Abstract
Of the several critical challenges present in environmental microbiology today, one is the assessment of the contribution of microorganisms in the carbon cycle in the Earth-climate system. Karstic subterranean ecosystems have been overlooked until recently. Covering up to 25% of the land surface and acting as a rapid CH4 sink and alternately as a CO2 source or sink, karstic subterranean ecosystems play a decisive role in the carbon cycle in terms of their contribution to the global balance of greenhouse gases. Recent data indicate that microbiota must play a significant ecological role in the biogeochemical processes that control the composition of the subterranean atmosphere, as well as in the availability of nutrients for the ecosystem. Nevertheless, there are still essential gaps in our knowledge concerning the budgets of greenhouse gases at the ecosystem scale and the possible feedback mechanisms between environmental-microclimatic conditions and the rates and type of activity of microbial communities in subterranean ecosystems. Another challenge is searching for bioactive compounds (antibiotics) used for treating human diseases. At present, there is a global health emergency and a strong need for novel biomolecules. In recent decades, great research efforts have been made to extract antibiotics from marine organisms. More recently, caves have been receiving considerable attention in search of novel antibiotics. Cave methanotrophic and heterotrophic bacteria are producers of bioactive compounds and may be potential sources of metabolites with antibacterial, antifungal or anticancer activities of interest in pharmacological and medical research, as well as enzymes with a further biotechnological use. Here we also show that bacteria isolated from mines, a still unexplored niche for scientists in search of novel compounds, can be a source of novel secondary metabolites.
Keywords:
karst; methane; carbon dioxide; greenhouse gases; methanotrophy; cave bacteria; bioactive compounds 1. Introduction
Karst is the term used to describe terrains underlain by soluble rock and characterized by the occurrence of caves, sinkholes, sinking streams, and an assortment of other landforms carved on the bedrock. Shallow karst ecosystems cover up to 25% of the Earth’s land surface [1] and differ from the surface environments because of their limited energy and available nutrients.
Caves, in general, are characterized by a constant temperature, humidity, and high carbon dioxide (CO2) concentration the year round, as well as absence of light and scarcity of nutrients. Microorganisms occupy all the niches of the biosphere, including the subsurface, as a part of the critical zone, the heterogeneous near surface environment in which complex interactions involve rock, soil, water, air, and living organisms [2].
Earth’s subsurface contains an active microbiota colonizing rock surfaces. In this environment, microorganisms are forced to adapt their metabolism for surviving in extreme conditions, and the low input of carbon, nitrogen and phosphorus as well as the chemical composition of the rock has a direct impact on the community diversity. In fact, one of the main reservoirs of microbial life, even at great depths, where life is not dependent on solar energy and photosynthesis for its primary energy supply is the terrestrial subsurface [3].
The colonization of substrates in caves is not homogeneous. Microorganisms colonize speleothems, host rock, detrital sediments, and/or speleosols with different compositions (clays, carbonate minerals, etc.) and/or textures (crystal habit, grain size, permeability, etc.). Microbial colonization is ultimately a complex and dynamic process that is determined and controlled by physicochemical properties (temperature, pH, redox potential, salinity) and biochemical factors (bioreceptivity, nutrient or electron acceptor availability, carbon, nitrogen and phosphorous concentrations, etc.) [4]. Therefore, the collective metabolic processes of microorganisms are decisive in the biogeochemical cycles of the biosphere: C and N fixation, CH4 metabolism, S oxide reduction, etc.
It is well-known that dissolution and precipitation of carbonates are the main processes involved in the mobilization of carbon in subterranean environments. Cave microorganisms are able to induce the precipitation of carbonates, via biomineralization processes [5] and also dissolution processes due to the excretion of acids [6]. There is a wide array of literature on the study of bio-induced mineral formations in subterranean environments [7] and on the microbial–rock interaction related to the CO2 uptake or release processes [8]. In this context, previous studies have confirmed that Actinobacteria biofilms developing on cave walls promote uptake of CO2, dissolve the rock, and produce calcite crystals in periods of lower humidity and/or CO2 [8]. However, the interactions of microbes with the air–water–rock interfaces in subterranean ecosystems and the biological mechanisms by which microorganisms adjust to new environments or changes in their current environment are poorly understood.
Low energy subsurface environments are uniquely positioned for examining minimum energetic requirements and adaptations for chemolithotrophic life and become a suitable environment to study the origins of life on Earth and may also serve as analogs to explore subsurface life in extraterrestrial bodies [9]. Furthermore, the microbiota from shallow subsurface environments (karst cavities, lava tubes) are becoming a target of increasing interest in different research fields, including biodiversity [10], mineral formation and dissolution [7], cultural and natural heritage conservation [11], and paleoclimatology [12]. In addition, other important uses of microorganisms are the production of bioactive compounds valuable for medicine and enzymes for bioremediation [13].
The extensive literature about microbial diversity and activity of cave microorganisms has been reviewed by many authors. The books “Microbial Life of Cave Systems” by Engel [14] and “Cave Ecology” by Moldovan et al. [15] are a rich source of information. In addition, other book chapters and review articles are relevant [16,17,18,19,20,21]. Because of the comprehensive scope of the literature on this topic, for this review we have selected two emerging research topics representing recent advances in environmental microbiology: (1) the control of greenhouse gas fluxes by cave microorganisms, and (2) the search of antibiotics produced by subsurface bacteria.
2. The Control of Greenhouse Gas Fluxes by Cave Microorganisms
Global changes in the Earth’s climate and its relationship to the increasing concentration of greenhouse gases (GHGs) in the atmosphere has received special attention since the last quarter of the 20th century. Etiope and Klusman [22] reported that the major sources for atmospheric methane (CH4) budget derive from the natural processes in the biosphere (modern microbial activity) and from fossil, radiocarbon-free CH4 emission, estimated at approximately 20% of atmospheric CH4, which is due to and mediated by anthropogenic activity. However, this estimation is higher than the estimates from statistical data of CH4 emission from fossil fuel and related anthropogenic sources. For these authors, geologic sources are more than enough to provide the amount of CH4 required to account for the suspected missing source of fossil CH4. In addition, Etiope and Lollar [23] distinguished between biotic and abiotic CH4, the latter produced in magmatic processes (volcanoes and high-temperature active hydrothermal vents) and postmagmatic processes at lower temperatures (gas–water–rock interactions).
A better understanding of the carbon cycle in the Earth-climate system is nowadays a crucial knowledge gap. The main research efforts are focused on identifying and characterizing all possible sources, reservoirs, and sinks of GHGs, mainly CO2 and CH4, in order to more accurately calculate the budgets, especially in the carbon cycle [24]. This issue is critical to understand the effects of changes in the carbon cycle on Earth’s climate, and to assess the level of effort required in order to adapt and mitigate climate change.
The interactions between geological, microbiological, and chemical processes are responsible for the physical-chemical properties of the atmosphere and especially for changes in its composition. Caves and other shallow vadose environments are populated by methanotrophic microorganisms and thus represent a CH4 sink. This subterranean CH4 sink is largely overlooked in the scientific literature. Understanding how cave microbiomes influence the systems in which they inhabit is proving to be an exceptional research challenge [25].
Methane is consumed from the atmosphere by methanotrophs in forests, grasslands, paddy, and other unsaturated soils, which represent the major terrestrial sinks. Environmental CH4 oxidation by bacteria is mainly carried out by Gammaproteobacteria, Alphaproteobacteria, and Verrucomicrobia [26], though there is also recent evidence for methanotrophy in Rokubacteria [27].
The presence of methanotrophic bacteria in caves has been widely studied in Movile Cave, Romania, by using isolation techniques, 13CH4-labelling, and 13C-DNA analysis, and the significant importance to the ecosystem development and primary productivity has been remarked upon [28,29,30,31]. Evidence of the occurrence of methanotrophs has also been found in other caves [10,32,33]. However, in these studies the microorganisms were not related with the sink of GHGs in caves.
Specific studies, both on the environmental-driven controls on microbial activity and, in turn, on the microbial role in composition changes of natural subterranean ecosystems, constitute a new research area of the highest potential with a pool of questions to solve. The starting hypothesis was that the subterranean microbiome plays a significant ecological role in the biogeochemical processes controlling the composition of the underground atmosphere, as well as in the availability of nutrients for the rest of the ecosystem’s biota.
Fernandez-Cortes et al. [34] evidenced for the first time that cave ecosystems act as effective natural sinks of atmospheric CH4 on seasonal and daily scales and this phenomenon may thus be relevant on a global scale in terms of its contribution to the global balance of GHGs. The potential methanotrophy in four Spanish caves was assessed by tracking the presence of methane-oxidizing bacteria using the particulate methane monooxygenase gene pmoA, which is a phylogenetic marker for identifying methanotroph-specific DNA sequences in the environment [35]. The study revealed the presence of the proteobacteria Methylocapsa aurea, Methylomicrobium album, Methylococcus capsulatus, and methanotrophs of the K1-1 and K3-16 groups in samples from Altamira, Sidron, and Ojo Guareña caves, mainly in locations where CH4 usually reaches concentrations near to the atmospheric background levels. These soil bacteria oxidize the atmospheric CH4 [36].
However, the analyses did not detect methanotrophs in remote subterranean locations or poorly ventilated caves, such as Castañar de Ibor Cave, where CH4 is absent or present in minimal concentrations (below the accuracy threshold) throughout the year. Fernandez-Cortes et al. [34] suggested that complete consumption of CH4 was favored in the subsurface atmosphere under near vapor-saturation conditions without significant intervention of methanotrophic bacteria. This led to the assumption that CH4 oxidation was induced by ions and •OH generated by the radioactive decay of radon (222Rn). In fact, one of the important •OH sources in cave air may be from radioactive 222Rn decay [34]. However, further research verified that the mechanism of CH4 consumption was seasonally changing and methane-oxidizing bacteria were primarily responsible for the widespread observations of CH4 depletion in subterranean environments, discarding any evidence of radiolysis contribution [37,38,39].
Schimmelmann et al. [37] tested, in controlled laboratory experiments, whether radiolysis could rapidly oxidize CH4 in sealed air with different relative humidity and elevated levels of radiation from Rn isotopes. No evidence of CH4 oxidation by radiolysis was found. On the contrary, a rapid loss of CH4 was found when moist soil in the absence of Rn was added to the container. This was consistent with the presence of methane-oxidizing bacteria, which were responsible for the widespread observations of CH4 depletion in subterranean environments.
