Abstract
Hyaluronic acid (HA) has anti-inflammatory and anti-edematous effects and, thus, could be promising in the treatment of oral lichen planus (OLP). The aim of the study was to evaluate the effects of topical hyaluronic acid, compared to placebo, on salivary levels of calprotectin, interleukin-6 (IL-6), and bacteria, as well as clinical and subjective parameters. Fourteen patients with confirmed OLP were included. After random selection, patients started with either 0.2% hyaluronic acid or a placebo gel for 6 weeks. Following a wash-out period, the groups changed the application. Whole saliva, clinical parameters, and questionnaires were evaluated before and after the intervention, as well as after the crossover phase. Salivary calprotectin, IL-6, and inflammation-related bacteria were determined by ELISA and PCR, respectively. There were no significant differences in clinical or subjective outcome parameters, salivary levels of IL-6, calprotectin, or bacteria after the application of hyaluronic acid, compared to placebo. However, only nine patients completed the study, as five out of seven patients starting with placebo were lost to follow-up. Significant effects of HA on inflammatory mediators and clinical parameters in OLP patients could not be proven, although a trend in clinical severity improvement could be observed.
1. Introduction
Oral lichen planus (OLP) affects around 2% of the adult population, with a higher prevalence in women [1]. Diagnosis is based on clinical appearance and histopathology [2]. OLP is considered an inflammatory mucocutaneous disease; however, the mechanisms are not entirely clarified. A genetic (HLA-DR2) background and infection as a predisposing or provoking factor are considered to be involved in the pathogenesis of OLP [3].
Patients often suffer from pain or burning sensations, and from psychosocial impairment linked to anxiety and the burden of a chronic, and potentially malignant, disease, even if the malignant transformation is only in a small subset of OLP patients (around 1.1%) [4]. Especially erosive oral mucosal conditions radically affect oral health-related quality of life, and are accompanied by a high frequency of psychological problems [2]. Pathognomonic reticular lesions (Wickham striae) are usually discovered incidentally during routine intraoral examination, and are mostly asymptomatic. However, erosive lesions may cause considerable discomfort, affecting quality of life and leading patients to seek medical care [5].
Depending on the severity of the clinical symptoms, various therapeutic options have been described: the first line, mainly symptomatic treatment targeting the alleviation of pain and erythema, is topical corticosteroids (TCS), which can be recommended as safe, efficacious, and cost-effective. Although TCS is usually successful, it has side-effects, including candidiasis, burning, or stinging sensation, mucosal atrophy, bad taste, nausea, sore throat, and dry or swollen mouth. However, a superior effectiveness of topical corticosteroids, compared to other therapeutics, has not been corroborated in systematic reviews [6].
For steroid-refractory OLP, there is a plethora of alternative topical treatment approaches. Evidence-based local treatments, as supported by randomized controlled trials (RCTs), comprise macrolide immunosuppressants (tacrolimus, pimecrolimus, cyclosporine A), retinoids, and low-level laser therapy [7,8]. In addition to standard protocols, systemic strategies with immunosuppressants or immunostimulants are well supported by research [9,10,11,12,13,14].
To minimize side effects, such as burning sensations, fungal infections [15,16], or risk of potentially malignant transformation [17,18,19], nutraceuticals with anti-inflammatory/antioxidative properties (e.g., aloe vera, honey, curcumin) have been investigated in RCTs with promising, but inconclusive, results [7,8,20,21].
Hyaluronic acid (HA), a naturally occurring glycosaminoglycan with high molecular weight, is a promising novel alternative agent to immunosuppressants. It is a major component of the extracellular matrix, and present in various body fluids. Its major features are a high hygroscopicity and viscoelasticity. Due to its anti-edematous, anti-inflammatory, bacteriostatic, fungistatic, and pro-angiogenetic properties, it has been shown to promote wound healing in several tissues, and, therefore, could be topically used in OLP [22,23,24]. Beneficial effects of HA in OLP have already been reported in recent studies [24] focusing mainly on subjective outcome parameters, such as the visual analogue scale (VAS) and clinical appearance [24,25,26,27].
Therapeutic interventions could also be monitored by the determination of various biomarkers possibly related to the pathogenesis of OLP. The pro-inflammatory cytokine interleukin-6 (IL-6) is thought to be of major pathobiological importance in OLP; therefore, it was shown that salivary IL-6 might represent a useful diagnostic and therapeutic biomarker in OLP [28]. Calprotectin, a protein released during inflammatory conditions by activated polymorphonuclear leukocytes, monocytes, and macrophages, was shown to be increased in the saliva of OLP patients, and might be also used as a monitoring marker [29,30].
The aim of the present study was to evaluate effects of topically applied HA in OLP patients on inflammation-related biomarkers and bacteria in saliva, as well as clinical appearance and subjective outcome parameters.
2. Materials and Methods
This was a single-center, prospective, double blind, and placebo-controlled, randomized, crossover study according to the principles stated in the Declaration of Helsinki. Ethical approval was obtained from the Ethics Committee of the Medical University of Vienna (EK Nr.1585/2012) and the Austrian Agency for Health and Food Safety Ltd. (AGES). All patients gave written consent for their participation. Patients were eligible if they had (1) the diagnosis of a symptomatic oral lichen planus (OLP) and were not undergoing therapy, (2) confirmation by histopathology and direct immunofluorescence assay, (3) dentures which were correctly fitting, (4) a minimum of 18 years of age, and (5) no nicotine abuse (cigarettes, cigars, chewing tobacco, or snuff). Exclusion criteria were malignant transformation, severe histopathologic dysplasia, carcinoma in situ, severe vitamin deficiency, pregnancy, age below 18 years, lactation period, nicotine abuse, the use of another therapeutic agent for OLP (e.g., corticosteroids, calcineurin inhibitors), the presence of asymptomatic OLP, or an oral mucositis of other origin (e.g., drug intake).
2.1. Patient Recruitment, Gel Application, and Saliva Sampling
The study was carried out at the University Clinic of Dentistry, Medical University of Vienna, Austria. Patients were recruited from the outpatient and inpatient clinic for mucosal diseases of the Division of Oral Surgery (Table 1). Diagnosis of OLP was made according to clinical appearance; lesions were documented by photographs, and confirmed by histopathology and direct immunofluorescence analysis. The gentle removal of the tissue sample for the histopathologic diagnosis and execution of direct immunofluorescence assay was performed by a single specially-trained surgeon (GD). A specialist (KR) in immune dermatology and mucosal histopathology performed these analyses. Sample size calculation was based on previous studies [31].

Table 1.
Patients included in the study at baseline clinical severity score (CSI), visual analogue scale (VAS), plaque index maximal value (PImax), gingival index highest value (GImax), Oral Health Impact Profile summary score German version (OHIP-G).
After obtaining informed consent, allocation to therapy was assigned by permuted block randomization to either group HA1st, starting with hyaluronic acid (HA), or group P1st, beginning with placebo. To eliminate bias, the order of placebo and hyaluronic acid treatment was randomized. Hyaluronic acid gel 0.2% (Bloxaphte®, Ricerfarma, Milano, Italy) was packed in neutral brown flasks containing 4 g of fluid to be used three times daily for two minutes, without drying the area before application. The placebo (methylcellulose 400) was packed in neutral brown flasks containing 4 grams of fluid to be used in the same manner. Patients changed therapy after 6 weeks, with a wash-out phase of 48 h in between therapies. Both patients and investigators were blinded to treatment.
Stimulated whole saliva was collected to perform bacterial and biomarker measurement according to a previous protocol for saliva analyses [32]. Patients were advised to refrain from intake of any food or beverage (water exempted) one hour before the test session. Smoking, chewing gum, and intake of coffee were also prohibited during this hour. Before sampling, patients were advised to rinse their mouth several times with deionized water and then to relax for five minutes. Unstimulated whole saliva was collected first for the determination of salivary flow (average 5 min) and pH [33]. pH was subsequently measured with conventional pH strips. The study participants then rinsed their mouth with an acidic extraction solution for stimulated whole saliva collection (Saliva Collection System, Greiner Bio-One, Kremsmuenster, Austria). All clinical procedures were performed by one trained and calibrated person (MM).
