Evaluation of the Cultivated Mushroom Pleurotus ostreatus Basidiocarps Using Vibration Spectroscopy and Chemometrics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Basidiocarps of Chosen Strains
2.2. Elemental Analysis And Determination of Proteins
2.3. Determination of Glucans
2.4. Analysis of Neutral Sugars Composition
2.5. Vibration Spectroscopy
2.6. Statistical Methods
3. Results and Discussion
3.1. Composition of Basidiocarps
3.2. Vibration Spectroscopy
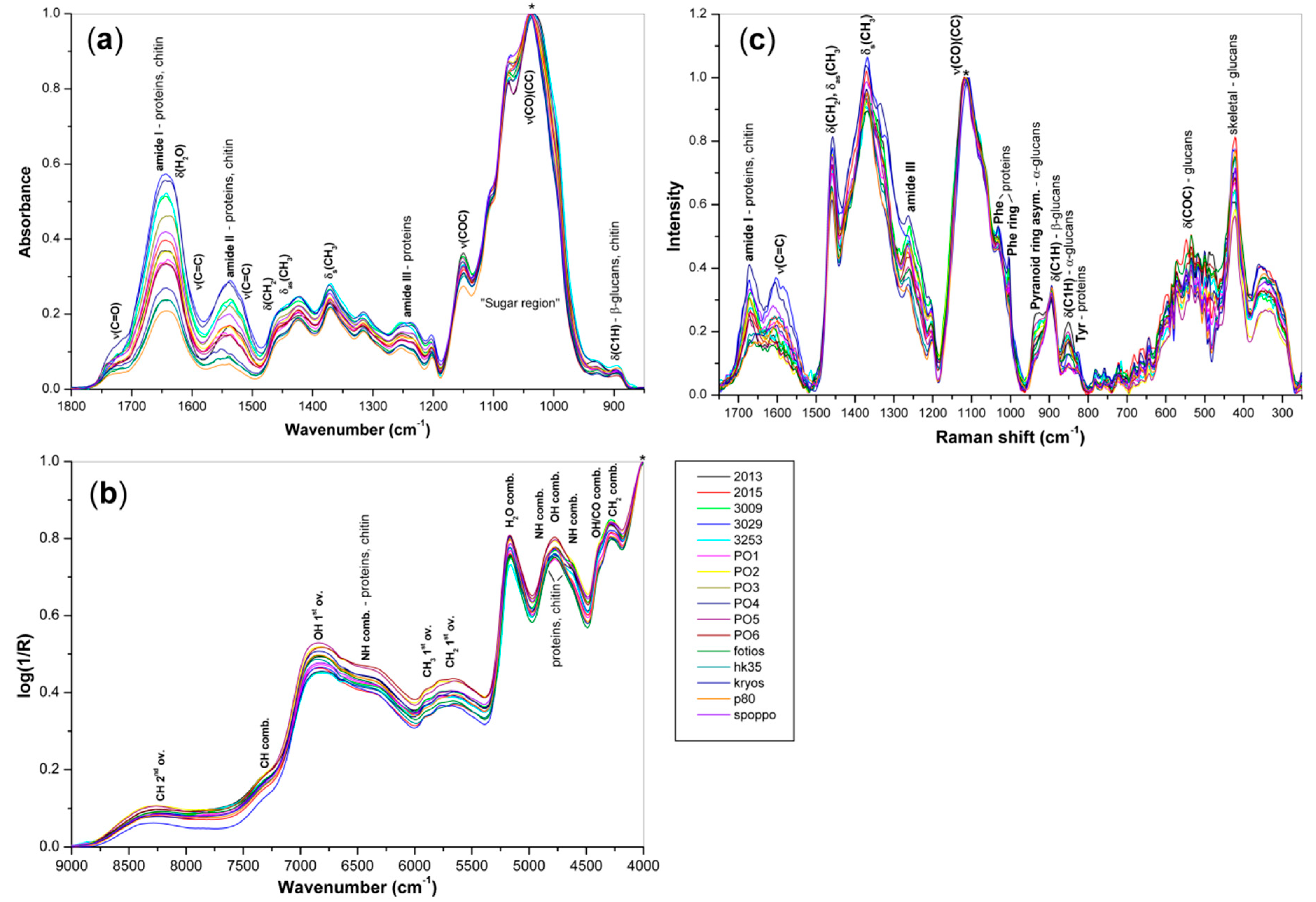
3.2.1. FT MIR
3.2.2. FT NIR
3.2.3. FT Raman
3.3. PCA of Spectroscopic Data
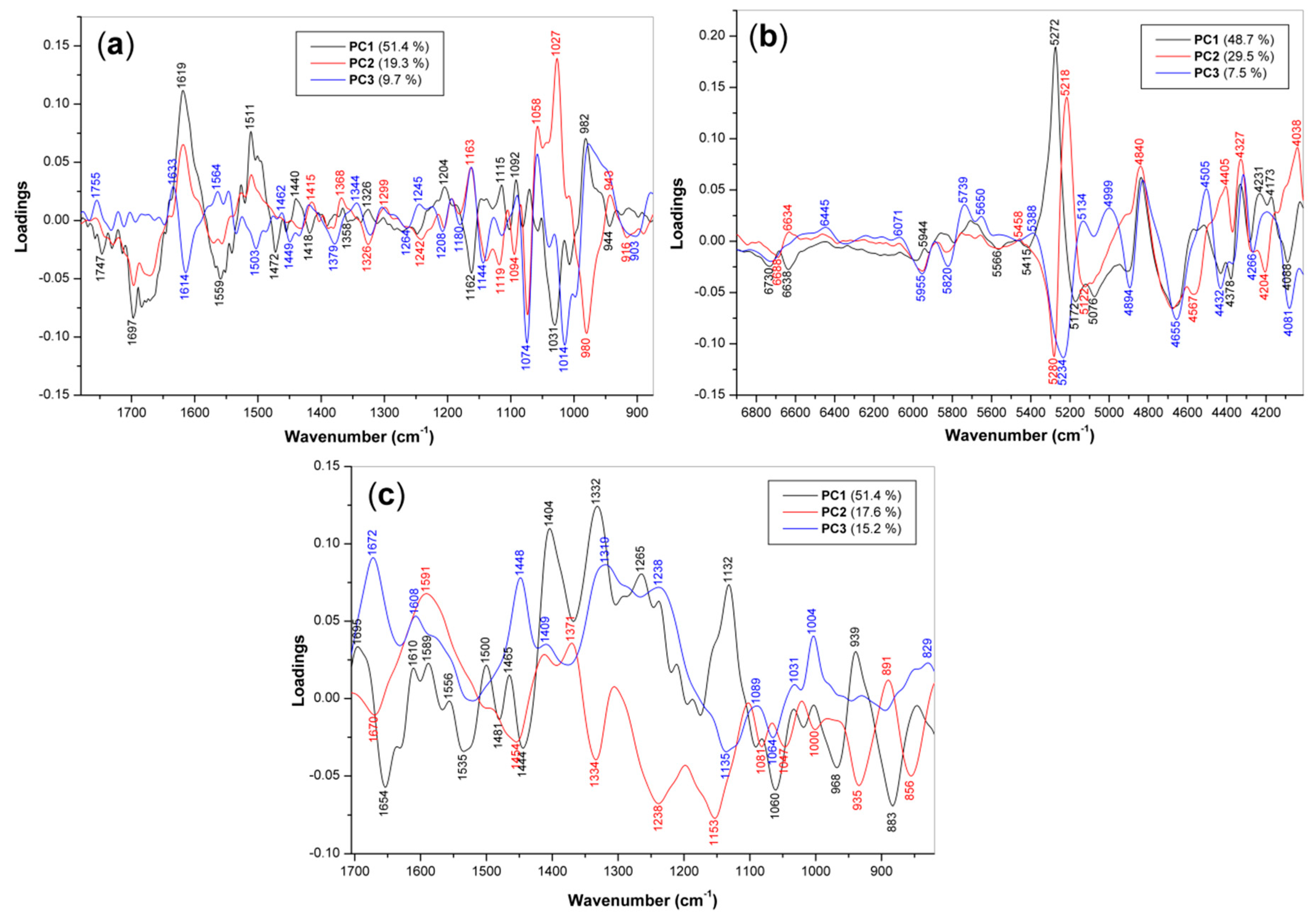
3.3.1. PCA Loadings
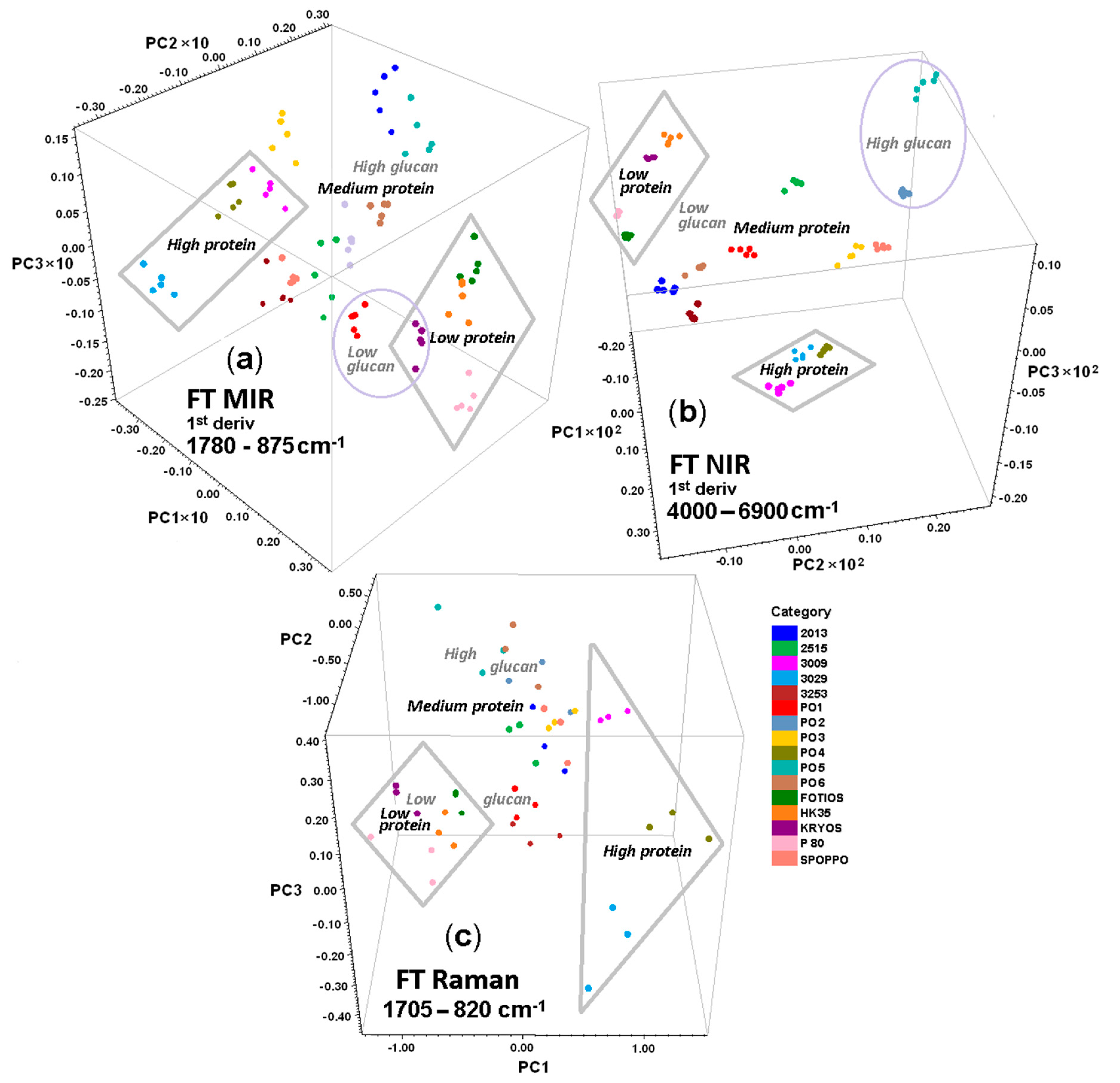
3.3.2. 3D Component Score
3.4. PLSR Models for Protein and Total Glucan Contents
3.5. Estimation of Proteins By Raman Band at 1004 cm−1
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- de Carvalho, C.S.M.; Sales-Campos, C.; de Andrade, M.C.N. Mushrooms of the Pleurotus genus: A review of cultivation techniques. Interciencia 2010, 35, 177–182. [Google Scholar]
- Corrêa, R.C.G.; Brugnari, T.; Bracht, A.; Peralta, R.M.; Ferreira, I.C. Biotechnological, nutritional and therapeutic uses of Pleurotus spp. (Oyster mushroom) related with its chemical composition: A review on the past decade findings. Trends Food Sci. Technol. 2016, 50, 103–117. [Google Scholar] [CrossRef] [Green Version]
- Carrasco-González, J.A.; Serna-Saldívar, S.O.; Gutiérrez-Uribe, J.A. Nutritional composition and nutraceutical properties of the Pleurotus fruiting bodies: Potential use as food ingredient. J. Food Compos. Anal. 2017, 58, 69–81. [Google Scholar] [CrossRef]
- Kalač, P. A review of chemical composition and nutritional value of wild-growing and cultivated mushrooms. J. Sci. Food Agric. 2013, 93, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Synytsya, A.; Míčková, K.; Jablonský, I.; Sluková, M.; Čopíková, J. Mushrooms of genus Pleurotus as a source of dietary fibres and glucans for food supplements. Czech J. Food Sci. 2009, 26, 441–446. [Google Scholar] [CrossRef] [Green Version]
- Patel, Y.; Naraian, R.; Singh, V.K. Medicinal properties of Pleurotus species (Oyster mushroom): A review. World J. Fungal Plant Biol. 2012, 3, 1–12. [Google Scholar]
- Zhu, F.; Du, B.; Bian, Z.; Xu, B. β-Glucans from edible and medicinal mushrooms: Characteristics, physicochemical and biological activities. J. Food Compos. Anal. 2015, 41, 165–173. [Google Scholar] [CrossRef]
- Rop, O.; Mlcek, J.; Jurikova, T. β-Glucans in higher fungi and their health effects. Nutr. Rev. 2009, 67, 624–631. [Google Scholar] [CrossRef]