Since the pioneering work of Fernandez-Cortes et al. [34], a few authors, based on studies in caves from Australia, the USA, Vietnam, and Spain, additionally supported CH4 oxidation by methanotrophic bacteria [38,39,40,41,42].
Webster et al. [38] reported that the concentrations and stable isotopic compositions of CH4, CO2, and Rn in cave air overlapped and diverged from those of the atmosphere, as the majority of cave air samples were depleted in CH4 and enriched in CO2 relative to the local atmosphere. These differences indicate that atmospheric and internal cave processes influenced the composition of cave air. Therefore, the authors, on the basis of CH4 concentrations, δ13CCH4, and δ2HCH4 values measured in 33 caves in the USA and three caves in New Zealand, suggested that microbial methanotrophy within caves is the primary CH4 consumption mechanism. Furthermore, the stable isotopic composition of CH4 in the studied caves suggested that, in addition to atmospheric CH4, at least two additional CH4 sources were present in some caves: CH4 produced from acetate fermentation, and from CO2 reduction, processes occurring over a wide scale in the environment.
Lennon et al. [39] also proposed that biological processes, largely oxidation by methanotrophic bacteria, cause a depletion of CH4 in caves. They conducted a field mesocosm experiment to test whether or not microbial methanotrophy has the potential to act as a daily sink for CH4 in two fairly well-ventilated Vietnamese caves with low Rn concentrations (75–115 Bq/m3), temperatures of 19–21 °C, and relative humidity ranging between 85 and 95%, depending on the airflow and location within the cave. The data suggested that biological processes have the potential to deplete atmospheric levels of CH4 (~2 ppmv) via methanotrophy on a daily basis, as an 87% reduction in CH4 concentrations was observed.
It appears that CH4 depletion is a seasonal phenomenon, as reported by several authors. Fernandez-Cortes et al. [34] found significant seasonal and even daily variations in the gas composition of cave air, which involves the exchange of large amounts of other GHGs, in addition to CO2(g), with the lower troposphere. Waring et al. [40] performed a continuous 3-year record of CH4 and other trace gases in an Australian cave and found a seasonal cycle of extreme CH4 depletion, from ambient ~1775 ppb to near zero during summer and to ~800 ppb in winter.
Ojeda et al. [41] found methanotrophic bacteria from the families Methylococcaceae (Gammaproteobacteria) and Methylocystaceae (Alphaproteobacteria) in 67% of the samples collected in Nerja Cave, Spain. In a recent innovative research, Cuezva et al. [42] confirmed that microbial action in caves plays a crucial role in the processes of production, consumption and storage of GHGs (CO2 and CH4) and largely determines the strong variations of these major GHGs in natural underground ecosystems. This study was developed in three Spanish caves (Pindal, Castañar de Ibor and La Garma) as a first approach to systematically characterize the role of cave sediments in the production and transport of CO2 and CH4 in the subterranean environment.
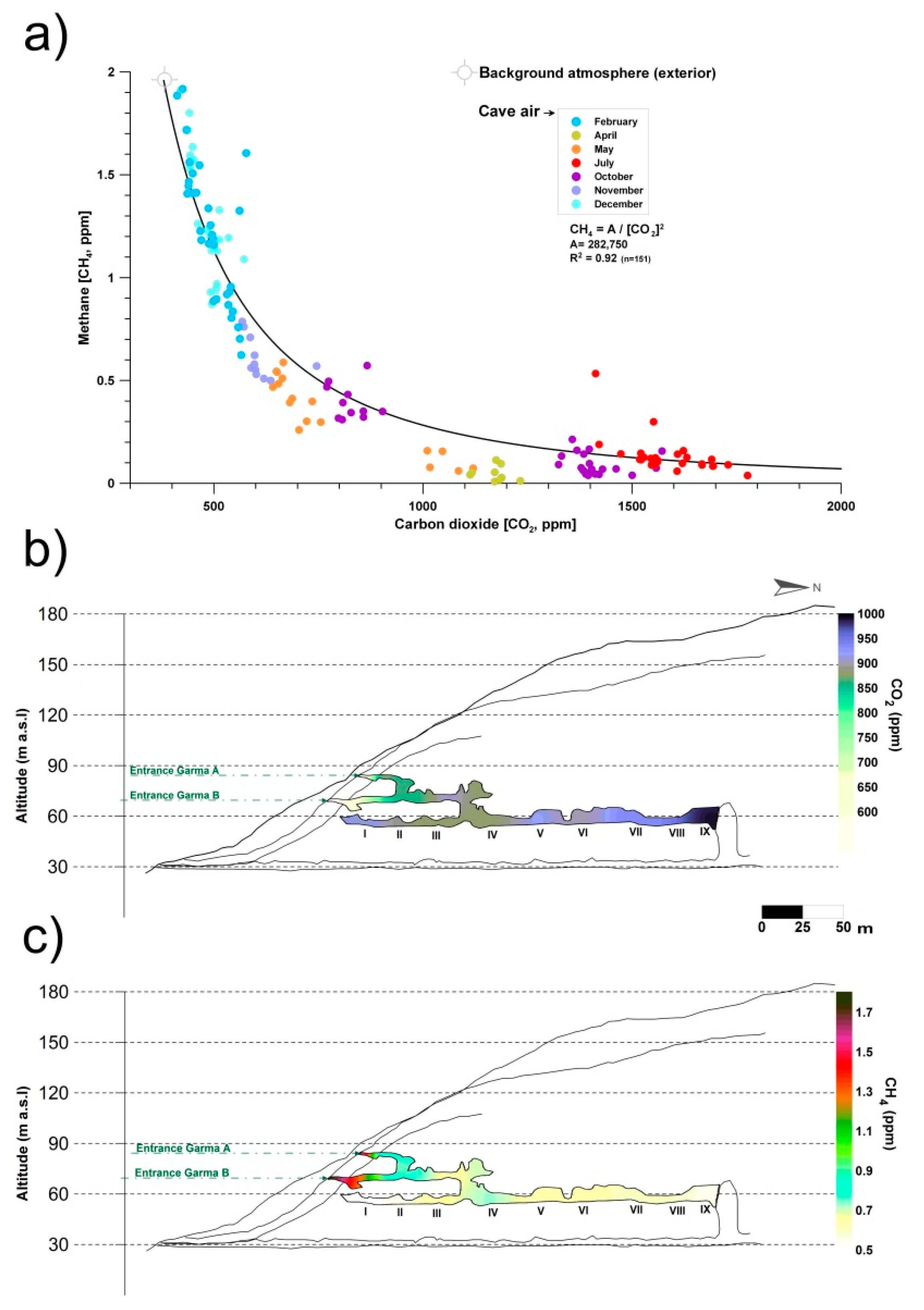
Monitoring and sampling for more than two years in La Garma Cave showed that during the stages with greater ventilation, air circulates daily and there is a continual contribution of external air to the cave, which has lower CO2 content and CH4 levels close to the atmospheric background. Therefore, CH4 depletion rises with slight changes in CO2. Conversely, in stages with a low ventilation rate, CO2 reaches high concentrations in the cave because air exchange with the external atmosphere is negligible. Thus, the removed CH4 is not rapidly replenished. As a result, CH4 depletion rate tends to become negligible as the CO2 content of cave air rises (Figure 1a).
Figure 1.
(a) Monthly co-variations in the concentrations of carbon dioxide (CO2) and methane (CH4) in La Garma Cave (Cantabria, northern Spain), a dynamically ventilated cave. (b) Spatial distribution of average concentrations of CO2. (c) Spatial distribution of average concentrations of CH4. Data from October 2014 to July 2017.
Figure 1b,c shows the spatial distribution of air CO2 and CH4 concentrations, respectively, in the air from La Garma Cave. Data for each contour map correspond to mean values from a set of bimonthly spot air samplings, conducted from October 2014 to July 2017, in a pre-established network of 11 points covering up to three levels of cave passages along an altitude gradient.
The average CO2 and CH4 concentrations of cave air were 894 and 0.65 ppm, respectively. Both GHGs depend on the rate of cave air exchange with the local atmosphere, which is controlled by climate-driven processes (primarily advection), and it is a very good indicator of the levels of matter and energy exchange with the exterior, showing the isolated areas and those with a prevailing connection with the exterior. Thus, a remarkable spatial pattern is distinguished; the highest average values of CO2 concentration and the lowest CH4 were found in the sectors of the lower gallery furthest from the main cave entrances (Garma A and Garma B, Figure 1b,c). Therefore, these cave maps with the contoured CO2 and CH4 levels reveal the importance of cave morphology in complex subterranean systems which control the gaseous composition of cave air, particularly in terms of gas variations due to the occurrence of elevation changes, multiple entrances or presence of dead-end passages. In the case of CH4, its average concentration decreased drastically below 0.7 ppm from the connection of the intermediate gallery with the lower gallery and was practically null (<0.5 ppm) in the most distant sectors of the cave entrances (Figure 1c). This CH4 pattern results from a decreasing percentage of mixing with the exterior and, consequently, a more effective methanotrophic activity of bacterial origin.
