A Systematic Comparative Study of the Toxicity of Semiconductor and Graphitic Carbon-Based Quantum Dots Using In Vitro Cell Models
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Equipment
2.3. Methods
2.3.1. Synthesis of Carbon-Based Quantum Dots
2.3.2. Cell Culture
2.3.3. Exposure of Cells to CQDs and SQDs
2.3.4. MTT Cytotoxicity Assay
2.3.5. Immunofluorescence and Confocal Microscopy
2.3.6. Statistical Analysis
3. Results and Discussion
3.1. Results
3.1.1. Selection and Optical Characterization of CQDs and SQDs
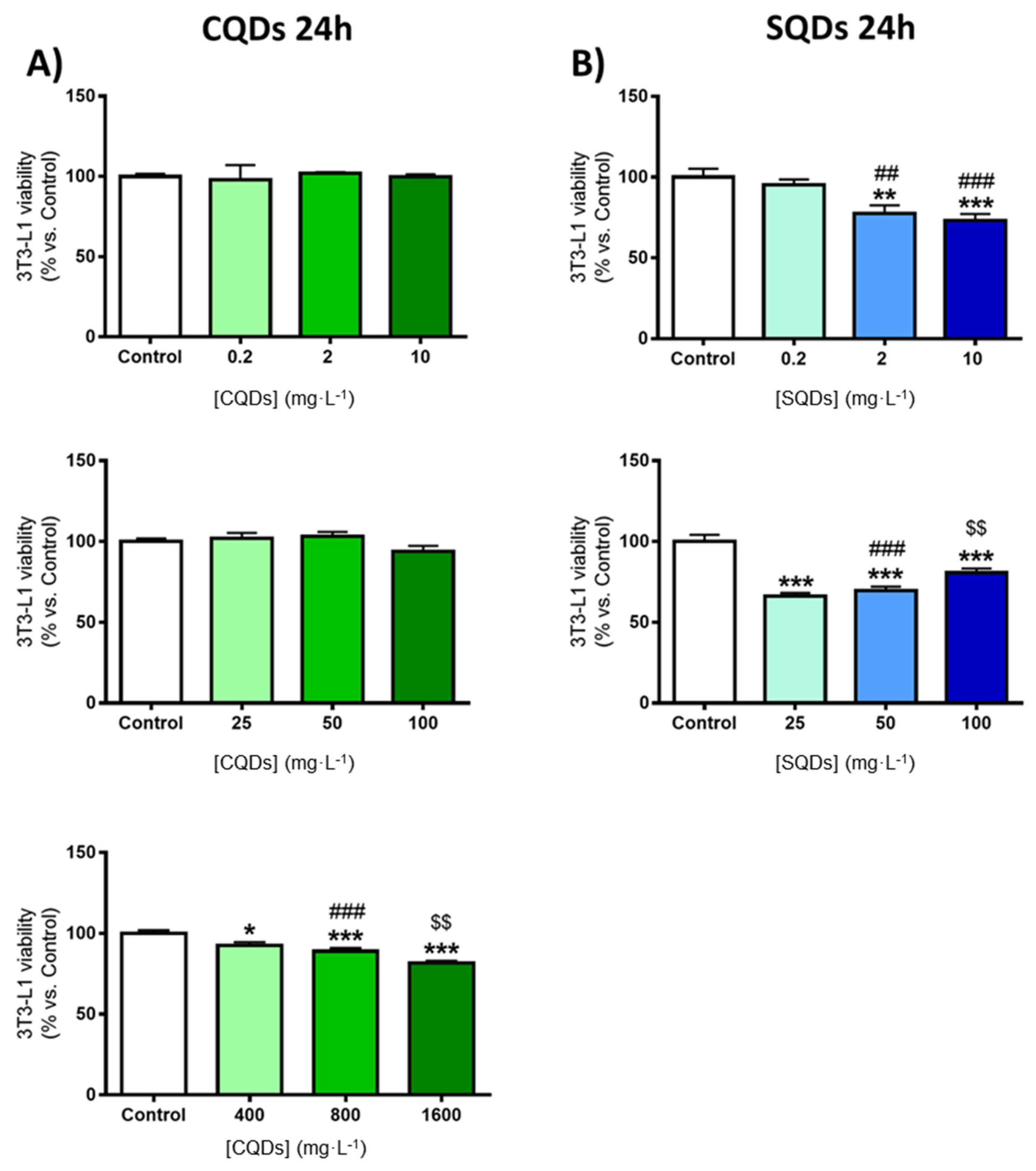
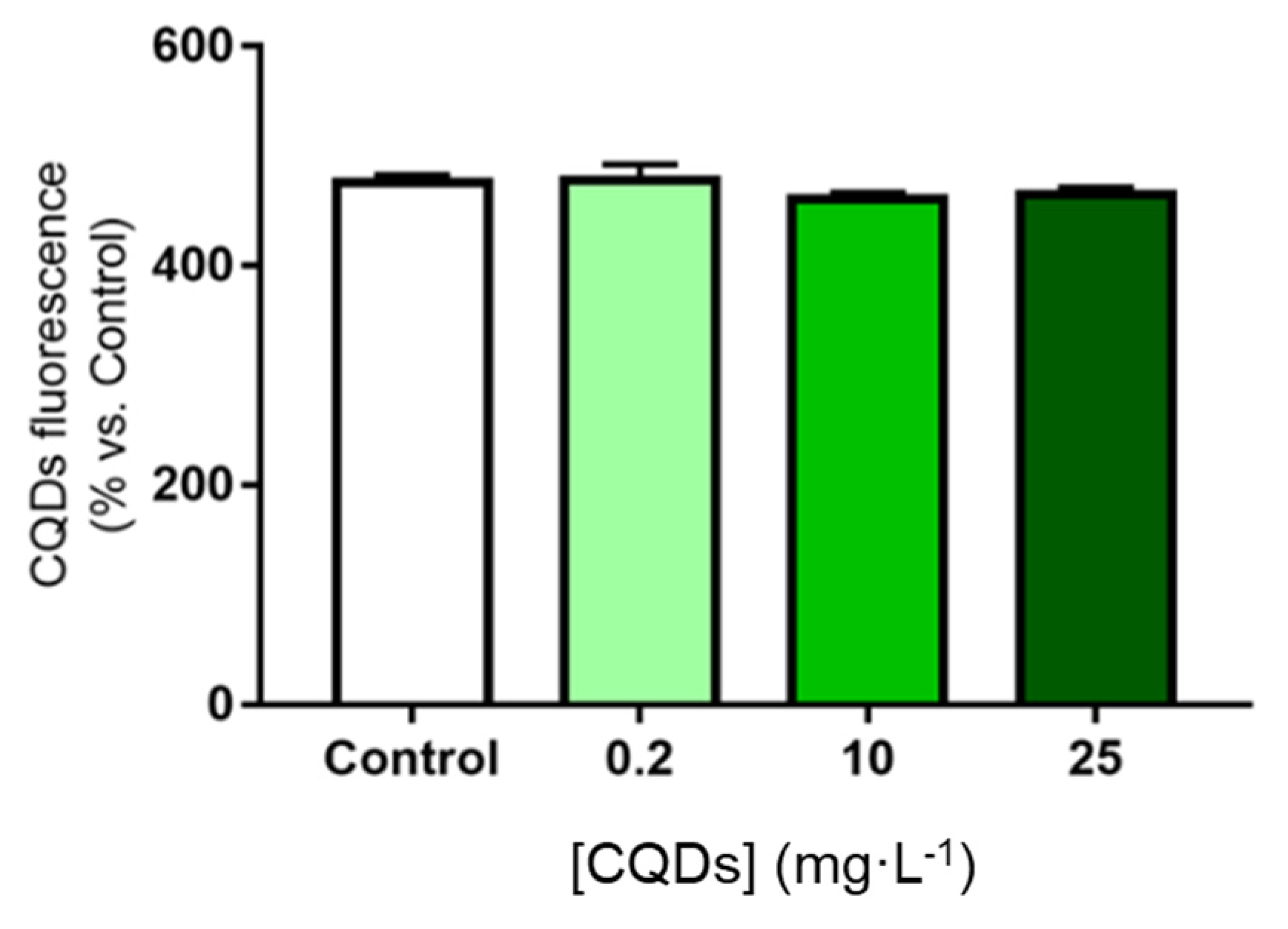
3.1.2. Evaluation of Toxicity of CQDs and SQDs
Toxicity of CQDs and SQDs in 3T3-L1 Cells
- Defining the optimal cellular conditions prior to cytotoxicity assay.
- i.
- MTT cell viability assay.
- ii.
