Experimental Devices to Investigate the Long-Term Stability of Phase Change Materials under Application Conditions
Abstract
:1. Introduction
2. Comparison of Experimental Devices
2.1. Summary of Technical Specifications
2.2. Descriptions of Testing Devices
2.2.1. Type A—Thermal Cycling Stability Tests
CIEMAT I
CIEMAT II
CIEMAT III
EHU
HSLU
ISE Capsules
ISE Peltier
LAMTE
LGCgE
LTTT
UDL-GREiA II
UDL-GREiA III
ZAE
Summary of Type A Tests
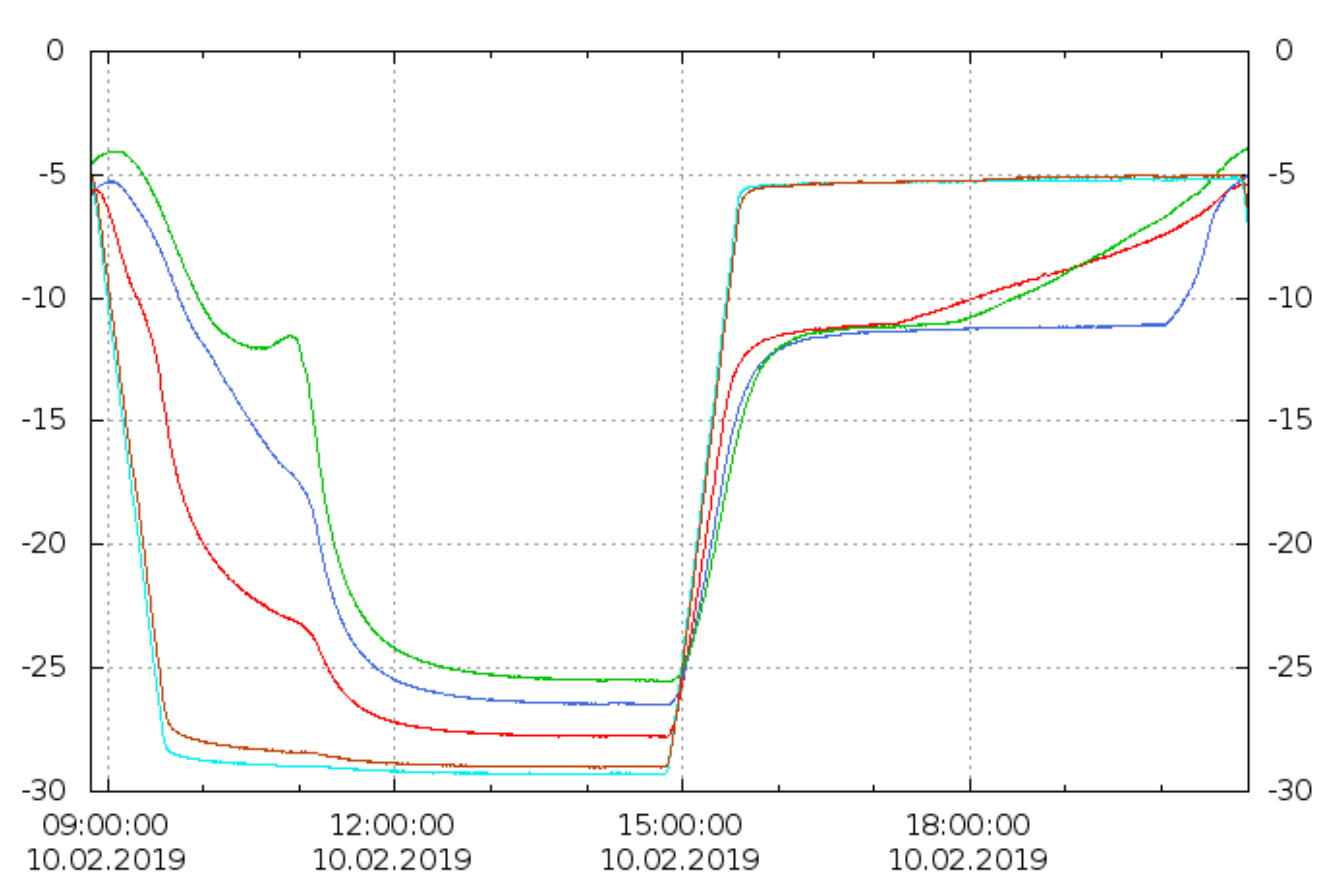
2.2.2. Type B—Tests on Supercooled PCM
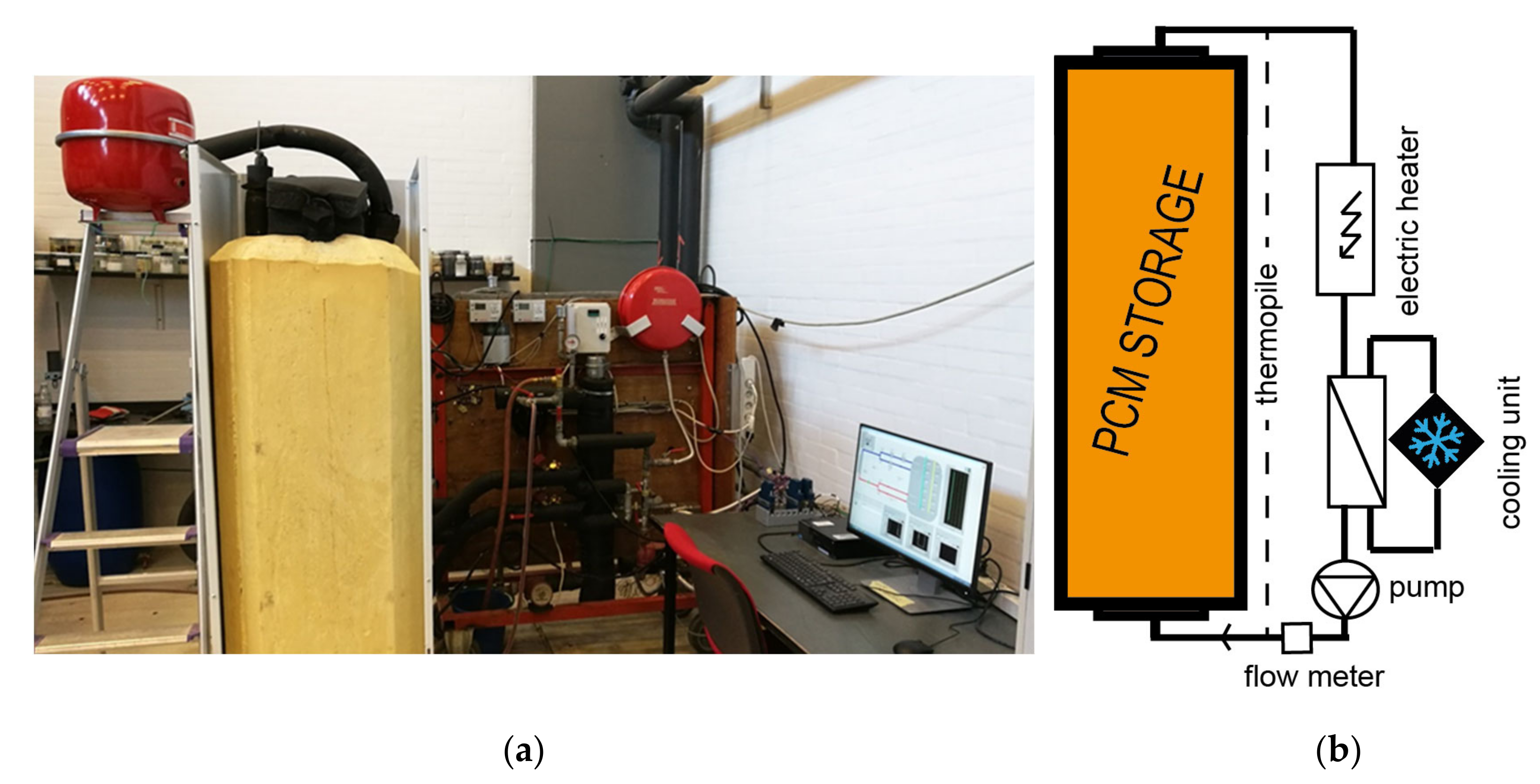
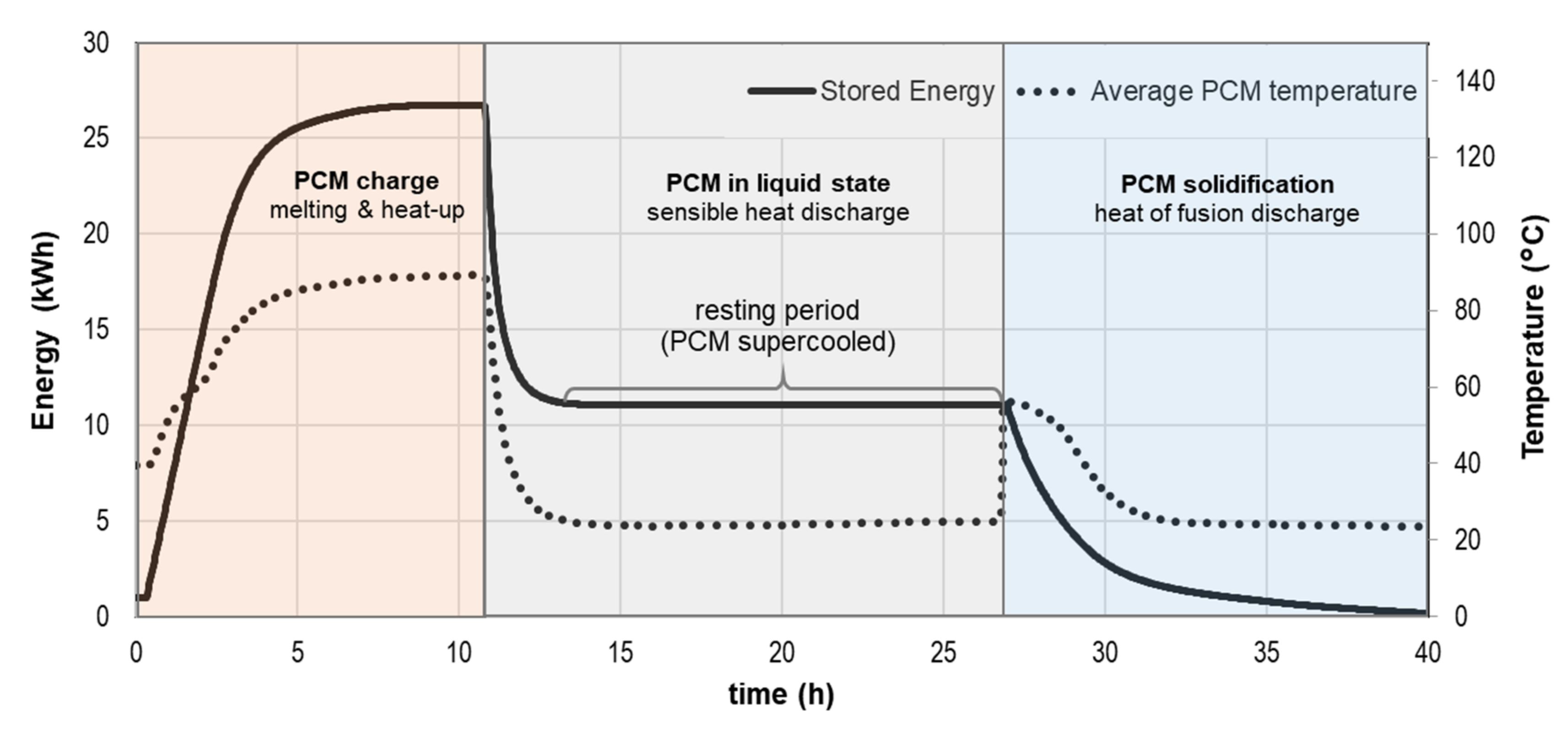
DTU Full-Scale
DTU Heat Loss Method
DTU Multiple Storage
Summary of Type B Tests
2.2.3. Type C—Tests on Phase Change Slurries (PCS)
ISE PCS
Summary of Type C Tests
3. Experimental Techniques to Check a Possible Degradation of PCM
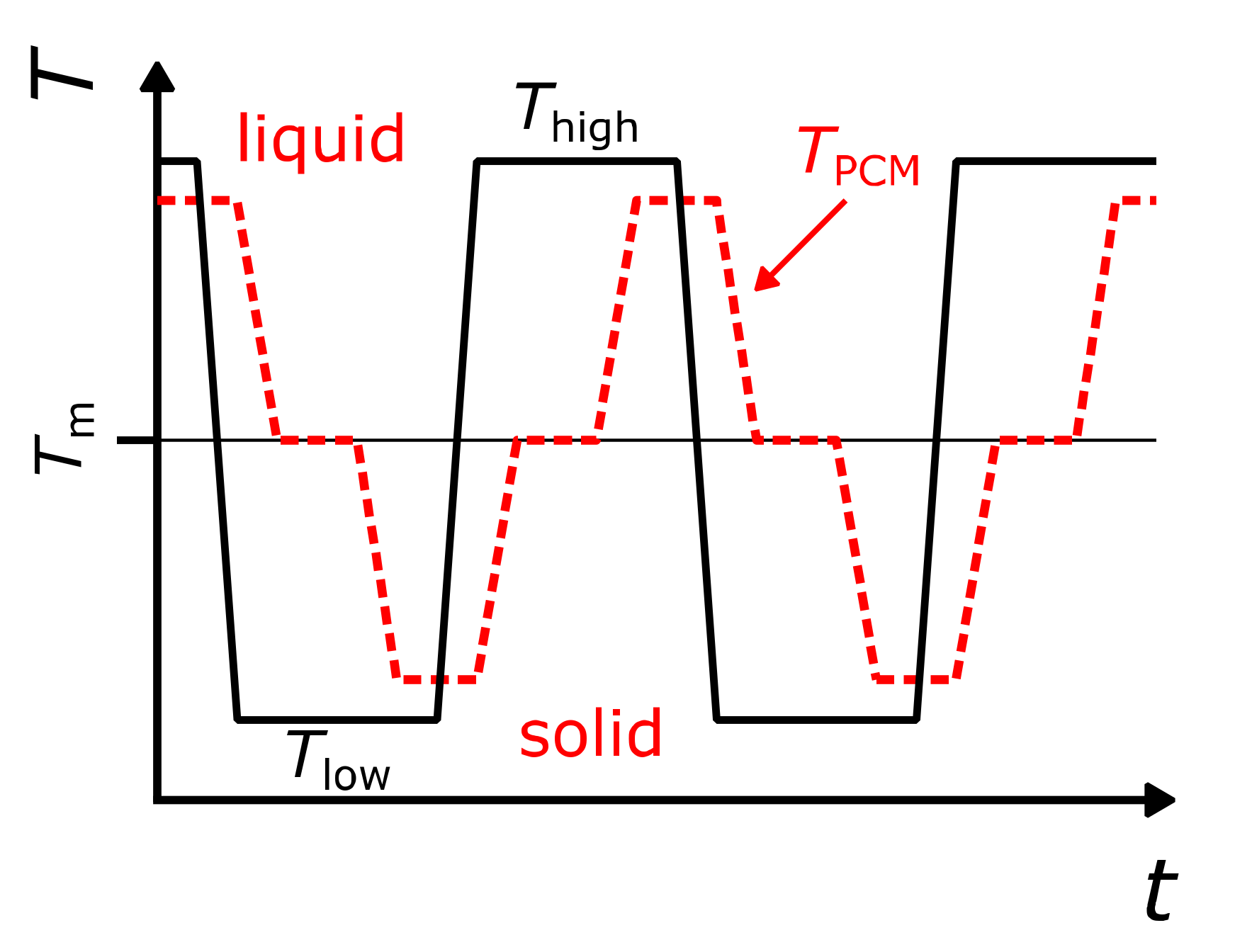
3.1. Comparison of Temperature–Time Curves of Different Cycles (“T(t)cycles”)
3.2. Temperature Profile During Material Solidification (“T(t)solid”)
3.3. Differential Scanning Calorimetry (“DSC”)
3.4. Sample Mass Monitoring after Certain Number of Cycles or Time (“m(t)”)
3.5. Thermogravimetric Analysis (“TGA”)
3.6. Comparison of the Thermal Energy Content in Repeated Storage Cycles (“Δhcycles”)
3.7. Visually (“vis.”)
3.8. X-ray Powder Diffraction (“XRD”)
3.9. Fourier Transform Infrared Spectroscopy (FT-IR)
3.10. Reflected Light Microscopy (RLM)
3.11. Pressure Loss of Cooling Heat Exchanger (ΔpHX)
3.12. Particle Size Analysis (PSA)
3.13. Viscosity Measurement (μ)
