Modeling the Ecosystem Services Related to Phytoextraction: Carbon Sequestration Potential Using Willow and Poplar
Abstract
:1. Introduction
2. Materials and Methods
2.1. Description of the Experimental Site
2.2. Determination of the Actual Phytoextraction Rate
2.3. Trace Element Assessment in Soil and Evaluation of Phytoextraction
2.4. Estimation of Ecosystem Services Provided by Forest
2.5. Economic Evaluation of Carbon Dioxide Sequestration
- CSi = carbon dioxide sequestration value of i-th trees species (EUR ha−1);
- Bi = carbon content (expressed as CO2) in the dried biomass of i-th trees species (Mg ha−1);
- Pc = pricing carbon dioxide (EUR Mg−1).
2.6. Statistical Analysis
3. Results
3.1. Biomass Yield of Willow and Poplar
3.2. Phytoextraction and Soil Quality
3.3. Carbon Dioxide Sequestration Value
- Cn = value of carbon dioxide sequestration at the end (year n) of our analysis (2018) (EUR ha−1);
- C0 = value of carbon dioxide sequestration at different years (i.e., 2015, 2016, 2017, 2018) (EUR ha−1);
- q = 1 + r (r is the market discount rate);
- n = length of the period (time between C0 and Cn).
4. Discussion
4.1. Biomass Yield
4.2. Phytoextraction
4.3. Carbon Dioxide Sequestration
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pérez, A.P.; Eugenio, N.R. Status of Local Soil Contamination in Europe: Revision of the indicator Progress in the management Contaminated Sites in Europe; EUR 29124 EN; Publications Office of the European Union: Luxembourg, 2018; ISBN 978-92-79-80072-6. [Google Scholar] [CrossRef]
- Perez, J. The Soil Remediation Industry in Europe: The Recent Past and Future Perspectives Context of the Soil Remediation in Member States Overview of the Regulatory Context Situation of Soil Remediation Market; Young, Ernst & Young: London, UK, 2012. [Google Scholar]
- Järup, L. Hazards of heavy metal contamination. Br. Med. Bull. 2003, 68, 167–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- E.P.A. Recent Developments for In Situ Treatment of Metal Contaminated Soils; US Environ. Prot. Agency: Washington, DC, USA, 1997; 64p.
- Pilon-Smits, E. Phytoremediation. Annu. Rev. Plant Biol. 2005, 56, 15–39. [Google Scholar] [CrossRef] [PubMed]
- Sheoran, V.; Sheoran, A.S.; Poonia, P. Factors Affecting Phytoextraction: A Review. Pedosphere 2016, 26, 148–166. [Google Scholar] [CrossRef]
- Baker, A.J.M.; Brooks, R.R. Terrestrial higher plants which hyperaccumulate metallic elements-a review of their distribution, ecology and phytochemistry. Biorecovery 1989, 1, 81–126. [Google Scholar]
- Krzciuk, K.; Gałuszka, A. Prospecting for hyperaccumulators of trace elements: A review. Crit. Rev. Biotechnol. 2015, 35, 522–532. [Google Scholar] [CrossRef]
- Rockwood, D.L.; Naidu, C.V.; Carter, D.R.; Rahmani, M.; Spriggs, T.A.; Lin, C.; Alker, G.R.; Isebrands, J.G.; Segrest, S.A. Short-rotation woody crops and phytoremediation: Opportunities for agroforestry? Agrofor. Syst. 2004, 61–62, 51–63. [Google Scholar] [CrossRef]
- Komives, T.; Gullner, G. Dendroremediation: The Use of Trees in Cleaning up Polluted Soils. Phytoremediation Rhizoremediation 2006, 23–31. [Google Scholar] [CrossRef]
- Guidi Nissim, W.; Palm, E.; Mancuso, S.; Azzarello, E. Trace element phytoextraction from contaminated soil: A case study under Mediterranean climate. Environ. Sci. Pollut. Res. 2018, 25, 9114–9131. [Google Scholar] [CrossRef]
- Guidi Nissim, W.; Cincinelli, A.; Martellini, T.; Alvisi, L.; Palm, E.; Mancuso, S.; Azzarello, E. Phytoremediation of sewage sludge contaminated by trace elements and organic compounds. Environ. Res. 2018, 164, 356–366. [Google Scholar] [CrossRef]
- Zalesny, J.A.; Zalesny, R.S.; Coyle, D.R.; Hall, R.B. Growth and biomass of Populus irrigated with landfill leachate. For. Ecol. Manag. 2007, 248, 143–152. [Google Scholar] [CrossRef]