2.2. Outcome Parameters
The following outcome parameters were evaluated at the beginning, after 6 weeks, and after 12 weeks: total amount and pH of saliva; the salivary markers calprotectin and interleukin-6 measured by ELISA assays (S100A8/A9, Buehlmann, Schoenenbuch, Switzerland for Calprotectin; Human IL-6 Quantikine HS ELISA Kit #HS600B, R&D Systems, Minneapolis, MN, USA for IL-6). Periodontopathic bacteria Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis, Tannerella forsythia, Treponema denticola, Prevotella intermedia, Parvimonas micra, Campylobacter rectus, Eubacterium nodatum, Eikenella corrodens, Capnocytophaga species, and Fusobacterium nucleatum were determined by 16S ribosomal RNA-based polymerase chain reaction (ParoCheck®, Greiner Bio-One, Kremsmuenster, Austria) according to the protocol used in previous studies [34].
For clinical parameters, the Clinical Severity Index (CSI) [35] for the differentiation of the area affected by OLP, a gingival index (GI) [36], and a plaque index (PI) [37] for the assessment of gingival inflammation and plaque deposits, respectively, were evaluated.
Subjective outcome parameters were evaluated as follows:
To analyze oral health-related quality of life, a standardized questionnaire validated in cross-sectional, as well as longitudinal, studies was used. The Oral Health Impact Profile questionnaire German version (OHIP-G) contains 53 questions concerning the previous four weeks [38]. Answers are given on a Likert-type five-point scale (0 = never; 1 = hardly ever; 2 = occasionally; 3 = fairly often; and 4 = very often). In this analysis, the total summary OHIP-G53 score was utilized, calculated by summing the score of the answers to each question. Higher scores indicate lower oral health-related quality of life. Four dimensions (psychosocial impact, orofacial pain, oral functions, and appearance) serve as principal components of oral health-related quality of life. The dimensions were calculated as described previously [2].
For measuring pain intensity, a visual analogue scale (VAS), a 100 mm numeric rating scale, was used; patients can rate their pain on a scale between 0 and 10. Scores of 0 imply “no pain”, while 10 means the “worst pain imaginable”. A recommendation for interpretation of the ratings suggest 0 to 4 mm to be read as no pain, 5 to 44 mm as mild pain, 45 to 74 mm as moderate pain, and 75 to 100 mm as severe pain. With this subjective evaluation method, it is possible to compare pain levels at every visit, to see score changes over time [39].
2.3. Statistical Analysis
According to power analysis based on a power of 80% and a two-sided significance level of 5% that there will be a significant improvement with therapy, 12 patients should have been included. The drop-out rate was assumed to be about 10%, thus 14 patients were included.
For a study with a double crossover design, a case number of 12 patients was calculated, which appears realistic in comparison with similar studies with 13 patients per group [31].
The calculation was carried out with R Version 2.10.1 (R Foundation for Statistical Computing, Vienna, Austria). The “crossdes” package was used to calculate the number of cases and to create the design. The function of the MOLS, which is a generalized Youden design, was used for the construction. In order to be able to reveal with a thickness (power) of 80% and a bilateral significance level of 5% that there is a significant improvement through the therapies, a case number of 12 patients was calculated.
Parameters were described by initial means and standard deviation, under hyaluronic acid treatment vs. placebo. Categorical data were described by frequencies and percentages. For differences between treatment groups of ordinal data, paired Wilcoxon rank sum tests were used. Significance tests were performed two-sided, and a p-value of ≤0.05 was considered as significant. Bonferroni correction was used for multiple testing.
3. Results
Nine out of 14 patients (8 females, 1 male, mean age 48 (18–71) years) complied with the follow-up and treatment crossover, and completed the study. Five patients, all from the P1st group (n = 7) quit the study because of non-compliance or the use of a different treatment, which was an exclusion criterion (dropout rate 35.7%). The chance to quit the study was significantly increased in the P1st group (Fisher’s exact test for count data: OR = ∞, 95% CI = 1.4–∞, p = 0.021, adjusted p = 0.231).
3.1. Salivary Parameter
Salivary parameters did not differ significantly concerning the amount (paired Wilcoxon rank sum test with continuity correction: W = 39, p-value = 0.9314, adjusted p = 1.0) after HA and placebo, nor did pH (paired Wilcoxon rank sum test with continuity correction: W = 40.5, p-value = 1, adjusted p = 1.0). Baseline amount of saliva was 1.0 ± 0.8 g, which increased by 0.3 ± 0.5 g in the HA group and 0.3 ± 0.5 g in the placebo group. The baseline pH was slightly sour, with an average value of 6.7 ± 0.4, which decreased by −0.4 ± 0.4 in the HA group and −0.4 ± 0.8 in the placebo group.
3.2. Cytokine Levels
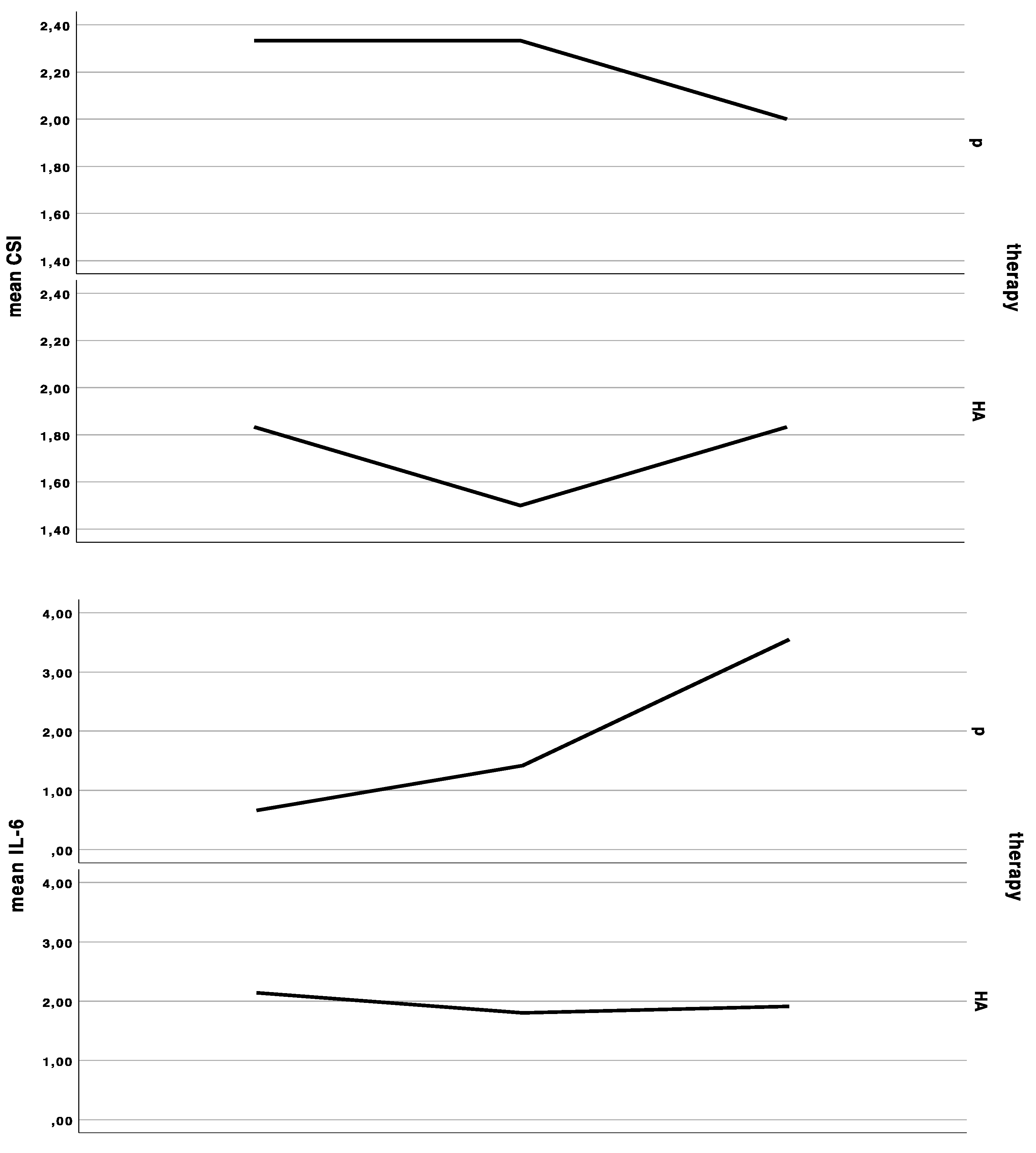
Salivary IL-6 (paired Wilcoxon rank sum test: V = 4, p-value = 0.7728, adjusted p = 1.0) and calprotectin (paired Wilcoxon rank sum test: V = 17, p-value = 0.5703, adjusted p = 1.0) levels were not significantly different between the groups. The baseline IL-6 level of 1.6 ± 1.6 pg/mL increased by 0.2 ± 0.4 pg/mL under HA treatment, and 0.4 ± 0.4 pg/mL during control. The baseline calprotectin levels of 12.9 ± 13.2 pg/mL decreased by 3.0 ± 18.1 under HA treatment, and 4.0 ± 9.7 pg/mL during placebo (Figure 1).