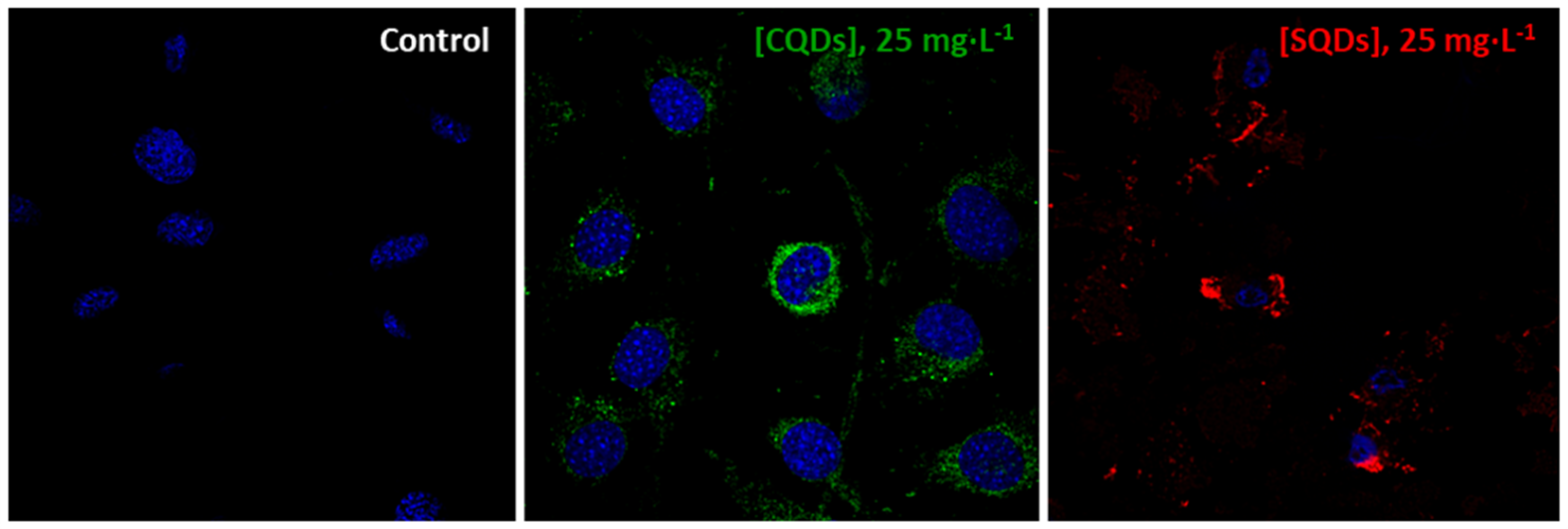
- Imaging assay.
- iii.
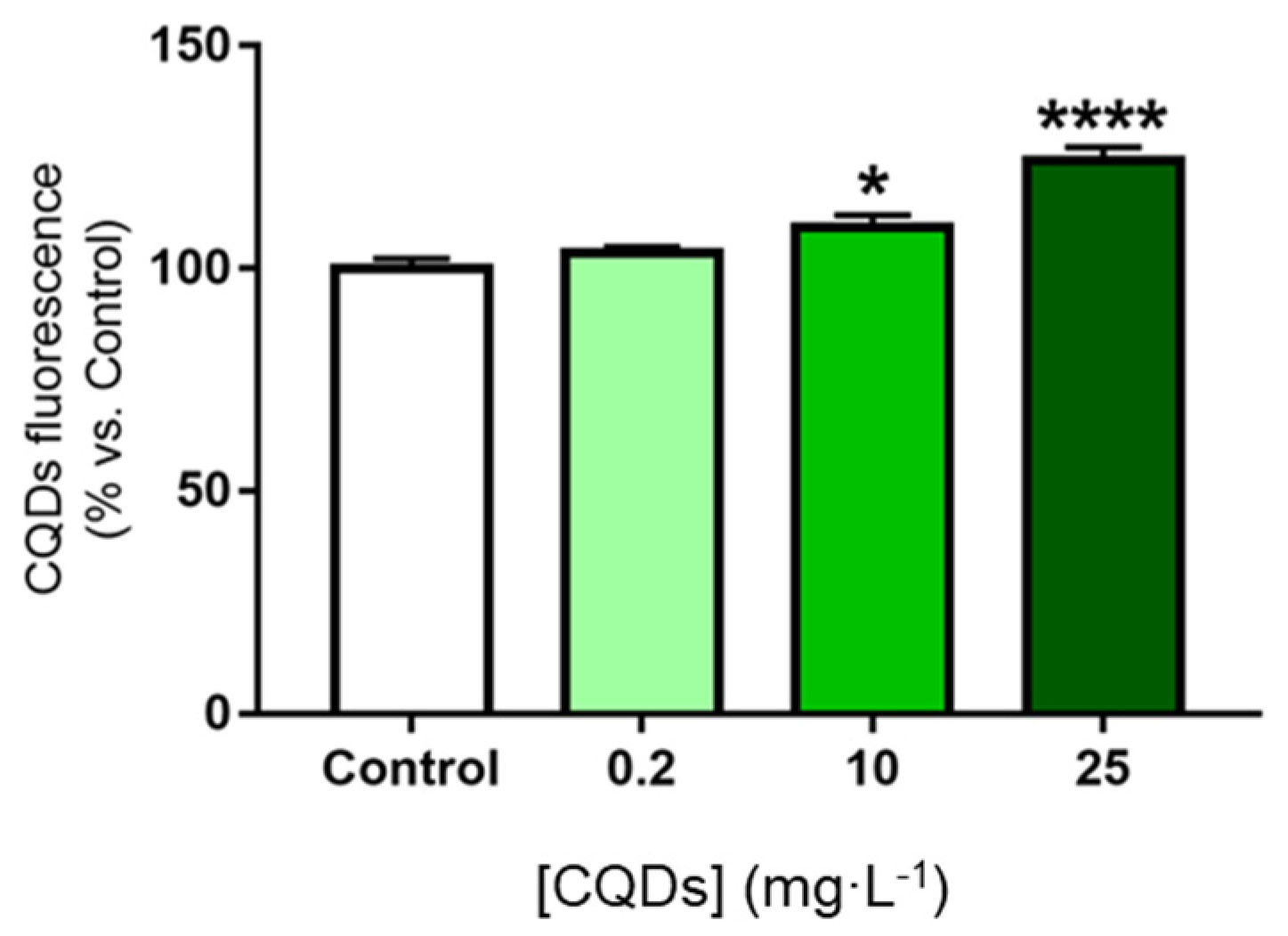
- Cell uptake assay.
- i.
- MTT cell viability assay.
- ii.
- Cell uptake assay.
Toxicity of CQDs and SQDs in HepG2 Cells
- Defining the optimal cellular conditions prior to cytotoxicity assay.
- i.
- MTT cell viability assay.
Extracellular–Quantum Dot Interaction Studies. Influence of the Culture Media on Nanodot Behavior
3.2. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ekimov, A.I.; Onushchenko, A.A. Quantum size effect in the optical-spectra of semiconductor micro-crystals. Sov. Phys. Semiconduct. 1982, 16, 775–778. [Google Scholar]
- Cayuela, A.; Soriano, M.L.; Carrillo-Carrion, C.; Valcárcel, M. Semiconductor and carbon-based fluorescent nanodots: The need for consistency. Chem. Commun. 2016, 52, 1311–1326. [Google Scholar] [CrossRef]
- Wu, X.; Liu, H.; Liu, J.; Haley, K.N.; Treadway, J.A.; Larson, J.P.; Ge, N.; Peale, F.; Bruchez, M.P. Immunofluorescent labeling of cancer marker Her2 and other cellular targets with semiconductor quantum dots. Nat. Biotechnol. 2003, 21, 41–46. [Google Scholar] [CrossRef]
- Srinivasan, C.; Lee, J.; Papadimitrakopoulos, F.; Silbart, L.K.; Zhao, M.; Burgess, D.J. Labeling and intracellular tracking of functionally active plasmid DNA with semiconductor quantum dots. Mol. Ther. 2006, 14, 192–201. [Google Scholar] [CrossRef]
- Liu, Y.; Steiniger, S.C.; Kim, Y.; Kaufmann, G.F.; Felding-Habermann, B.; Janda, K.D. Mechanistic studies of a peptidic GRP78 ligand for cancer cell-specific drug delivery. Mol. Pharm. 2007, 4, 435–447. [Google Scholar] [CrossRef]
- Jamieson, T.; Bakhshi, R.; Petrova, D.; Pocock, R.; Imani, M.; Seifalian, A.M. Biological applications of quantum dots. Biomaterials 2007, 28, 4717–4732. [Google Scholar] [CrossRef]
- Rosenthal, S.J.; Chang, J.C.; Kovtun, O.; McBride, J.R.; Tomlinson, I.D. Biocompatible quantum dots for biological applications. Chem. Biol. 2011, 18, 10–24. [Google Scholar] [CrossRef]
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef]
- Xu, Y.; Jia, X.-H.; Yin, X.-B.; He, X.-W.; Zhang, Y.-K. Carbon quantum dot stabilized gadolinium nanoprobe prepared via a one-pot hydrothermal approach for magnetic resonance and fluorescence dual-modality bioimaging. Anal. Chem. 2014, 86, 12122–12129. [Google Scholar] [CrossRef]