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| DSC | Differential scanning calorimetry |
| FT-IR | Fourier transform infrared spectroscopy |
| HTF | Heat transfer fluid |
| PCM | Phase change material |
| PCS | Phase change slurry |
| PMMA | Poly (methyl methacrylate) |
| PSA | Particle size analysis |
| RLM | Reflected light microscope |
| SAT | Sodium acetate trihydrate |
| TES | Thermal energy storage |
| TGA | Thermogravimetric analysis |
| TCM | Thermochemical material |
| XRD | X-ray diffraction spectroscopy |
| h | Specific enthalpy/J∙g−1 |
| m | Mass/kg |
| μ | Viscosity/Pa∙s |
| T | Temperature/°C |
| t | Time/s |
References
- Mehling, H.; Cabeza, L.F. Heat and Cold Storage with PCM; Springer: Berlin, Germany, 2008. [Google Scholar]
- Zalba, B.; Marın, J.M.; Cabeza, L.F.; Mehling, H. Review on thermal energy storage with phase change: Materials, heat transfer analysis and applications. Appl. Therm. Eng. 2003, 23, 251–283. [Google Scholar]
- Cabeza, L.F.; Castell, A.; Barreneche, C.D.; De Gracia, A.; Fernández, A.I. Materials used as PCM in thermal energy storage in buildings: A review. Renew. Sustain. Energy Rev. 2011, 15, 1675–1695. [Google Scholar]
- Zsembinszki, G.; Fernández, A.G.; Cabeza, L.F. Selection of the Appropriate Phase Change Material for Two Innovative Compact Energy Storage Systems in Residential Buildings. Appl. Sci. 2020, 10, 2116. [Google Scholar]
- IEA ES Annex 33/SHC Task 58 “Material and Component Development for Compact Thermal Energy Storage”. Available online: https://task58.iea-shc.org/ (accessed on 17 August 2020).