- Ma, G.; Yang, W.; Mariga, A.M.; Fang, Y.; Ma, N.; Pei, F.; Hu, Q. Purification, characterization and antitumor activity of polysaccharides from Pleurotus eryngii residue. Carbohydr. Polym. 2014, 114, 297–305. [Google Scholar] [CrossRef]
- Zhang, Y.; Dai, L.; Kong, X.; Chen, L. Characterization and in vitro antioxidant activities of polysaccharides from Pleurotus ostreatus. Int. J. Biol. Macromol. 2012, 51, 259–265. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, T.; Zhou, H.; Jin, G.; Yang, Y. Antidiabetic effect of polysaccharides from Pleurotus ostreatus in streptozotocin-induced diabetic rats. Int. J. Biol. Macromol. 2016, 83, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Smiderle, F.; Olsen, L.; Carbonero, E.; Marcon, R.; Baggio, C.; Freitas, C.; Santos, A.; Torri, G.; Gorin, P.; Iacomini, M. A 3-O-methylated mannogalactan from Pleurotus pulmonarius: Structure and antinociceptive effect. Phytochemistry 2008, 69, 2731–2736. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-X.; Liu, J. Purification, structure and immunobiological activity of a water-soluble polysaccharide from the fruiting body of Pleurotus ostreatus. Bioresour. Technol. 2009, 100, 983–986. [Google Scholar] [CrossRef] [PubMed]
- Synytsya, A.; Míčková, K.; Synytsya, A.; Jablonský, I.; Spěváček, J.; Erban, V.; Kováříková, E.; Čopíková, J. Glucans from fruit bodies of cultivated mushrooms Pleurotus ostreatus and Pleurotus eryngii: Structure and potential prebiotic activity. Carbohydr. Polym. 2009, 76, 548–556. [Google Scholar] [CrossRef]
- Deepalakshmi, K.; Sankaran, M. Pleurotus ostreatus: An oyster mushroom with nutritional and medicinal properties. J. Biochem. Technol. 2014, 5, 718–726. [Google Scholar]
- Alam, N.; Yoon, K.N.; Lee, T.S.; Lee, U.Y. Hypolipidemic Activities of Dietary Pleurotus ostreatus in Hypercholesterolemic Rats. Mycobiology 2011, 39, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Schneider, I.; Kressel, G.; Meyer, A.; Krings, U.; Berger, R.G.; Hahn, A. Lipid lowering effects of oyster mushroom (Pleurotus ostreatus) in humans. J. Funct. Foods 2011, 3, 17–24. [Google Scholar] [CrossRef]
- Vasudewa, N.; Abeytunga, D.; Ratnasooriya, W. Antinociceptive Activity of Pleurotus ostreatus, an Edible Mushroom, in Rats. Pharm. Biol. 2007, 45, 533–540. [Google Scholar] [CrossRef]
- Jayakumar, T.; Ramesh, E.; Geraldine, P. Antioxidant activity of the oyster mushroom, Pleurotus ostreatus, on CCl4-induced liver injury in rats. Food Chem. Toxicol. 2006, 44, 1989–1996. [Google Scholar] [CrossRef]
- Jedinak, A.; Dudhgaonkar, S.; Wu, Q.; Simon, J.E.; Sliva, D. Anti-inflammatory activity of edible oyster mushroom is mediated through the inhibition of NF-κB and AP-1 signaling. Nutr. J. 2011, 10, 52. [Google Scholar] [CrossRef] [Green Version]
