applications in Biology; WH Freeman and Company: New York, NY, USA, 2000. [Google Scholar]
- Fernandez-Lopez, M.; Pedroche, J.; Valverde, J.; Sanchez-Silva, L. Simulation of the gasification of animal wastes in a dual gasifier using Aspen Plus®. Energy Convers. Manag. 2017, 140, 211–217. [Google Scholar] [CrossRef]
- Pala, L.P.R.; Wang, Q.; Kolb, G.; Hessel, V. Steam gasification of biomass with subsequent syngas adjustment using shift reaction for syngas production: An Aspen Plus model. Renew. Energy 2017, 101, 484–492. [Google Scholar] [CrossRef]
- Burr, B.; Lyddon, L. A Comparison of Physical Solvents for Acid Gas Removal; Gas Processors’ Association Convention: Grapevine, TX, USA, 2008. [Google Scholar]
- Trop, P.; Anicic, B.; Goricanec, D. Production of methanol from a mixture of torrefied biomass and coal. Energy 2014, 77, 125–132. [Google Scholar] [CrossRef]




| Proximate Analysis (wt.%) | |
| Moisture content | 32.5–43.7 |
| Ultimate analysis (%) | |
| Carbon | 30.4–37.7 |
| Hydrogen | 7.6–7.9 |
| Nitrogen | <0.27 |
| Oxygen | 54.4–61.7 |
| Sulfur | <0.01 |
| Flow Sheet Block ID | Aspen Plus Block ID | Description |
|---|---|---|
| R1 | RStoic | Reactor with known conversion rate—used to extract moisture form pyrolysis oil. Operation at 150 °C. |
| SEP-1 | Sep | Used to separate the moisture from the pyrolysis oil. |
| R2 | RYield | Yield reactor—used to decompose nonconventional pyrolysis oil into its elemental components by FORTRAN statement. Operation at 500 °C. |
| R3 | RGibbs | Gibbs free energy reactor—used to complete chemical equilibrium by minimizing Gibbs-free energy. Operation at 800 °C. |
| R4 | RGibbs | Gibbs free energy reactor—used to calculate syngas composition by minimizing Gibbs-free energy. Operation temperature ranges from 400 °C to 1200 °C. |
| Flow Sheet Block ID | Aspen Plus Block ID | Description |
|---|---|---|
| SEP-2 | SSplit | Used to separate ash from the syngas. Operation at 800 °C. |
| C-1 | Heater | Used to decrease syngas temperature to 80 °C. |
| P-1 | Pump | Used to increase syngas pressure to 40 bar |
| RECTISOL | Radfrac | Used to remove acid syngas and partial carbon dioxide |
| SEP-4 | Flash 2 | Used to separate part of CO2 and impurities syngas from methanol and H2O |
| SEP-5 | Sep2 | Used to separate H2O from methanol |
| Flow Sheet Block ID | Aspen Plus Block ID | Description |
|---|---|---|
| H-1 | Heater | Used to heat up syngas to 250 °C |
| COMPR | MCompr | Used to compress syngas to 50 bar |
| R5 | RPlug | Used to simulate methanol synthesis reactor |
| C-2 | Heater | Used to cool down the outlet syngas of methanol synthesis reactors |
| SEP-3 | Flash 2 | Used to separate produced methanol and residual syngas |
| Syngas Composition (Mole %) | Experiment | Model |
|---|---|---|
| H2 | 55.5 | 55.9 |
| CO | 19.3 | 24.0 |
| CO2 | 19.0 | 19.8 |
| CH4 | 5.4 | 0.3 |
| C2H6 | 1.0 | trace |
| H2S | - | trace |
| NH3 | - | trace |
| Component Dry Basis | Feed Syngas | Purified Syngas Experimental Results | Simulated Results |
|---|---|---|---|
| Pressure (MPa) | 7.8 | 7.6 | 7.8 |
| H2 | 62.5% | 95.3% | 95.9% |
| N2 + Ar | 0.5% | 0.8% | 0.7% |
| CO + CH4 | 2.7% | 4.0% | 3.4% |
| CO2 | 34.1% | 20 ppm | trace |
| H2S + COS | 0.3% | 0.1 ppm | trace |
| Flow rate (kmol/h) | 6021.1 | 3936.1 | 3890.3 |
| Feed | Outlet Stream (Industry) | Outlet Stream (Simulation) | |
|---|---|---|---|
| Temperature (°C) | 225 | 255 | 225 |
| Pressure (bar) | 69.7 | 66.7 | 40 |
| Mass flow rate (kg/h) | 57,282.8 | 57,282.8 | 57,282.8 |
| Components flow rate (kg/h) | |||
| CO | 10,727.9 | 4921.0 | 4068.7 |
| CO2 | 23,684.2 | 18,316.4 | 19,577.3 |
| H2 | 9586.5 | 8013.7 | 8063.6 |
| H2O | 108.8 | 2309.3 | 1789.9 |
| Methanol | 756.7 | 11,283.1 | 11,364.6 |
| CH4 | 4333.1 | 4333.1 | 4333.1 |
| N2 | 8072.0 | 8071.9 | 8072.0 |
| Ethanol | 0.6 | 8.7 | 0.6 |
| Propanol | - | 0.1 | - |
| Methyl formate | 13.0 | 25.6 | 13.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Delcroix, B.; Rezazgui, O.; Mangin, P. Methanol Production from Pyrolysis Oil Gasification—Model Development and Impacts of Operating Conditions. Appl. Sci. 2020, 10, 7371. https://doi.org/10.3390/app10207371
Zhang Z, Delcroix B, Rezazgui O, Mangin P. Methanol Production from Pyrolysis Oil Gasification—Model Development and Impacts of Operating Conditions. Applied Sciences. 2020; 10(20):7371. https://doi.org/10.3390/app10207371
Chicago/Turabian StyleZhang, Zhihai, Benoit Delcroix, Olivier Rezazgui, and Patrice Mangin. 2020. "Methanol Production from Pyrolysis Oil Gasification—Model Development and Impacts of Operating Conditions" Applied Sciences 10, no. 20: 7371. https://doi.org/10.3390/app10207371
APA StyleZhang, Z., Delcroix, B., Rezazgui, O., & Mangin, P. (2020). Methanol Production from Pyrolysis Oil Gasification—Model Development and Impacts of Operating Conditions. Applied Sciences, 10(20), 7371. https://doi.org/10.3390/app10207371