- Ferrer, G.; Solé, A.; Barreneche, C.; Martorell, I.; Cabeza, L.F. Review on the methodology used in thermal stability characterization of phase change materials. Renew. Sustain. Energy Rev. 2015, 50, 665–685. [Google Scholar]
- Rathgeber, C.; Grisval, A.; Schmit, H.; Hoock, P.; Hiebler, S. Concentration dependent melting enthalpy, crystallization velocity, and thermal cycling stability of pinacone hexahydrate. Thermochim. Acta 2018, 670, 142–147. [Google Scholar]
- Bayón, R.; Rojas, E. Development of a new methodology for validating thermal storage media: Application to phase change materials. Int. J. Energy Res. 2019, 43, 6521–6541. [Google Scholar]
- RAL Quality Association PCM. Quality and Testing Specifications for Phase Change Materials. 2018. Available online: http://www.pcm-ral.org/pcm/en/quality-testing-specifications-pcm/ (accessed on 17 August 2020).
- Englmair, G.; Jiang, Y.; Dannemand, M.; Moser, C.; Schranzhofer, H.; Furbo, S.; Fan, J. Crystallization by local cooling of supercooled sodium acetate trihydrate composites for long-term heat storage. Energy Build. 2018, 180, 159–171. [Google Scholar]
- Desgrosseilliers, L. Design and Evaluation of a Modular, Supercooling Phase Change Heat Storage Device for Indoor Heating. Ph.D. Thesis, Dalhousie University, Halifax, NS, Canada, 2016. Available online: https://dalspace.library.dal.ca/handle/10222/73309 (accessed on 17 August 2020).
- Englmair, G.; Moser, C.; Furbo, S.; Dannemand, M.; Fan, J. Design and functionality of a segmented heat-storage prototype utilizing stable supercooling of sodium acetate trihydrate in a solar heating system. Appl. Energy 2018, 221, 522–534. [Google Scholar]
- Englmair, G.; Kong, W.; Berg, J.B.; Furbo, S.; Fan, J. Demonstration of a solar combi-system utilizing stable supercooling of sodium acetate trihydrate for heat storage. Appl. Therm. Eng. 2020, 166, 114647. [Google Scholar]
- Englmair, G.; Moser, C.; Schranzhofer, H.; Fan, J.; Furbo, S. A solar combi-system utilizing stable supercooling of sodium acetate trihydrate for heat storage: Numerical performance investigation. Appl. Energy 2019, 242, 1108–1120. [Google Scholar]
- Duquesne, M.; Del Barrio, E.P.; Godin, A. Nucleation triggering of highly undercooled Xylitol using an air lift reactor for seasonal thermal energy storage. Appl. Sci. 2019, 9, 267. [Google Scholar]
- Puupponen, S.; Mikkola, V.; Ala-Nissila, T.; Seppälä, A. Novel microstructured polyol-polystyrene composites for seasonal heat storage. Appl. Energy 2016, 172, 96–106. [Google Scholar]