- Karácsonyi, S.; Kuniak, L. Polysaccharides of Pleurotus ostreatus: Isolation and structure of pleuran, an alkali-insoluble β-d-glucan. Carbohydr. Polym. 1994, 24, 107–111. [Google Scholar] [CrossRef]
- Jesenak, M.; Majtan, J.; Rennerova, Z.; Kyselovic, J.; Banovcin, P.; Hrubisko, M. Immunomodulatory effect of pleuran (β-glucan from Pleurotus ostreatus) in children with recurrent respiratory tract infections. Int. Immunopharmacol. 2013, 15, 395–399. [Google Scholar] [CrossRef]
- Tong, H.; Xia, F.; Feng, K.; Sun, G.; Gao, X.; Sun, L.; Jiang, R.; Tian, D.; Sun, X. Structural characterization and in vitro antitumor activity of a novel polysaccharide isolated from the fruiting bodies of Pleurotus ostreatus. Bioresour. Technol. 2009, 100, 1682–1686. [Google Scholar] [CrossRef] [PubMed]
- Curvetto, N.; Figlas, D.; Devalis, R.; Delmastro, S. Growth and productivity of different Pleurotus ostreatus strains on sunflower seed hulls supplemented with N–NH4+ and/or Mn(II). Bioresour. Technol. 2002, 84, 171–176. [Google Scholar] [CrossRef]
- Meenu, M.; Xu, B. Application of vibrational spectroscopy for classification, authentication and quality analysis of mushroom: A concise review. Food Chem. 2019, 289, 545–557. [Google Scholar] [CrossRef]
- Baeva, E.; Bleha, R.; Lavrova, E.; Sushytskyi, L.; Čopíková, J.; Jablonsky, I.; Klouček, P.; Synytsya, A. Polysaccharides from Basidiocarps of Cultivating Mushroom Pleurotus ostreatus: Isolation and Structural Characterization. Molecules 2019, 24, 2740. [Google Scholar] [CrossRef] [Green Version]
- Bekiaris, G.; Tagkouli, D.; Koutrotsios, G.; Kalogeropoulos, N.; Zervakis, G.I. Pleurotus Mushrooms Content in Glucans and Ergosterol Assessed by ATR-FTIR Spectroscopy and Multivariate Analysis. Foods 2020, 9, 535. [Google Scholar] [CrossRef]
- Mikola, E.; Geösel, A.; Stefanovits-Bányai, É.; Fodor, M. Quantitative determination of macro components and classification of some cultivated mushrooms using near-infrared spectroscopy. J. Food Process. Preserv. 2020, 44, 14540. [Google Scholar] [CrossRef]
- Gomba, G.K.; Synytsya, A.; Švecová, P.; Čopíková, J.; Coimbra, M. Distinction of fungal polysaccharides by N/C ratio and mid infrared spectroscopy. Int. J. Biol. Macromol. 2015, 80, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Oberle, J.; Dighton, J.; Arbuckle-Keil, G.A. Comparison of methodologies for separation of fungal isolates using Fourier transform infrared (FTIR) spectroscopy and Fourier transform infrared-attenuated total reflectance (FTIR-ATR) microspectroscopy. Fungal Biol. 2015, 119, 1100–1114. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.-M.; Zhang, J.; Liu, H.-G.; Li, T.; Wang, Y. Fourier transform mid-infrared spectroscopy and chemometrics to identify and discriminate Boletus edulis and Boletus tomentipes mushrooms. Int. J. Food Prop. 2017, 20, S56–S68. [Google Scholar] [CrossRef] [Green Version]