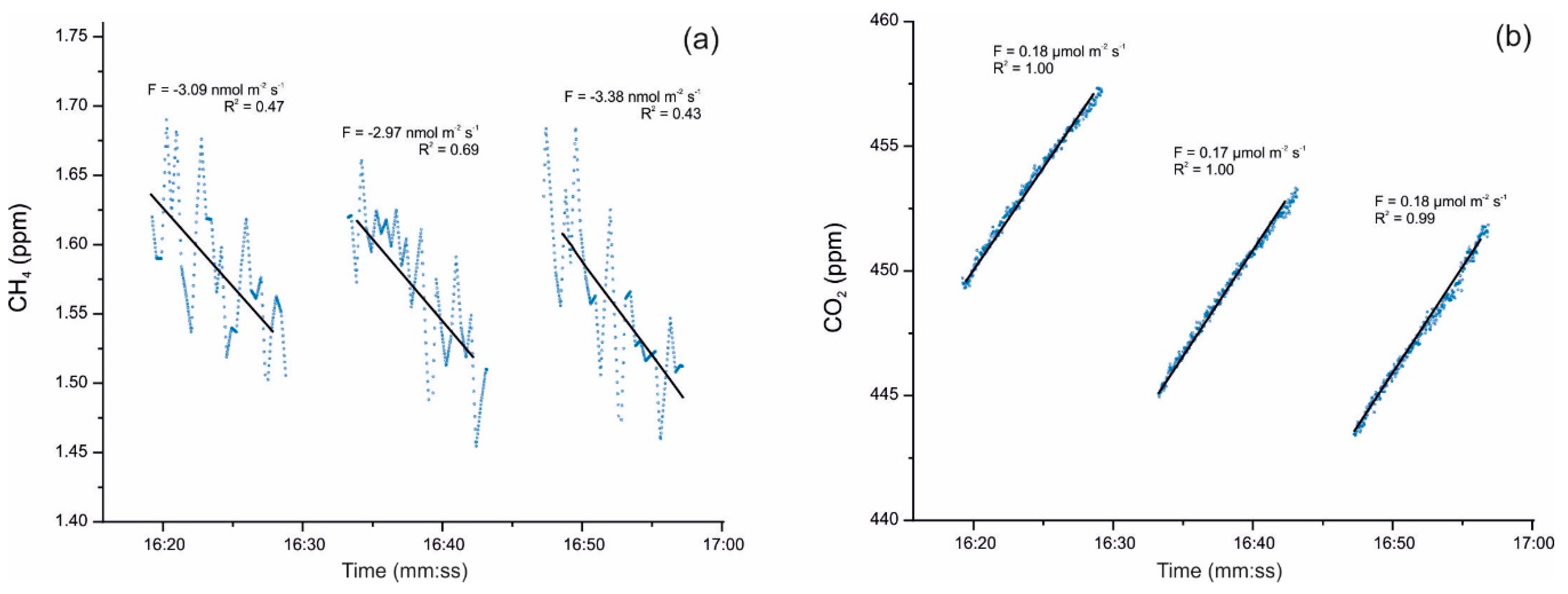
Cuezva et al. [42] are developing seasonal campaigns for CH4 and CO2 daily fluxes with continuous monitoring by a closed chamber-based gas exchange system (LI-COR Automated Soil Gas Flux System), in conjunction with a compatible Gasmet Fourier Transform Infrared (FTIR) gas analyzer and combined with δ13C geochemical tracing by cavity ring-down spectroscopy (CRDS) to understand the underlying mechanisms in cave sediments. Moreover, an autonomous piece of equipment monitored the main microenvironmental parameters of the local subsurface-soil-atmosphere system. Preliminary results showed net CO2 emissions from cave sediments resulting from respiration by chemolithotrophic microorganisms. The results also revealed simultaneous net CH4 uptake from cave sediments on a daily scale, with no significant level of variations along the day (Figure 2). Anaerobic oxidation of CH4 coupled to nitrite reduction is produced by members of the phylum Rokubacteria. These bacteria have also been found in Pindal Cave [42] and in an Alpine cave [43].
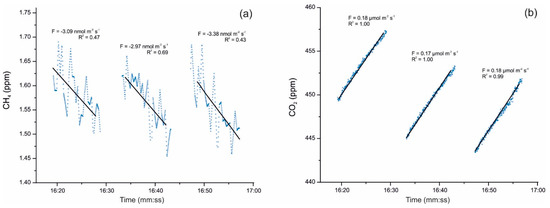
Figure 2.
(a) Detail of CH4 uptake fluxes with an average of −3.15 nmol m−2 s−1 and (b) simultaneous CO2 emission fluxes uptake fluxes with an average of +0.17 µmol m−2 s−1, monitored on 17 December 2019 directly above sediments inside Pindal Cave (Asturias, Spain). The value of the diffusive flux (F) and the corresponding exponential adjustment (R2) of each measurement are indicated.
Other studies combining the depletion of CH4 with other GHGs (N2O and NO2) were carried out in Vapor Cave, Southeast Spain. This is a hypogenic cave formed by the upwelling of hydrothermal CO2-rich fluids in which anomalous concentrations of nitrogen oxides can be found [44]. The cave is characterized by a combination of rising warm air with large CO2 outgassing and highly diluted CH4 of endogenous origin. Additionally, extreme environmental conditions were observed, such as high air temperatures (38–43 °C) and 100% relative humidity, hypoxic conditions (17% O2), CO2 concentrations that exceed 1%, 222Rn activity with values above 50 kBq/m3, and a vertical thermal gradient of 3.2 °C/100 m [45]. These conditions, associated with the combined effects of tectonic activity and hydrothermalism, make this cave a remarkable site for the study of uncommon or extremophilic microbial communities. In Vapor Cave, the depletion of CH4 was quantified to account for more than 60% removal of the deep endogenous component of this gas [45].
Martin-Pozas et al. [44] collected different cave air and sediment samples from −2 to −80 m in Vapor Cave. The analyses were conducted by taking advantage of technological advances in high-precision field-deployable CRDS and FTIR spectrometers, which allowed to measure target tracer gases (NO2, N2O, CH4, and CO2) and δ13C of both carbon-GHGs in situ. The δ13CCO2 data (−4.5 to −7.5‰) suggested a mantle-rooted CO2 likely generated by the thermal decarbonation of underlying marine carbonates, combined with degassing from CO2-rich groundwater. CH4 molar fractions and their δD (−77 to −48‰) and δ13C values (−52 to −30‰) indicated that the CH4 reaching Vapor Cave is the remnant of a larger and deep-sourced CH4, which was likely generated by the microbial reduction in carbonates. This CH4 was affected by a postgenetic depletion during its migration through the cave environment as a component of the rising warm air.
CH4 concentrations and δ13CH4 varied with depth. At −80 m, higher concentrations were found but above −30 m depth lower CH4 concentrations were found and heavier δ13C values were found near the cave entrance. This was consistent with a methane oxidation mediated by microorganisms and in fact, next generation sequencing (NGS) analysis of sediments showed a relative abundance of Candidatus Methylomirabilis 4 to 5 times higher in the deepest sample (−80 m) with respect to −30 and −15 m. Candidatus Methylomirabilis oxyfera (Rokubacteria) is an anaerobic denitrifying methanotroph [46]. It must be noticed that Isobe et al. [47] found that members of the uncultivated candidate phylum Rokubacteria responded positively to elevate CO2 concentrations.
In a similar way, Cappelleti et al. [48] studied an area of agricultural soils in Italy with anomalously high temperatures (up to ≅ 50 °C) and found emissions of biogenic CO2 linked to CH4 oxidation at a depth of 0.7 m from the surface. A strong biological methane-oxidizing activity in these soils was found and an examination of the pmoA clone libraries revealed the large biodiversity of methanotrophs including Methylomonas, Methylococcus, Methylocystis, and Methylocaldum.
Regarding the nitrogen gases, Martin-Pozas et al. [44] stated that the analysis of the ecological functions and metabolism of the microbiota from cave sediments suggested that N2O is mainly produced in the deepest areas of Vapor Cave (below −15 m depth). In these areas, high CO2 concentrations and low O2 levels within the sediments determine a prevailing hypoxic and acidic environment that promotes the release of nitrite, nitric oxide, and hydroxylamine as products of the metabolism of ammonia-oxidizing archaea and nitrate reduction. In fact, at −15 m depth, the archaeal communities were dominated by the class Nitrososphaeria (69.0% of the total Archaea), with a majority of uncultured members and only two identified genera, Nitrososphaera and Nitrosotenuis. This is consistent with the abundant occurrence of these Archaea in deep sediments and better survival under conditions of low dissolved oxygen.
To summarize, considerable advances have been reached in recent years regarding processes of production, consumption, and storage of greenhouse gases (CO2, CH4 and NxOx) by cave microorganisms in subterranean vadose ecosystems. Recent and current research has shown that cave Actinobacteria are active agents in the fixation of CO2, capturing CO2 from air and forming calcium carbonate polymorphs [8]. In particular, direct CO2 flux measurements in areas heavily colonized by bacteria indicate that they were promoting the uptake of this gas. Subterranean environments act as sinks or net sources of soil-derived carbon dioxide (CO2) on annual and even daily scales, reaching up to ten times higher than the mean atmospheric CO2 content, which involves the exchange of large amounts of CO2(g) with the lower troposphere and its role as a depot and/or emitter. In a very recent in-situ experimental work (Pindal Cave, Spain) with a closed chamber-based gas exchange system—research in progress—we have verified negative CH4 fluxes (uptake) from microbial communities, simultaneously linked to positive CO2 fluxes (emission) directly related to microbial methanotrophy. The most recent data from direct measurements of gas exchange fluxes indicate that both gases are inextricably linked in these microbial-induced processes.
3. The Search of Antibiotics Produced by Subsurface Bacteria
Methanotrophic bacteria not only perform consumption of CH4 in caves, but also have other capabilities. Methanotrophs produce methanobactins, bioactive compounds which have a high affinity for copper, but can bind additional metal ions, suggesting that these compounds might play a role in the protection against toxicity of metal ions other than copper [49]. Methanobactins exhibited antibiotic activity against Gram-positive bacteria and have been investigated as a treatment for Wilson disease, a human disorder involving toxic copper accumulation. For more detailed information, the reader is referred to the review of Kenney and Rosenzweig [49] (and references therein). Other uses of methanotrophs rely on their application and potential value in bioremediation, namely methane removal from landfills and coal mines, as well as biodegradation of toxic compounds [50,51].
In the biosphere, methanotrophs share niches with other bacterial groups, namely Proteobacteria and Actinobacteria [43,44]. In fact, several reports have shown the interactions between methanotrophs and heterotrophs and clear beneficial associations promoting growth were observed among them [52,53,54]. Most of these heterotrophs are well-known producers of bioactive compounds (antibiotics).
3.1. Why Is There a Need of New Antibiotics?
According to the World Health Organization (WHO) [55], there is a serious lack of new antibiotics to combat the growing threat of antimicrobial resistance of pathogenic bacteria as well as an urgent need for more investment in research and development to fight against antibiotic-resistant infections. De Kraker et al. [56] predicted more than ten million deaths of people infected with the antibiotic-resistant bacteria worldwide within the next 30 years if urgent measures are not taken now.
