Histopathology and Genetic Biomarkers of Choroidal Melanoma
Abstract
:1. Introduction
2. Eye Anatomy
- (i).
- the outer layer (sclera and cornea): the sclera is a fibrous connective tissue mainly composed of non-parallel-oriented, dense type 1 collagen fibers; the cornea consists of type I collagen fibers oriented in parallel and lined by a non-keratinized, stratified squamous epithelium.
- (ii).
- the uveal tract (choroid, iris and ciliary bodies): the choroid is made up of a dense network of blood vessels, embedded in loose connective tissue. The iris is composed of connective fibrovascular tissue, into which the sphincter and dilator pupillae muscles are inserted, and lined by a pigmented epithelium. Ciliary bodies separate the vitreous from the remaining posterior segment structures and are composed of the ciliary muscle that regulates the accommodation of the lens, and the ciliary epithelium, that produces aqueous humor.
- (iii).
- the inner layer (lens, vitreous and retina): the retina constitutes the intraocular nervous tissue, consisting of multiple layers (retinal pigment epithelium, outer limiting membrane, outer nuclear layer, outer plexiform layer, inner nuclear layer, inner plexiform layer, ganglion cell layer, nerve fiber layer and internal limiting membrane).
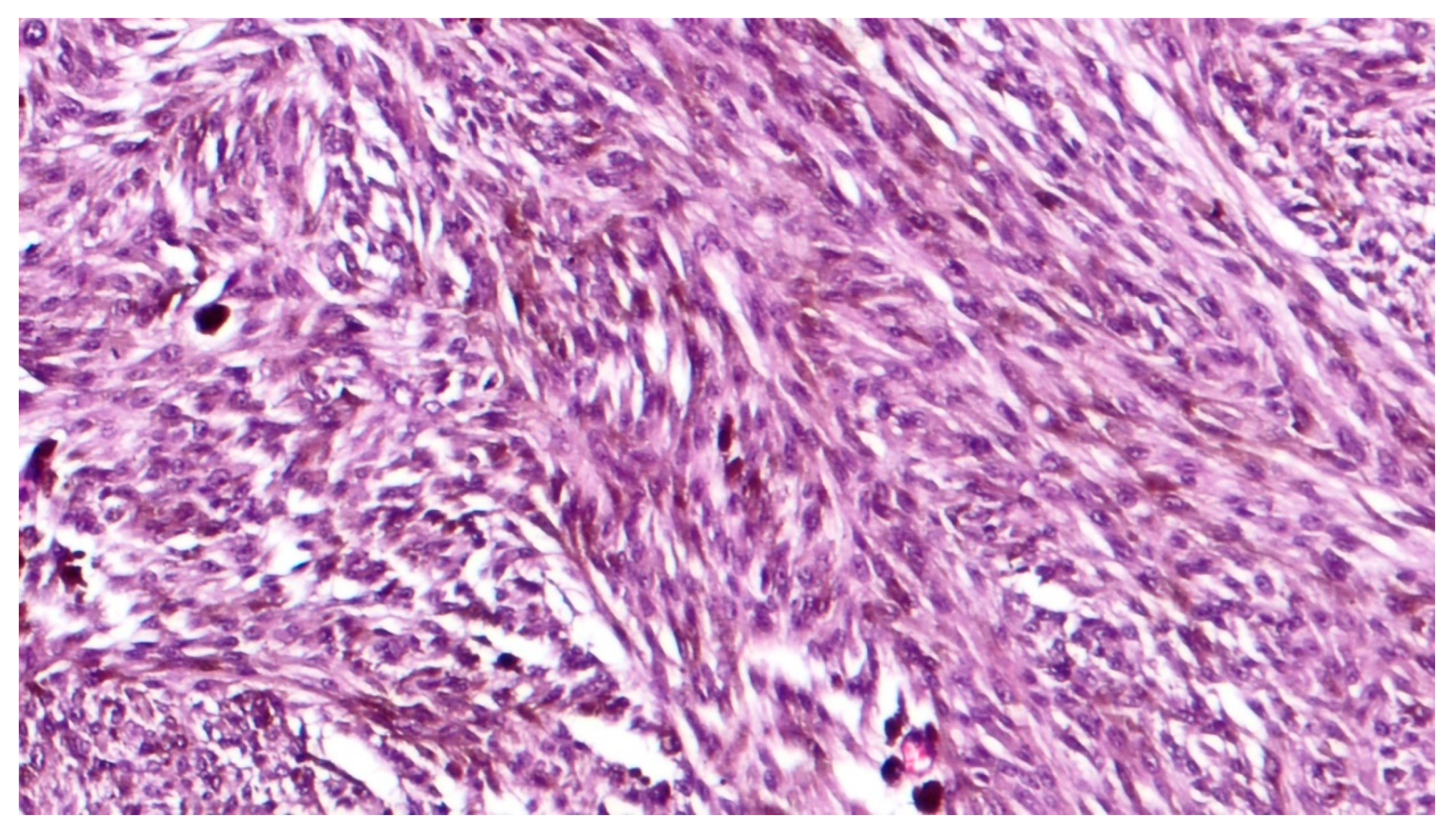
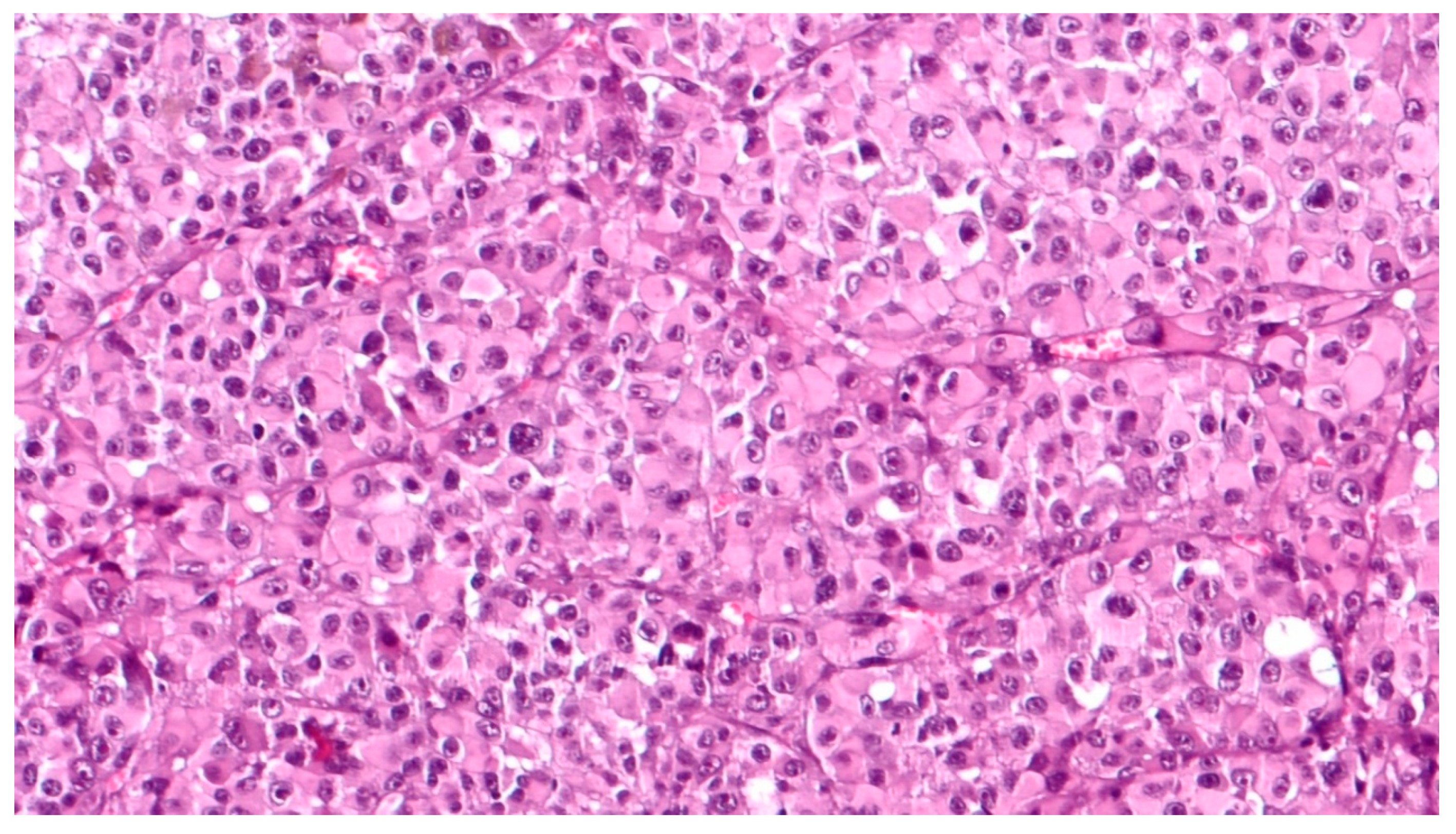
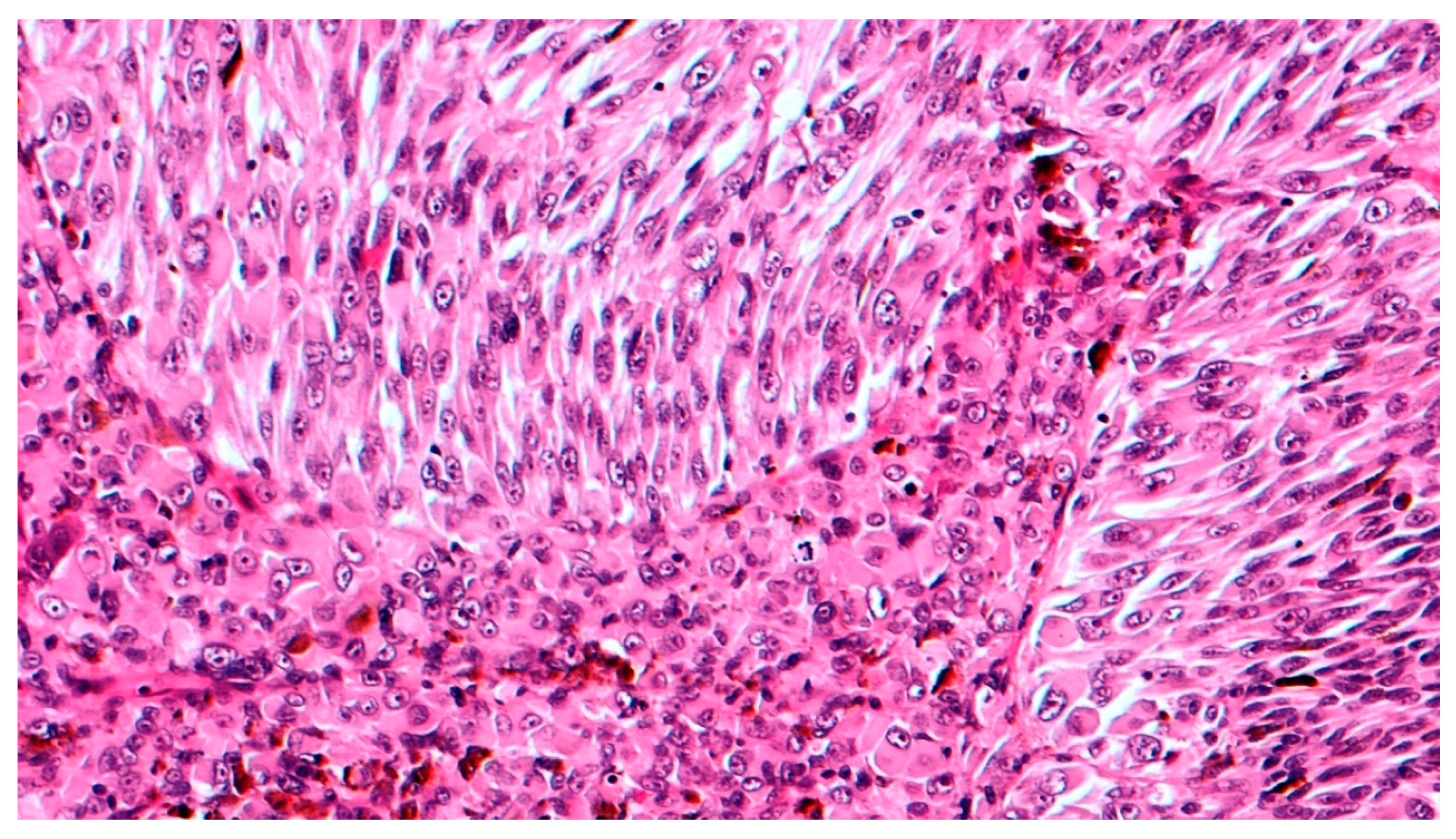
3. Histopathological Features
4. Immunohistochemical Features
5. Genetic Profile
6. Prognostic Factors
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Spagnolo, F.; Caltabiano, G.; Queirolo, P. Uveal melanoma. Cancer. Treat. Rev. 2012, 38, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Jager, M.J.; Shields, C.L.; Cebulla, C.M.; Abdel-Rahman, M.H.; Grossniklaus, H.E.; Stern, M.H.; Carvajal, R.D.; Belfort, R.N.; Jia, R.; Shields, J.A.; et al. Uveal melanoma. Nat. Rev. Dis. Primers 2020, 6, 24. [Google Scholar] [CrossRef] [PubMed]
- Mahendraraj, K.; Lau, C.S.; Lee, I.; Chamberlain, R.S. Trends in incidence, survival, and management of uveal melanoma: A population-based study of 7516 patients from the surveillance, epidemiology, and end results database (1973–2012). Clin. Ophthalmol. 2016, 10, 2113–2119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Callender, G.R. Malignant melanotic tumors of the eye: A study of histologic types in 111 cases. Trans. Am. Acad. Ophthalmol. Otolaryngol. 1931, 131, 131–142. [Google Scholar]
- Paul, E.V.; Parnell, B.L.; Fraker, M. Prognosis of malignant melanomas of the choroid and ciliary body. Int. Ophthalmol. Clin. 1962, 2, 387–402. [Google Scholar] [CrossRef]
- McLean, I.W.; Foster, W.D.; Zimmerman, L.E.; Gamel, J.W. Modifications of Callender’s Classification of Uveal Melanoma at the Armed Forces Institute of Pathology. Am. J. Ophthalmol. 2018, 195, lvi–lx. [Google Scholar] [CrossRef]
- McLean, I.W.; Foster, W.D.; Zimmerman, L.E.; Gamel, J.W. Modifications of Callender’s classification of uveal melanoma at the Armed Forces Institute of Pathology. Am. J. Ophthalmol. 1983, 96, 502–509. [Google Scholar] [CrossRef]
- Kivela, T.; Simpson, E.R.; Grossniklaus, H.E.; Jager, M.J.; Sough, A.D.; Caminal, J.M. Uveal melanoma. In AJCC Cancer Staging Manual, 8th ed.; Amin, E.B., Edge, S., Greene, F., Byrd, D.R., Brookland, K.R., Washington, M.K., Eds.; Springer: New York, NY, USA; pp. 805–817.