- Fujihara, S.; Kasuga, A.; Aoyagi, Y.; Sugahara, T. Nitrogen-to-Protein Conversion Factors for Some Common Edible Mushrooms. J. Food Sci. 1995, 60, 1045–1047. [Google Scholar] [CrossRef]
- Braaksma, A.; Schaap, D. Protein analysis of the common mushroom Agaricus bisporus. Postharvest Biol. Technol. 1996, 7, 119–127. [Google Scholar] [CrossRef]
- Wiercigroch, E.; Szafraniec, E.; Czamara, K.; Pacia, M.Z.; Majzner, K.; Kochan, K.; Kaczor, A.; Barańska, M.; Malek, K. Raman and infrared spectroscopy of carbohydrates: A review. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 185, 317–335. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, A.; Prieto, A.; Martínez, M.J. Structural characterization of extracellular polysaccharides produced by fungi from the genus Pleurotus. Carbohydr. Res. 1996, 281, 143–154. [Google Scholar] [CrossRef]
- Šandula, J.; Kogan, G.; Kačuráková, M.; Machová, E. Microbial (1→3)-β-d-glucans, their preparation, physico-chemical characterization and immunomodulatory activity. Carbohydr. Polym. 1999, 38, 247–253. [Google Scholar] [CrossRef]
- Unursaikhan, S.; Xu, X.; Zeng, F.; Zhang, L. Antitumor activities of O-sulphonated derivatives of (1→3)-glucans from different Lentinus edodes. Biosci. Biotechnol. Biochem. 2006, 70, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Deng, L.; Li, S.; Tan, T. Structural characterization of a water insoluble α-(1→3)-d-glucan isolated from the Penicillium chrysogenum. Carbohydr. Polym. 2007, 67, 133–137. [Google Scholar] [CrossRef]
- Liu, G.; Song, D.; Zhao, D.; Liu, J.-H.; Zhou, Y.; Ou, J.; Sun, S. A study of the mushrooms of boletes by Fourier transform infrared spectroscopy. In Proceedings of the ICO20: Biomedical Optics, Changchun, China, 2 February 2006; Volume 6026. [Google Scholar] [CrossRef]
- Cárdenas, G.; Cabrera, G.; Taboada, E.; Miranda, S.P. Chitin characterization by SEM, FTIR, XRD, and13C cross polarization/mass angle spinning NMR. J. Appl. Polym. Sci. 2004, 93, 1876–1885. [Google Scholar] [CrossRef]
- Kong, J.; Yu, S. Fourier Transform Infrared Spectroscopic Analysis of Protein Secondary Structures. Acta Biochim. Biophys. Sin. 2007, 39, 549–559. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, M.; Nada, A.; Kamal, D.E. Density functional theory and FTIR spectroscopic study of carboxyl group. Ind. J. Pure Appl. Phys. 2005, 43, 911–917. [Google Scholar]
- Miyazawa, M.; Sonoyama, M. Second Derivative near Infrared Studies on the Structural Characterisation of Proteins. J. Near Infrared Spectrosc. 1998, 6, A253–A257. [Google Scholar] [CrossRef]