- Guidi Nissim, W.; Jerbi, A.; Lafleur, B.; Fluet, R.; Labrecque, M. Willows for the treatment of municipal wastewater: Performance under different irrigation rates. Ecol. Eng. 2015, 81, 395–404. [Google Scholar] [CrossRef]
- Gerhardt, K.E.; Huang, X.D.; Glick, B.R.; Greenberg, B.M. Phytoremediation and rhizoremediation of organic soil contaminants: Potential and challenges. Plant Sci. 2009, 176, 20–30. [Google Scholar] [CrossRef]
- Weir, E.; Doty, S. Social acceptability of phytoremediation: The role of risk and values. Int. J. Phytoremediation 2016, 18, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.H. Ecological restoration of mine degraded soils, with emphasis on metal contaminated soils. Chemosphere 2003, 50, 775–780. [Google Scholar] [CrossRef]
- Nehnevajova, E.; Herzig, R.; Erismann, K.H.; Schwitzguébel, J.P. In vitro breeding of Brassica juncea L. to enhance metal accumulation and extraction properties. Plant Cell Rep. 2007, 26, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Grenier, V.; Pitre, F.E.; Guidi Nissim, W.; Labrecque, M. Genotypic differences explain most of the response of willow cultivars to petroleum-contaminated soil. Trees-Struct. Funct. 2015, 29, 871–881. [Google Scholar] [CrossRef]
- Guidi Nissim, W.; Palm, E.; Mancuso, S.; Azzarello, E. Trace element partitioning in a poplar phytoextraction stand in relation to stem size. J. Environ. Manag. 2019, 247, 688–697. [Google Scholar] [CrossRef]
- Jan, S.; Rashid, B.; Azooz, M.M.; Hossain, M.A.; Ahmad, P. Genetic Strategies for Advancing Phytoremediation Potential in Plants: A Recent Update. Plant Met. Interact. Emerg. Remediat. Tech. 2015, 431–454. [Google Scholar] [CrossRef]
- Lebeau, T.; Braud, A.; Jézéquel, K. Performance of bioaugmentation-assisted phytoextraction applied to metal contaminated soils: A review. Environ. Pollut. 2008, 153, 497–522. [Google Scholar] [CrossRef]
- Shahid, M.; Austruy, A.; Echevarria, G.; Arshad, M.; Sanaullah, M.; Aslam, M.; Nadeem, M.; Nasim, W.; Dumat, C. EDTA-Enhanced Phytoremediation of Heavy Metals: A Review. Soil Sediment Contam. 2014, 23, 389–416. [Google Scholar] [CrossRef]
- USEPA. Green Remediation: Incorporating Sustainable Environmental Practices into Remediation of Contaminated Sites; United States Environmental Protection Agency: Cincinnati, OH, USA, 2008; Volume EPA 542-R-08-002, ISBN 3016043408.
- Schröder, P.; Herzig, R.; Bojinov, B.; Ruttens, A.; Nehnevajova, E.; Stamatiadis, S.; Memon, A.; Vassilev, A.; Caviezel, M.; Vangronsveld, J. Bioenergy to save the world: Producing novel energy plants for growth on abandoned land. Environ. Sci. Pollut. Res. 2008, 15, 196–204. [Google Scholar] [CrossRef]
- Witters, N.; Mendelsohn, R.O.; Van Slycken, S.; Weyens, N.; Schreurs, E.; Meers, E.; Tack, F.; Carleer, R.; Vangronsveld, J. Phytoremediation, a sustainable remediation technology? Conclusions from a case study. I: Energy production and carbon dioxide abatement. Biomass Bioenergy 2012, 39, 454–469. [Google Scholar] [CrossRef]
- Pearce, D.W. The Economic Value of Forest Ecosystems. Ecosyst. Heal. 2001, 7, 284–296. [Google Scholar] [CrossRef]
- Blattert, C.; Lemm, R.; Thees, O.; Lexer, M.J.; Hanewinkel, M. Management of ecosystem services in mountain forests: Review of indicators and value functions for model based multi-criteria decision analysis. Ecol. Indic. 2017, 79, 391–409. [Google Scholar] [CrossRef]
- Bottalico, F.; Pesola, L.; Vizzarri, M.; Antonello, L.; Barbati, A.; Chirici, G.; Corona, P.; Cullotta, S.; Garfì, V.; Giannico, V.; et al. Modeling the influence of alternative forest management scenarios on wood production and carbon storage: A case study in the Mediterranean region. Environ. Res. 2016, 144, 72–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciccarese, L.; Crosti, R.; Cascone, C.; Cipollaro, S.; Ballarin Denti, A.; Fontanarosa, E.; Masiero, M.; Pizzuto Antinoro, M.; Veca, L.M.D. Status Report of Forest Biomass Use in the Mediterranean Region. Proforbiomed Report. Case-Study: Italy; ISPRA: Rome, Italy, 2012.