- Biswas, J.; Raghavendra, R.; Ratra, V.; Krishnakumar, S.; Gopal, L.; Shanmugam, M.P. Diffuse malignant melanoma of the choroid simulating metastatic tumour in the choroid. Indian. J. Ophthalmol. 2000, 48, 137–140. [Google Scholar]
- Grossniklaus, H.E.; Albert, D.M.; Green, W.R.; Conway, B.P.; Hovland, K.R. Clear cell differentiation in choroidal melanoma. COMS report no. 8. Collaborative Ocular Melanoma Study Group. Arch. Ophthalmol. 1997, 115, 894–898. [Google Scholar] [CrossRef]
- Khalil, M.K. Balloon cell malignant melanoma of the choroid: Ultrastructural studies. Br. J. Ophthalmol. 1983, 67, 579–584. [Google Scholar] [CrossRef]
- Bronkhorst, I.H.; Jager, M.J. Inflammation in uveal melanoma. Eye 2013, 27, 217–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mlecnik, B.; Bindea, G.; Pagès, F.; Galon, J. Tumor immunosurveillance in human cancers. Cancer Metastasis Rev. 2011, 30, 5–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Folberg, R.; Pe’er, J.; Gruman, L.M.; Woolson, R.F.; Jeng, G.; Montague, P.R.; Moninger, T.O.; Yi, H.; Moore, K.C. The morphologic characteristics of tumor blood vessels as a marker of tumor progression in primary human uveal melanoma: A matched case-control study. Hum. Pathol. 1992, 23, 1298–1305. [Google Scholar] [CrossRef]
- Fernandes, B.F.; Odashiro, A.N.; Saraiva, V.S.; Logan, P.; Antecka, E.; Burnier, M.N., Jr. Immunohistochemical expression of melan-A and tyrosinase in uveal melanoma. J. Carcinog. 2007, 6, 6. [Google Scholar] [CrossRef]
- Mouriaux, F.; Vincent, S.; Kherrouche, Z.; Maurage, C.A.; Planque, N.; Monté, D.; Labalette, P.; Saule, S. Microphthalmia transcription factor analysis in posterior uveal melanomas. Exp. Eye Res. 2003, 76, 653–661. [Google Scholar] [CrossRef]
- Iwamoto, S.; Burrows, R.C.; Kalina, R.E.; George, D.; Boehm, M.; Bothwell, M.A.; Schmidt, R. Immunophenotypic differences between uveal and cutaneous melanomas. Arch. Ophthalmol. 2002, 120, 466–470. [Google Scholar] [CrossRef] [Green Version]
- Miettinen, M.; McCue, P.A.; Sarlomo-Rikala, M.; Biernat, W.; Czapiewski, P.; Kopczynski, J.; Thompson, L.D.; Lasota, J.; Wang, Z.; Fetsch, J.F. Sox10--a marker for not only schwannian and melanocytic neoplasms but also myoepithelial cell tumors of soft tissue: A systematic analysis of 5134 tumors. Am. J. Surg. Pathol. 2015, 39, 826–835. [Google Scholar] [CrossRef] [Green Version]
- Harbhajanka, A.; Chahar, S.; Miskimen, K.; Silverman, P.; Harris, L.; Williams, N.; Varadan, V.; Gilmore, H. Clinicopathological, immunohistochemical and molecular correlation of neural crest transcription factor SOX10 expression in triple-negative breast carcinoma. Hum. Pathol. 2018, 80, 163–169. [Google Scholar] [CrossRef]
- Van Raamsdonk, C.D.; Griewank, K.G.; Crosby, M.B.; Garrido, M.C.; Vemula, S.; Wiesner, T.; Obenauf, A.C.; Wackernagel, W.; Green, G.; Bouvier, N.; et al. Mutations in GNA11 in uveal melanoma. N. Engl. J. Med. 2010, 363, 2191–2199. [Google Scholar] [CrossRef] [Green Version]
- Decatur, C.L.; Ong, E.; Garg, N.; Anbunathan, H.; Bowcock, A.M.; Field, M.G.; Harbour, J.W. Driver Mutations in Uveal Melanoma: Associations With Gene Expression Profile and Patient Outcomes. JAMA Ophthalmol. 2016, 134, 728–733. [Google Scholar] [CrossRef] [Green Version]