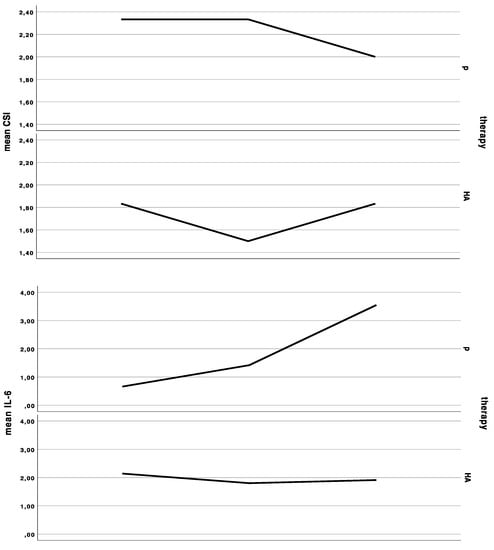
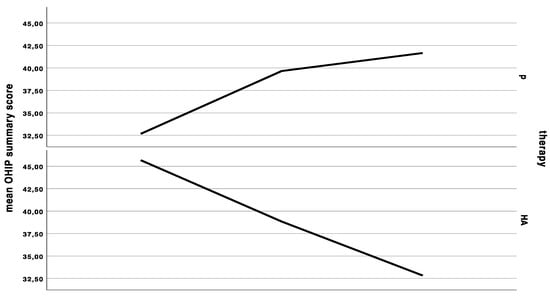
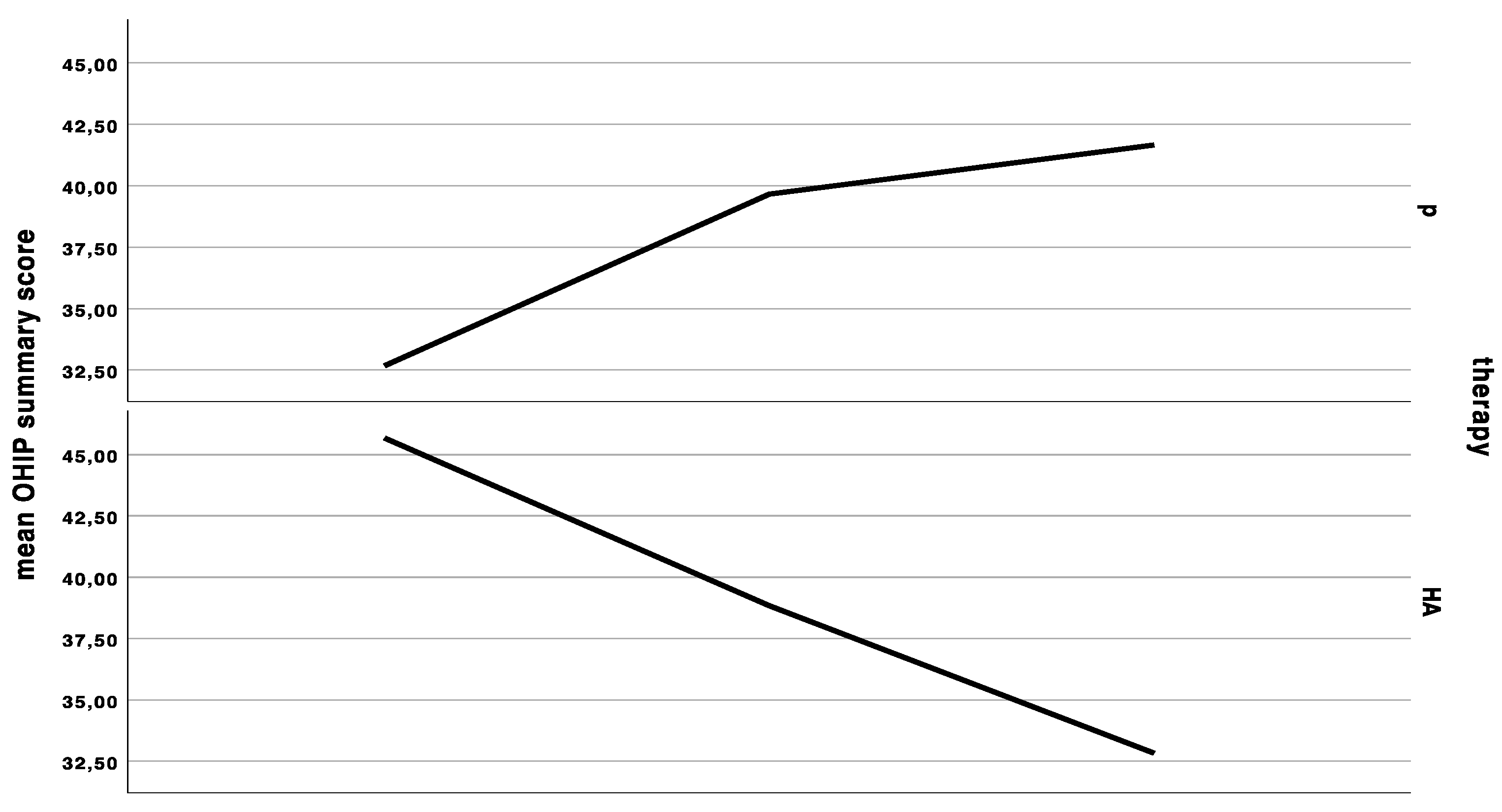
Figure 1.
Curves indicating the mean clinical severity index (CSI), mean interleukin-6 (IL-6) levels in saliva, mean Oral Health Impact Profile (OHIP) summary score at the first baseline, cross-over, and last appointment in the group starting with placebo (p), and in the group starting with hyaluronic acid (HA), either for 6 weeks and a wash-out period of 48 h in between treatments.
3.3. Periodontal Microbiota
Aggregatibacter actinomycetemcomitans was detectable at an amount of 103 only in one patient at baseline and after HA. This was the only patient with higher IL-6 values after HA treatment. Porphyromonas gingivalis was detectable at the amount of 104 in two patients at two different time points, once at baseline and once after HA treatment. Tannerella forsythia was detectable at the amount of 104 in one patient at one time point after HA treatment. Treponema denticola was detectable at the amount of 104 in one patient at baseline only. Prevotella intermedia, Eubacterium nodatum, Eikenella corrodens, and Parvimonas micra were not identified. Fusobacterium nucleatum was identified in all patients (104 ± 1.0 ranging from 103 to 105 bacteria); there was no significant (paired Wilcoxon rank sum test: V = 3, p-value = 0.371, adjusted p = 1.0) difference under HA treatment (104.2 ± 1.0) or placebo (104.0 ± 1.0). Capnocytophaga species were identified at the amount of 104 in three patients only after HA treatment.
3.4. Periodontal Parameters
The baseline average GI was 0.8 ± 0.3(0.3–1.3), the corresponding baseline PI was 0.4 ± 0.3 (0.0–0.9). There was no significant difference of mean GI change under HA and placebo treatment (Wilcoxon rank sum test with continuity correction: V = 33, p-value = 0.25, adjusted p = 1.0) or of mean PI change between HA and placebo (V = 16, p-value = 0.4768, adjusted p = 1.0). The mean GI change was 0.0 ± 0.3 under the HA treatment, and −0.1 ± 0.4 in the placebo treatment. The mean PI change was −0.1 ± 0.3 under HA treatment versus 0.0 ± 0.3 under placebo.