- Wang, J.; Sowa, M.G.; Ahmed, M.K.; Mantsch, H.H. Photoacoustic Near-Infrared Investigation of Homo-Polypeptides. J. Phys. Chem. 1994, 98, 4748–4755. [Google Scholar] [CrossRef]
- Workmanjr, J. Interpretive Spectroscopy for Near-Infrared. Appl. Spectrosc. Rev. 1996, 31, 251–320. [Google Scholar] [CrossRef]
- López, M.G.; García-González, A.S.; Franco-Robles, E. Carbohydrate Analysis by NIRS-Chemometrics. In Developments in Near-Infrared Spectroscopy; IN TECH: München, Germany, 2017; Volume 10, p. 67208. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; He, H.; Wu, J.; Wang, C.; Chao, K.; Huang, Q. Assessment of Polysaccharides from Mycelia of genus Ganoderma by Mid-Infrared and Near-Infrared Spectroscopy. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Rygula, A.; Majzner, K.; Marzec, K.M.; Kaczor, A.; Pilarczyk, M.; Baranska, M. Raman spectroscopy of proteins: A review. J. Raman Spectrosc. 2013, 44, 1061–1076. [Google Scholar] [CrossRef]
- Noothalapati, H.; Sasaki, T.; Kaino, T.; Kawamukai, M.; Ando, M.; Hamaguchi, H.-O.; Yamamoto, T. Label-free Chemical Imaging of Fungal Spore Walls by Raman Microscopy and Multivariate Curve Resolution Analysis. Sci. Rep. 2016, 6, 27789. [Google Scholar] [CrossRef] [Green Version]
- Synytsya, A.; Novák, M. Structural diversity of fungal glucans. Carbohydr. Polym. 2013, 92, 792–809. [Google Scholar] [CrossRef]
- Ruthes, A.C.; Smiderle, F.R.; Iacomini, M. Mushroom heteropolysaccharides: A review on their sources, structure and biological effects. Carbohydr. Polym. 2016, 136, 358–375. [Google Scholar] [CrossRef]


| Sample | Strain | Supplier |
|---|---|---|
| 2013 | 2013 | Mycelia nv (Deinze, Belgium) |
| 2515 | 2515 | Mycelia nv (Deinze, Belgium) |
| 3009 | 3009 | Sylvan Inc. (Kittanning, PA, USA) |
| 3029 | 3029 | Sylvan Inc. (Kittanning, PA, USA) |
| 3253 | 3253 | Sylvan Inc. (Kittanning, PA, USA) |
| fotios | fotios | Sylvan Inc. (Kittanning, PA, USA) |
| hk35 | HK35 | Sylvan Inc. (Kittanning, PA, USA) |
| kryos | KRYOS | Sylvan Inc. (Kittanning, PA, USA) |
| spoppo | SPOPPO | Sylvan Inc. (Kittanning, PA, USA) |
| po1 | PO1 | Unknown |
| po2 | PO2 | Unknown |
| po3 | PO3 | Unknown |
| po4 | PO4 | Unknown |
| po5 | PO5 | Unknown |
| po6 | PO6 | Unknown |
| p80 | P80 | Hollander Spawn BV (Horst, Holland) |
| Sample | Organic Elements (% w/w) | Proteins 2 (% w/w) | |||
|---|---|---|---|---|---|
| N | C | H | S | ||
| 2013 | 3.05 ± 0.30 | 40.55 ± 0.37 | 6.69 ± 0.09 | 0.14 ± 0.02 | 12.17 ± 1.20 bcdef |
| 2515 | 2.90 ± 0.14 | 40.33 ± 0.56 | 6.78 ± 0.05 | 0.14 ± 0.02 | 11.57 ± 0.56 abcde |
| 3009 | 4.09 ± 0.38 | 41.14 ± 0.31 | 6.73 ± 0.07 | 0.19 ± 0.04 | 16.32 ± 1.52 ef |
| 3029 | 4.17 ± 0.16 | 41.03 ± 0.44 | 6.79 ± 0.09 | 0.22 ± 0.01 | 16.64 ± 0.64 ef |