- Fernando, K.A.; Sahu, S.; Liu, Y.; Lewis, W.K.; Guliants, E.A.; Jafariyan, A.; Wang, P.; Bunker, C.E.; Sun, Y.P. Carbon quantum dots and applications in photocatalytic energy conversion. ACS Appl. Mater. Interfaces 2015, 7, 8363–8376. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, A. Carbon quantum dots: Synthesis, properties and applications. J. Mater. Chem. C 2014, 2, 6921–6939. [Google Scholar] [CrossRef]
- Kargbo, O.; Jin, Y.; Ding, S.-N. Recent advances in luminescent carbon dots. Curr. Anal. Chem. 2015, 11, 4–21. [Google Scholar] [CrossRef]
- Liu, W.; Li, C.; Ren, Y.; Sun, X.; Pan, W.; Li, Y.; Wang, J.W.; Wang, W. Carbon dots: Surface engineering and applications. J. Mater. Chem. B 2016, 4, 5772–5788. [Google Scholar] [CrossRef]
- Molaei, M.J.A. review on nanostructured carbon quantum dots and their applications in biotechnology, sensors, and chemiluminescence. Talanta 2019, 196, 456–478. [Google Scholar] [CrossRef]
- Panwar, N.; Soehartono, A.M.; Chan, K.K.; Zeng, S.; Xu, G.; Qu, J.; Coquet, P.; Yong, K.-T.; Chen, X. Nanocarbons for biology and medicine: Sensing, imaging, and drug delivery. Chem. Rev. 2019, 16, 9559–9656. [Google Scholar] [CrossRef]
- Tian, X.-T.; Yin, X.-B. Carbon Dots, Unconventional preparation strategies, and applications beyond photoluminescence. Small 2019, 1901803. [Google Scholar] [CrossRef]
- Ray, S.C.; Saha, A.; Jana, N.R.; Sarkar, R. Fluorescent carbon NPs: Synthesis, characterization, and bioimaging application. J. Phys. Chem. C 2009, 113, 18546–18551. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, P.; Zhai, X.; Tian, F.; Li, W.; Yang, J.; Liu, Y.; Wang, H.; Wang, W.; Liu, W. Nano-carrier for gene delivery and bioimaging based on carbon dots with PEI-passivation enhanced fluorescence. Biomaterials 2012, 33, 3604–3613. [Google Scholar] [CrossRef]
- Wu, Y.-F.; Wu, H.-C.; Kuan, C.J. Multi-functionalized carbon dots as theranostic nanoagent for gene delivery in lung cancer therapy. Sci. Rep. 2016, 6, 21170. [Google Scholar] [CrossRef]
- Wang, K.; Gao, Z.; Gao, G.; Wo, Y.; Wang, Y.; Shen, G.; Cui, D. Systematic safety evaluation on photoluminescent carbon dots. Nanoscale Res. Lett. 2013, 8, 122. [Google Scholar] [CrossRef]
- Yan, J.; Hou, S.; Yu, Y.; Qiao, Y.; Xiao, T.; Mei, Y.; Zhang, Z.; Wang, B.; Huang, C.C.; Lin, C.H.; et al. The effect of surface charge on the cytotoxicity and uptake of carbon quantum dots in human umbilical cord derived mesenchymal stem cells. Colloids Surf. B Biointerfaces 2018, 171, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Blas-Garcia, A.; Baldovi, H.G.; Polo, M.; Victor, V.M.; Garcia, H.; Herance, J.R. Toxicological properties of two fluorescent carbon quantum dots with onion ring morphology and their usefulness as bioimaging agents. RSC Adv. 2016, 6, 30611–30622. [Google Scholar] [CrossRef]