In 2017, the WHO [57] reported a list of 12 bacteria for which novel antibiotics were urgently required. The list comprises three categories: critical, high and medium priority, according to the urgency of need for new antibiotics. In critical priority were included Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacteriaceae. High priority was assigned to Enterococcus faecium, Staphylococcus aureus, Helicobacter pylori, Campylobacter spp., Salmonellae, and Neisseria gonorrhoeae, and finally the category of medium priority encompasses Streptococcus pneumoniae, Haemophilus influenzae, and Shigella spp. It is remarkable that only three out of the twelve bacteria from the list are Gram-positive, which denotes the interest in having antibiotics active against Gram-negative bacteria.
The clinical pipeline for new antibiotics currently includes only a small number of novel compounds in development. In the past 20 years, only two new antibiotic classes, both only active against Gram-positive bacteria, have received global regulatory approval by the US Food and Drug Administration and European Medicines Agency [58]. In the same time period, no new antibiotics against Gram-negative bacteria have been approved, and the quinolones, discovered in 1962, represent the last novel drug class identified to be active against Gram-negative bacteria. In addition, only two completely new drugs for multidrug-resistant tuberculosis treatment have reached the market in over 70 years [58].
Currently, the clinical pipeline is dominated by improvements of existing products with similar chemical structures, but some level of cross-resistance and fast adaptation of target bacteria can be expected. Resistance to one specific antibiotic agent can lead to resistance to a whole related class, through exchange of genetic material between different bacteria, and can affect antibiotic treatment of a wide range of infections and diseases [59]. Ideally, research should be focused on entirely new classes of compounds, targets, and modes of action to avoid cross-resistance to existing antibiotics [60].
3.2. Antibiotics from Subsurface Bacteria
About two-thirds of all known antibiotics were produced by Actinobacteria, particularly by species of the genus Streptomyces [61,62]. Historically, the decades from the 1930s to 1980s represented the golden age of antibiotic discovery. The starting point was the exhaustive screening on soil Actinobacteria carried out by Waksman and collaborators, which led to the discovery of actinomycin and streptomycin [63,64]. Further screenings of soil bacteria produced thousands of bioactive compounds, such as the well-known chloramphenicol, tetracycline, erythromycin, vancomycin, gentamicin, etc. However, in subsequent searches the same or similar compounds already known were recurrently obtained from soil bacteria. Even different species produced the same compounds [65]. Therefore, the investigations adopted different strategies.
A few authors stated that new bioactive compounds can be found in Actinobacteria that have been previously studied to the extent that re-examination of known microorganisms, already in storage, should provide novel compounds [66,67]. In this context, Takahashi and Nakashima [67] tested lyophilized actinobacterial strains from the Kitasato University Microbial Library, Japan, most of them more than 35 years old, and found that 330 strains were producers of useful bioactive compounds. In this work, a strain of Streptomyces griseus, isolated from a soil sample in 1971, and producing streptomycin, was cultivated in four different media and revealed to yield two new N-containing compounds, iminimycin A and B, both with antimicrobial activity against Gram-positive and Gram-negative bacteria. This finding was possible due to the methodology adopted, a physico-chemical screening of culture broths involving the use of a reagent to identify nitrogen-containing metabolites and a routine analysis by liquid chromatography–mass spectrometry, liquid chromatography–UV detection, and polarity and their comparison with existing databases.
It is amazing that after Schatz et al. [64] discovered streptomycin in a S. griseus strain, about 200 compounds have been reported from other strains identified as S. griseus, and still new secondary metabolites are being discovered, pointing to Actinobacteria as an inexhaustible source of naturally occurring antibiotics [67].
In a search for bioactive compounds, some authors moved to different or unexplored ecosystems other than soils. In this respect, the exploration of marine organisms is conducted to discover novel bioactive compounds—such as two nucleosides extracted from the sponge Tectitethya crypta: spongothymidine and spongouridine [68], or more recently trabectedin, obtained from the tunicate Ecteinascidia turbinata [69]. Another approach was to increase the efforts in screening rare Actinobacteria genera [70] or explore unknown niches [71].
In the biosphere there is a little-explored niche which can provide promising results: the subterranean environment. The study of the microbiology of caves is an interesting line of research and allows the search of a great diversity of unknown bacteria and fungi and the possible production of new bioactive compounds, as reported by Cheeptham [72] and Cheeptham and Saiz-Jimenez [73].
Caves are colonized by complex bacteria communities and are an excellent reservoir for new species of Actinobacteria [71,74,75]. Research on this topic has not been considered with all the dedication it deserves, to the point that the microbiology of most caves is relatively unknown.
Nowadays, two main approaches are adopted in the study of cave bacteria. One is the isolation of the organism and the subsequent assay of the antimicrobial activity of extracts against pathogenic bacteria. Numerous studies can be found in the literature, for which only a few representative papers are cited here [76,77,78,79,80,81]. In most papers, attempts to identify the bioactive compounds through chemical and structural analyses were not accomplished. The screening of only antimicrobial activity without a structural elucidation of the involved metabolites may not be useful for the discovery of new antibiotics.
A few examples of studies where the bioactive compounds were fully identified are those of Herold et al. [82], who provided the chemical structure of cervimycin A-D, a polyketide glycoside complex obtained from Streptomyces tendae, isolated from Grotta dei Cervi, in Italy, and the huanglongmycin A-C complex, synthesized by a strain of Streptomyces found in a Chinese cave [83].
Axenov-Gibanov et al. [84] isolated a Streptomyces sp. from a cave moonmilk which produced cyclodysidin D and chaxalactin B. Another 120 metabolites were observed in the liquid extract, from which a total of 102 compounds could not be identified and appeared to be novel. These three cases exemplify how caves are a research field with high potential for novel drug discovery.
In addition to conventional isolation techniques, several authors explored the possibilities of increasing the recovery of novel or interesting bacteria by improving isolation and cultivation techniques in order to extend the number of bacteria producing bioactive compounds under laboratory conditions [85]. Some of these methodologies include pretreating samples under different conditions—air drying, dry heating, moist incubation, desiccation, setting different pH, design of new culture media mimicking nature conditions, etc.—for an effective isolation of rare Actinobacteria [71,86,87]. A list of non-specific and specific methods that enhance the production of bioactive compounds can be found in Manteca and Yagüe [88].
Another approach is represented by NGS techniques in combination with genome mining which revolutionized the field of antibiotic research. According to Nett et al. [89] genome mining analyses suggest that less than 10% of the genetic potential of antibiotic producers is currently being used, which indicates that there is a huge untapped genetic reservoir waiting to be exploited for drug discovery.
Currently, more than 1555 completed genome sequences of Streptomyces are available in EzBiocloud [90] and, for instance, Streptomyces coelicolor harbors 22 secondary metabolite gene clusters but really produces only four of the encoded metabolites under standard laboratory conditions [58].
Screening for biosynthetic genes is an effective strategy to characterize bioactivity. For Bukelskis et al. [91] information on the expression of biosynthetic genes encoding for various bioactive compounds in cave bacteria is either limited or missing, and genome mining for PKS and NRPS genes in parallel with transcriptional analysis of the identified genes would be the more effective strategy to analyze and exploit the bioactivity of cave bacterial strains.
Along with genomic mining, the combination of culturing techniques and transcriptomics would complement the systematic investigation for bioactive compounds in bacteria. These techniques have been recently used to investigate the production of the antibiotic andrimid in the marine bacterium Vibrio coralliilyticus [92]. In this study, the authors reported the differential expression of five biosynthetic gene clusters as well as an overproduction of andrimid in the presence of chitin rather than glucose in the culture medium. Alteration of cultivation parameters, such as, solid/liquid culturing, the presence/absence of nutrients, variations of pH and temperature, or changes in aeration supplying, would lead to the activation/inactivation of metabolic pathways involved in the biosynthesis of antimicrobials. These conditions or phenotypic variations are used in transcriptomic analyses to identify the clusters of genes differentially expressed in every condition, and afterwards, expressed in heterologous hosts by means of vectors such as plasmids, cosmids, fosmids, and artificial bacterial chromosomes or bioactive compounds. Methodology based on heterologous expression and subsequent screening has been widely implemented in several species of the genus Streptomyces and other actinobacteria for secondary metabolism expression and subsequent identification of bioactive compounds [93].
Culturing and isolation of microorganisms has been the primary methodology in new antibiotics discovery but, although nowadays it is extensively used, this approach is biased by the impossibility of extrapolating the subsurface microbiome to the laboratory. In fact, the majority of bacterial species cannot grow in laboratory conditions, an issue that could have led to disregarding a huge amount of new antibiotics synthetized by uncultured bacteria [87]. To amend the culture-dependent bias, metagenomic mining and metatranscriptomics should raise the chance to acquire novel bioactive compounds directly from the subterranean ecosystems.
Metagenomics allows the study of genomes from non-culturable bacteria by means of sequencing the in-situ collected DNA and the subsequent bioinformatic analyses for assembly, binning, and annotation of the genomes of bacteria present in the sample. As a result of the treatment of metagenomic data, potential biosynthetic gene clusters are set in libraries and expressed by heterologous hosts to evaluate and validate their bioactivity.
Metatranscriptomics have a double impact in microbiome studies—on one hand, RNA sequencing can focus on the metabolically active bacteria, discriminating the ancient DNA and latent or inactive bacteria that could add “noise” to the study, since the variation of relative abundance and presence of bacterial communities has been observed, even at the phylum level, in rRNA 16S studies when comparing total and metabolically active communities using both cloning and NGS analyses [33,94]. Thus, efforts of bioactivity analyses could be more exhaustive and accurate. On the other hand, interactions inter- and intraspecies are the best frames to investigate the bioactivity of bacteria in competition with other species sharing the same niche.
In the last decade, a few projects have been funded by the European Commission and National Organizations. They are SeaBioTech (https://spider.science.strath.ac.uk/seabiotech/index.php), Marex (https://www.marex.fi/), PharmaSea (http://www.pharma-sea.eu/pharmasea/), FucoSan (https://www.fucosan.eu/en/), Tascmar (https://cordis.europa.eu/project/id/634674), and Probio (https://www.vliz.be/en/news?p=show&id=8386). All of them focused their activities on marine organisms (bacteria, algae, invertebrates, etc.). Activities and results can be found in their respective web pages. As far as we know no project on terrestrial microorganisms is ongoing, other than a recent research project launched with the aim of studying the biodiversity of extreme environments such as abandoned and active mines in Euroregion A3 (Alentejo, Algarve, and Andalucia) [95].