- Pearce, D.; Turner, K.; Bateman, I. Economia Ambientale; Il Mulino: Bologna, Italy, 2003. [Google Scholar]
- Stanturf, J.A. Future landscapes: Opportunities and challenges. New For. 2015, 46, 615–644. [Google Scholar] [CrossRef]
- Marta-Pedroso, C.; Laporta, L.; Gama, I.; Domingos, T. Economic valuation and mapping of ecosystem services in the context of protected area management (Natural park of Serra de São Mamede, Portugal). One Ecosyst. 2018, 3. [Google Scholar] [CrossRef] [Green Version]
- Costanza, R.; de Groot, R.; Braat, L.; Kubiszewski, I.; Fioramonti, L.; Sutton, P.; Farber, S.; Grasso, M. Twenty years of ecosystem services: How far have we come and how far do we still need to go? Ecosyst. Serv. 2017, 28, 1–16. [Google Scholar] [CrossRef]
- Bateman, I.J.; Mace, G.M.; Fezzi, C.; Atkinson, G.; Turner, K. Economic analysis for ecosystem service assessments. Environ. Resour. Econ. 2011, 48, 177–218. [Google Scholar] [CrossRef] [Green Version]
- Repubblica Italiana. Decreto Legislativo 3 aprile 2006, n. 152; Norme in materia ambientale. Gazz. Uff. 2006, 1, 172. [Google Scholar]
- Walkley, A.; Armstrong, B. An examination of the degtjareff method for determining soil organic matter. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Conyers, M.K.; Davey, B.G. Observations on some routine methods for soil pH determination. Soil Sci. 1988, 29–36. [Google Scholar] [CrossRef]
- Pansu, M.; Gautheyrou, J. Handbook of Soil Analysis; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Bremner, J.M. Determination of nitrogen in soil by the Kjeldahl method. J. Agric. Sci. 1960, 55, 11–33. [Google Scholar] [CrossRef]
- Bennett, J.W.; Pearce, D.W.; Turner, R.K. Economics of Natural Resources and the Environment. Am. J. Agric. Econ. 1991, 73, 227. [Google Scholar] [CrossRef]
- Tao, Z.; Yan, H.; Zhan, J. Economic Valuation of Forest Ecosystem Services in Heshui Watershed using Contingent Valuation Method. Procedia Environ. Sci. 2012, 13, 2445–2450. [Google Scholar] [CrossRef] [Green Version]
- Bernetti, I.; Sottini, V.A.; Marinelli, N.; Marone, E.; Menghini, S.; Riccioli, F.; Sacchelli, S.; Marinelli, A. Quantification of the total economic value of forest systems: Spatial analysis application to the region of Tuscany (Italy). Aestimum 2013, n.62, 29–65. [Google Scholar]
- Tempesta, T.; Thiene, M. Percezione e Valore del Paesaggio; Franco Angeli: Milan, Italy, 2006; ISBN 9788846479136. [Google Scholar]
- Riccioli, F.; Marone, E.; Boncinelli, F.; Tattoni, C.; Rocchini, D.; Fratini, R. The recreational value of forests under different management systems. New For. 2019, 50, 345–360. [Google Scholar] [CrossRef] [Green Version]
- Santos, P.Z.F.; Crouzeilles, R.; Sansevero, J.B.B. Can agroforestry systems enhance biodiversity and ecosystem service provision in agricultural landscapes? A meta-analysis for the Brazilian Atlantic Forest. For. Ecol. Manag. 2019, 433. [Google Scholar] [CrossRef]
- José, I.B.; Bastrup-Birk, A.; Teller, A.; Onaindia, M.; Fernández, d.M.B.; Madariaga, I.; Rodríguez-Loinaz, G.; Pinho, P.; Nunes, A.; Ramos, A.; et al. Mapping and Assessment of Forest Ecosystems and Their Services–Applications and Guidance for Decision Making in the Framework of MAES; European Commission: Brussels, Belgium, 2015. [Google Scholar]
- SAGOFF, M. On the Economic Value of Ecosystem Services. Environ. Values 2008, 17, 239–257. [Google Scholar] [CrossRef] [Green Version]
- Lal, R. Forest soils and carbon sequestration. For. Ecol. Manag. 2005, 220, 242–258. [Google Scholar] [CrossRef]
- Moise, I.; Moise, V. Algorithm for Carbon Capacity Storage of the Forest Species According to Soil Characteristics and Stands Age. J. Environ. Prot. Ecol. 2013, 14, 1651–1660. [Google Scholar]
- Riccioli, F.; Castiglione, F.; Casini, L.; El Asmar, J.; Fratini, R. Analysis of Ecosystem Services Provided by Forests: A Case Study in Southern Italy. Sci. Reg. 2019, 3. [Google Scholar] [CrossRef]
- Trexler, M. Minding the Carbon Store: Weighing US Forestry Strategies to Slow Global Warming; World Resources Institute: New York, NY, USA, 1991. [Google Scholar]
- Patton, D.; Bergstrom, J.C.; Moore, R.; Covich, A.P. Economic value of carbon storage in U.S. National Wildlife Refuge wetland ecosystems. Ecosyst. Serv. 2015, 16, 94–104. [Google Scholar] [CrossRef]
- Gren, I.M.; Carlsson, M. Economic value of carbon sequestration in forests under multiple sources of uncertainty. J. For. Econ. 2013, 19, 174–189. [Google Scholar] [CrossRef]
- Jerath, M.; Bhat, M.; Rivera-Monroy, V.H.; Castañeda-Moya, E.; Simard, M.; Twilley, R.R. The role of economic, policy, and ecological factors in estimating the value of carbon stocks in Everglades mangrove forests, South Florida, USA. Environ. Sci. Policy 2016, 66, 160–169. [Google Scholar] [CrossRef] [Green Version]
- Garzuglia, M.; Saket, M. Wood Volume and Woody Biomass: Review of FRA 2000 Estimates. Available online: http://www.fao.org/3/ae153e/AE153e00.htm#TopOfPage (accessed on 27 July 2020).