| 3253 | 3.27 ± 0.36 | 40.83 ± 0.44 | 6.75 ± 0.04 | 0.14 ± 0.02 | 13.05 ± 1.44 cdef |
| fotios | 2.13 ± 0.22 | 40.85 ± 0.35 | 6.79 ± 0.11 | 0.11 ± 0.01 | 8.50 ± 0.88 abc |
| hk35 | 1.55 ± 0.19 | 39.93 ± 0.28 | 6.75 ± 0.09 | 0.09 ± 0.02 | 6.18 ± 0.76 a |
| kryos | 1.56 ± 0.20 | 39.73 ± 0.36 | 6.74 ± 0.10 | 0.08 ± 0.01 | 6.22 ± 0.80 a |
| spoppo | 3.70 ± 0.23 | 40.81 ± 0.28 | 6.80 ± 0.05 | 0.11 ± 0.02 | 14.76 ± 0.92 def |
| po1 | 3.09 ± 0.32 | 40.56 ± 0.35 | 6.80 ± 0.05 | 0.14 ± 0.01 | 12.33 ± 1.28 bcdef |
| po2 | 3.27 ± 0.25 | 40.94 ± 0.41 | 6.77 ± 0.05 | 0.17 ± 0.02 | 13.05 ± 1.00 cdef |
| po3 | 3.47 ± 0.21 | 41.06 ± 0.39 | 6.78 ± 0.07 | 0.16 ± 0.03 | 13.85 ± 0.84 cdef |
| po4 | 4.38 ± 0.33 | 41.73 ± 0.40 | 6.78 ± 0.06 | 0.22 ± 0.03 | 17.48 ± 1.32 f |
| po5 | 2.48 ± 0.19 | 40.50 ± 0.13 | 6.79 ± 0.08 | 0.12 ± 0.02 | 9.90 ± 0.76 abcd |
| po6 | 2.81 ± 0.29 | 40.88 ± 0.36 | 6.77 ± 0.10 | 0.13 ± 0.02 | 11.21 ± 1.16 abcde |
| p80 | 1.73 ± 0.17 | 40.23 ± 0.23 | 6.81 ± 0.07 | 0.09 ± 0.01 | 6.90 ± 0.68 ab |
| Sample | Neutral Sugars (mol %) | ||||||
|---|---|---|---|---|---|---|---|
| Fucose | Arabinose | Mannose | Glucose | Galactose | Rhamnose | Xylose | |
| 2013 | trace | 0.14 ± 0.010 | 2.10 ± 0.44 | 92.99 ± 1.10 | 1.46 ± 0.19 | 0.31 ± 0.11 | 3.00 ± 0.84 |
| 2515 | 0.14 ± 0.09 | 0.16 ± 0.12 | 1.96 ± 0.004 | 91.20 ± 4.27 | 1.18 ± 0.03 | 0.27 ± 0.04 | 5.09 ± 2.20 |
| 3009 | trace | 0.45 ± 0.012 | 8.70 ± 0.25 | 71.26 ± 1.30 | 12.63 ± 0.43 | 0.17 ± 0.008 | 6.78 ± 1.98 |
| 3029 | 0.09 ± 0.03 | 0.24 ± 0.08 | 2.21 ± 0.02 | 93.39 ± 1.21 | 1.49 ± 0.03 | 0.24 ± 0.05 | 2.34 ± 0.32 |
| 3253 | trace | 0.07 ± 0.07 | 1.95 ± 0.05 | 87.54 ± 1.30 | 1.55 ± 0.24 | 0.16 ± 0.022 | 8.71 ± 0.12 |
| fotios | 0.08 ± 0.03 | 0.20 ± 0.11 | 1.64 ± 0.10 | 91.20 ± 1.27 | 0.87 ± 0.016 | 4.00 ± 0.05 | 2.01 ± 0.28 |
| hk35 | 0.29 ± 0.22 | 0.18 ± 0.09 | 2.14 ± 0.03 | 89.94 ± 1.26 | 0.56 ± 0.04 | 1.44 ± 1.28 | 5.44 ± 0.35 |
| kryos | trace | 0.61 ± 0.25 | 1.85 ± 0.07 | 91.31 ± 1.61 | 0.50 ± 0.015 | 2.75 ± 2.38 | 2.94 ± 0.94 |
| spoppo | 0.67 ± 0.19 | 0.49 ± 0.09 | 1.84 ± 0.11 | 87.19 ± 1.06 | 1.84 ± 0.09 | 3.08 ± 0.19 | 4.89 ± 0.40 |
| po1 | 0.09 ± 0.012 | trace | 2.56 ± 0.07 | 94.41 ± 1.21 | 1.08 ± 0.010 | 0.16 ± 0.003 | 1.68 ± 0.14 |
| po2 | 0.18 ± 0.16 | 0.13 ± 0.05 | 2.15 ± 0.13 | 94.27 ± 1.76 | 0.84 ± 0.07 | 0.34 ± 0.005 | 2.10 ± 1.06 |
| po3 | 0.07 ± 0.008 | 0.24 ± 0.09 | 1.69 ± 0.02 | 92.52 ± 2.30 | 1.14 ± 0.03 | 0.17 ± 0.03 | 4.17 ± 0.04 |
| po4 | 0.06 ± 0.05 | 0.11 ± 0.04 | 2.10 ± 0.010 | 91.00 ± 1.74 | 1.39 ± 0.007 | 0.93 ± 0.76 | 4.40 ± 0.06 |
| po5 | trace | 0.18 ± 0.18 | 1.16 ± 0.03 | 92.76 ± 1.62 | 0.67 ± 0.09 | 0.19 ± 0.012 | 5.02 ± 0.32 |