3.5. Clinical Severity Index
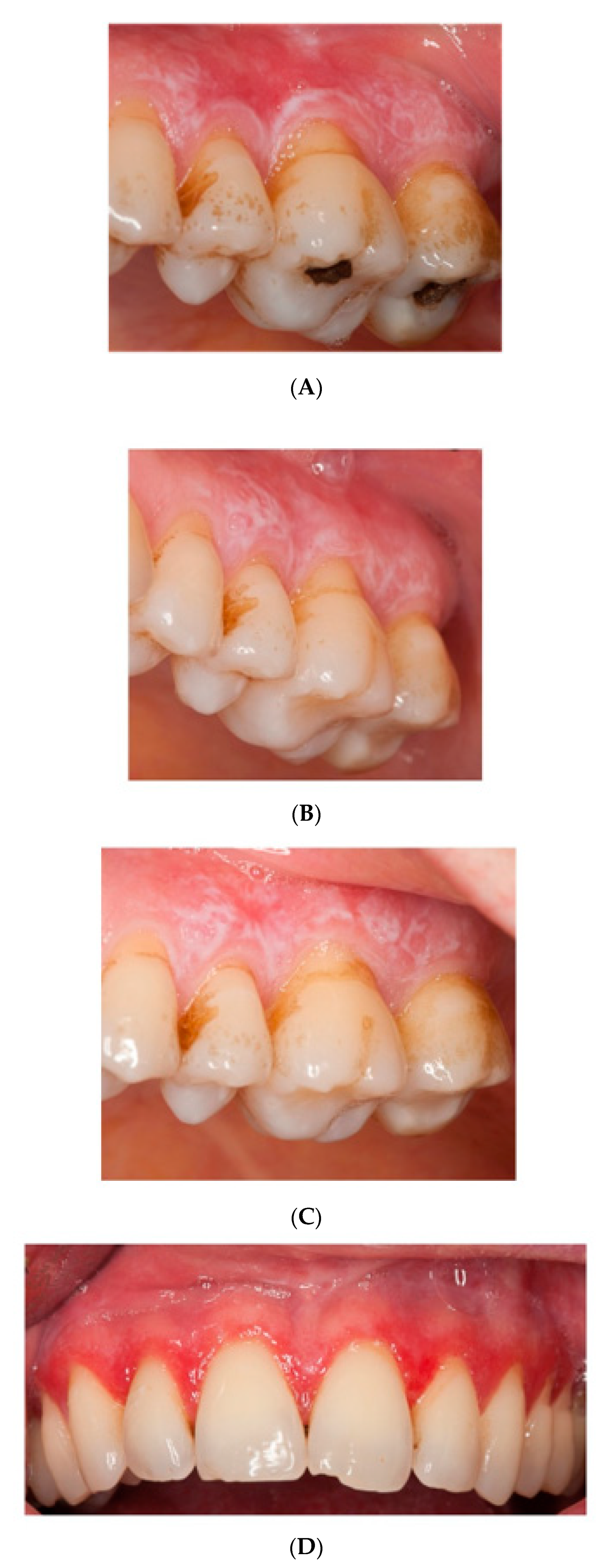
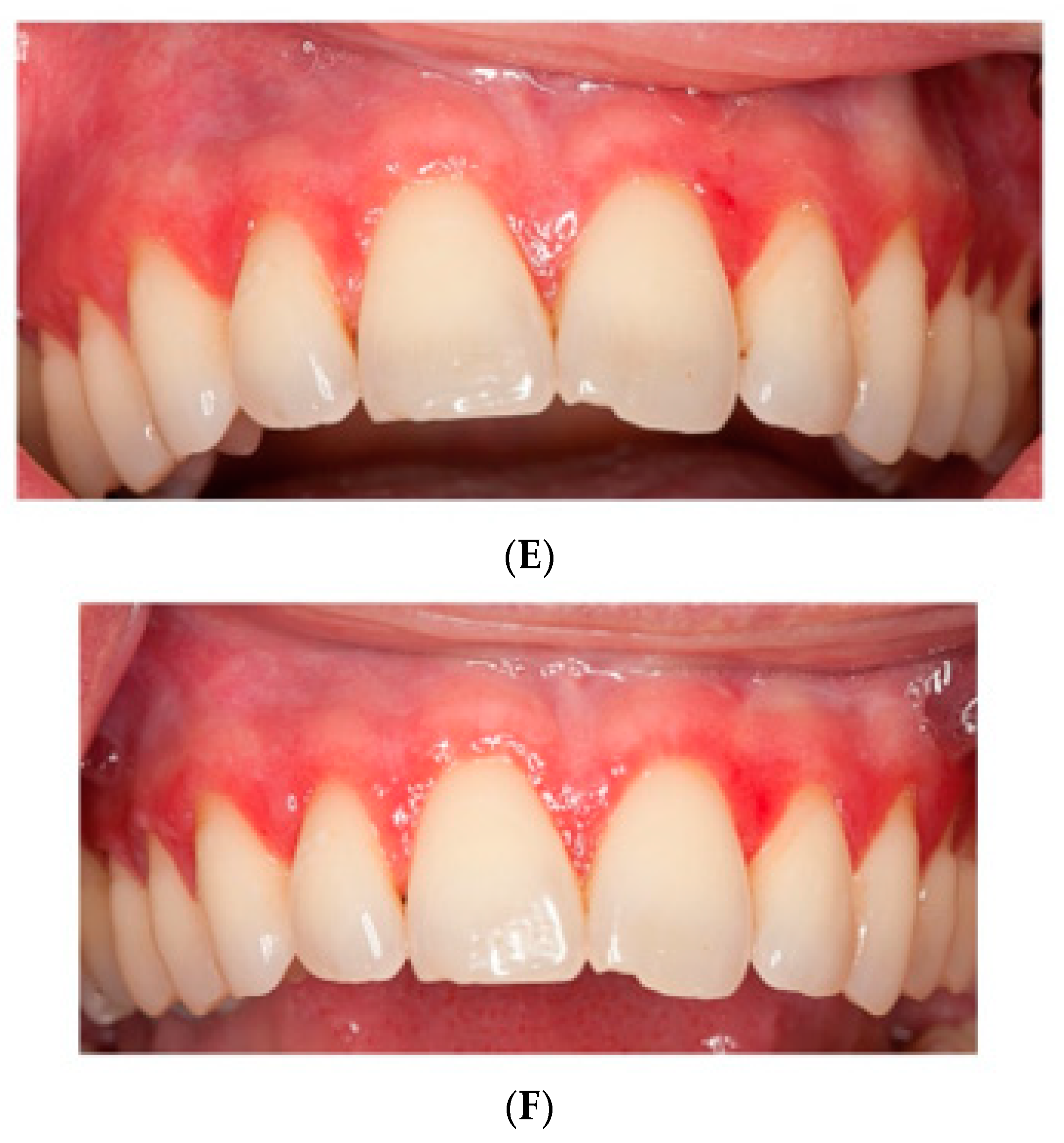
The baseline CSI of 2 ± 1 (range 1 to 4) did not significantly change after HA or placebo administration, respectively. There was an insignificant (Wilcoxon rank sum test with continuity correction: V = 0, p-value = 0.1489, adjusted p = 1.0) improvement (−0.3 ± 0.5) after HA in the CSI, and no change of CSI after placebo treatment (0.0 ± 0.5). In the HA1st (the group starting with hyaluronic acid), CSI showed an initial improvement, yet dropped back to baseline values after crossover to placebo. (Figure 1 and Figure 2).
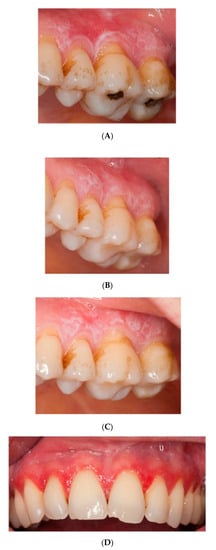
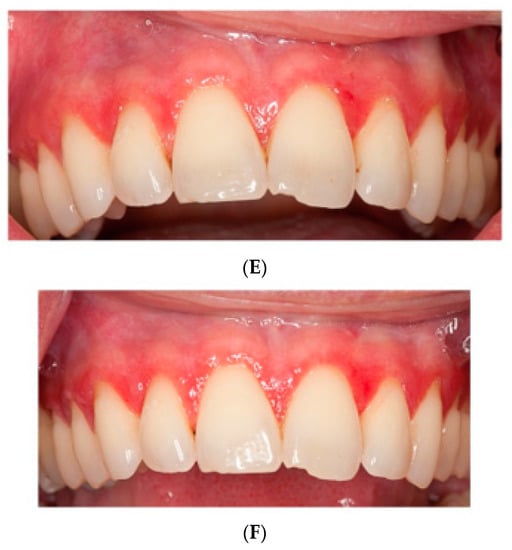
Figure 2.
Pictures of patients one and two at (A,D) baseline, (B,E) HA treatment, (C,F) placebo treatment.