- Rathod, M.K.; Banerjee, J. Thermal stability of phase change materials used in latent heat energy storage systems: A review. Renew. Sustain. Energy Rev. 2013, 18, 246–258. [Google Scholar]
- Bayón, R.; Biencinto, M.; Rojas, E.; Uranga, N. Study of hybrid dry cooling systems for STE plants based on latent storage. In Proceedings of the ISEC Conference, Graz, Austria, 3–5 October 2018. [Google Scholar]
- Rodríguez-García, M.M.; Rojas, E.; Bayón, R. Test campaign and performance evaluation of a spiral latent storage module with Hitec® as PCM. In Proceedings of the SWC 2017/SHC2017 Conference, Abu Dhabi, UAE, 29 October–2 November 2017. [Google Scholar]
- Rodríguez-García, M.M.; Bayón, R.; Rojas, E. Stability of D-mannitol upon melting/freezing cycles under controlled inert atmosphere. Energy Procedia 2016, 91, 218–225. [Google Scholar]
- Biedenbach, M.; Klünder, F.; Gschwander, S. Investigations on the stability of metallic cans for PCM macro-encapsulation. In Proceedings of the International Institute of Refrigeration (IIR), Birmingham, AL, USA, 2–5 September 2018. [Google Scholar]
- Kahwaji, S.; Johnson, M.B.; Kheirabadi, A.C.; Groulx, D.; White, M.A. Stable, low-cost phase change material for building applications: The eutectic mixture of decanoic acid and tetradecanoic acid. Appl. Energy 2016, 168, 457–464. [Google Scholar]
- Kheirabadi, A.C.; Groulx, D. Design of an automated thermal cycler for long-term phase change material phase transition stability studies. In Proceedings of the COMSOL Conference 2014, Boston, MA, USA, 8–10 October 2014. [Google Scholar]
- Kahwaji, S.; Johnson, M.B.; Kheirabadi, A.C.; Groulx, D.; White, M.A. Fatty acids and related phase change materials for reliable thermal energy storage at moderate temperatures. Sol. Energy Mater. Sol. Cells 2017, 167, 109–120. [Google Scholar]
- Kahwaji, S.; Johnson, M.B.; Kheirabadi, A.C.; Groulx, D.; White, M.A. A comprehensive study of properties of paraffin phase change materials for solar thermal energy storage and thermal management applications. Energy 2018, 162, 1169–1182. [Google Scholar]
- Beaupere, N. Pilotage de la Libération de Chaleur et Etude du Vieillissement de Matériaux à Changement de Phase. Ph.D. Thesis, Université d’Artois/CEA Grenoble, Grenoble, France, 2019. [Google Scholar]
- Orak, F.; König-Haagen, A.; Brüggemann, D. Effect of Nucleators on Heat Storage Properties of Salt Hydrates. In Proceedings of the 2017 11th International Renewable Energy Storage Conference (IRES 2017), Düsseldorf, Germany, 14–16 March 2017. [Google Scholar]