- Sendeco2-Carbon Prices. Available online: https://www.sendeco2.com/it/ (accessed on 27 July 2020).
- Lamlom, S.H.; Savidge, R.A. A reassessment of carbon content in wood: Variation within and between 41 North American species. Biomass Bioenergy 2003, 25, 381–388. [Google Scholar] [CrossRef]
- Carbon Pricing Leadership Coalition Understanding Carbon Pricing. Available online: https://www.carbonpricingleadership.org/what (accessed on 27 July 2020).
- Romm, J. A Big Source of Climate Confusion: The Factor of 3.67 Difference between Carbon vs. Carbon Dioxide–Think Progress. Available online: https://archive.thinkprogress.org/a-big-source-of-climate-confusion-the-factor-of-3-67-difference-between-carbon-vs-carbon-dioxide-9eb19bd2bb7c/ (accessed on 21 August 2020).
- Bredenberg, A. Carbon Dioxide: How Can a Little CO2 Molecule Be Such a Big Troublemaker? Available online: https://www.thomasnet.com/insights/carbon-dioxide-how-can-the-little-co2-molecule-be-such-a-big-troublemaker/ (accessed on 21 August 2020).
- Malagoli, L.; Bertoldo, M. Estimo Territoriale e Ambientale; Aracne, Ed.: Roma, Italy, 2007. [Google Scholar]
- Michieli, I.; Michieli, M. Trattato di Estimo; Edagricole: Bologna, Italy, 2011. [Google Scholar]
- Gallerani, V.; Zanni, G.; Viaggi, D. Manuale di Estimo, 2nd ed.; McGraw-Hill, Ed.: Milano, Italy, 2011; ISBN 978-88-386-6501-1. [Google Scholar]
- Adger, W.N.; Brown, K.; Cervigni, R.; Moran, D. Total economic value of forests in Mexico. Ambio 1995, 24, 286–296. [Google Scholar] [CrossRef]
- Krieger, D. The Economic Value of Forest Ecosystem Services: A Review; Wilderness Society: Washington, DC, USA, 2001. [Google Scholar]
- Ciancio, O.; Corona, P.; Marinelli, M.; Pettenella, D. Metodologia per la Valutazione Economica dei Danni da Incendi Boschivi; Accademia di Scienze Forestali: Firenze, Italy, 2007. [Google Scholar]
- Saltelli, A.; Tarantola, S.; Campolongo, F.; Ratto, M. Sensitivity Analysis in Practice: A Guide to Assessing Scientific Models|Wiley; Wiley: Hoboken, NJ, USA, 2004. [Google Scholar]
- Pappenberger, F.; Iorgulescu, I.; Beven, K.J. Sensitivity analysis based on regional splits and regression trees (SARS-RT). Environ. Model. Softw. 2006, 21, 976–990. [Google Scholar] [CrossRef]
- Nordhaus, W. Critical assumptions in the stern review on climate change. Science 2007, 317, 201–202. [Google Scholar] [CrossRef]
- Capuana, M. A review of the performance of woody and herbaceous ornamental plants for phytoremediation in urban areas. iForest-Biogeosciences For. 2020, 13, 139–151. [Google Scholar] [CrossRef] [Green Version]
- Dickmann, D.I.; Kuzovkina, J. Poplars and Willows in the World; FAO UN: Rome, Italy, 2008. [Google Scholar]
- Mitchell, C.P. Ecophysiology of short rotation forest crops. Biomass Bioenergy 1992, 2, 25–37. [Google Scholar] [CrossRef]
- Bédéneau, M.; Auclair, D. Effect of coppicing on hybrid poplar fine root dynamics. Ann. des Sci. For. 1989, 46, 294s–296s. [Google Scholar] [CrossRef] [Green Version]
- Labrecque, M.; Teodorescu, T.I. Field performance and biomass production of 12 willow and poplar clones in short-rotation coppice in southern Quebec (Canada). Biomass Bioenergy 2005, 29, 1–9. [Google Scholar] [CrossRef]
- Nassi, O.; Di Nasso, N.; Guidi, W.; Ragaglini, G.; Tozzini, C.; Bonari, E. Biomass production and energy balance of a 12-year-old short-rotation coppice poplar stand under different cutting cycles. GCB Bioenergy 2010, 2, 89–97. [Google Scholar] [CrossRef]
- Kopp, R.F.; Abrahamson, L.P.; White, E.H.; Volk, T.A.; Nowak, C.A.; Fillhart, R.C. Willow biomass production during ten successive annual harvests. Biomass Bioenergy 2001, 20, 1–7. [Google Scholar] [CrossRef]
- Facciotto, G.; Bergante, S.; Lioia, C.; Mughini, G.; Zenone, T. Produttività di cloni di pioppo e salice in piantagioni a turno breve. In Proceedings of the V Congresso Nazionale SISEF-“Foreste e Società: Cambiamenti, Conflitti, Sinergie”, Turin, Italy, 27–30 September 2005; Volume 1, pp. 27–30. [Google Scholar]