The mines (pyrite, manganese, copper, etc.) to be investigated are located in the Iberian Pyrite Belt. The Iberian Pyrite Belt is one of the World’s largest accumulations of mine wastes and acidic mine waters from drainages, which have caused severe pollution by the low pH and presence of dissolved metals. This acidity and metal pollution has caused the loss of most forms of aquatic life, with the exception of acidophilic microorganisms which inhabit these extreme environments [96]. In this scenario, microorganisms are subjected to stress and the need to develop a metabolic system capable of coping with oligotrophy (lack of organic nutrients) and the expression of genes that produce bioactive compounds to compete with other organisms for the scarce nutrients available in the acidic environment.
The inclusion of mines in this project represents a further step and an innovative research area, since as far as we know there are no previous reports considering mine microbiomes as a source of bioactive compounds.
Preliminary results (Table 1) showed that about 14% of the bacteria isolated from caves and mines produced bioactive compounds against the tested pathogens. It was noteworthy that bacteria isolated from sediments of two submarine cave and marine organisms inhabiting the caves, located in the Algarve coast, only yielded two positive strains, in contrast with the higher number of terrestrial isolates. In addition, it is remarkable that 29% of bacteria isolated from the Iberian Pyrite Belt mines showed inhibitory activity against pathogens, which doubled the percentage of cave bacteria. Most of the bacteria showed high activity against Gram-positive bacteria and lower against Gram-negative bacteria. This is a reason to focus the search on having antibiotics active against Gram-negative bacteria, as demanded by the WHO.

Table 1.
Screening of bacteria from mines and marine caves in Alentejo, Algarve, and Andalucia, as well as from the IRNAS-CSIC cave bacterial collection.
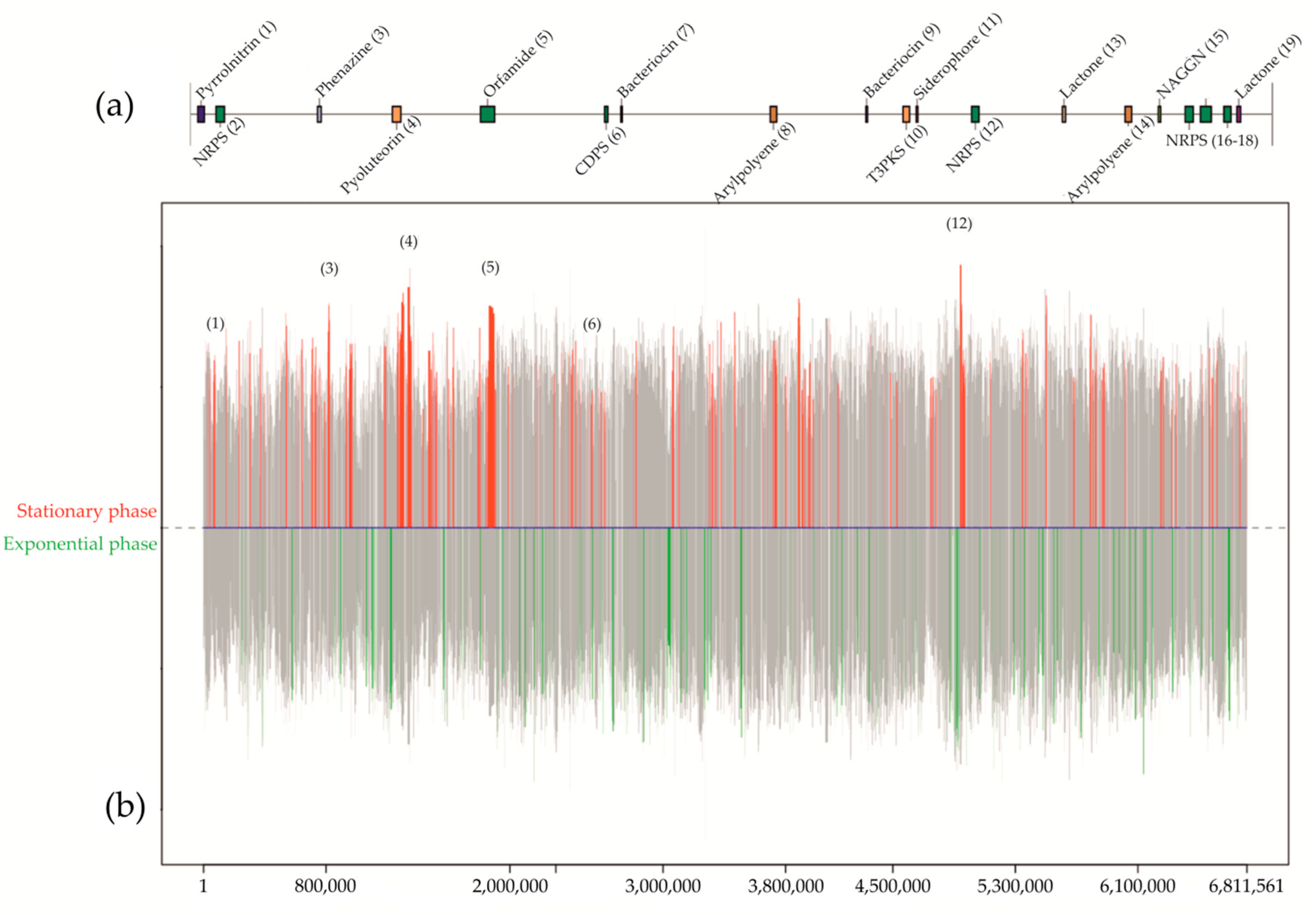
A complete screening of bacteria from air, water, and sediments from mines provided a bacterium collected with an air sampler [97] inside the mine of Lousal (Portugal). Based on the study of the 16S rRNA gene and subsequent sequencing of the genome, the strain was identified as a species within the genus Pseudomonas and showed a high bioactivity against all the tested pathogenic bacteria. Genome mining analysis focused on the secondary metabolism with antiSMASH [98] which resulted in the prediction of 19 biosynthetic gene clusters from different domains such as polyketide synthases, non-ribosomal peptide synthases, tRNA-dependent cyclodipeptide synthases, aryl polyenes, phenazines, bacteriocins, N-acetylglutaminylglutamine amides, betalactones, flavin-dependent tryptophan halogenases, homoserine lactones, and siderophores (Figure 3).
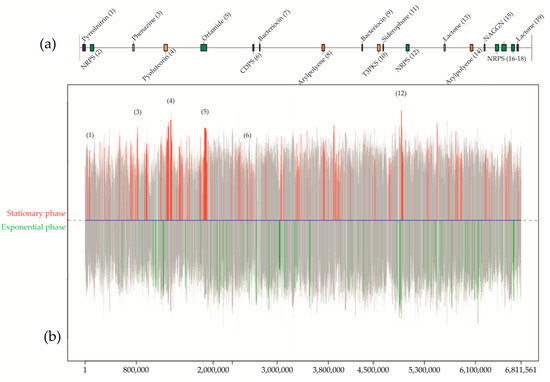
Figure 3.
Graphic example from a differential gene expression analysis for Pseudomonas sp., isolated from a mine in Lousal (Portugal). Culturing conditions were established in solid medium with nutrient agar and 2% of glycerol. The prediction and location of the biosynthetic gene clusters in the 6.8 Mbp genome (a) were used to map the differentially expressed genes (b) between the stationary phase and the exponential along the chromosome. Colored peaks represent the variability of expressed genes between both conditions with a 99% of probability. Red peaks show those genes differentially expressed in the stationary phase, in opposition to the green peaks, which are referred to the differential expression in the exponential phase. Grey peaks represent those genes differentially expressed below the 99% threshold, or simply not differentially expressed between conditions. From the predicted secondary metabolites, only six of them were differentially expressed in the stationary phase. Considering the bioactivity analyses in this medium and the study of antibiotic resistance mechanisms on genomes of pathogenic bacteria, no expression of genes involved in the synthesis of antibiotics against Gram-negative bacteria occurred for the described solid media culture.
An “One Strain Many Compounds” (OSMAC) approach [99] was used to check the bioactivity of Pseudomonas sp. with the aim of performing a comparative analysis of gene expression by means of transcriptomics techniques. A variation of pattern in the inhibition of pathogens was observed between solid and liquid cultures. The bioactivity of Pseudomonas sp. was limited to Gram-positive bacteria in a solid culture with nutrient agar and 2% of glycerol, whereas for the liquid version of the same culture, the inhibition of pathogens was total, for both Gram-positive and Gram-negative bacteria. Inhibition of growth in pathogens was only observed during the stationary phase. Thus, prediction of gene clusters involved in the synthesis of secondary metabolites, comparison of analyses based on the gene expressions, and the study of the antibiotic’s resistance mechanisms, or resistome, from the pathogenic bacteria genomes allowed the identification of specific genetic mechanisms involved in the antimicrobial bioactivity for the tested Pseudomonas sp. strain.
For a correct management and understanding of genomics and transcriptomics data, advances of bioinformatics have empowered the development of these omics sciences. Software tools addressing quality checking, correction, assembly, mapping, and annotation of data represent the basic procedure, but the availability of hardware with the capacity to carry out high-level operations is essential. However, these analyses and processes are harder and tedious for functional metagenomics and metatranscriptomics because of a higher presence of data coming from hundred or even thousand bacterial genomes present in the same sample. In this sense, the continuous improvement of bioinformatics tools and workflows are needed to achieve a deeper understanding of biological processes [100].
The improvement in NGS platforms and the rising use of omics sciences has stimulated the falling cost of sequencing and the appearance of better computational methodologies. Nevertheless, although these techniques are presented as a prominent stimulus in new antibiotics research, few studies have focused on cave microbiome have used metagenomic and metatranscriptomic analyses so far. Beyond the inherent bias to the application of nucleic acid-based techniques such as a low concentration of DNA/RNA, partial fragmentation, and presence of enzyme inhibitors [101], current limitations are focused on the training of computational scientists to analyze the complex data as well as sequencing enough samples to get a powerful study [102].