- Damato, B.; Duke, C.; Coupland, S.E.; Hiscott, P.; Smith, P.A.; Campbell, I.; Douglas, A.; Howard, P. Cytogenetics of uveal melanoma: A 7-year clinical experience. Ophthalmology 2007, 114, 1925–1931. [Google Scholar] [CrossRef] [PubMed]
- Prescher, G.; Bornfeld, N.; Horsthemke, B.; Becher, R. Chromosomal aberrations defining uveal melanoma of poor prognosis. Lancet 1992, 339, 691–692. [Google Scholar] [CrossRef]
- Malaponte, G.; Libra, M.; Gangemi, P.; Bevelacqua, V.; Mangano, K.; D’Amico, F.; Mazzarino, M.C.; Stivala, F.; McCubrey, J.A.; Travali, S. Detection of BRAF gene mutation in primary choroidal melanoma tissue. Cancer. Biol. Ther. 2006, 5, 225–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallander, M.L.; Layfield, L.J.; Emerson, L.L.; Mamalis, N.; Davis, D.; Tripp, S.R.; Holden, J.A. KIT mutations in ocular melanoma: Frequency and anatomic distribution. Mod. Pathol. 2011, 24, 1031–1035. [Google Scholar] [CrossRef] [PubMed]
- Eletr, Z.M.; Wilkinson, K.D. An emerging model for BAP1’s role in regulating cell cycle progression. Cell Biochem. Biophys. 2011, 60, 3–11. [Google Scholar] [CrossRef] [Green Version]
- Harbour, J.W.; Onken, M.D.; Roberson, E.D.; Duan, S.; Cao, L.; Worley, L.A.; Council, M.L.; Matatall, K.A.; Helms, C.; Bowcock, A.M. Frequent mutation of BAP1 in metastasizing uveal melanomas. Science 2010, 330, 1410–1413. [Google Scholar] [CrossRef] [Green Version]
- Onken, M.D.; Li, J.; Cooper, J.A. Uveal melanoma cells utilize a novel route for transendothelial migration. PLoS ONE 2014, 9, e115472. [Google Scholar] [CrossRef] [Green Version]
- Koopmans, A.E.; Verdijk, R.M.; Brouwer, R.W.; van den Bosch, T.P.; van den Berg, M.M.; Vaarwater, J.; Kockx, C.E.; Paridaens, D.; Naus, N.C.; Nellist, M.; et al. Clinical significance of immunohistochemistry for detection of BAP1 mutations in uveal melanoma. Mod. Pathol. 2014, 27, 1321–1330. [Google Scholar] [CrossRef] [Green Version]
- Harbour, J.W.; Roberson, E.D.; Anbunathan, H.; Onken, M.D.; Worley, L.A.; Bowcock, A.M. Recurrent mutations at codon 625 of the splicing factor SF3B1 in uveal melanoma. Nat. Genet. 2013, 45, 133–135. [Google Scholar] [CrossRef]
- Yavuzyigitoglu, S.; Koopmans, A.E.; Verdijk, R.M.; Vaarwater, J.; Eussen, B.; van Bodegom, A.; Paridaens, D.; Kilic, E.; de Klein, A.; Rotterdam Ocular Melanoma Study Group. Uveal Melanomas with SF3B1 Mutations: A Distinct Subclass Associated with Late-Onset Metastases. Ophthalmology 2016, 123, 1118–1128. [Google Scholar] [CrossRef]
- Martin, M.; Maßhöfer, L.; Temming, P.; Rahmann, S.; Metz, C.; Bornfeld, N.; van de Nes, J.; Klein-Hitpass, L.; Hinnebusch, A.G.; Horsthemke, B.; et al. Exome sequencing identifies recurrent somatic mutations in EIF1AX and SF3B1 in uveal melanoma with disomy 3. Nat. Genet. 2013, 45, 933–936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onken, M.D.; Worley, L.A.; Ehlers, J.P.; Harbour, J.W. Gene expression profiling in uveal melanoma reveals two molecular classes and predicts metastatic death. Cancer Res. 2004, 64, 7205–7209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gill, H.S.; Char, D.H. Uveal melanoma prognostication: From lesion size and cell type to molecular class. Can. J. Ophthalmol. 2012, 47, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Robertson, A.G.; Shih, J.; Yau, C.; Gibb, E.A.; Oba, J.; Mungall, K.L.; Hess, J.M.; Uzunangelov, V.; Walter, V.; Danilova, L.; et al. Integrative Analysis Identifies Four Molecular and Clinical Subsets in Uveal Melanoma. Cancer Cell 2017, 32, 204–220.e15, Erratum in 2018, 33, 151. [Google Scholar] [CrossRef] [PubMed]