- Yu, G.; Jiang, P.; Fu, X.; Liu, J.; Sunahara, G.I.; Chen, Z.; Xiao, H.; Lin, F.; Wang, X. Phytoextraction of cadmium-contaminated soil by Celosia argentea Linn.: A long-term field study. Environ. Pollut. 2020, 266. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Li, Z.; Wang, J.; Liu, H.; Li, N.; Wu, L.; Hu, P.; Luo, Y.; Christie, P. Long-term field phytoextraction of zinc/cadmium contaminated soil by Sedum plumbizincicola under different agronomic strategies. Int. J. Phytoremediation 2016, 18, 134–140. [Google Scholar] [CrossRef]
- Zacchini, M.; Pietrini, F.; Scarascia Mugnozza, G.; Iori, V.; Pietrosanti, L.; Massacci, A. Metal tolerance, accumulation and translocation in poplar and willow clones treated with cadmium in hydroponics. Water. Air. Soil Pollut. 2009, 197, 23–34. [Google Scholar] [CrossRef]
- Dos Santos Utmazian, M.N.; Wieshammer, G.; Vega, R.; Wenzel, W.W. Hydroponic screening for metal resistance and accumulation of cadmium and zinc in twenty clones of willows and poplars. Environ. Pollut. 2007, 148, 155–165. [Google Scholar] [CrossRef]
- Kacálková, L.; Tlustoš, P.; Száková, J. Phytoextraction of Risk Elements by Willow and Poplar Trees. Int. J. Phytoremediation 2015, 17, 414–421. [Google Scholar] [CrossRef]
- Sebastiani, L.; Scebba, F.; Tognetti, R. Heavy metal accumulation and growth responses in poplar clones Eridano (Populus deltoides × maximowiczii) and I-214 (P. × euramericana) exposed to industrial waste. Environ. Exp. Bot. 2004, 52, 79–88. [Google Scholar] [CrossRef]
- Hammer, D.; Kayser, A.; Keller, C. Phytoextraction of Cd and Zn with Salix viminalis in field trials. Soil Use Manag. 2003, 19, 187–192. [Google Scholar] [CrossRef]
- Meers, E.; Lamsal, S.; Vervaeke, P.; Hopgood, M.; Lust, N.; Tack, F.M.G. Availability of heavy metals for uptake by Salix viminalis on a moderately contaminated dredged sediment disposal site. Environ. Pollut. 2005, 137, 354–364. [Google Scholar] [CrossRef]
- King, R.F.; Royle, A.; Putwain, P.D.; Dickinson, N.M. Changing contaminant mobility in a dredged canal sediment during a three-year phytoremediation trial. Environ. Pollut. 2006, 143, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Van Nevel, L.; Mertens, J.; Oorts, K.; Verheyen, K. Phytoextraction of metals from soils: How far from practice? Environ. Pollut. 2007, 150, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Seidl, R.; Rammer, W.; Jäger, D.; Currie, W.S.; Lexer, M.J. Assessing trade-offs between carbon sequestration and timber production within a framework of multi-purpose forestry in Austria. For. Ecol. Manag. 2007, 248, 64–79. [Google Scholar] [CrossRef]
- Sandhu, H.S.; Wratten, S.D.; Cullen, R.; Case, B. The future of farming: The value of ecosystem services in conventional and organic arable land. An experimental approach. Ecol. Econ. 2008, 64, 835–848. [Google Scholar] [CrossRef]
- Kumar Lal, A. Economic Worth of Carbon stored in above ground Biomass of Indian Forests. In Proceedings of the XII World Forestry Congress, Québec City, QC, Canada, 21–28 September 2003. [Google Scholar]
- Ibarra, A.A.; Zambrano, L.; Valiente, E.L.; Ramos-Bueno, A. Enhancing the potential value of environmental services in urban wetlands: An agro-ecosystem approach. Cities 2013, 31, 438–443. [Google Scholar] [CrossRef]
- IFM The Case for Carbon Taxation–IMF F&D|DECEMBER 2019. Available online: https://www.imf.org/external/pubs/ft/fandd/2019/12/the-case-for-carbon-taxation-and-putting-a-price-on-pollution-parry.htm (accessed on 27 July 2020).
- IFM Fiscal Monitor: How to Mitigate Climate Change. Available online: https://www.imf.org/en/Publications/FM/Issues/2019/09/12/fiscal-monitor-october-2019 (accessed on 27 July 2020).