4. Conclusions
Subterranean ecosystems constitute a huge subsurface reactor of the global biogeochemical cycle with a potential and regular buffering effect on long-term increments of atmospheric GHGs linked to climate change. The subterranean microbiome plays a primary regulatory role in its gas composition, controlling the uptake-fixation-production of CO2, CH4 and NxOx gases, as well as their coupled evolution during their migration through the critical zone to the lower troposphere. Recent studies apply an innovative and multidisciplinary combination of a broad suite of cutting-edge technologies -GHG flux monitoring, isotopic geochemical tracing, biogeochemistry, metagenomics, etc.—to quantify the GHG fluxes controlled by microbial-induced processes and directly exchanged with the cave atmosphere in several temporal scales (daily, seasonal, annual pattern). The next step should be to establish the feedback mechanisms between environmental-microclimatic conditions and rates and type of activity of microbial communities in subterranean ecosystems.
The recent in-depth study on the microbial activity of the subterranean microbiota has not only provided qualitative and quantitative data on the regulation of the concentration of CO2 and CH4, but has also shown that methanotrophs and heterotrophs can interact and stimulate the growth of each other. This growth results in the production of bioactive compounds. Although the literature describes the isolation of many cave bacteria with inhibitory properties against pathogens, only a few studies provide the identification and chemical structure of the metabolites. The advent of massive sequencing technologies or NGS has promoted the development of the so-called omics sciences, offering a holistic view of the inter- and intracommunity relationships of microorganisms. Genomics, transcriptomics, and the consequent massive generation of data have favored the development of bioinformatics as an essential interdisciplinary field, in continuous progress, for the interpretation of biological processes. This set of techniques and disciplines has not only allowed a more exhaustive knowledge of the biology of microorganisms, but it has made possible to overcome the barrier of extrapolating the ideal conditions for microbial growth in the natural environment to the laboratory, and in this way it has also been made possible to identify new genetic mechanisms involved in the synthesis of bioactive compounds.
Author Contributions
Conceptualization, S.S.-M., S.C. and C.S.-J.; investigation, T.M.-P., S.C., V.J., J.L.G.-P., I.D.-M. and J.C.C.; writing—original draft preparation, T.M.-P., S.S.-M., J.L.G.-P., A.F.-C. and C.S.-J.; writing—review and editing, C.S.-J. All authors have read and agreed to the published version of the manuscript.
Funding
Financial support was obtained through project 0483_PROBIOMA_5_E, co-financed by the European Regional Development Fund within the framework of the Interreg V-A Spain-Portugal program (POCTEP) 2014–2020. This work was also supported by the Spanish Ministry of Economy and Competitiveness through projects CGL2016-75590-P and PID2019-110603RB-I00, AEI/FEDER, UE.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ford, D.C.; Williams, P.W. Karst Hydrogeology and Geomorphology; Wiley: Chichester, UK, 2007. [Google Scholar]
- Giardino, J.R.; Houser, C. (Eds.) Introduction to the critical zone. In Principles and Dynamics of the Critical Zone; Elsevier: Amsterdam, The Netherlands, 2015; pp. 1–13. [Google Scholar]
- Gold, T. The deep, hot biosphere. Proc. Natl. Acad. Sci. USA 1992, 89, 6045–6049. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.A.; Bennett, P.C. Mineral ecology: Surface specific colonization and geochemical drivers of biofilm accumulation, composition, and phylogeny. Front. Microbiol. 2017, 8, 491. [Google Scholar] [CrossRef] [PubMed]
- Cuezva, S.; Sanchez-Moral, S.; Saiz-Jimenez, C.; Cañaveras, J.C. Microbial communities and associated mineral fabrics in Altamira Cave, Spain. Int. J. Speleol. 2009, 38, 83–92. [Google Scholar] [CrossRef]
- Northup, D.E.; Lavoie, K.H. Geomicrobiology of caves: A review. Geomicrobiol. J. 2001, 18, 199–222. [Google Scholar] [CrossRef]
- Sánchez-Moral, S.; Portillo, M.D.C.; Janices, I.; Cuezva, S.; Cortés, Ángel, F.; Cañaveras, J.C.; Gonzalez, J.M. The role of microorganisms in the formation of calcitic moonmilk deposits and speleothems in Altamira Cave. Geomorphology 2012, 139–140, 285–292. [Google Scholar] [CrossRef][Green Version]
- Cuezva, S.; Fernandez-Cortes, A.; Porca, E.; Pašić, L.; Jurado, V.; Hernández, M.; Serrano-Ortiz, P.; Hermosin, B.; Cañaveras, J.C.; Sanchez-Moral, S.; et al. The biogeochemical role of Actinobacteria in Altamira Cave, Spain. FEMS Microbiol. Ecol. 2012, 81, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.M.; Goordial, J.M.; Orcutt, B.N. Low energy subsurface environments as extraterrestrial analogs. Front. Microbiol. 2018, 9, 1605. [Google Scholar] [CrossRef]
- Lavoie, K.H.; Winter, A.S.; Read, K.J.H.; Hughes, E.M.; Spilde, M.N.; Northup, D.E. Comparison of bacterial communities from lava cave microbial mats to overlying surface soils from Lava Beds National Monument, USA. PLoS ONE 2017, 12, e0169339. [Google Scholar] [CrossRef]
- Miller, A.Z.; Garcia-Sanchez, A.M.; Martin-Sanchez, P.M.; Pereira, M.F.C.; Spangenberg, J.E.; Jurado, V.; Dionísio, A.; Afonso, M.J.; Chaminé, H.I.I.S.; Hermosin, B.; et al. Origin of abundant moonmilk deposits in a subsurface granitic environment. Sedimentology 2018, 65, 1482–1503. [Google Scholar] [CrossRef]
- Sasowsky, I.D.; Mylroie, J. Studies of Cave Sediments. Physical and Chemical Records of Paleoclimate; Springer: Dordrecht, The Netherlands, 2012. [Google Scholar]
- Ghosh, S.; Kuisiene, N.; Cheeptham, N. The cave microbiome as a source for drug discovery: Reality or pipe dream? Biochem. Pharmacol. 2017, 134, 18–34. [Google Scholar] [CrossRef]
- Engel, A.S. Microbial Life of Cave Systems; De Gruiter: Berlin, Germany, 2015. [Google Scholar]
- Moldovan, O.T.; Kovac, L.; Halse, S. Cave Ecology; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Jones, B. Microbial activity in caves—A geological perspective. Geomicrobiol. J. 2001, 18, 345–357. [Google Scholar] [CrossRef]
- Barton, H.A.; Northup, D.E. Geomicrobiology in cave environments: Past, current and future perspectives. J. Cave Karst Stud. 2007, 69, 163–178. [Google Scholar]
- Engel, A.S. Microbial diversity of cave ecosystems. In Geomicrobiology: Molecular and Environmental Perspectives; Barton, L.L., Mandl, M., Loy, A., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 219–238. [Google Scholar]
- Tomczyk-Żak, K.; Zielenkiewicz, U. Microbial diversity in caves. Geomicrobiol. J. 2016, 33, 20–38. [Google Scholar] [CrossRef]
- Jones, D.S.; Macalady, J.L. The snotty and the stringy: Energy for subsurface life in caves. In Their World: A Diversity of Microbial Environments; Hurst, C.J., Ed.; Springer: Cham, Switzerland, 2016; pp. 203–224. [Google Scholar]
- De Mandal, S.; Chatterjee, R.; Kumar, N.S. Dominant bacterial phyla in caves and their predicted functional roles in C and N cycle. BMC Microbiol. 2017, 17, 90. [Google Scholar] [CrossRef]
- Etiope, G.; Klusman, R.W. Geologic emissions of methane to the atmosphere. Chemosphere 2002, 49, 777–789. [Google Scholar] [CrossRef]
- Etiope, G.; Lollar, B.S. Abiotic methane on earth. Rev. Geophys. 2013, 51, 276–299. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change. Carbon and other biogeochemical cycles. In Climate Change 2013: The Physical Science Basis; Cambridge Univ. Press: Cambridge, UK, 2014; pp. 465–570. [Google Scholar]
- Hall, E.K.; Bernhardt, E.S.; Bier, R.L.; Bradford, M.A.; Boot, C.M.; Cotner, J.B.; Del Giorgio, P.A.; Evans, S.E.; Graham, E.B.; Jones, S.E.; et al. Understanding how microbiomes influence the systems they inhabit. Nat. Microbiol. 2018, 3, 977–982. [Google Scholar] [CrossRef]
- Op den Camp, H.J.M.; Islam, T.; Stott, M.B.; Harhangi, H.R.; Hynes, A.; Schouten, S.; Jetten, M.S.M.; Birkeland, N.-K.; Pol, A.; Dunfield, P.F. Environmental, genomic and taxonomic perspectives on methanotrophic Verrucomicrobia. Environ. Microbiol. Rep. 2009, 1, 293–306. [Google Scholar] [CrossRef]
- Butterfield, C.N.; Li, Z.; Andeer, P.F.; Spaulding, S.; Thomas, B.C.; Singh, A.; Hettich, R.L.; Suttle, K.B.; Probst, A.J.; Tringe, S.G.; et al. Proteogenomic analyses indicate bacterial methylotrophy and archaeal heterotrophy are prevalent below the grass root zone. PeerJ 2016, 4, e2687. [Google Scholar] [CrossRef]