- Havrdova, M.; Hola, K.; Skopalik, J.; Tomankova, K.; Petr, K.C.M.; Polakova, K.; Tucek, J.; Bourlinos, A.B.; Zboril, R. Toxicity of carbon dots—Effect of surface functionalization on the cell viability, reactive oxygen species generation and cell cycle. Carbon 2016, 99, 238–248. [Google Scholar] [CrossRef]
- Jung, Y.K.; Shin, E.; Kim, B.S. Cell nucleus-targeting zwitterionic carbon dots. Sci. Rep. 2015, 5, 18807. [Google Scholar] [CrossRef]
- Cayuela, A.; Soriano, M.L.; Valcárcel, M. Strong luminescence of carbon dots induced by acetone passivation: Efficient sensor for a rapid analysis of two different pollutants. Anal. Chim. Acta 2013, 804, 246–251. [Google Scholar] [CrossRef]
- Caballero-Díaz, E.; Guzmán-Ruiz, R.; Malagón, M.M.; Simonet, B.M.; Valcárcel, M. Effects of the interaction of single-walled carbon nanotubes with 4-nonylphenol on their in vitro toxicity. J. Hazard. Mater. 2014, 275, 107–115. [Google Scholar] [CrossRef]
- Nguyen, K.C.; Willmore, W.G.; Tayabali, A.F. Cadmium telluride quantum dots cause oxidative stress leading to extrinsic and intrinsic apoptosis in hepatocellular carcinoma HepG2 cells. Toxicology 2013, 306, 114–123. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Poon, W.; Tavares, A.J.; McGilvray, I.D.; Chan, W.C.W. Nanoparticle-liver interactions: Cellular uptake and hepatobiliary elimination. J. Control. Release 2016, 240, 332–348. [Google Scholar] [CrossRef]
- Sciortino, A.; Cayuela, A.; Soriano, M.L.; Gelardi, F.M.; Cannas, M.; Valcárcel, M.; Messina, F. Different natures of Surface electronic transitions of carbon NPs. Phys. Chem. Chem. Phys. 2017, 19, 22670–22677. [Google Scholar] [CrossRef]
- Lesniak, A.; Fenaroli, F.; Monopoli, M.P.; Åberg, C.; Dawson, K.A.; Salvati, A. Effects of the presence or absence of a protein corona on silica nanoparticle uptake and impact on cells. ACS Nano 2012, 6, 5845–5857. [Google Scholar] [CrossRef]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular uptake of NPs: Journey inside the cell. Chem. Soc. Rev. 2017, 46, 4218–4244. [Google Scholar] [CrossRef] [PubMed]