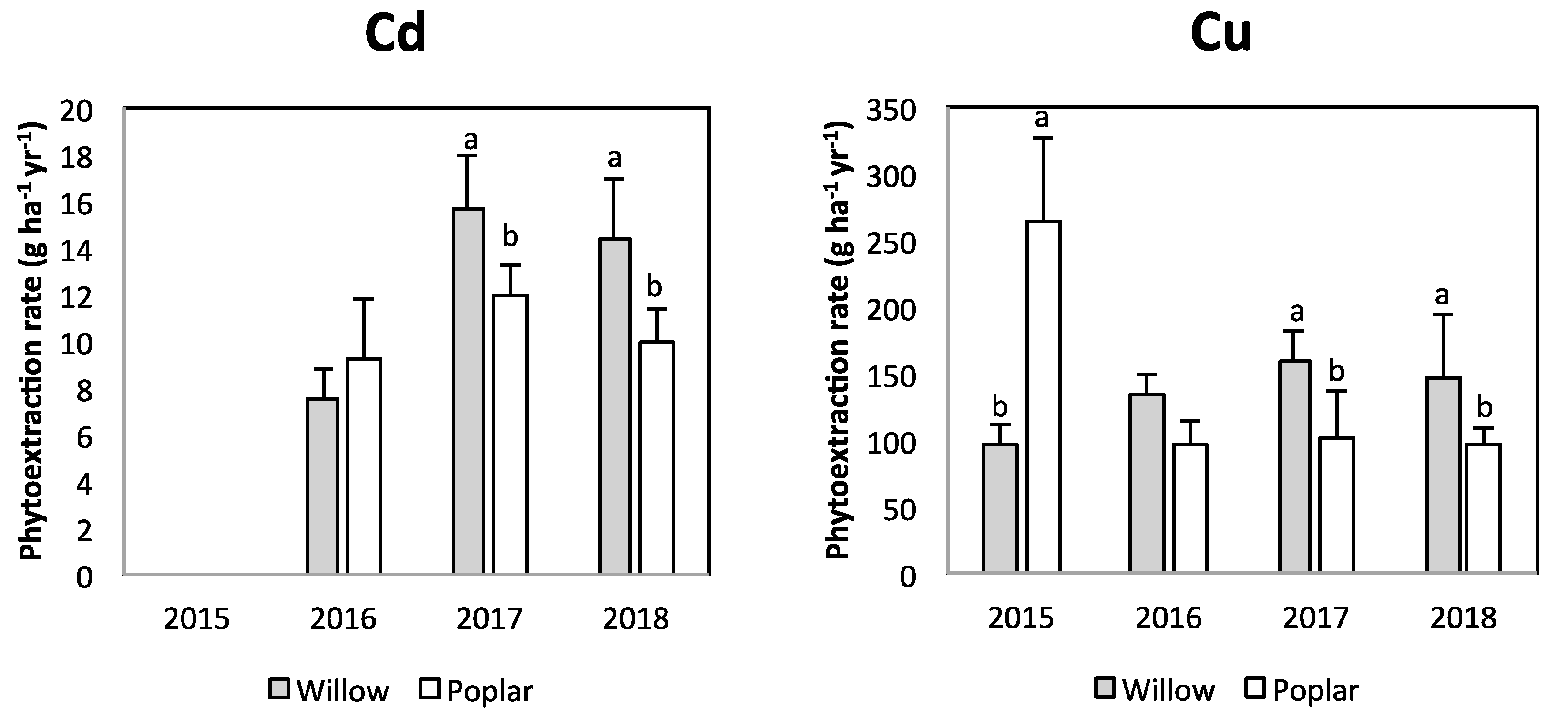
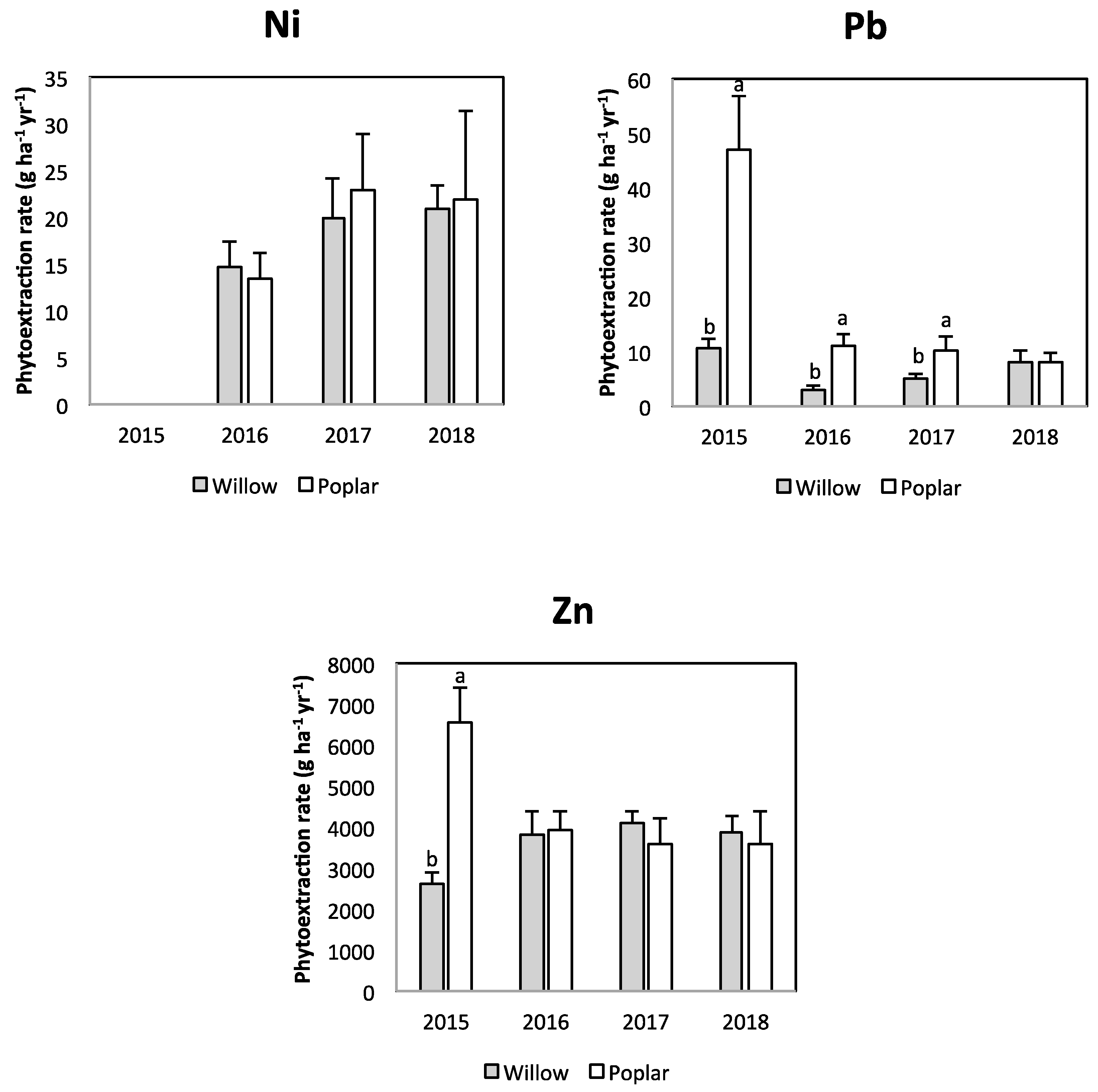

| Parameter | Unit | Value | Parameter | Unit | Value | |
|---|---|---|---|---|---|---|
| pH | 8.5 | Cd | Total | mg kg−1 | 1.3 | |
| O.M. | % | 7.0 | DTPA | 0.2 | ||
| Sand | % | 83.9 | Cu | Total | 81.5 | |
| Silt | % | 6.9 | DTPA | 11.7 | ||
| Clay | % | 9.2 | Ni | Total | 28.9 | |
| Total N | g kg−1 | 0.10 | DTPA | 0.4 | ||
| Total P | % ss | 0.07 | Pb | Total | 117.9 | |
| Total K | g kg−1 | 3.5 | DTPA | 30.2 | ||
| C.E.C. | meq 100 g −1 | 20.7 | Zn | Total | 389.3 | |
| E.C. | μS cm−1 | 145.9 | DTPA | 75.3 | ||
| Elements | Standard Material (mg kg−1) | Observed in the Current Study (mg kg−1) | Recovery (%) | ||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||||
| Cd | 0.12 | ± | 0.01 | 0.11 | ± | 0.02 | 91.7 |
| Cu | 10.2 | ± | 0.5 | 9.2 | ± | 0.6 | 90.3 |
| Ni | 15.2 | ± | 0.6 | 13.9 | ± | 0.5 | 91.2 |
| Pb | 1.67 | ± | 0.11 | 1.53 | ± | 0.13 | 91.6 |
| Zn | 30.5 | ± | 1.1 | 29.1 | ± | 0.9 | 95.5 |
| Species | Element | Paired Differences (T0-Tf) | (Δ%) | 95% Confidence Interval of the difference | t | df | Sig. (2-tailed) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SEM | Lower | Upper | |||||||
| Poplar | Cd | Total | 0.05 | 0.03 | 19.2 | −0.03 | 0.14 | 1.94 | 3 | 0.147 |
| DTPA | 0.06 | 0.00 | 61.8 | 0.05 | 0.08 | 13.71 | 3 | 0.001 | ||
| Cu | Total | 7.28 | 2.38 | 26.1 | −0.30 | 14.85 | 3.06 | 3 | 0.055 | |
| DTPA | 3.80 | 2.64 | 23.9 | −4.60 | 12.20 | 1.44 | 3 | 0.246 | ||
| Ni | Total | 3.96 | 1.93 | 14.0 | −2.17 | 10.08 | 2.05 | 3 | 0.132 | |
| DTPA | 0.30 | 0.07 | 57.2 | 0.07 | 0.52 | 4.18 | 3 | 0.025 | ||
| Pb | Total | 5.14 | 10.56 | 8.1 | −28.45 | 38.73 | 0.49 | 3 | 0.660 | |