- Algazi, A.P.; Tsai, K.K.; Shoushtari, A.N.; Munhoz, R.R.; Eroglu, Z.; Piulats, J.M.; Ott, P.A.; Johnson, D.B.; Hwang, J.; Daud, A.I.; et al. Clinical outcomes in metastatic uveal melanoma treated with PD-1 and PD-L1 antibodies. Cancer 2016, 122, 3344–3353. [Google Scholar] [CrossRef]
- Terai, M.; Londin, E.; Rochani, A.; Link, E.; Lam, B.; Kaushal, G.; Bhushan, A.; Orloff, M.; Sato, T. Expression of Tryptophan 2,3-Dioxygenase in Metastatic Uveal Melanoma. Cancers 2020, 12, 405. [Google Scholar] [CrossRef] [Green Version]
- Pilotte, L.; Larrieu, P.; Stroobant, V.; Colau, D.; Dolusic, E.; Frédérick, R.; De Plaen, E.; Uyttenhove, C.; Wouters, J.; Masereel, B.; et al. Reversal of tumoral immune resistance by inhibition of tryptophan 2,3-dioxygenase. Proc. Natl. Acad. Sci. USA 2012, 109, 2497–2502. [Google Scholar] [CrossRef] [Green Version]
- Kivelä, T.; Kujala, E. Prognostication in eye cancer: The latest tumor, node, metastasis classification and beyond. Eye 2013, 27, 243–252. [Google Scholar] [CrossRef] [Green Version]
- Shields, C.L.; Kaliki, S.; Furuta, M.; Fulco, E.; Alarcon, C.; Shields, J.A. American Joint Committee on Cancer classification of posterior uveal melanoma (tumor size category) predicts prognosis in 7731 patients. Ophthalmology 2013, 120, 2066–2071. [Google Scholar] [CrossRef]
- Shields, C.L.; Kaliki, S.; Furuta, M.; Shields, J.A. Diffuse versus nondiffuse small (≤3 MM thickness) choroidal melanoma: Comparative analysis in 1,751 cases. The 2012 F. Phinizy Calhoun lecture. Retina 2013, 33, 1763–1776. [Google Scholar] [CrossRef]
- Brierley, J.D.; Gospodarowicz, M.K.; Wittekind, C. (Eds.) Classification of Malignant Tumours; Wiley-Blackwell: Oxford, UK; Hoboken, NJ, USA, 2017. [Google Scholar]
- Mooy, C.M.; Luyten, G.P.; de Jong, P.T.; Luider, T.M.; Stijnen, T.; van de Ham, F.; van Vroonhoven, C.C.; Bosman, F.T. Immunohistochemical and prognostic analysis of apoptosis and proliferation in uveal melanoma. Am. J. Pathol. 1995, 147, 1097–1104. [Google Scholar] [PubMed]
- Salvatorelli, L.; Puzzo, L.; Russo, A.; Reibaldi, M.; Longo, A.; Ragusa, M.; Aldo, C.; Rappazzo, G.; Caltabiano, R.; Salemi, M. Immunoexpression of SPANX-C in metastatic uveal melanoma. Pathol. Res. Pract. 2019, 215, 152431. [Google Scholar] [CrossRef] [PubMed]
- Salvatorelli, L.; Puzzo, L.; Bartoloni, G.; Palmucci, S.; Longo, A.; Russo, A.; Reibaldi, M.; Li Volti, G.; Caltabiano, R. Immunoexpression of macroH2A in uveal melanoma. Appl. Sci. 2019, 9, 3244. [Google Scholar] [CrossRef] [Green Version]
- Broggi, G.; Musumeci, G.; Puzzo, L.; Russo, A.; Reibaldi, M.; Ragusa, M.; Longo, A.; Caltabiano, R. Immunohistochemical expression of ABCB5 as a potential prognostic factor in uveal melanoma. Appl. Sci. 2019, 9, 1316. [Google Scholar] [CrossRef] [Green Version]