- Hutchens, E.; Radajewski, S.; Dumont, M.G.; McDonald, I.R.; Murrell, J.C. Analysis of methanotrophic bacteria in Movile Cave by stable isotope probing. Environ. Microbiol. 2004, 6, 111–120. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, L.; Boden, R.; Hillebrand, A.; Kumaresan, D.; Moussard, H.; Baciu, M.; Lu, Y.; Murrell, J.C. Life without light: Microbial diversity and evidence of sulfur- and ammonium-based chemolithotrophy in Movile Cave. ISME J. 2009, 3, 1093–1104. [Google Scholar] [CrossRef] [PubMed]
- Kumaresan, D.; Wischer, D.; Stephenson, J.; Hillebrand-Voiculescu, A.; Murrell, J.C. Microbiology of Movile Cave—A chemolithoautotrophic ecosystem. Geomicrobiol. J. 2014, 31, 186–193. [Google Scholar] [CrossRef]
- Kumaresan, D.; Stephenson, J.; Doxey, A.C.; Bandukwala, H.; Brooks, E.; Hillebrand-Voiculescu, A.; Whiteley, A.S.; Murrell, J.C. Aerobic proteobacterial methylotrophs in Movile Cave: Genomic and metagenomic analyses. Microbiome 2018, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Saiz-Jimenez, C. The microbiology of show caves, mines tunnels and tombs: Implications for management and conservation. In Microbial Life of Cave Systems; Engel, A.S., Ed.; De Gruiter: Berlin, Germany, 2015; pp. 231–261. [Google Scholar]
- Gonzalez-Pimentel, J.L.; Miller, A.Z.; Jurado, V.; Laiz, L.; Pereira, M.F.C.; Saiz-Jimenez, C. Yellow coloured mats from lava tubes of La Palma (Canary Islands, Spain) are dominated by metabolically active Actinobacteria. Sci. Rep. 2018, 8, 1944. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Cortes, A.; Cuezva, S.; Alvarez-Gallego, M.; Garcia-Anton, E.; Pla, C.; Benavente, D.; Jurado, V.; Saiz-Jimenez, C.; Sanchez-Moral, S. Subterranean atmospheres may act as daily methane sinks. Nat. Commun. 2015, 6, 7003. [Google Scholar] [CrossRef] [PubMed]
- McDonald, I.R.; Murrell, J.C. The particulate methane monooxygenase gene pmoA and its use as a functional gene probe for methanotrophs. FEMS Microbiol. Lett. 1997, 156, 205–210. [Google Scholar] [CrossRef]
- Tveit, A.T.; Hestnes, A.G.; Robinson, S.L.; Schintlmeister, A.; Dedysh, S.N.; Jehmlich, N.; Von Bergen, M.; Herbold, C.; Wagner, M.; Richter, A.; et al. Widespread soil bacterium that oxidizes atmospheric methane. Proc. Natl. Acad. Sci. USA 2019, 116, 8515–8524. [Google Scholar] [CrossRef]
- Schimmelmann, A.; Fernandez-Cortes, A.; Cuezva, S.; Streil, T.; Lennon, J.T. Radiolysis via radioactivity is not responsible for rapid methane oxidation in subterranean air. PLoS ONE 2018, 13, e0206506. [Google Scholar] [CrossRef]
- Webster, K.D.; Drobniak, A.; Etiope, G.; Mastalerz, M.; Sauer, P.E.; Schimmelmann, A. Subterranean karst environments as a global sink for atmospheric methane. Earth Planet. Sci. Lett. 2018, 485, 9–18. [Google Scholar] [CrossRef]
- Lennon, J.T.; Nguyễn-Thùy, D.; Phạm, T.M.; Drobniak, A.; Tạ, P.H.; Phạm, N.Ð.; Streil, T.; Webster, K.D.; Schimmelmann, A. Microbial contributions to subterranean methane sinks. Geobiology 2016, 15, 254–258. [Google Scholar] [CrossRef]
- Waring, C.L.; Hankin, S.I.; Griffith, D.W.T.; Kertesz, M.A.; Kobylski, V.; Wilson, N.L.; Coleman, N.V.; Kettlewell, G.; Zlot, R.; Bosse, M.; et al. Seasonal total methane depletion in limestone caves. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ojeda, L.; Vadillo, I.; Etiope, G.; Benavente, J.; Liñán, C.; del Rosal, Y.; Tapia, S.T.; Moríñigo, M.Á.; Carrasco, F. Methane sources and sinks in karst systems: The Nerja cave and its vadose environment (Spain). Geochim. Cosmochim. Acta 2019, 259, 302–315. [Google Scholar] [CrossRef]
- Cuezva, S.; Martin-Pozas, T.; Fernandez-Cortes, A.; Cañaveras, J.C.; Janssens, I.; Sanchez-Moral, S. On the role of cave-soil in the carbon cycle. A first approach. In Proceedings of the EGU General Assembly 2020, Online, 4–8 May 2020. Abstract 21793. [Google Scholar] [CrossRef]
- Jurado, V.; Gonzalez-Pimentel, J.L.; Miller, A.Z.; Hermosin, B.; D’Angeli, I.M.; Tognini, P.; De Waele, J.; Saiz-Jimenez, C. Microbial communities in vermiculation deposits from an Alpine cave. Front Earth Sci. 2020, in press. [Google Scholar]
- Martin-Pozas, T.; Sánchez-Moral, S.; Cuezva, S.; Jurado, V.; Saiz-Jimenez, C.; López, R.P.; Carrey, R.; Otero, N.; Giesemann, A.; Well, R.; et al. Biologically mediated release of endogenous N2O and NO2 gases in a hydrothermal, hypoxic subterranean environment. Sci. Total Environ. 2020, 747, 141218. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Cortes, A.; Perez-Lopez, R.; Cuezva, S.; Calaforra, J.M.; Cañaveras, J.C.; Sanchez-Moral, S. Geochemical fingerprinting of rising deep endogenous gases in an active hypogenic karst system. Geofluids 2018, 2018, 1–19. [Google Scholar] [CrossRef]
- He, Z.; Cai, C.; Wang, J.; Xu, X.; Zheng, P.; Jetten, M.S.M.; Hu, B. A novel denitrifying methanotroph of the NC10 phylum and its microcolony. Sci. Rep. 2016, 6, srep32241. [Google Scholar] [CrossRef]
- Isobe, K.; Bouskill, N.J.; Brodie, E.L.; Sudderth, E.A.; Martiny, J.B.H. Phylogenetic conservation of soil bacterial responses to simulated global changes. Philos. Trans. R. Soc. B 2020, 375, 20190242. [Google Scholar] [CrossRef]
- Cappelletti, M.; Ghezzi, D.; Zannoni, D.; Capaccioni, B.; Fedi, S. Diversity of methane-oxidizing bacteria in soils from “Hot Lands of Medolla” (Italy) featured by anomalous high-temperatures and biogenic CO2 emission. Microbes Environ. 2016, 31, 369–377. [Google Scholar] [CrossRef]
- Kenney, G.E.; Rosenzweig, A.C. Methanobactins: Maintaining copper homeostasis in methanotrophs and beyond. J. Biol. Chem. 2018, 293, 4606–4615. [Google Scholar] [CrossRef]
- Jiang, H.; Chen, Y.; Jiang, P.; Zhang, C.; Smith, T.J.; Murrell, J.C.; Xing, X.-H. Methanotrophs: Multifunctional bacteria with promising applications in environmental bioengineering. Biochem. Eng. J. 2010, 49, 277–288. [Google Scholar] [CrossRef]
- Pandey, V.C.; Singh, J.S.; Singh, D.P.; Singh, R.P. Methanotrophs: Promising bacteria for environmental remediation. Int. J. Environ. Sci. Technol. 2014, 11, 241–250. [Google Scholar] [CrossRef]
- Iguchi, H.; Yurimoto, H.; Sakai, Y. Stimulation of methanotrophic growth in cocultures by cobalamin excreted by Rhizobia. Appl. Environ. Microbiol. 2011, 77, 8509–8515. [Google Scholar] [CrossRef] [PubMed]
- Stock, M.; Hoefman, S.; Kerckhof, F.-M.; Boon, N.; De Vos, P.; De Baets, B.; Heylen, K.; Waegeman, W. Exploration and prediction of interactions between methanotrophs and heterotrophs. Res. Microbiol. 2013, 164, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- Veraart, A.J.; Garbeva, P.; Van Beersum, F.; Ho, A.; Hordijk, C.A.; Meima-Franke, M.; Zweers, A.J.; Bodelier, P.L.E. Living apart together—Bacterial volatiles influence methanotrophic growth and activity. ISME J. 2018, 12, 1163–1166. [Google Scholar] [CrossRef]
- World Health Organization. Global Action Plan on Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- De Kraker, M.E.; Stewardson, A.J.; Harbarth, S. Will 10 million people die a year due to antimicrobial resistance by 2050? PLoS Med. 2016, 13, e1002184. [Google Scholar] [CrossRef]
- World Health Organization. Prioritization of Pathogens to Guide Discovery, Research and Development of New Antibiotics for Drug-Resistant Bacterial Infections, Including Tuberculosis; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- World Health Organization. Antibacterial Agents in Clinical Development: An Analysis of the Antibacterial Clinical Development Pipeline; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Theuretzbacher, U. Antibiotic innovation for future public health needs. Clin. Microbiol. Infect. 2017, 23, 713–717. [Google Scholar] [CrossRef]
- Barka, E.A.; Vatsa, P.; Sanchez, L.; Gaveau-Vaillant, N.; Jacquard, C.; Klenk, H.-P.; Clément, C.; Ouhdouch, Y.; Van Wezel, G.P. Taxonomy, physiology, and natural products of Actinobacteria. Microbiol. Mol. Biol. Rev. 2015, 80, 1–43. [Google Scholar] [CrossRef]
- Mast, Y.; Stegmann, E. Actinomycetes: The antibiotics producers. Antibiotics 2018, 8, 105. [Google Scholar] [CrossRef]
- Waksman, S.A.; Woodruff, H.B. Bacteriostatic and bactericidal substances produced by a soil Actinomyces. Exp. Biol. Med. 1940, 45, 609–614. [Google Scholar] [CrossRef]
- Schatz, A.; Bugle, E.; Waksman, S.A. Streptomycin, a substance exhibiting antibiotic activity against gram-positive and gram-negative bacteria. Exp. Biol. Med. 1944, 55, 66–69. [Google Scholar] [CrossRef]
- Nakashima, T.; Anzai, K.; Suzuki, R.; Kuwahara, N.; Takeshita, S.; Kanamoto, A.; Ando, K. Productivity of bioactive compounds in Streptomyces species isolated from Nagasaki marine environments. Actinomycetologica 2009, 23, 16–20. [Google Scholar] [CrossRef]