3.6. Pain as Measured by the VAS
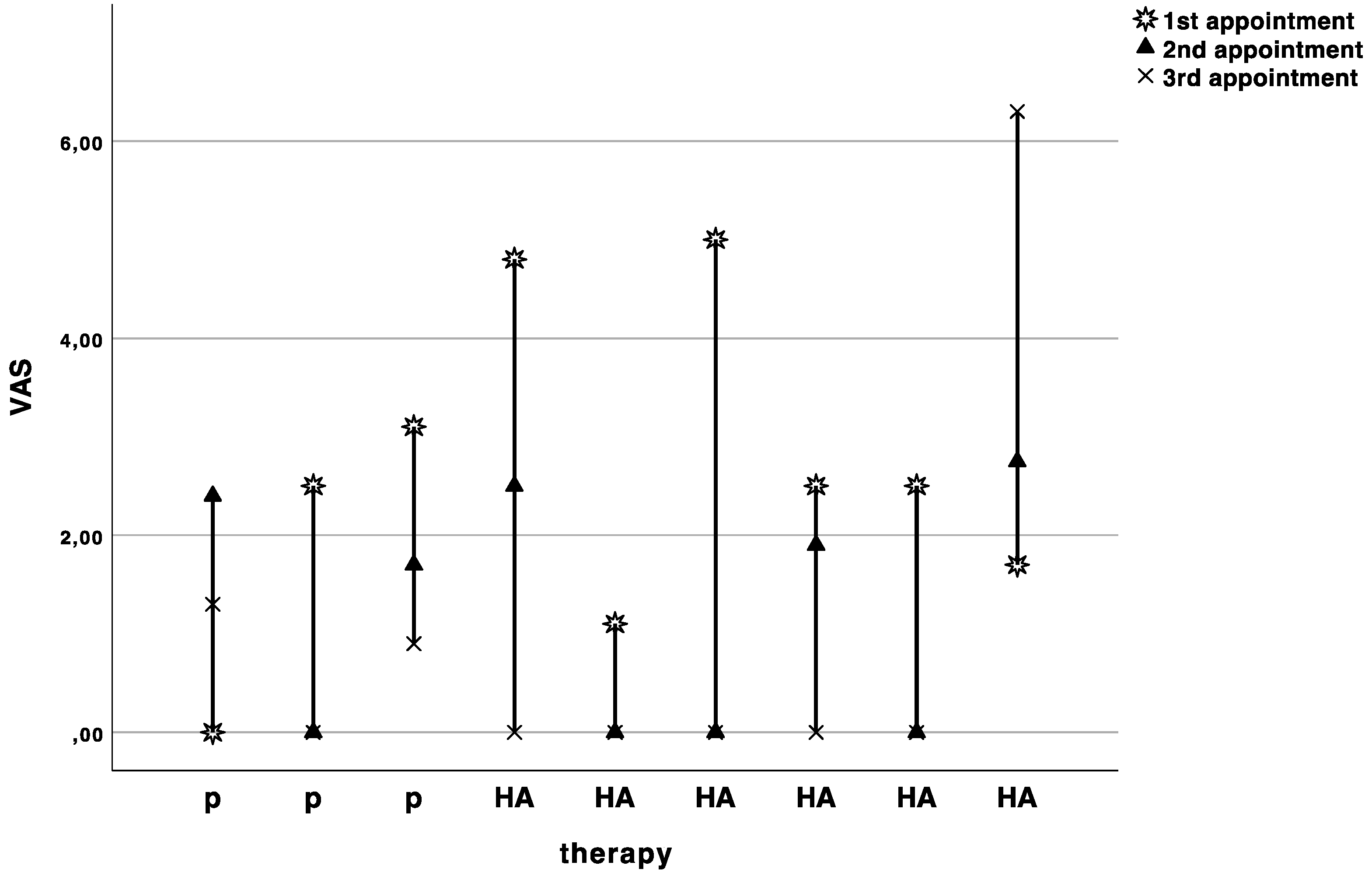
The baseline pain level on the visual analogue scale was 2.6 ± 1.6. The pain levels slightly decreased with each appointment, but no significant difference (Wilcoxon rank sum test with continuity correction: V = 9, p-value = 0.7874, adjusted p = 1.0) was observed between HA (−1.4 ± 2.2) and placebo (−1.5 ± 2.9) treatment (Figure 3).
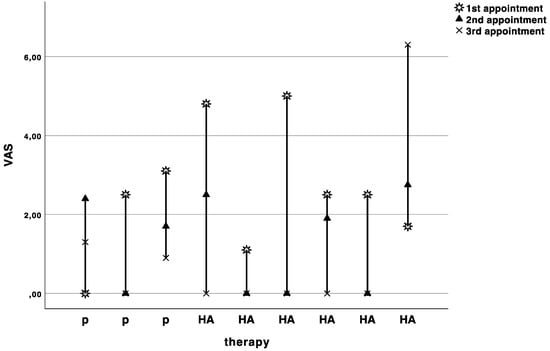
Figure 3.
Curves indicating the visual analogue scale values in cm for each patient at baseline (1st appointment), cross over (2nd appointment), and recall (3rd appointment). P indicates the patients starting with placebo, and HA indicates the patients starting with hyaluronic acid.
3.7. Oral Health Impact Profile (OHIP-G53)
The baseline OHIP was 41.3 ± 13.7. OHIP decreased by significantly less (Wilcoxon rank sum test with continuity correction: V = 44, p-value = 0.01269, adjusted p = 0.152) under HA treatment (OHIP change compared to baseline: −0.4 ± 14.6) compared to placebo treatment (OHIP change compared to baseline: −7.3 ± 14.7) (Figure 1). The baseline dimension oral function was 1.8 ± 1.8, which increased by 0.3 ± 1.5 under HA treatment and did not change under placebo treatment (0.0 ± 1.9). There was no significant difference between HA and P (Wilcoxon rank sum test with continuity correction: V = 8, p-value = 0.345, adjusted p = 1.0). The baseline dimension “orofacial pain” was 9.8 ± 4.9, which dropped by −0.6 ± 5.7 under HA and –2.4 ± 5.8 under placebo treatment (Wilcoxon rank sum test with continuity correction: V = 25, p-value = 0.3592, adjusted p = 1.0). The baseline dimension “psychosocial impact” was 2.8 ± 2.6, which did not significantly differ (Wilcoxon rank sum test with continuity correction: V = 1.5, p-value = 0.269, adjusted p = 1.0) after HA treatment (0.0 ± 1.2) and P treatment (0.6 ± 2.1). The baseline dimension “appearance” was 3.8 ± 2.1, which significantly differed between both groups (Wilcoxon rank sum test with continuity correction: V = 26.5, p-value = 0.039, adjusted p = 0.385). The appearance score increased under HA treatment 0.8 ± 1.6 and decreased under p treatment −0.4 ± 1.9.
4. Discussion
In the present prospective, randomized, controlled study with crossover design, the topical application of HA had no significant beneficial effect in the treatment of OLP, compared to a placebo.
The first line of medical therapy is aimed at eliminating atrophic and ulcerative lesions, alleviating symptoms, and eventually decreasing the risk of malignant transformation [5]. New therapeutic modalities have been tested, due to the adverse effects of standard immunosuppressive treatments. Due to its beneficial properties for tissue regeneration and wound healing, topical HA has been shown to be a useful adjuvant in the treatment of gingivitis and chronic periodontitis, as well as during the postoperative period for faster healing and to reduce patients’ discomfort [22]. Data obtained from a review by Casale et al., comprising 20 clinical studies, demonstrate that regarding tissue repair and wound healing, topical administration of HA could play a role not only in postoperative dental surgery, but also in the treatment of patients affected by inflammatory disease, with a significant improvement in quality of life [40].
These HA-related improvements could not be reproduced in the present study; the findings considering clinical appearance, pain, and gingival inflammation could not be verified either. Despite its promising properties, data for the treatment of OLP are scarce and remain conflicting.
Salivary IL-6 was suggested as a diagnostic and therapeutic biomarker in OLP patients [28]. However, although possible confounders (smoking, periodontal disease, caries) were avoided by the inclusion criteria in our study, we could not observe a meaningful trend of IL-6 in neither the HA nor in the placebo group. These conflicting results may have several reasons: while some studies reported IL-6 level changes with types and severity of OLP [41,42], a systematic review found no influence between patients with erosive OLP and patients with nonerosive OLP [43]. Furthermore, different kits have been used for salivary IL-6 quantification [44], and most studies for the evaluation of salivary IL-6 levels have used small sample sizes prone to heterogeneity.