- Caltabiano, R.; Puzzo, L.; Barresi, V.; Ieni, A.; Loreto, C.; Musumeci, G.; Castrogiovanni, P.; Ragusa, M.; Foti, P.; Russo, A.; et al. ADAM 10 expression in primary uveal melanoma as prognostic factor for risk of metastasis. Pathol. Res. Pract. 2016, 212, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Caltabiano, R.; Puzzo, L.; Barresi, V.; Cardile, V.; Loreto, C.; Ragusa, M.; Russo, A.; Reibaldi, M.; Longo, A. Expression of Raf Kinase Inhibitor Protein (RKIP) is a predictor of uveal melanoma metastasis. Histol. Histopathol. 2014, 29, 1325–1334. [Google Scholar] [CrossRef]
- Russo, D.; Di Crescenzo, R.M.; Broggi, G.; Merolla, F.; Martino, F.; Varricchio, S.; Ilardi, G.; Borzillo, A.; Carandente, R.; Pignatiello, S.; et al. Expression of P16INK4a in Uveal Melanoma: New Perspectives. Front. Oncol. 2020, 10, 562074. [Google Scholar] [CrossRef]


| Gene Symbol | Gene Name |
|---|---|
| UpRegulated in Class 2 Uveal Melanomas | |
| CDH1 | E-cadherin |
| ECM1 | Extracellular matrix protein 1 |
| HTR2B | 5-Hydroxytryptamine (serotonin) receptor 2B |
| RAB31 RAB31 | member of the RAS oncogene family |
| DownRegulated in Class 2 Uveal Melanomas | |
| EIF1B | Eukaryotic translation initiation factor 1B |
| FXR1 | Fragile X mental retardation, autosomal homolog 1 |
| ID2 | Inhibitor of DNA binding 2 |
| LMCD1 | LIM and cysteine-rich domains 1 |
| LTA4H | Leukotriene A4 hydrolase |
| MTUS1 | Microtubule-associated tumor suppressor 1 |
| ROBO1 | Roundabout, axon guidance receptor, 1 |
| SATB1 | SATB homeobox 1 |
| Control Genes | |
| MRPS21 | Mitochondrial ribosomal protein S21 |
| RBM23 | RNA-binding motif protein 23 |
| SAP130 | Sin3A-associated protein, 130 kDa |
| Pathologic Prognostic Factors of Choroidal Melanoma |
|---|
| Cell type (epithelioid, spindle, mixed cell type) |
| Tumor location |
| TNM staging (T-stage based on largest tumor basal diameter and thickness) |
| Extraocular invasion |
| Mitotic index |
| Ki67 proliferation rate |
| Intratumoral inflammatory infiltrates (tumor-infiltrating lymphocytes and tumor-associated macrophages) |
| PAS-positive peritumoral loops |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Broggi, G.; Russo, A.; Reibaldi, M.; Russo, D.; Varricchio, S.; Bonfiglio, V.; Spatola, C.; Barbagallo, C.; Foti, P.V.; Avitabile, T.; et al. Histopathology and Genetic Biomarkers of Choroidal Melanoma. Appl. Sci. 2020, 10, 8081. https://doi.org/10.3390/app10228081
Broggi G, Russo A, Reibaldi M, Russo D, Varricchio S, Bonfiglio V, Spatola C, Barbagallo C, Foti PV, Avitabile T, et al. Histopathology and Genetic Biomarkers of Choroidal Melanoma. Applied Sciences. 2020; 10(22):8081. https://doi.org/10.3390/app10228081
Chicago/Turabian StyleBroggi, Giuseppe, Andrea Russo, Michele Reibaldi, Daniela Russo, Silvia Varricchio, Vincenza Bonfiglio, Corrado Spatola, Cristina Barbagallo, Pietro Valerio Foti, Teresio Avitabile, and et al. 2020. "Histopathology and Genetic Biomarkers of Choroidal Melanoma" Applied Sciences 10, no. 22: 8081. https://doi.org/10.3390/app10228081
APA StyleBroggi, G., Russo, A., Reibaldi, M., Russo, D., Varricchio, S., Bonfiglio, V., Spatola, C., Barbagallo, C., Foti, P. V., Avitabile, T., Longo, A., & Caltabiano, R. (2020). Histopathology and Genetic Biomarkers of Choroidal Melanoma. Applied Sciences, 10(22), 8081. https://doi.org/10.3390/app10228081