| po6 | 0.07 ± 0.012 | 0.28 ± 0.004 | 2.08 ± 0.04 | 90.54 ± 1.54 | 1.19 ± 0.35 | 0.21 ± 0.010 | 5.62 ± 1.91 |
| p80 | 0.35 ± 0.15 | 0.09 ± 0.019 | 1.46 ± 0.017 | 86.07 ± 2.13 | 0.74 ± 0.019 | 5.29 ± 0.93 | 6.01 ± 1.57 |
| Sample | Glucans (% w/w) 1 | ||
|---|---|---|---|
| Total | α-Glucans | β-Glucans | |
| 2013 | 48.44 ± 2.01 c | 8.98 ± 0.27 cd | 39.46 ± 1.61 bcde |
| 2515 | 45.99 ± 1.13 c | 13.85 ± 0.14 e | 32.14 ± 0.82 bc |
| 3009 | 55.31 ± 1.56 c | 8.66 ± 0.17 c | 46.65 ± 1.35 def |
| 3029 | 43.54 ± 2.80 bc | 4.89 ± 0.52 ab | 38.79 ± 1.97 bcde |
| 3253 | 52.51 ± 1.44 c | 11.42 ± 0.30 cde | 41.10 ± 3.06 cdef |
| fotios | 45.45 ± 2.30 bc | 5.45 ± 0.43 b | 40.03 ± 1.96 bcde |
| hk35 | 49.22 ± 2.55 c | 5.22 ± 0.61 ab | 44.00 ± 1.75 cdef |
| kryos | 18.79 ± 3.39 a | 2.94 ± 0.46 ab | 15.85 ± 2.51 a |
| spoppo | 50.58 ± 2.85 c | 11.76 ± 0.93 de | 38.86 ± 2.29 bcde |
| po1 | 30.39 ± 2.95 ab | 3.15 ± 0.25 ab | 27.23 ± 2.25 ab |
| po2 | 56.17 ± 2.79 c | 4.63 ± 0.81 ab | 51.54 ± 1.80 ef |
| po3 | 48.32 ± 3.22 c | 9.03 ± 0.14 cd | 39.29 ± 2.77 bcde |
| po4 | 45.98 ± 2.08 c | 12.43 ± 0.30 e | 33.56 ± 1.57 bcd |
| po5 | 58.17 ± 3.14 c | 9.55 ± 0.68 cd | 48.62 ± 1.80 ef |
| po6 | 50.84 ± 3.39 c | 16.72 ± 0.48 f | 34.12 ± 2.37 bcd |
| p80 | 57.89 ± 2.60 c | 2.52 ± 0.19 a | 55.36 ± 2.33 f |
| Method | Wavenumber (cm−1) | Origin 1 | |||
|---|---|---|---|---|---|
| Loadings | Spectra | ||||
| PC1 | PC2 | PC3 | |||
| FT MIR | ↓1697/↑1619 | ↓1697/↑1619 | ↑1633/↓1614 | 1642 | amide I—proteins, chitin |
| ↓1559/↑1511 | ↓1556/↑1511 | ↑1564/↓1503 | 1536 | amide II—proteins, chitin | |
| ↓1472/↑1440 | ↑1462/↓1449 | 1455sh | δ(CH2), δas(CH3) | ||
| ↓1418 | ↑1415 | ↑1418 | 1421 | δ(CHO)(COH)—glucans | |
| ↓1382/↑1368 | ↓1379 | 1375 | δs(CH3)—proteins, chitin | ||
| ↑1326 | ↓1326/↑1299 | ↓1324/↑1300 | 1316 | amide III—chitin | |
| ↓1246 | ↓1242 | ↑1245 | 1236 | amide III—proteins | |
| ↑1204 | ↓1206 | ↓1208 | 1202 | β-d-glucans | |
| ↓1162/↑1144 | ↑1163/↓1142 | ↑1163/↓1144 | 1150 | ν(COC)—glucans, chitin | |
| ↑1115 | ↓1119 | ↓1114 | 1100 | ν(CO)(CC)—glucans, chitin | |
| ↑1070 | ↓1074/↑1058 | ↓1074/↑1058 | 1075 | ν(CO)(CC)—glucans, chitin | |
| ↓1031/↑982 | ↑1027/↓980 | ↓1014/↑980 | 1040 | ν(CO)(CC)—glucans, chitin | |
| ↓944 | ↑943 | ↑946 | 930 | α-d-glucans | |
| FT NIR | ↓6730, ↓6638 | ↓6688/↑6634 | ↓6700 | 6600sh | NH comb.—proteins, chitin |
| ↑6445 | 6470sh | NH 1st ov.—proteins, chitin | |||
| ↓5955, ↓5820 | ↓5955, ↓5820 | CH, CH2, CH3 1st ov. | |||
| ↑5739, ↑5850 | CH, CH2, CH3 1st ov. | ||||
| ↑5272/↓5172 | ↓5280/↑5218 | ↓5234 | 5165 | OH comb.–glucans, chitin | |
| ↓5076 | ↓5122 | ↓5134, ↓4999 | OH comb.–glucans, chitin | ||
| ↓4894/↑4840 | ↑4900, ↑4840 | ↓4894/↑4840 | amide A/II comb.–proteins | ||