Elevated levels of extracellular calprotectin are present in diverse inflammatory disorders. This makes it a potential biomarker for inflammation [45,46]. Salivary calprotectin concentration is positively correlated with the intensity of fungal infection [47] with the presence of Sjogren’s syndrome [48], with periodontitis and the presence of Treponema denticola [32]. Our findings did not allow useful comparison or discrimination between groups or treatment phases, although there was a decrease with HA and placebo. This is somehow surprising, as calprotectin is overexpressed at sites of inflammation. Accordingly, levels found in stimulated saliva of patients with aggressive periodontitis [32] or Sjogren’s syndrome [48] were 106 higher than our findings. The sampling procedure and site of collection may be of major importance [49]. Still, a possible future role as a diagnostic marker in OLP patients is underpinned by the fact that for differentiation of bowel diseases, already-validated commercial tests are available [50].
Two cross-sectional studies found higher amounts of periodontal pathogens in OLP patients compared to patients with plaque-induced gingivitis, with subgingival sampling method, and suggested a possible association between periodontal pathogens and OLP [51,52]. In our study, the microbiologic analysis was performed from saliva, as this has been shown to be a promising alternative [34]. Interestingly, patients in our study presented with amounts of putative pathogens mostly below therapeutic thresholds before therapy, and levels did not differ significantly between groups. Hence, it was clear that the inflammatory parameters, Il-6 and calprotectin, did not origin from microorganisms. Also, the microflora changes under HA treatment, which is antimicrobial, could not be observed. This is in accordance with the minimal amount of plaque, as measured with PI. Although dental plaque is not the prevalent etiologic factor of OLP, several authors reported improvement of clinical parameters and severity after plaque reduction [53,54]. In comparison with other studies [55], patients in our cohort had low levels of gingival inflammation, which did not change significantly in either study phase (Figure 2).
An improvement in clinical severity was observed in the HA group, which dropped to baseline levels after placebo treatment, but without statistical significance. In a study evaluating the efficacy of a 0.2% topical hyaluronic acid gel preparation in the management of OLP patients, the test group showed only transient improvements for up to 4 hours post-application. There were no differences between treatment groups at any of the time intervals, and HA, as well as placebo, had no effect on the extent and severity [25]. These data corroborate our findings. In contrast, another placebo-controlled trial, with a 0.2% preparation applied three times a day for 14 days in 25 patients, reported a significant reduction in the HA group for the degree of erythema on day 14, 21, and 28, but not on day 7. For mean area of lesion and VAS scores, the difference was significant at all time points [27]. Recently, Hashem et al. compared the therapeutic effects of a 0.2% HA preparation with triamcinolone (TCS) 0.1% for 28 days, and reported that both HA and TCS reduced the VAS score, the degree of erythema, and size of the lesions after treatment, with TCS showing a faster relief [56]. In our study, the pain level improved in both groups, starting from a level that may be qualified as mild pain. As mentioned before, PI and GI were low, which corroborates the hypothesis that pain could interfere with oral hygiene procedures, and thus negatively influence periodontal conditions.
OHIP values of perceived quality of life were better in the placebo group, as well as the subjective outcome in the dimension “appearance”. However, a high drop-out rate (36%, five out of seven patients) was observed in the group that started with the placebo treatment. This could be due to unsatisfying effects of the placebo, which might have led patients to quit the study; however, this hypothesis would need further clarification. Furthermore, comparison of OHIP-G53 data with results derived from the use of the OHIP-14 is difficult, also due to a reference time of 4 weeks, in contrast to 3 months in the English version. However, others were able to show significant improvement of patient quality of life after treatment using OHIP-14 scores [57,58].
The present study provided a long application period for both HA and placebo, so effects should become visible. However, half-life of HA is very short, and patients came to recall appointments after the wash-out period. Hence, the effect could have been of very short time, and a relapse may have been recorded.
The concentration of HA might be responsible for different clinical effects. Galli et al. evaluated the efficiency of HA in improving the healing of surgical incisions in the oral cavity, and concluded that a single dose of 0.8% HA placed over surgical incisions in the oral cavity does not appear to improve wound healing [59]. In contrast, Yildirim et al. discussed the effect of topically-applied HA on pain and palatal epithelial wound healing, and found that 0.2% HA gel demonstrated better complete epithelization results on day 14, compared with 0.8% HA gel [60]. Thus, efficacy studies of different concentrations of high-molecular HA remain to be evaluated in detailed future studies.
While some authors related the positive immediate effect on pain in OLP [25] or patients with oral ulcers to a barrier effect of HA [61], Romeo et al. found no difference in pain perception in biopsy wounds treated with a gel containing amino acids and 1.33% HA versus no gel, despite faster healing [62]. Interestingly, proteins essential for lubrication and viscoelasticity were missing in saliva from OLP patients [63], possibly rendering them more sensitive to the beneficial effects of HA.
One limitation of our study is the high dropout rate and the small sample size. Sample size calculation took into account previous prospective trials of topical agents comprising similar numbers of participants [31]. However, the relatively small sample size in the current investigation can be outweighed by its prospective crossover design, with histologically confirmed OLP cases only [64]. Power calculation had foreseen a drop-out rate of only ten percent. It has to be pointed out that the high number in the placebo group resulted from the need for alternative treatments (cyclosporine A or topical steroids) due to unsustainable pain. The improved subjective outcome in the dimension “appearance” of this group might be due to a positive selection bias as a consequence of spontaneous remission.
For further studies, the ideal composition and concentration of HA should be determined, and standardized methods for evaluating the severity of the disease, such as cytotomorphometric analysis, and criteria for determining levels of improvement and patient-centered outcomes should be established [65]. Protein-based techniques in the past have been used to identify valid biomarkers for monitoring OLP, especially to be able to predict OLP malignant transformation [63]. Recently, proteomic profiling and a multiplex analysis of whole saliva identified 17 proteins triggering cytokines in OLP patients [66]. Thus, in the future, more complex methods might be necessary to reveal pathological functions and disease activity in OLP.
Author Contributions
Conceptualization: C.B. and G.D.; methodology: G.D.; formal analysis: R.S. and X.R.-F.; investigation: C.B. and K.R.; resources: G.D., X.R.-F., C.B.; data curation: R.S.; writing—original draft preparation: H.H., C.B., and G.D.; writing—review and editing: H.H., G.D., and C.B.; project administration: C.B. and G.D.; funding acquisition: C.B. and G.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received hyaluronic acid and placebo samples from Ricerfarma.
Acknowledgments
The authors would like to thank Marlene Muehlbachler (Division of Conservative Dentistry and Periodontology, University Clinic of Dentistry, Medical University of Vienna, Vienna, Austria) for her help in the clinical part of the study as well as Phuong Quynh Nguyen (Competence Centre for Periodontal Research, University Clinic of Dentistry, Medical University of Vienna, Vienna, Austria) for biomarker analyses.
Conflicts of Interest
The author(s) declare no potential conflict of interest with respect to the research, authorship, and/or publication of this article. The HA manufacturer (Ricerfarma) provided only the HA and placebo samples.