- Navarro, L.; Solé, A.; Martín, M.; Barreneche, C.; Olivieri, L.; Tenorio, J.A.; Cabeza, L.F. Benchmarking of useful phase change materials for a building application. Energy Build. 2019, 182, 45–50. [Google Scholar]
- Oró, E.; Gil, A.; Miró, L.; Peiró, G.; Álvarez, S.; Cabeza, L.F. Thermal energy storage implementation using phase change materials for solar cooling and refrigeration applications. Energy Procedia 2012, 30, 947–956. [Google Scholar]
- Gil, A.; Oró, E.; Castell, A.; Cabeza, L.F. Experimental analysis of the effectiveness of a high temperature thermal storage tank for solar cooling applications. Appl. Therm. Eng. 2013, 54, 521–527. [Google Scholar]
- Gil, A.; Oró, E.; Miró, L.; Peiró, G.; Ruiz, A.; Salmerón, J.M.; Cabeza, L.F. Experimental analysis of hydroquinone used as phase change material (PCM) to be applied in solar cooling refrigeration. Int. J. Refrig. 2014, 39, 95–103. [Google Scholar] [CrossRef]
- Peiró, G.; Gasia, J.; Miró, L.; Cabeza, L.F. Experimental evaluation at pilot plant scale of multiple PCMs (cascaded) vs. single PCM configuration for thermal energy storage. Renew. Energy 2015, 83, 729–736. [Google Scholar] [CrossRef] [Green Version]
- Prieto, C.; Miró, L.; Peiró, G.; Oró, E.; Gil, A.; Cabeza, L.F. Temperature distribution and heat losses in molten salts tanks for CSP plants. Sol. Energy 2016, 135, 518–526. [Google Scholar] [CrossRef] [Green Version]
- Peiró, G.; Gasia, J.; Miró, L.; Prieto, C.; Cabeza, L.F. Experimental analysis of charging and discharging processes, with parallel and counter flow arrangements, in a molten salts high temperature pilot plant scale setup. Appl. Energy 2016, 178, 394–403. [Google Scholar] [CrossRef] [Green Version]
- Gasia, J.; de Gracia, A.; Peiró, G.; Arena, S.; Cau, G.; Cabeza, L.F. Use of partial load operating conditions for latent thermal energy storage management. Appl. Energy 2018, 216, 234–242. [Google Scholar] [CrossRef] [Green Version]
- Peiró, G.; Prieto, C.; Gasia, J.; Jové, A.; Miró, L.; Cabeza, L.F. Two-tank molten salts thermal energy storage system for solar power plants at pilot plant scale: Lessons learnt and recommendations for its design, start-up and operation. Renew. Energy 2018, 121, 236–248. [Google Scholar] [CrossRef]
- Gil, A.; Peiró, G.; Oró, E.; Cabeza, L.F. Experimental analysis of the effective thermal conductivity enhancement of PCM using finned tubes in high temperature bulk tanks. Appl. Therm. Eng. 2018, 142, 736–744. [Google Scholar] [CrossRef]
- Gasia, J.; de Gracia, A.; Zsembinszki, G.; Cabeza, L.F. Influence of the storage period between charge and discharge in a latent heat thermal energy storage system working under partial load operating conditions. Appl. Energy 2019, 235, 1389–1399. [Google Scholar] [CrossRef]