- Koomsiri, W.; Inahashi, Y.; Kimura, T.; Shiomi, K.; Takahashi, Y.; Ōmura, S.; Thamchaipenet, A.; Nakashima, T. Bisoxazolomycin A: A new natural product from ‘Streptomyces subflavus subsp. irumaensis’ AM-3603. J. Antibiot. 2017, 70, 1142–1145. [Google Scholar] [CrossRef]
- Takahashi, Y.; Nakashima, T. Actinomycetes, an inexhaustible source of naturally occurring antibiotics. Antibiotics 2018, 7, 45. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, W.; Burke, D.C. Marine products. XXXIX. The nucleosides of sponges. III. Spongothymidine and spongouridine. J. Org. Chem. 1955, 20, 1501–1507. [Google Scholar] [CrossRef]
- Lindequist, U. Marine-derived pharmaceuticals—Challenges and opportunities. Biomol. Ther. 2016, 24, 561–571. [Google Scholar] [CrossRef]
- Tiwari, K.; Gupta, R.K. Diversity and isolation of rare actinomycetes: An overview. Crit. Rev. Microbiol. 2013, 39, 256–294. [Google Scholar] [CrossRef]
- Fang, B.-Z.; Salam, N.; Han, M.-X.; Jiao, J.-Y.; Cheng, J.; Wei, D.-Q.; Xiao, M.; Li, W.-J. Insights on the effects of heat pretreatment, pH, and calcium salts on isolation of rare Actinobacteria from karstic caves. Front. Microbiol. 2017, 8, 1535. [Google Scholar] [CrossRef]
- Cheeptham, N. Cave Microbiomes: A Novel Resource for Drug Discovery; Springer: New York, NY, USA, 2013. [Google Scholar]
- Cheeptham, N.; Saiz-Jimenez, C. New sources of antibiotics: Caves. In Antibiotics. Current Innovations and Future Trends; Sánchez, S., Demain, A.L., Eds.; Caister Academic Press: Portland, Oregon, 2015; pp. 213–227. [Google Scholar]
- Groth, I.; Vettermann, R.; Schuetze, B.; Schumann, P.; Saiz-Jimenez, C. Actinomycetes in Karstic caves of northern Spain (Altamira and Tito Bustillo). J. Microbiol. Methods 1999, 36, 115–122. [Google Scholar] [CrossRef]
- Jurado, V.; Laiz, L.; Rodríguez-Nava, V.; Boiron, P.; Hermosin, B.; Sanchez-Moral, S.; Saiz-Jimenez, C. Pathogenic and opportunistic microorganisms in caves. Int. J. Speleol. 2010, 39, 15–24. [Google Scholar] [CrossRef]
- Cheeptham, N.; Sadoway, T.; Rule, D.; Watson, K.; Moote, P.; Soliman, L.C.; Azad, N.; Donkor, K.K.; Horne, D. Cure from the cave: Volcanic cave actinomycetes and their potential in drug discovery. Int. J. Speleol. 2013, 42, 35–47. [Google Scholar] [CrossRef]
- Belyagoubi, L.; Belyagoubi-Benhammou, N.; Jurado, V.; Dupont, J.; Lacoste, S.; Djebbah, F.; Ounadjela, F.Z.; Benaissa, S.; Habi, S.; Abdelouahid, D.E.; et al. Antimicrobial activities of culturable microorganisms (actinomycetes and fungi) isolated from Chaabe Cave, Algeria. Int. J. Speleol. 2018, 47, 189–199. [Google Scholar] [CrossRef]
- Yasir, M. Analysis of bacterial communities and characterization of antimicrobial strains from cave microbiota. Braz. J. Microbiol. 2018, 49, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Rangseekaew, P.; Pathom-Aree, W. Cave Actinobacteria as producers of bioactive metabolites. Front. Microbiol. 2019, 10, 387. [Google Scholar] [CrossRef] [PubMed]
- Avguštin, J.A.; Petrič, P.; Pašić, L. Screening the cultivable cave microbial mats for the production of antimicrobial compounds and antibiotic resistance. Int. J. Speleol. 2019, 48, 295–303. [Google Scholar] [CrossRef]
- Voytsekhovskaya, I.V.; Axenov-Gribanov, D.V.; Murzina, S.A.; Pekkoeva, S.N.; Protasov, E.S.; Gamaiunov, S.V.; Timofeyev, M. Estimation of antimicrobial activities and fatty acid composition of actinobacteria isolated from water surface of underground lakes from Badzheyskaya and Okhotnichya caves in Siberia. PeerJ 2018, 6, e5832. [Google Scholar] [CrossRef]
- Herold, K.; Gollmick, F.A.; Groth, I.; Roth, M.; Menzel, K.-D.; Möllmann, U.; Gräfe, U.; Hertweck, C. Cervimycin A-D: A polyketide glycoside complex from a cave bacterium can defeat vancomycin resistance. Chem. Eur. J. 2005, 11, 5523–5530. [Google Scholar] [CrossRef]
- Jiang, L.; Pu, H.; Xiang, J.; Su, M.; Yan, X.; Yang, D.; Zhu, X.; Shen, B.; Duan, Y.; Huang, Y. Huanglongmycin A-C, cytotoxic polyketides biosynthesized by a putative type ii polyketide synthase from Streptomyces sp. CB09001. Front. Chem. 2018, 6, 254. [Google Scholar] [CrossRef]
- Axenov-Gibanov, D.; Voytsekhovskaya, I.V.; Tokovenko, B.T.; Protasov, E.S.; Gamaiunov, S.V.; Rabets, Y.V.; Luzhetskyy, A.N.; Timofeyev, M.A. Actinobacteria isolated from an underground lake moonmilk speleothem from the biggest conglomeratic karstic cave in Siberia as sources of novel biologically active compounds. PLoS ONE 2016, 11, e0149216. [Google Scholar] [CrossRef]
- Bollmann, A.; Lewis, K.; Epstein, S.S. Incubation of environmental samples in a diffusion chamber increases the diversity of recovered isolates. Appl. Environ. Microbiol. 2007, 73, 6386–6390. [Google Scholar] [CrossRef]
- Hug, J.J.; Bader, C.D.; Remškar, M.; Cirnski, K.; Müller, R. Concepts and methods to access novel antibiotics from Actinomycetes. Antibiotics 2018, 7, 44. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K.; Epstein, S.S.; D’Onofrio, A.; Ling, L.L. Uncultured microorganisms as a source of secondary metabolites. J. Antibiot. 2010, 63, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Manteca, A.; Yagüe, P. Streptomyces differentiation in liquid cultures as a trigger of secondary metabolism. Antibiotics 2018, 7, 41. [Google Scholar] [CrossRef] [PubMed]
- Nett, M.; Ikeda, H.; Moore, B.S. Genomic basis for natural product biosynthetic diversity in the actinomycetes. Nat. Prod. Rep. 2009, 26, 1362–1384. [Google Scholar] [CrossRef]
- Yoon, S.-H.; Ha, S.-M.; Kwon, S.; Lim, J.; Kim, Y.; Seo, H.; Chun, J. Introducing EzBioCloud: A taxonomically united database of 16S rRNA and whole genome assemblies. Int. J. Syst. Evol. Microbiol. 2017, 67, 1613–1617. [Google Scholar] [CrossRef]
- Bukelskis, D.; Dabkeviciene, D.; Lukoseviciute, L.; Bucelis, A.; Kriaučiūnas, I.; Lebedeva, J.; Kuisiene, N. Screening and transcriptional analysis of polyketide synthases and non-ribosomal peptide synthetases in bacterial strains from Krubera-Voronja Cave. Front. Microbiol. 2019, 10, 2149. [Google Scholar] [CrossRef]
- Giubergia, S.; Phippen, C.; Nielsen, K.F.; Gram, L. Growth on chitin impacts the transcriptome and metabolite profiles of antibiotic-producing Vibrio coralliilyticus S2052 and Photobacterium galatheae S2753. mSystems 2017, 2, e00141-16. [Google Scholar] [CrossRef]
- Ahmed, Y.; Rebets, Y.; Estévez, M.R.; Zapp, J.; Myronovskyi, M.; Luzhetskyy, A. Engineering of Streptomyces lividans for heterologous expression of secondary metabolite gene clusters. Microb. Cell Fact. 2020, 19, 5. [Google Scholar] [CrossRef]
- Paun, V.I.; Icaza, G.; Lavin, P.; Marin, C.; Tudorache, A.; Perşoiu, A.; Dorador, C.; Purcarea, C. Total and potentially active bacterial communities entrapped in a late glacial through holocene ice core from Scarisoara Ice Cave, Romania. Front. Microbiol. 2019, 10, 1193. [Google Scholar] [CrossRef]
- Interreg project. 0483_PROBIOMA_5_E: Prospecting Underground Environments for Microbial Bioactive Compounds with Potential Use in Medicine, Agriculture and Environment. Available online: https://probioma.org (accessed on 15 November 2020).
- Sánchez España, J. Acid mine drainage in the Iberian Pyrite Belt: An overview with special emphasis on generation mechanisms, aqueous composition and associated mineral phases. Macla 2008, 10, 34–43. [Google Scholar]
- Porca, E.; Jurado, V.; Martin-Sanchez, P.M.; Hermosin, B.; Bastian, F.; Alabouvette, C.; Saiz-Jimenez, C. Aerobiology: An ecological indicator for early detection and control of fungal outbreaks in caves. Ecol. Indic. 2011, 11, 1594–1598. [Google Scholar] [CrossRef]
- Blin, K.; Shaw, S.; Steinke, K.; Villebro, R.; Ziemert, N.; Lee, S.Y.; Medema, M.H.; Weber, T. antiSMASH 5.0: Updates to the secondary metabolite genome mining pipeline. Nucleic Acids Res. 2019, 47, W81–W87. [Google Scholar] [CrossRef] [PubMed]
- Romano, S.; Jackson, S.A.; Patry, S.; Dobson, A.D.W. Extending the “One Strain Many Compounds” (OSMAC) principle to marine microorganisms. Mar. Drugs 2018, 16, 244. [Google Scholar] [CrossRef] [PubMed]
- Jünemann, S.; Kleinbölting, N.; Jaenicke, S.; Henke, C.; Hassa, J.; Nelkner, J.; Stolze, Y.; Albaum, S.P.; Schlüter, A.; Goesmann, A.; et al. Bioinformatics for NGS-based metagenomics and the application to biogas research. J. Biotechnol. 2017, 261, 10–23. [Google Scholar] [CrossRef]
- Alain, K.; Callac, N.; Ciobanu, M.C.; Reynaud, Y.; Duthoit, F.; Jebbar, M. DNA extractions from deep subsea floor sediments: Novel cryogenic mill-based procedure and comparison to existing protocols. J. Microbiol. Methods 2011, 87, 355–362. [Google Scholar] [CrossRef]
- Quince, C.; Walker, A.W.; Simpson, J.T.; Loman, N.J.; Segata, N. Shotgun metagenomics, from sampling to analysis. Nat. Biotechnol. 2017, 35, 833–844. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).