References
- Le Cleach, L.; Chosidow, O. Clinical practice. Lichen planus. N. Engl. J. Med. 2012, 366, 723–732. [Google Scholar] [CrossRef] [PubMed]
- McCartan, B.E.; Healy, C.M. The reported prevalence of oral lichen planus: A review and critique. J. Oral Pathol. Med. 2008, 37, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Fadler, A.; Hartmann, T.; Bernhart, T.; Monshi, B.; Rappersberger, K.; Hof, M.; Dvorak, G. Effect of personality traits on the oral health-related quality of life in patients with oral mucosal disease. Clin. Oral Investig. 2015, 19, 1245–1250. [Google Scholar] [CrossRef] [PubMed]
- Giannetti, L.; Dello Diago, A.M.; Spinas, E. Oral Lichen planus. J. Biol. Regul. Homeost. Agents 2018, 32, 391–395. [Google Scholar] [PubMed]
- Aghbari, S.M.H.; Abushouk, A.I.; Attia, A.; Elmaraezy, A.; Menshawy, A.; Ahmed, M.S.; Elsaadany, B.A.; Ahmed, E.M. Malignant transformation of oral lichen planus and oral lichenoid lesions: A meta-analysis of 20095 patient data. Oral Oncol. 2017, 68, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Eisen, D. The clinical manifestations and treatment of oral lichen planus. Dermatol. Clin. 2003, 21, 79–89. [Google Scholar] [CrossRef]
- Lodi, G.; Carrozzo, M.; Furness, S.; Thongprasom, K. Interventions for treating oral lichen planus: A systematic review. Br. J. Dermatol. 2012, 166, 938–947. [Google Scholar] [CrossRef]
- Yang, H.; Wu, Y.; Ma, H.; Jiang, L.; Zeng, X.; Dan, H.; Zhou, Y.; Chen, Q. Possible alternative therapies for oral lichen planus cases refractory to steroid therapies. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2016, 121, 496–509. [Google Scholar] [CrossRef]
- Gupta, S.; Ghosh, S.; Gupta, S. Interventions for the management of oral lichen planus: A review of the conventional and novel therapies. Oral Dis. 2017, 23, 1029–1042. [Google Scholar] [CrossRef]
- Machado, A.C.; Sugaya, N.N.; Migliari, D.A.; Matthews, R.W. Oral Lichen planus. Clinical aspects and management in fifty-two Brazilian patients. West Indian Med. J. 2004, 53, 113–117. [Google Scholar]
- Lozada-Nur, F.; Miranda, C. Oral lichen planus: Topical and systemic therapy. Semin. Cutan. Med. Surg. 1997, 16, 295–300. [Google Scholar] [CrossRef]
- Woo, T.Y. Systemic isotretinoin treatment of oral and cutaneous lichen planus. Cutis 1985, 35, 385. [Google Scholar] [PubMed]
- Mousavi, F.; Sherafati, S.; Mojaver, Y.N. Ignatia in the treatment of oral lichen planus. Homeopathy 2009, 98, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Won, T.H.; Park, S.Y.; Kim, B.S.; Seo, P.S.; Park, S.D. Levamisole monotherapy for oral lichen planus. Ann. Dermatol. 2009, 21, 250–254. [Google Scholar] [CrossRef]
- Chaitanya, N.C.; Chintada, S.; Kandi, P.; Kanikella, S.; Kammari, A.; Waghamare, R.S. Zinc therapy in treatment of symptomatic oral Lichen planus. Indian Dermatol. Online J. 2019, 10, 174–177. [Google Scholar] [CrossRef]
- Edens, M.H.; Carpenter, M.D.; Napenas, J.J.; Brennan, M.T. Impact of salivary hypofunction on incidence of orofungal infections with use of topical steroids for management of oral lichen planus and xerostomia. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 126, 501–505. [Google Scholar] [CrossRef]
- Agha-Hosseini, F.; Mirzaii-Dizgah, I.; Mohammadpour, N. Muscarinic cholinergic receptors (MR3) in saliva of patients with oral lichen planus. Arch. Dermatol. Res. 2016, 308, 481–486. [Google Scholar] [CrossRef]
- Becker, J.C.; Houben, R.; Vetter, C.S.; Brocker, E.B. The carcinogenic potential of tacrolimus ointment beyond immune suppression: A hypothesis creating case report. BMC Cancer 2006, 6, 7. [Google Scholar] [CrossRef]
- Gorsky, M.; Epstein, J.B. Oral Lichen planus: Malignant transformation and human papilloma virus: A review of potential clinical implications. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2011, 111, 461–464. [Google Scholar] [CrossRef]
- Lopez-Jornet, P.; Camacho-Alonso, F.; Salazar-Sanchez, N. Topical tacrolimus and pimecrolimus in the treatment of oral lichen planus: An update. J. Oral Pathol. Med. 2010, 39, 201–205. [Google Scholar] [CrossRef]
- Zaslansky, R.; Schramm, C.; Stein, C.; Guthoff, C.; Schmidt-Westhausen, A.M. Topical application of morphine for wound healing and analgesia in patients with oral lichen planus: A randomized, double-blind, placebo-controlled study. Clin. Oral Investig. 2018, 22, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Tvarijonaviciute, A.; Aznar-Cayuela, C.; Rubio, C.P.; Tecles, F.; Ceron, J.J.; Lopez-Jornet, P. Salivary antioxidant status in patients with oral Lichen planus: Correlation with clinical signs and evolution during treatment with Chamaemelum nobile. Biomed. Res. Int. 2018, 2018, 5187549. [Google Scholar] [CrossRef] [PubMed]
- Bertl, K.; Bruckmann, C.; Isberg, P.E.; Klinge, B.; Gotfredsen, K.; Stavropoulos, A. Hyaluronan in non-surgical and surgical periodontal therapy: A systematic review. J. Clin. Periodontol. 2015, 42, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Al-Shammari, N.M.; Shafshak, S.M.; Ali, M.S. Effect of 0.8% hyaluronic acid in conventional treatment of moderate to severe chronic periodontitis. J. Contemp. Dent. Pract. 2018, 19, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Hashem, A.S.; Issrani, R.; Elsayed, T.E.E.; Prabhu, N. Topical hyaluronic acid in the management of oral lichen planus: A comparative study. J. Investig. Clin. Dent. 2019, 10, e12385. [Google Scholar] [CrossRef] [PubMed]
- Nolan, A.; Badminton, J.; Maguire, J.; Seymour, R.A. The efficacy of topical hyaluronic acid in the management of oral lichen planus. J. Oral Pathol. Med. 2009, 38, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Radwan-Oczko, M. Topical application of drugs used in treatment of oral lichen planus lesions. Adv. Clin. Exp. Med. 2013, 22, 893–898. [Google Scholar]
- Shetty, R.R.; Burde, K.N.; Guttal, K.S. The efficacy of topical hyaluronic acid 0.2% in the management of symptomatic oral lichen planus. J. Clin. Diagn. Res. 2016, 10, ZC46–ZC50. [Google Scholar] [CrossRef]
- Mozaffari, H.R.; Sharifi, R.; Sadeghi, M. Interleukin-6 levels in the serum and saliva of patients with oral lichen planus compared with healthy controls: A meta-analysis study. Cent. Eur. J. Immunol. 2018, 43, 103–108. [Google Scholar] [CrossRef]
- Eversole, L.R.; Miyasaki, K.T.; Christensen, R.E. Keratinocyte expression of calprotectin in oral inflammatory mucosal diseases. J. Oral Pathol. Med. 1993, 22, 303–307. [Google Scholar] [CrossRef]
- Ross, K.F.; Herzberg, M.C. Calprotectin expression by gingival epithelial cells. Infect. Immun. 2001, 69, 3248–3254. [Google Scholar] [CrossRef] [PubMed]
- Rhodus, N.L.; Cheng, B.; Bowles, W.; Myers, S.; Miller, L.; Ondrey, F. Proinflammatory cytokine levels in saliva before and after treatment of (erosive) oral lichen planus with dexamethasone. Oral Dis. 2006, 12, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Haririan, H.; Andrukhov, O.; Pablik, E.; Neuhofer, M.; Moritz, A.; Rausch-Fan, X. Comparative analysis of calcium-binding myeloid-related protein-8/14 in saliva and serum of patients with periodontitis and healthy individuals. J. Periodontol. 2016, 87, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Navazesh, M.; Kumar, S.K. Measuring salivary flow: Challenges and opportunities. J. Am. Dent. Assoc. 2008, 139, 35S–40S. [Google Scholar] [CrossRef]
- Haririan, H.; Andrukhov, O.; Bertl, K.; Lettner, S.; Kierstein, S.; Moritz, A.; Rausch-Fan, X. Microbial analysis of subgingival plaque samples compared to that of whole saliva in patients with periodontitis. J. Periodontol. 2014, 85, 819–828. [Google Scholar] [CrossRef]