- Mselle, B.D.; Verez, D.; Zsembinszki, G.; Borri, E.; Cabeza, L.F. Performance Study of Direct Integration of Phase Change Material into an Innovative Evaporator of a Simple Vapour Compression System. Appl. Sci. 2020, 10, 4649. [Google Scholar] [CrossRef]
- Dannemand, M.; Dragsted, J.; Fan, J.; Johansen, J.B.; Kong, W.; Furbo, S. Experimental investigations on prototype heat storage units utilizing stable supercooling of sodium acetate trihydrate mixtures. Appl. Energy 2016, 169, 72–80. [Google Scholar] [CrossRef] [Green Version]
- Dannemand, M.; Johansen, J.B.; Kong, W.; Furbo, S. Experimental investigations on cylindrical latent heat storage units with sodium acetate trihydrate composites utilizing supercooling. Appl. Energy 2016, 177, 591–601. [Google Scholar] [CrossRef] [Green Version]
- Englmair, G.; Furbo, S.; Dannemand, M.; Fan, J. Experimental investigation of a tank-in-tank heat storage unit utilizing stable supercooling of sodium acetate trihydrate. Appl. Therm. Eng. 2020, 167, 114709. [Google Scholar] [CrossRef]
- Kong, W.; Dannemand, M.; Johansen, J.B.; Fan, J.; Dragsted, J.; Englmair, G.; Furbo, S. Experimental investigations on heat content of supercooled sodium acetate trihydrate by a simple heat loss method. Sol. Energy 2016, 139, 249–257. [Google Scholar] [CrossRef] [Green Version]
- Dannemand, M.; Furbo, S. Supercooling stability of sodium acetate trihydrate composites in multiple heat storage units. Refrig. Sci. Technol. 2018, 227–231. [Google Scholar] [CrossRef]
- Niedermaier, S.; Biedenbach, M.; Gschwander, S. Characterisation and enhancement of phase change slurries. Energy Procedia 2016, 99, 64–71. [Google Scholar] [CrossRef] [Green Version]
- Peiró, G. High Temperature Thermal Energy Storage for Solar Cooling and Solar Thermal Power Plants Applications. Ph.D. Thesis, University of Lleida, Lleida, Spain, 2017. Available online: http://hdl.handle.net/10803/462071 (accessed on 17 August 2020).
- Deng, J.; Furbo, S.; Kong, W.; Fan, J. Thermal performance assessment and improvement of a solar domestic hot water tank with PCM in the mantle. Energy Build. 2018, 172, 10–21. [Google Scholar] [CrossRef]
- Gschwander, S.; Haussmann, T.; Hagelstein, G.; Barreneche, C.; Ferrer, G.; Cabeza, L.; Diarce, G.; Hohenauer, W.; Lager, D.; Rathgeber, C.; et al. Standardization of pcm characterization via DSC. Refrig. Sci. Technol. 2016, 70–75. [Google Scholar] [CrossRef]