| ↓4675/↑4515 | ↓4675, ↓4567 | ↓4655/↑4505 | amide B/II comb.–proteins | ||
| ↓4432, ↓4378 | ↑4405 | ↓4432 | OH/CO comb.–glucans, chitin | ||
| ↑4231 | ↑4327/↓4266 | ↑4335/↓4266 | 4285 | CH, CH2, CH3 comb. | |
| ↑4173/↓4088 | ↓4204/4038 | ↑4150/↓4081 | CH, CH2, CH3 comb. | ||
| FT Raman | ↓1654 | ↓1670 | ↑1672 | 1670 | amide I–proteins, chitin |
| ↑1610, ↑1589 | ↑1591 | ↑1608 | 1604 | νas(COO-)–proteins | |
| ↓1535 | ν(C=C)–phenolic compounds | ||||
| ↓1444 | ↓1454 | ↑1448 | 1457 | δ(CH2), δas(CH3) | |
| ↑1404 | ↑1409 | ↑1409 | νs(COO-)–proteins | ||
| ↑1371 | δs(CH3)–proteins, chitin | ||||
| ↑1332 | ↓1334 | ↑1310 | 1335 | δ(CH), δ(OH) | |
| ↑1265 | ↓1238 | ↑1238 | 1263 | amide III–proteins | |
| ↑1132 | ↓1153 | ↓1135 | 1116 | ν(CO)(CC)–glucans, chitin | |
| ↓1060 | ↓1081, 1047 | ↓1064 | ν(CO)(CC)–glucans, chitin | ||
| ↓1000 | ↑1031 | 1033 | Phe ring–proteins | ||
| ↑1004 | 1004 | Phe ring–proteins | |||
| ↓968, ↑939 | ↓935 | α-d-glucans | |||
| ↓883 | ↑891 | 894 | δ(C1H)–β-d-glucans, chitin | ||
| ↓856 | δ(C1H)–α-d-glucans | ||||
| ↑845, ↑829 | Tyr ring–proteins | ||||
| Parameters | Proteins | Total Glucans | ||||
|---|---|---|---|---|---|---|
| FT MIR | FT NIR | FT Raman | FT MIR | FT NIR | FT Raman | |
| Spectroscopic data | 1st deriv. | 1st deriv. | spectra | 1st deriv. | 1st deriv. | spectra |
| Region (cm−1) | 1800–845 | 6800–6550 5150–4000 | 1735–220 | 1800–845 | 6800–6550 5150–4000 | 1555–380 |
| Number of factors 1 | 10 | 5 | 5 | 7 | 12 | 8 |
| R2cal | 0.994 | 0.994 | 0.981 | 0.908 | 0.996 | 0.984 |
| R2cv | 0.910 | 0.970 | 0.901 | 0.370 | 0.804 | 0.599 |
| RMSEcal | 0.269 | 0.275 | 0.485 | 2.98 | 0.612 | 1.24 |
| RMSEcv | 1.08 | 0.608 | 1.11 | 8.50 | 4.66 | 6.61 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baeva, E.; Bleha, R.; Sedliaková, M.; Sushytskyi, L.; Švec, I.; Čopíková, J.; Jablonsky, I.; Klouček, P.; Synytsya, A. Evaluation of the Cultivated Mushroom Pleurotus ostreatus Basidiocarps Using Vibration Spectroscopy and Chemometrics. Appl. Sci. 2020, 10, 8156. https://doi.org/10.3390/app10228156
Baeva E, Bleha R, Sedliaková M, Sushytskyi L, Švec I, Čopíková J, Jablonsky I, Klouček P, Synytsya A. Evaluation of the Cultivated Mushroom Pleurotus ostreatus Basidiocarps Using Vibration Spectroscopy and Chemometrics. Applied Sciences. 2020; 10(22):8156. https://doi.org/10.3390/app10228156
Chicago/Turabian StyleBaeva, Ekaterina, Roman Bleha, Markéta Sedliaková, Leonid Sushytskyi, Ivan Švec, Jana Čopíková, Ivan Jablonsky, Pavel Klouček, and Andriy Synytsya. 2020. "Evaluation of the Cultivated Mushroom Pleurotus ostreatus Basidiocarps Using Vibration Spectroscopy and Chemometrics" Applied Sciences 10, no. 22: 8156. https://doi.org/10.3390/app10228156