- Thongprasom, K.; Chaimusig, M.; Korkij, W.; Sererat, T.; Luangjarmekorn, L.; Rojwattanasirivej, S. A randomized-controlled trial to compare topical cyclosporin with triamcinolone acetonide for the treatment of oral lichen planus. J. Oral Pathol. Med. 2007, 36, 142–146. [Google Scholar] [CrossRef]
- Silness, J.; Loe, H. Periodontal disease in pregnancy II. Correlation between oral hygiene and periodontal condtion. Acta Odontol. Scand. 1964, 22, 121–135. [Google Scholar] [CrossRef]
- Loe, H.; Silness, J. Periodontal disease in pregnancy I. Prevalence and severity. Acta Odontol. Scand. 1963, 21, 533–551. [Google Scholar] [CrossRef]
- John, M.T.; LeResche, L.; Koepsell, T.D.; Hujoel, P.; Miglioretti, D.L.; Micheelis, W. Oral health-related quality of life in Germany. Eur. J. Oral Sci. 2003, 111, 483–491. [Google Scholar] [CrossRef]
- Thong, I.S.K.; Jensen, M.P.; Miro, J.; Tan, G. The validity of pain intensity measures: What do the NRS, VAS, VRS, and FPS-R measure? Scand. J. Pain 2018, 18, 99–107. [Google Scholar] [CrossRef]
- Casale, M.; Vella, P.; Moffa, A.; Oliveto, G.; Sabatino, L.; Grimaldi, V.; Ferrara, P.; Salvinelli, F. Hyaluronic acid and upper airway inflammation in pediatric population: A systematic review. Int. J. Pediatr. Otorhinolaryngol. 2016, 85, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Gu, G.M.; Martin, M.D.; Darveau, R.P.; Truelove, E.; Epstein, J. Oral and serum IL-6 levels in oral lichen planus patients. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2004, 98, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Jacobs, R. Proinflammatory cytokine levels in oral lichen planus, oral leukoplakia, and oral submucous fibrosis. J. Korean Assoc. Oral Maxillofac. Surg. 2015, 41, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Shi, Q.; Yang, S.; Wang, Q.; Xu, J.; Guo, B. The relationship between levels of salivary and serum interleukin-6 and oral lichen planus: A systematic review and meta-analysis. J. Am. Dent. Assoc. 2017, 148, 743–749. [Google Scholar] [CrossRef]
- Brailo, V.; Vucicevic-Boras, V.; Cekic-Arambasin, A.; Alajbeg, I.Z.; Milenovic, A.; Lukac, J. The significance of salivary interleukin 6 and tumor necrosis factor alpha in patients with oral leukoplakia. Oral Oncol. 2006, 42, 370–373. [Google Scholar] [CrossRef]
- Pruenster, M.; Vogl, T.; Roth, J.; Sperandio, M. S100A8/A9: From basic science to clinical application. Pharmacol. Ther. 2016, 167, 120–131. [Google Scholar] [CrossRef]
- Foell, D.; Frosch, M.; Sorg, C.; Roth, J. Phagocyte-specific calcium-binding S100 proteins as clinical laboratory markers of inflammation. Clin. Chim. Acta 2004, 344, 37–51. [Google Scholar] [CrossRef]
- Kleinegger, C.L.; Stoeckel, D.C.; Kurago, Z.B. A comparison of salivary calprotectin levels in subjects with and without oral candidiasis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2001, 92, 62–67. [Google Scholar] [CrossRef]
- Cuida, M.; Halse, A.K.; Johannessen, A.C.; Tynning, T.; Jonsson, R. Indicators of salivary gland inflammation in primary Sjogren’s syndrome. Eur. J. Oral Sci. 1997, 105, 228–233. [Google Scholar] [CrossRef]
- Cuida, M.; Brun, J.G.; Tynning, T.; Jonsson, R. Calprotectin levels in oral fluids: The importance of collection site. Eur. J. Oral Sci. 1995, 103, 8–10. [Google Scholar] [CrossRef]
- Waugh, N.; Cummins, E.; Royle, P.; Kandala, N.B.; Shyangdan, D.; Arasaradnam, R.; Clar, C.; Johnston, R. Faecal calprotectin testing for differentiating amongst inflammatory and non-inflammatory bowel diseases: Systematic review and economic evaluation. Health Technol. Assess. 2013, 17, 1–211. [Google Scholar] [CrossRef] [PubMed]
- Ertugrul, A.S.; Arslan, U.; Dursun, R.; Hakki, S.S. Periodontopathogen profile of healthy and oral lichen planus patients with gingivitis or periodontitis. Int. J. Oral Sci. 2013, 5, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Arduino, P.G.; Romano, F.; Sasia, D.; Broccoletti, R.; Ricceri, F.; Barbui, A.M.; Brossa, S.; Cipriani, R.; Cricenti, L.; Cabras, M.; et al. Subgingival microbiota in white patients with desquamative gingivitis: A cross-sectional study. J. Periodontol. 2017, 88, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Holmstrup, P.; Schiotz, A.W.; Westergaard, J. Effect of dental plaque control on gingival lichen planus. Oral Surg. Oral Med. Oral Pathol. 1990, 69, 585–590. [Google Scholar] [CrossRef]
- Stone, S.J.; McCracken, G.I.; Heasman, P.A.; Staines, K.S.; Pennington, M. Cost-effectiveness of personalized plaque control for managing the gingival manifestations of oral lichen planus: A randomized controlled study. J. Clin. Periodontol. 2013, 40, 859–867. [Google Scholar] [CrossRef]
- Rai, N.P.; Kumar, P.; Mustafa, S.M.; Divakar, D.D.; Kheraif, A.A.; Ramakrishnaiah, R.; Vellapally, S.; Dalati, M.H.; Parine, N.R.; Anil, S. Relation between periodontal status and pre-cancerous condition (oral lichen planus): A pilot study. Adv. Clin. Exp. Med. Off. Organ Wroclaw Med. Univ. 2016, 25, 763–766. [Google Scholar] [CrossRef]
- Lopez-Jornet, P.; Camacho-Alonso, F.; Lucero Berdugo, M. Measuring the impact of oral mucosa disease on quality of life. Eur. J. Dermatol. 2009, 19, 603–606. [Google Scholar] [CrossRef]
- Lee, Y.C.; Shin, S.Y.; Kim, S.W.; Eun, Y.G. Intralesional injection versus mouth rinse of triamcinolone acetonide in oral lichen planus: A randomized controlled study. Otolaryngol. Head Neck Surg. 2013, 148, 443–449. [Google Scholar] [CrossRef]
- Galli, F.; Zuffetti, F.; Capelli, M.; Fumagalli, L.; Parenti, A.; Testori, T.; Esposito, M. Hyaluronic acid to improve healing of surgical incisions in the oral cavity: A pilot multicentre placebo-controlled randomised clinical trial. Eur. J. Oral Implantol. 2008, 1, 199–206. [Google Scholar]
- Yildirim, S.; Ozener, H.O.; Dogan, B.; Kuru, B. Effect of topically-applied hyaluronic-acid on pain and palatal epithelial wound healing: An examiner-blind, randomized, controlled clinical trial. J. Periodontol. 2017, 1–14. [Google Scholar] [CrossRef]
- Nolan, A.; Baillie, C.; Badminton, J.; Rudralingham, M.; Seymour, R.A. The efficacy of topical hyaluronic acid in the management of recurrent aphthous ulceration. J. Oral Pathol. Med. 2006, 35, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Romeo, U.; Libotte, F.; Palaia, G.; Galanakis, A.; Gaimari, G.; Tenore, G.; Del Vecchio, A.; Polimeni, A. Oral soft tissue wound healing after laser surgery with or without a pool of amino acids and sodium hyaluronate: A randomized clinical study. Photomed. Laser Surg. 2014, 32, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo-Pouso, A.I.; Perez-Sayans, M.; Bravo, S.B.; Lopez-Jornet, P.; Garcia-Vence, M.; Alonso-Sampedro, M.; Carballo, J.; Garcia-Garcia, A. Protein-based salivary profiles as novel biomarkers for oral diseases. Dis. Markers 2018, 2018, 6141845. [Google Scholar] [CrossRef] [PubMed]
- Suchmacher, M.; Geller, M. Chapter 1—Study type determination. In Practical Biostatistics; Suchmacher, M., Geller, M., Eds.; Academic Press: San Diego, CA, USA, 2012; pp. 3–15. [Google Scholar] [CrossRef]
- Ní Ríordáin, R.; Shirlaw, P.; Alajbeg, I.; Al Zamel, G.Y.; Fung, P.L.; Yuan, A.D.; McCreary, C.; Stoopler, E.T.; De Rossi, S.S.; Lodi, G.; et al. World Workshop on Oral Medicine VI: Patient-reported outcome measures and oral mucosal disease: Current status and future direction. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2015, 120, 152–160. [Google Scholar] [CrossRef]
- Souza, M.M.; Florezi, G.P.; Nico, M.; de Paula, F.; Paula, F.M.; Lourenco, S.V. Salivary proteomics in lichen planus: A relationship with pathogenesis? Oral Dis. 2018, 24, 784–792. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).