| DTPA | 15.30 | 2.33 | 54.6 | 7.87 | 22.73 | 6.55 | 3 | 0.007 | ||
| Zn | Total | 75.60 | 25.50 | 43.9 | −5.57 | 156.77 | 2.96 | 3 | 0.059 | |
| DTPA | 38.08 | 9.64 | 70.7 | 7.41 | 68.74 | 3.95 | 3 | 0.029 | ||
| Willow | Cd | Total | 1.81 | 2.20 | 16.6 | −5.20 | 8.82 | 0.82 | 3 | 0.471 |
| DTPA | 0.31 | 0.24 | 39.3 | −0.47 | 1.08 | 1.26 | 3 | 0.298 | ||
| Cu | Total | 42.38 | 28.94 | 19.2 | −49.74 | 134.49 | 1.46 | 3 | 0.239 | |
| DTPA | 3.73 | 0.82 | 38.4 | 1.11 | 6.35 | 4.54 | 3 | 0.020 | ||
| Ni | Total | 8.12 | 1.31 | 27.1 | 3.96 | 12.28 | 6.21 | 3 | 0.123 | |
| DTPA | 0.23 | 0.17 | 34.8 | −0.31 | 0.77 | 1.38 | 3 | 0.262 | ||
| Pb | Total | 44.90 | 28.39 | 13.1 | −45.45 | 135.24 | 1.58 | 3 | 0.212 | |
| DTPA | 12.08 | 5.07 | 38.5 | −4.07 | 28.22 | 2.38 | 3 | 0.098 | ||
| Zn | Total | 312.23 | 173.31 | 41.2 | −239.32 | 863.77 | 1.80 | 3 | 0.169 | |
| DTPA | 89.80 | 31.04 | 76.1 | −8.99 | 188.59 | 2.89 | 3 | 0.063 | ||
| Unplanted | Cd | Total | 0.03 | 0.05 | 2.7 | −0.15 | 0.20 | 0.48 | 3 | 0.663 |
| DTPA | 0.04 | 0.05 | 7.7 | −0.14 | 0.21 | 0.67 | 3 | 0.550 | ||
| Cu | Total | 4.80 | 1.82 | 16.0 | −1.01 | 10.61 | 2.63 | 3 | 0.078 | |
| DTPA | 1.04 | 0.65 | 15.7 | −1.03 | 3.12 | 1.60 | 3 | 0.208 | ||
| Ni | Total | 2.44 | 5.27 | 0.1 | −14.35 | 19.22 | 0.46 | 3 | 0.675 | |
| DTPA | 0.08 | 0.08 | 14.5 | −0.16 | 0.32 | 1.04 | 3 | 0.374 | ||
| Pb | Total | 1.33 | 1.66 | 2.1 | −3.95 | 6.61 | 0.80 | 3 | 0.480 | |
| DTPA | 5.09 | 6.91 | −13.8 | −16.91 | 27.09 | 0.74 | 3 | 0.515 | ||
| Zn | Total | 27.78 | 26.05 | 11.8 | −55.14 | 110.69 | 1.07 | 3 | 0.365 | |
| DTPA | 12.10 | 21.85 | −12.0 | −57.43 | 81.63 | 0.55 | 3 | 0.618 | ||
| Carbon Dioxide Stock | Year | |||
|---|---|---|---|---|
| 2015 | 2016 | 2017 | 2018 | |