- Bekale, L.; Agudelo, D.; Tajmir-Riahi, H.A. The role of polymer size and hydrophobic end-group in PEG-protein interaction. Colloids Surf. B Biointerfaces 2015, 130, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Tsoi, K.M.; Dai, Q.; Alman, B.A.; Chan, W.C. Are quantum dots toxic? Exploring the discrepancy between cell culture and animal studies. Acc. Chem. Res. 2013, 46, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Grabowska-Jadach, I.; Zuchowska, A.; Olesik, M.; Drozd, M.; Pietrzak, M.; Malinowska, E.; Brzozka, Z. Cytotoxicity studies of selected cadmium-based quantum dots on 2D vs. 3D cell cultures. New J. Chem. 2018, 42, 12787–12795. [Google Scholar] [CrossRef]
- Papaioannou, N.; Marinovic, A.; Yoshizawa, N.; Goode, A.E.; Fay, M.; Khlobystov, A.; Titirici, M.M.; Sapelkin, A. Structure and solvents effects on the optical properties of sugar-derived carbon nanodots. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Madani, S.Y.; Shabani, F.; Dwek, M.V.; Seifalian, A.M. Conjugation of quantum dots on carbon nanotubes for medical diagnosis and treatment. Int. J. Nanomed. 2013, 8, 941–950. [Google Scholar] [CrossRef][Green Version]
- Walkey, C.D.; Chan, W.C.W. Understanding and controlling the interaction of nanomaterials with proteins in a physiological environment. Chem. Soc. Rev. 2012, 41, 2780–2799. [Google Scholar] [CrossRef] [PubMed]
- Ehrenberg, M.S.; Friedman, A.E.; Finkelstein, J.N.; Oberdörster, G. The influence of protein adsorption on nanoparticle association with cultured endothelial cells. Biomaterials 2009, 30, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Feng, D.; Shi, W.; Li, X.; Ma, H. Parallel comparative studies on the toxic effects of unmodified CdTe quantum dots, gold NPs, and carbon nanodots on live cells as well as green gram sprouts. Talanta 2013, 116, 237–244. [Google Scholar] [CrossRef]
- McNamara, K.; Tofail, S.A.M. NPs in biomedical applications. Adv. Phys. 2017, 2, 54–88. [Google Scholar] [CrossRef]
- Bradburne, C.E.; Delehanty, J.B.; Gemmill, B.K.; Mei, B.C.; Mattoussi, H.; Susumu, K.; Blanco-Canosa, J.B.; Dawson, P.E.; Medintz, I.L. Cytotoxicity of quantum dots used for in vitro cellular labelling: Role of QD surface ligand, delivery modality, cell type, and direct comparison to organic fluorophores. Bioconjug. Chem. 2013, 24, 1570–1583. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.; Liu, R.; Gemill, K.B.; Bilal, M.; Cohen, Y.; Medintz, I.L. Meta-analysis of cellular toxicity for cadmium containing quantum dots. Nat. Nanotech. 