- Rathgeber, C.; Schmit, H.; Miró, L.; Cabeza, L.F.; Gutierrez, A.; Ushak, S.N.; Hiebler, S. Enthalpy-temperature plots to compare calorimetric measurements of phase change materials at different sample scales. J. Storage Mater. 2018, 15, 32–38. [Google Scholar] [CrossRef] [Green Version]
- Rathgeber, C.; Miró, L.; Cabeza, L.F.; Hiebler, S. Measurement of enthalpy curves of phase change materials via DSC and T-History: When are both methods needed to estimate the behaviour of the bulk material in applications? Thermochim. Acta 2014, 596, 79–88. [Google Scholar] [CrossRef]
- Bayón, R.; Rojas, R. Feasibility study of D-mannitol as phase change material for thermal storage. AIMS Energy 2017, 5, 404–424. [Google Scholar] [CrossRef]
- Doyle, C.D. Estimating isothermal life from thermogravimetric data. J. Appl. Polym. Sci. 1962, 6, 639–642. [Google Scholar] [CrossRef]
- Solé, A.; Neumann, H.; Niedermaier, S.; Martorell, I.; Schossig, P.; Cabeza, L.F. Stability of sugar alcohols as PCM for thermal energy storage. Sol. Energy Mater. Sol. Cells 2014, 126, 125–134. [Google Scholar] [CrossRef]



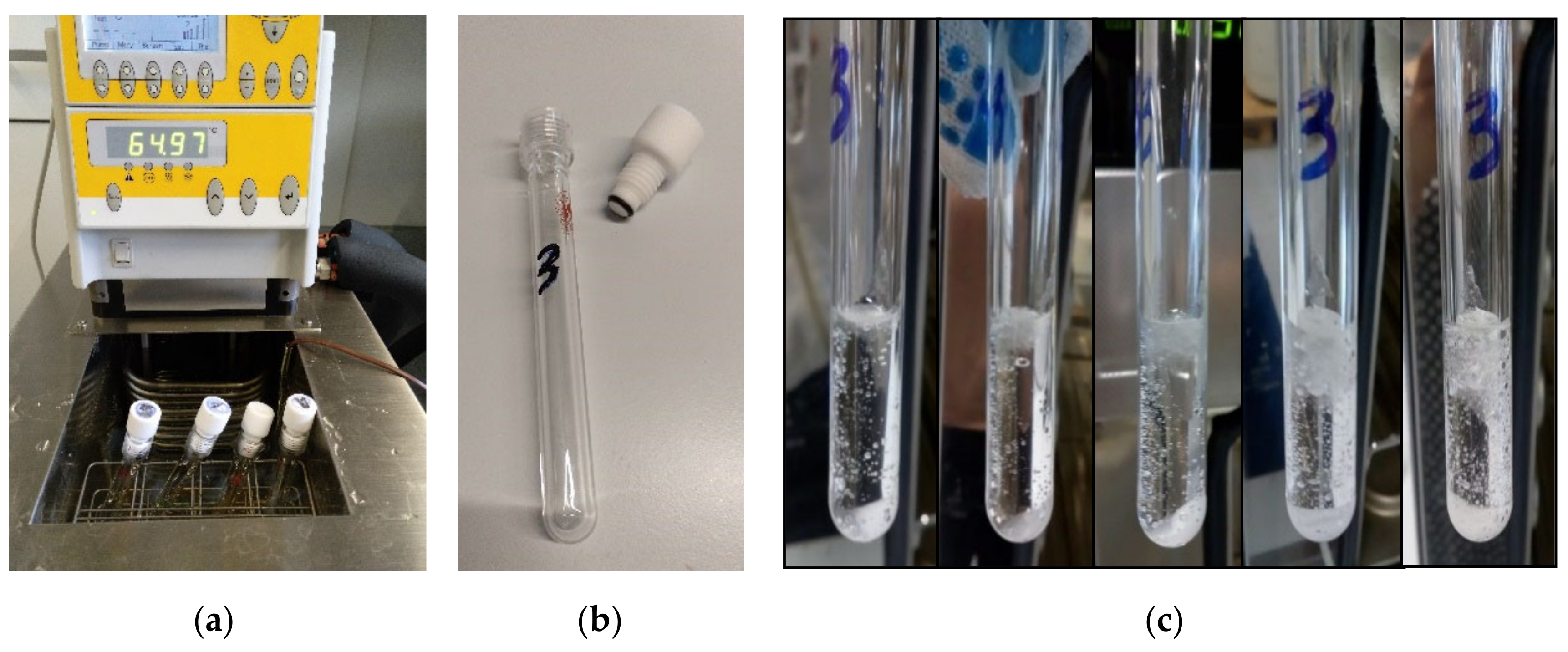

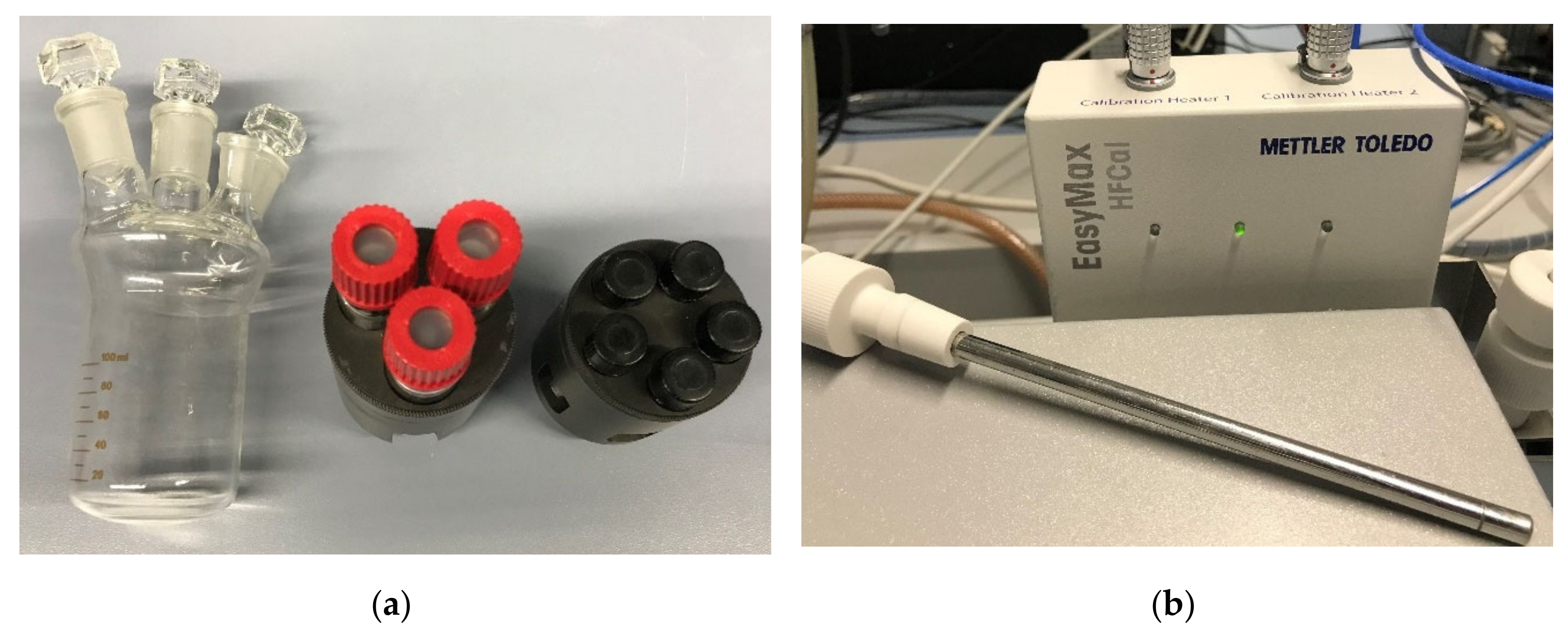
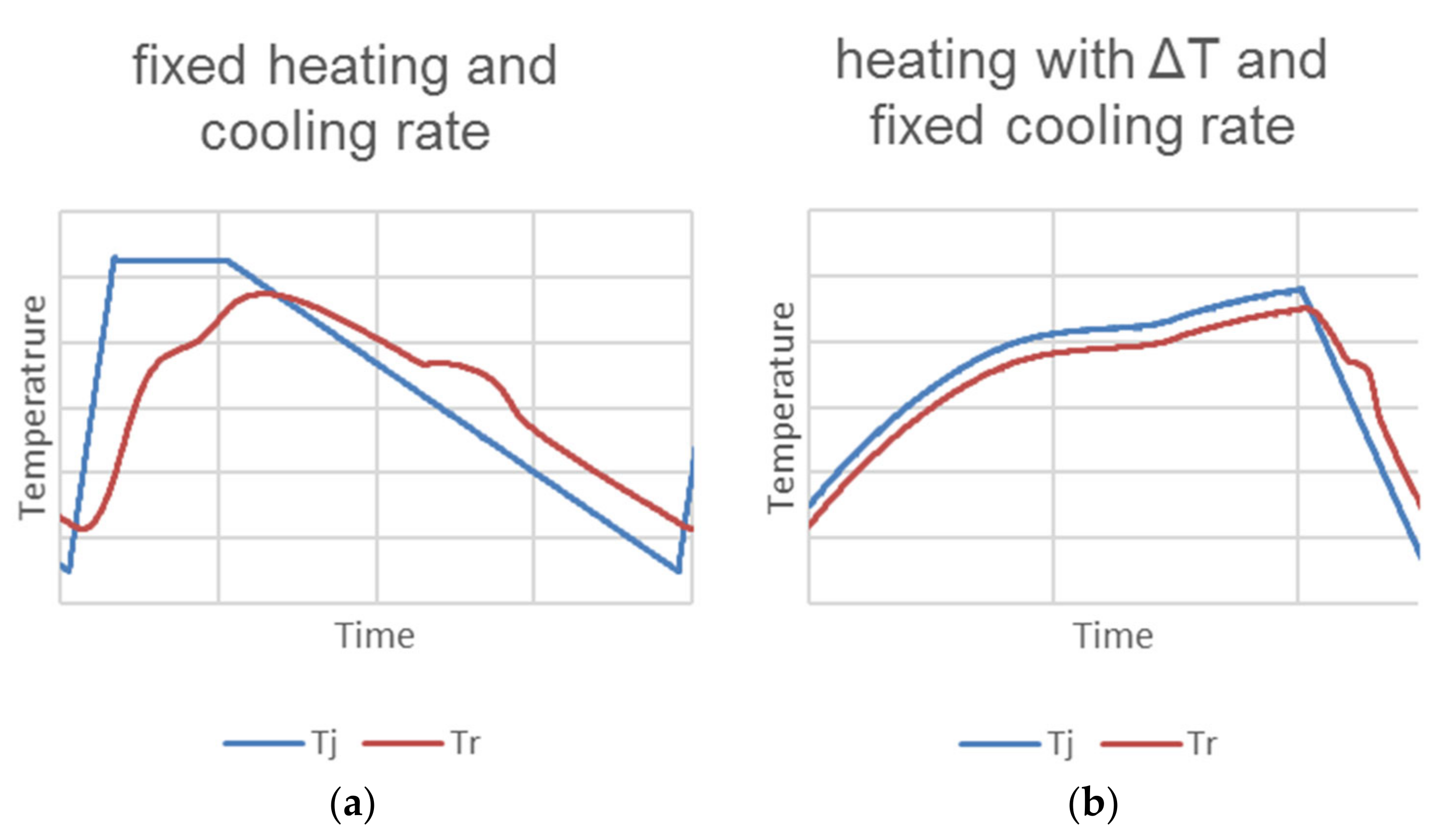



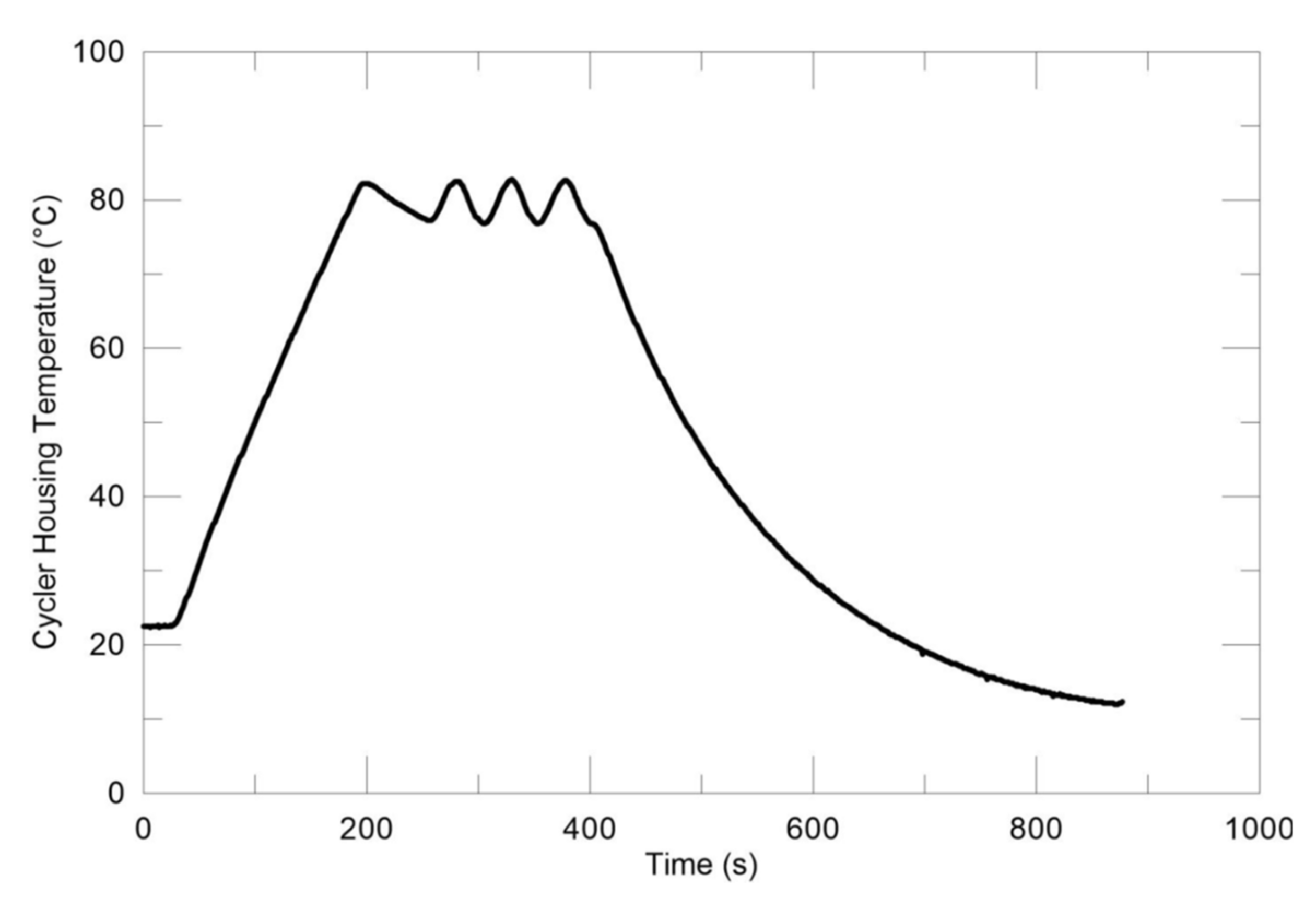
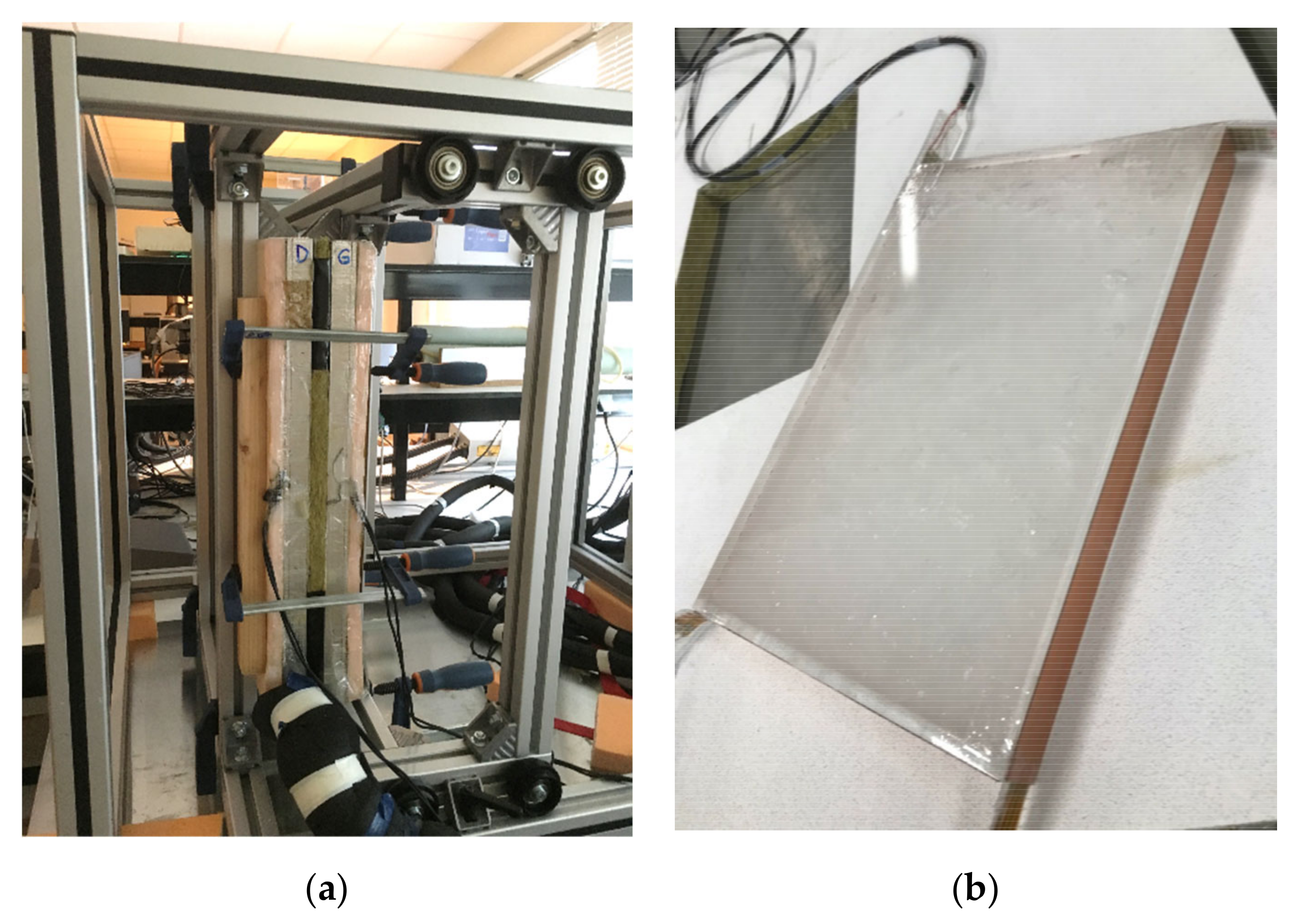
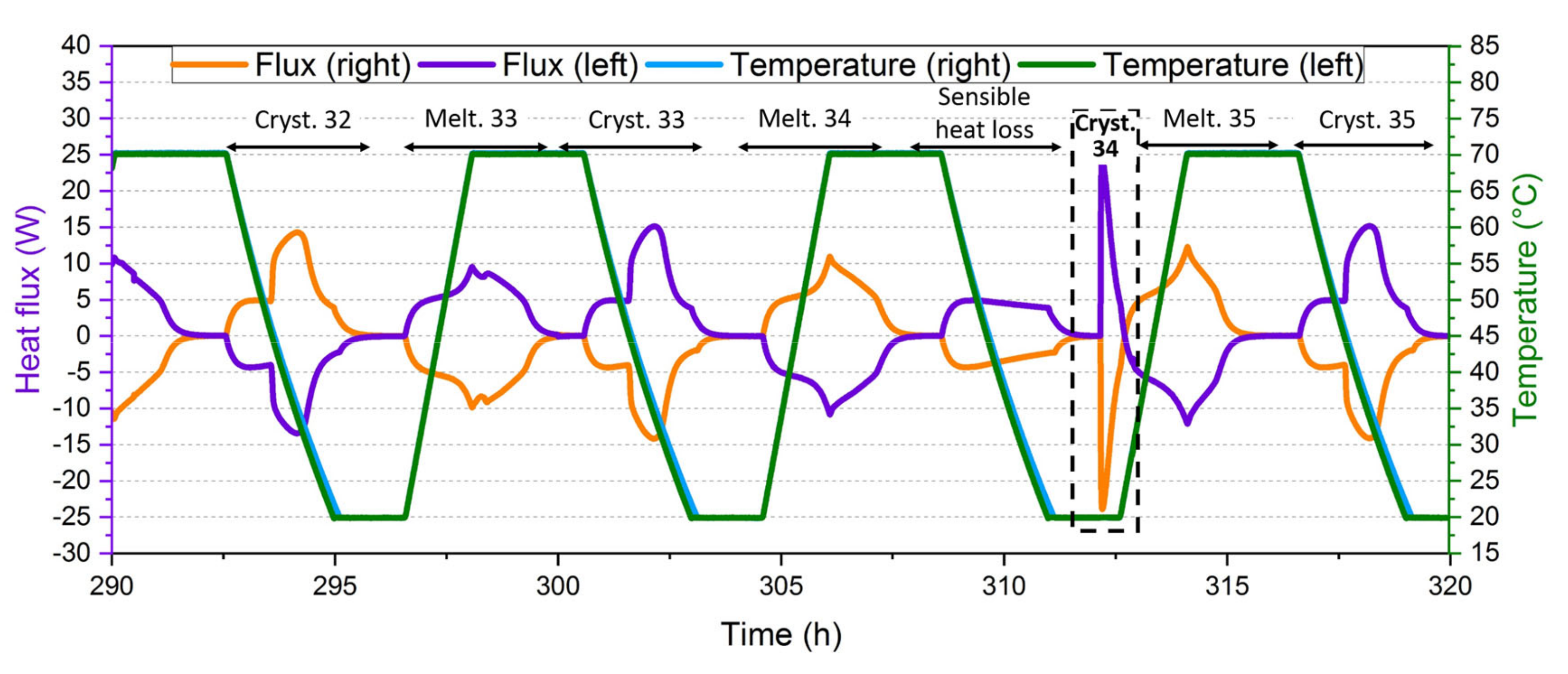


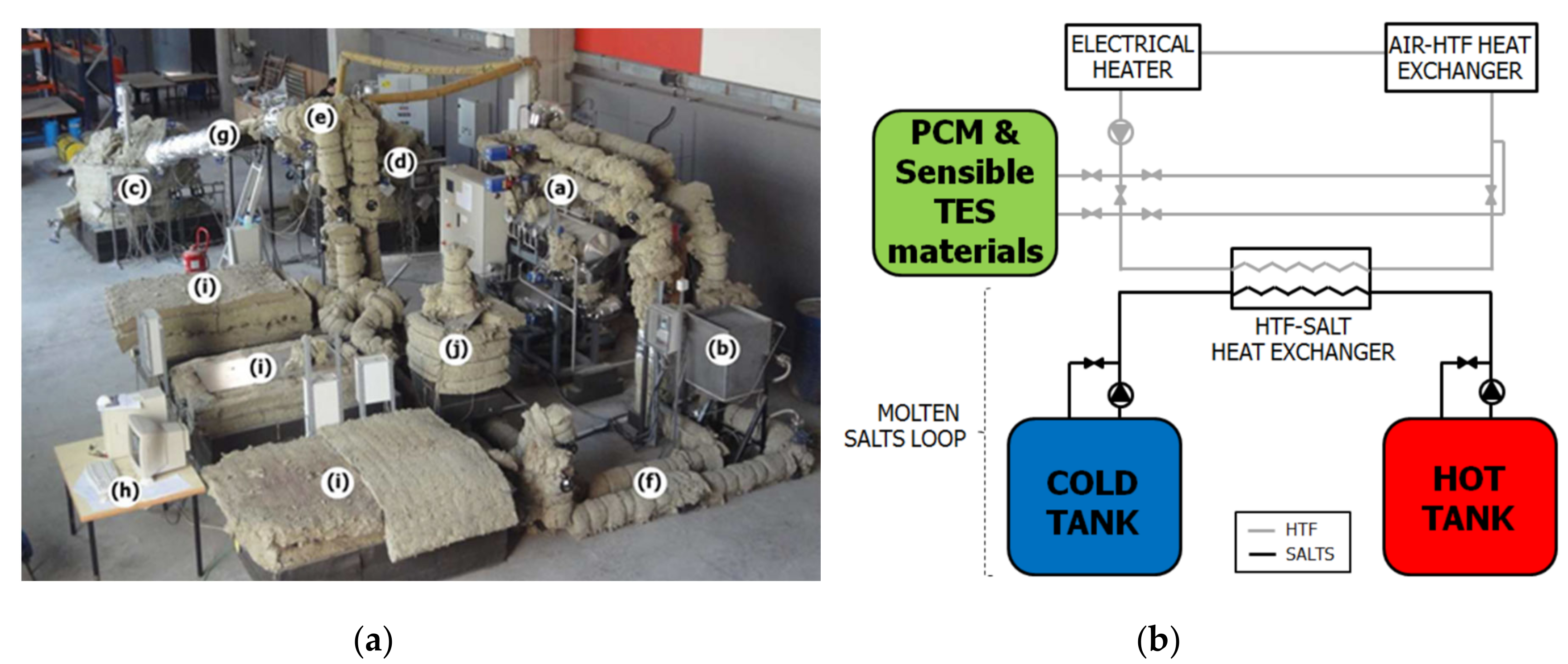


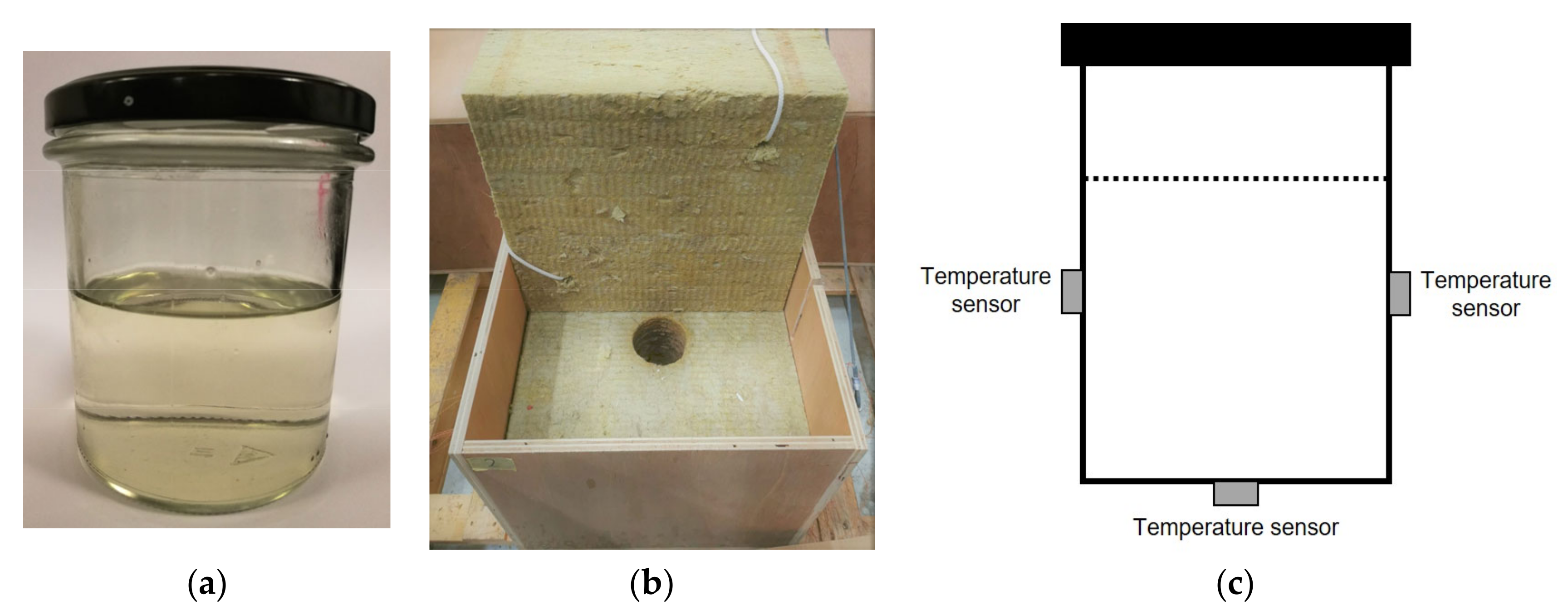
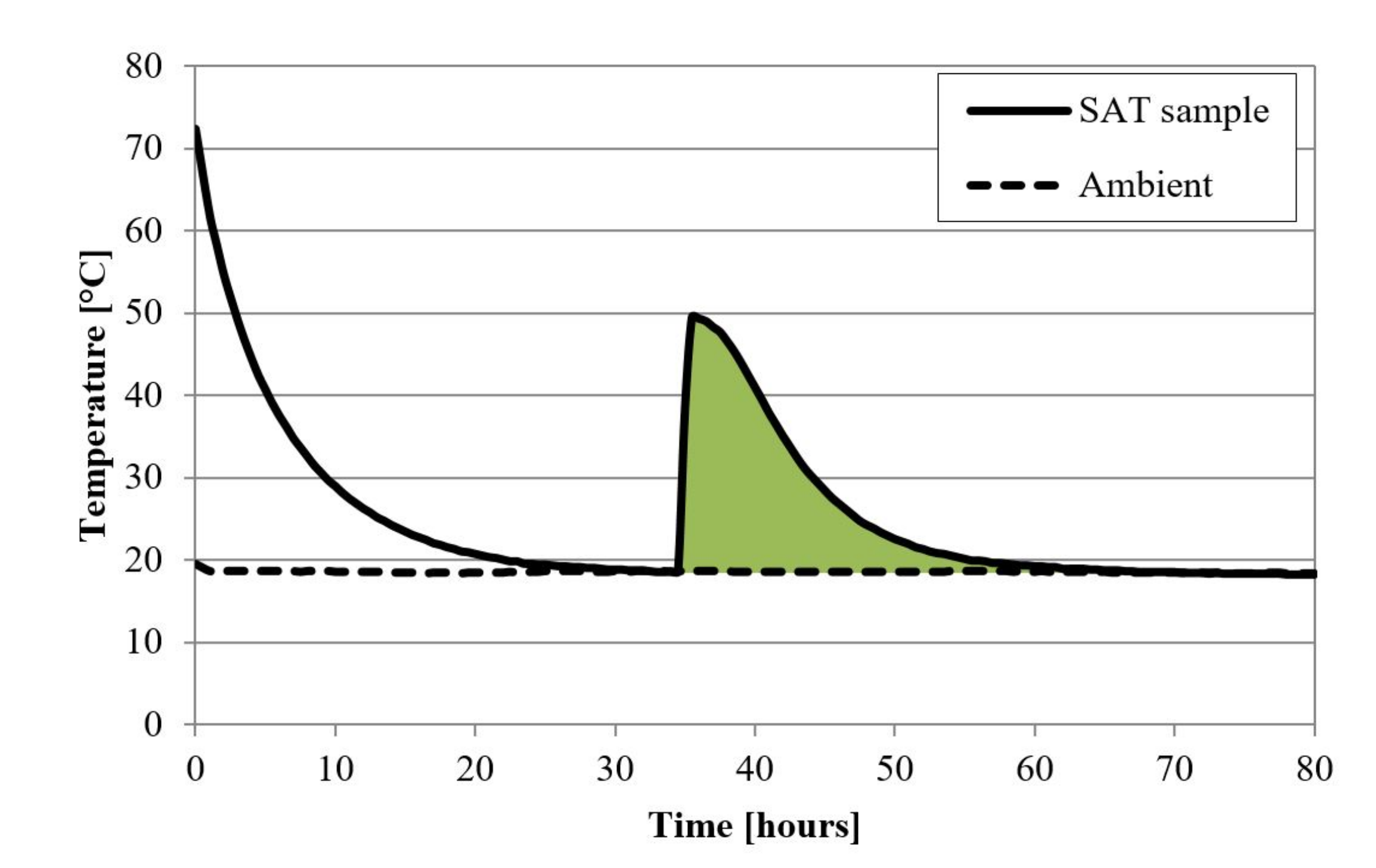


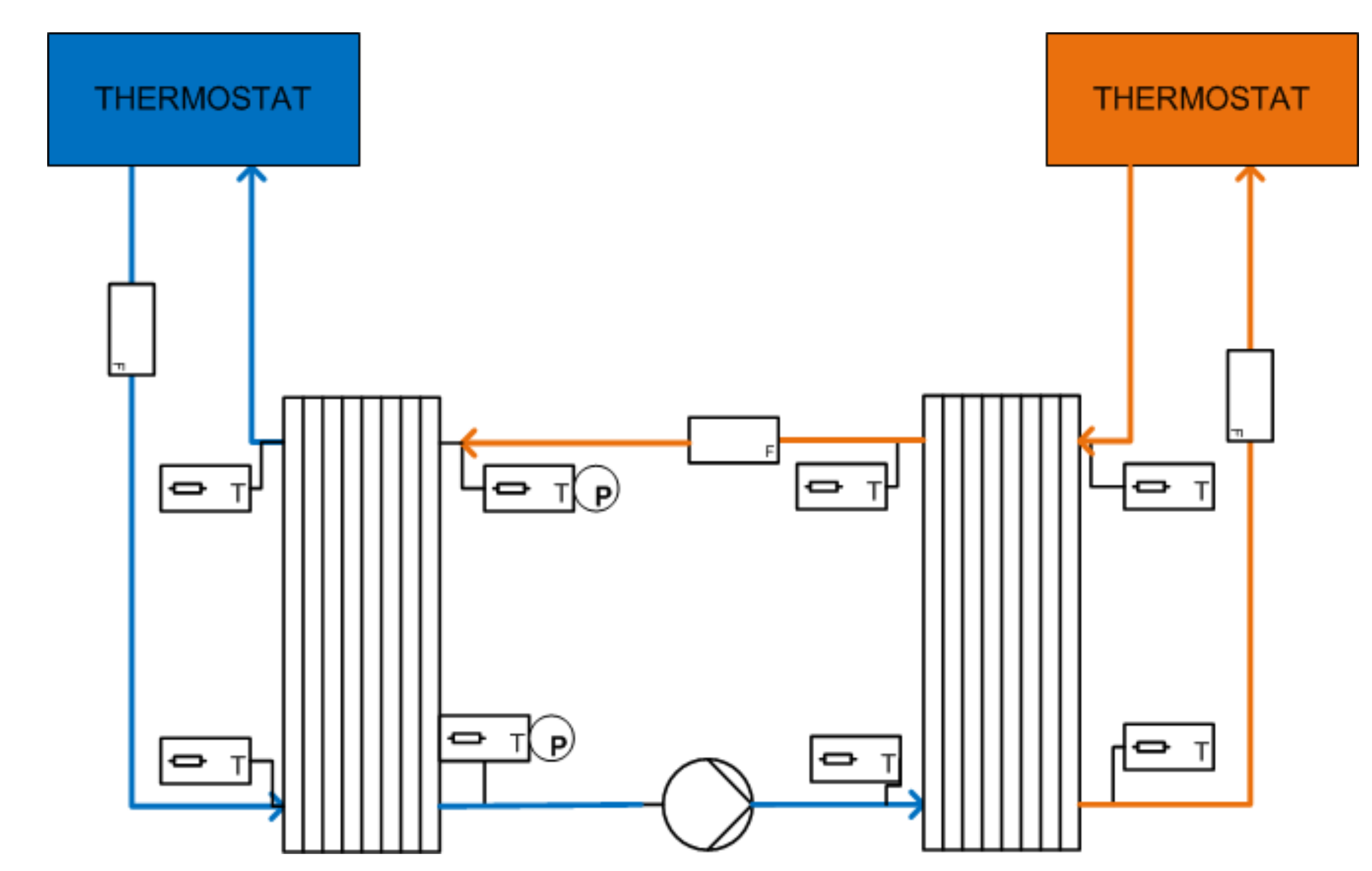
| Device Name (Type) | Sample Size | ΔT/°C | Heating, Cooling Rate/K∙min−1 | Cycles Per Day | Atmosphere | Stability Tests | Sample Container | Ref. |
|---|---|---|---|---|---|---|---|---|
| CIEMAT I (A) | 60–100 mL | RT 1–350 | 1–20 | 1 | air | T(t)cycles, DSC, m(t) | glass, ceramic | [18,19] |
| CIEMAT II (A) | 10 mL | RT–500 | 1–20, natural | 1 | air | T(t)cycles, DSC, m(t) | ceramic | [18,19] |
| CIEMAT III (A) | 60 mL | RT–500 | 1–20, natural | 1 | air, N2, Ar | T(t)cycles, DSC, m(t) | ceramic | [20] |
| EHU (A) | 5 g | −45–200 | variable | variable | air, N2, Ar | DSC, vis., XRD, FTIR | glass, metal | - |
| HSLU (A) | 1 × 100 mL, 3 × 25 mL, 5 × 8 mL | −40–180 | 10; 0.5 | 10 | air | T(t)cycles, DSC, vis. | glass | - |
| ISE capsules (A) | <22 L 3 | −10–80 | 1; 1 | 10 | air | DSC, m(t), RLM | polystyrene | [21] |
| ISE Peltier (A) | 10–100 mL | −30–200 | variable | <24 | air | DSC, vis. | stainless steel | |
| LAMTE (A) | 3–6 mL | −5–125 | 13; 7 | <140 | air | DSC | glass, plastic | [22,23,24,25] |
| LGCgE (A) | 270 mL | 5–80 | 0.05–0.3 | 2–3 | air, vacuum | T(t)cycles, Δhcycles, T(t)solid. | PMMA | [26] |