| Willow B (Mg ha−1) | 15.40 | 30.43 | 29.88 | 23.83 |
| Poplar B (Mg ha−1) | 34.28 | 32.45 | 25.85 | 22.37 |
| Carbon dioxide price Cs (EUR Mg−1) | 7.68 | 5.35 | 5.83 | 15.88 |
| Willow Pc (EUR ha−1) | 118.27 | 162.82 | 174.22 | 378.47 |
| Poplar Pc (EUR ha−1) | 263.29 | 173.60 | 150.70 | 355.18 |
| Species | Discount Rate | |||||||
|---|---|---|---|---|---|---|---|---|
| 1% | 2% | 3% | 4% | 5% | 6% | 7% | 8% | |
| Willow | 842.37 | 851.07 | 859.88 | 868.79 | 877.81 | 886.94 | 896.17 | 905.51 |
| Poplar | 955.75 | 968.92 | 982.28 | 995.84 | 1009.61 | 1023.57 | 1037.73 | 1052.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riccioli, F.; Guidi Nissim, W.; Masi, M.; Palm, E.; Mancuso, S.; Azzarello, E. Modeling the Ecosystem Services Related to Phytoextraction: Carbon Sequestration Potential Using Willow and Poplar. Appl. Sci. 2020, 10, 8011. https://doi.org/10.3390/app10228011
Riccioli F, Guidi Nissim W, Masi M, Palm E, Mancuso S, Azzarello E. Modeling the Ecosystem Services Related to Phytoextraction: Carbon Sequestration Potential Using Willow and Poplar. Applied Sciences. 2020; 10(22):8011. https://doi.org/10.3390/app10228011
Chicago/Turabian StyleRiccioli, Francesco, Werther Guidi Nissim, Matteo Masi, Emily Palm, Stefano Mancuso, and Elisa Azzarello. 2020. "Modeling the Ecosystem Services Related to Phytoextraction: Carbon Sequestration Potential Using Willow and Poplar" Applied Sciences 10, no. 22: 8011. https://doi.org/10.3390/app10228011
APA StyleRiccioli, F., Guidi Nissim, W., Masi, M., Palm, E., Mancuso, S., & Azzarello, E. (2020). Modeling the Ecosystem Services Related to Phytoextraction: Carbon Sequestration Potential Using Willow and Poplar. Applied Sciences, 10(22), 8011. https://doi.org/10.3390/app10228011