2016, 11, 479–493. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, B.; McNiven, M.A. Importance of endocytic pathways in liver function and disease. Compr. Physiol. 2014, 4, 1403–1417. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Xu, M.J.; Gao, B. Hepatocytes: A key cell type for innate immunity. Cell. Mol. Immunol. 2016, 13, 301–315. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.T.; Tang, F.M.A.; Li, J.J.; Ong, C.; Yung, L.L.Y.; Bay, B.H. Clathrin-mediated endocytosis of gold NPs In Vitro. Anat. Rec. 2015, 298, 418–427. [Google Scholar] [CrossRef] [PubMed]




| CQDs (0.2–100 mg·L−1) | SQDs (0.2–100 mg·L−1) | SWCNTs-PEG (2.6–13 mg·L−1) | |
|---|---|---|---|
| λexcitation/λ emission (nm) | 365/450 | 390/650 | - |
| Cell localization in fibroblasts | Intracellular, Absence of cell death | Intracellular, DNA decondensation Cell membrane damage | - |
| % Cell viability 3T3-L1 fibroblasts (-FBS) | 94–100% | 66–95% | 85–95% |
| % Cell viability 3T3-L1 fibroblasts (+FBS) | 87–99% | 33–89% | 95–100% |
| % Cell viability HepG2 hepatocytes (+FBS) | 93–98% | 84–91% | - |
| Reference | This work | [26] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navarro-Ruiz, M.C.; Cayuela, A.; Soriano, M.L.; Guzmán-Ruiz, R.; Malagón, M.M.; Valcárcel, M. A Systematic Comparative Study of the Toxicity of Semiconductor and Graphitic Carbon-Based Quantum Dots Using In Vitro Cell Models. Appl. Sci. 2020, 10, 8845. https://doi.org/10.3390/app10248845
Navarro-Ruiz MC, Cayuela A, Soriano ML, Guzmán-Ruiz R, Malagón MM, Valcárcel M. A Systematic Comparative Study of the Toxicity of Semiconductor and Graphitic Carbon-Based Quantum Dots Using In Vitro Cell Models. Applied Sciences. 2020; 10(24):8845. https://doi.org/10.3390/app10248845
Chicago/Turabian StyleNavarro-Ruiz, Maria Carmen, Angelina Cayuela, María Laura Soriano, Rocio Guzmán-Ruiz, Maria M. Malagón, and Miguel Valcárcel. 2020. "A Systematic Comparative Study of the Toxicity of Semiconductor and Graphitic Carbon-Based Quantum Dots Using In Vitro Cell Models" Applied Sciences 10, no. 24: 8845. https://doi.org/10.3390/app10248845
APA StyleNavarro-Ruiz, M. C., Cayuela, A., Soriano, M. L., Guzmán-Ruiz, R., Malagón, M. M., & Valcárcel, M. (2020). A Systematic Comparative Study of the Toxicity of Semiconductor and Graphitic Carbon-Based Quantum Dots Using In Vitro Cell Models. Applied Sciences, 10(24), 8845. https://doi.org/10.3390/app10248845