| LTTT (A) | 10–100 ml | −20–180 | variable | variable | air | T(t)cycles, DSC | glass, metal, plastic | [27] |
| UDL-GREiA I (A) | 18 × 0.5 mL | 4–99 | 50; 50 | 100 | air | DSC, FT-IR, TGA | polypropylene | [4,28] |
| UDL-GREiA II (A) | 154 L | 20–400 | 1; natural | 1 | air | DSC, FT-IR, TGA | stainless steel | [29,30,31,32,33,34,35,36,37,38] |
| UDL-GREiA III (A) | 8 L | −10–80 | 0.5; 1 | 2 | air | DSC, FT-IR, TGA | aluminum, stainless steel | [39] |
| ZAE (A) | 60 ml | −30–220 | 1 | 2 | air | T(t)cycles, DSC, vis. | glass, stainless steel | [7] |
| DTU full-scale (B) | 100–200 L | 20–90 | 1; 0.5 | 0.2 (0.4 2) | air | T(t)cycles, Δhcycles | metal, plastic | [40,41,42] |
| DTU heat loss (B) | 200 g | 20–90 | 1; natural | 0.25 (0.5 2) | air | T(t)cycles, Δhcycles, T(t)solid. | glass jar, metal lid | [43] |
| DTU multiple (B) | 10 × 30 L | 8–93 | 0.15; 3–4 | 0.25 | air | T(t)cycles, Δhcycles | stainless steel | [44] |
| ISE PCS (C) | 3.5 L | −10–80 | 140; 100 4 | 1300 4 | - −5 | DSC, ΔpHX, PSA, μ | stainless steel | [45] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rathgeber, C.; Hiebler, S.; Bayón, R.; Cabeza, L.F.; Zsembinszki, G.; Englmair, G.; Dannemand, M.; Diarce, G.; Fellmann, O.; Ravotti, R.; et al. Experimental Devices to Investigate the Long-Term Stability of Phase Change Materials under Application Conditions. Appl. Sci. 2020, 10, 7968. https://doi.org/10.3390/app10227968
Rathgeber C, Hiebler S, Bayón R, Cabeza LF, Zsembinszki G, Englmair G, Dannemand M, Diarce G, Fellmann O, Ravotti R, et al. Experimental Devices to Investigate the Long-Term Stability of Phase Change Materials under Application Conditions. Applied Sciences. 2020; 10(22):7968. https://doi.org/10.3390/app10227968
Chicago/Turabian StyleRathgeber, Christoph, Stefan Hiebler, Rocío Bayón, Luisa F. Cabeza, Gabriel Zsembinszki, Gerald Englmair, Mark Dannemand, Gonzalo Diarce, Oliver Fellmann, Rebecca Ravotti, and et al. 2020. "Experimental Devices to Investigate the Long-Term Stability of Phase Change Materials under Application Conditions" Applied Sciences 10, no. 22: 7968. https://doi.org/10.3390/app10227968
APA StyleRathgeber, C., Hiebler, S., Bayón, R., Cabeza, L. F., Zsembinszki, G., Englmair, G., Dannemand, M., Diarce, G., Fellmann, O., Ravotti, R., Groulx, D., Kheirabadi, A. C., Gschwander, S., Höhlein, S., König-Haagen, A., Beaupere, N., & Zalewski, L. (2020). Experimental Devices to Investigate the Long-Term Stability of Phase Change Materials under Application Conditions. Applied Sciences, 10(22), 7968. https://doi.org/10.3390/app10227968










