Nickel Binding Affinity with Size-Fractioned Sediment Dissolved and Particulate Organic Matter and Correlation with Optical Indicators
Abstract
:1. Introduction
2. Methods and Materials
2.1. Sampling Site and Samples Collection
2.2. AEOM Extraction and Separation
2.3. UV/Vis and Fluorescent Measurement
2.4. Optical Indicator
2.5. Statistical Analysis
3. Results and Discussion
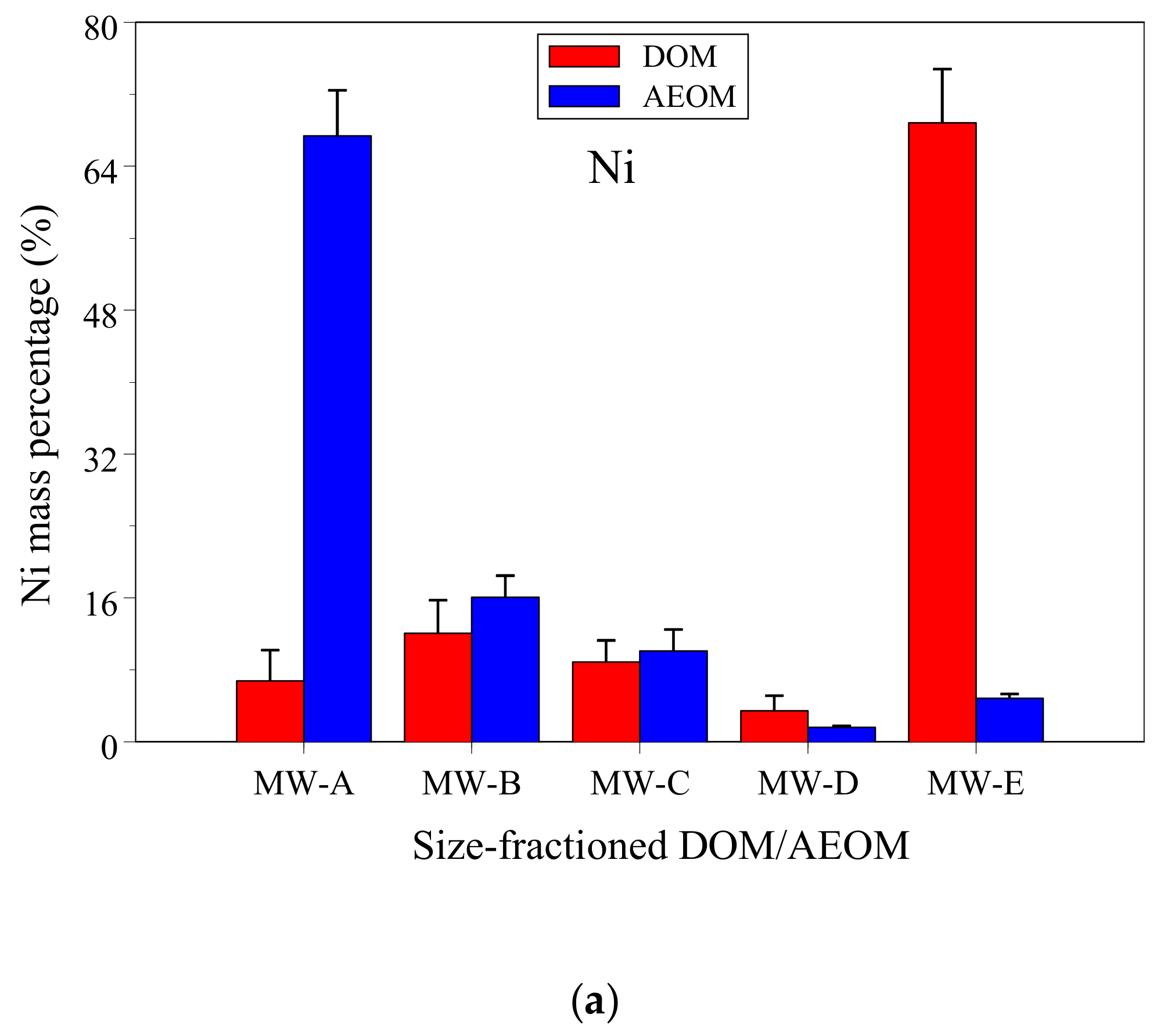
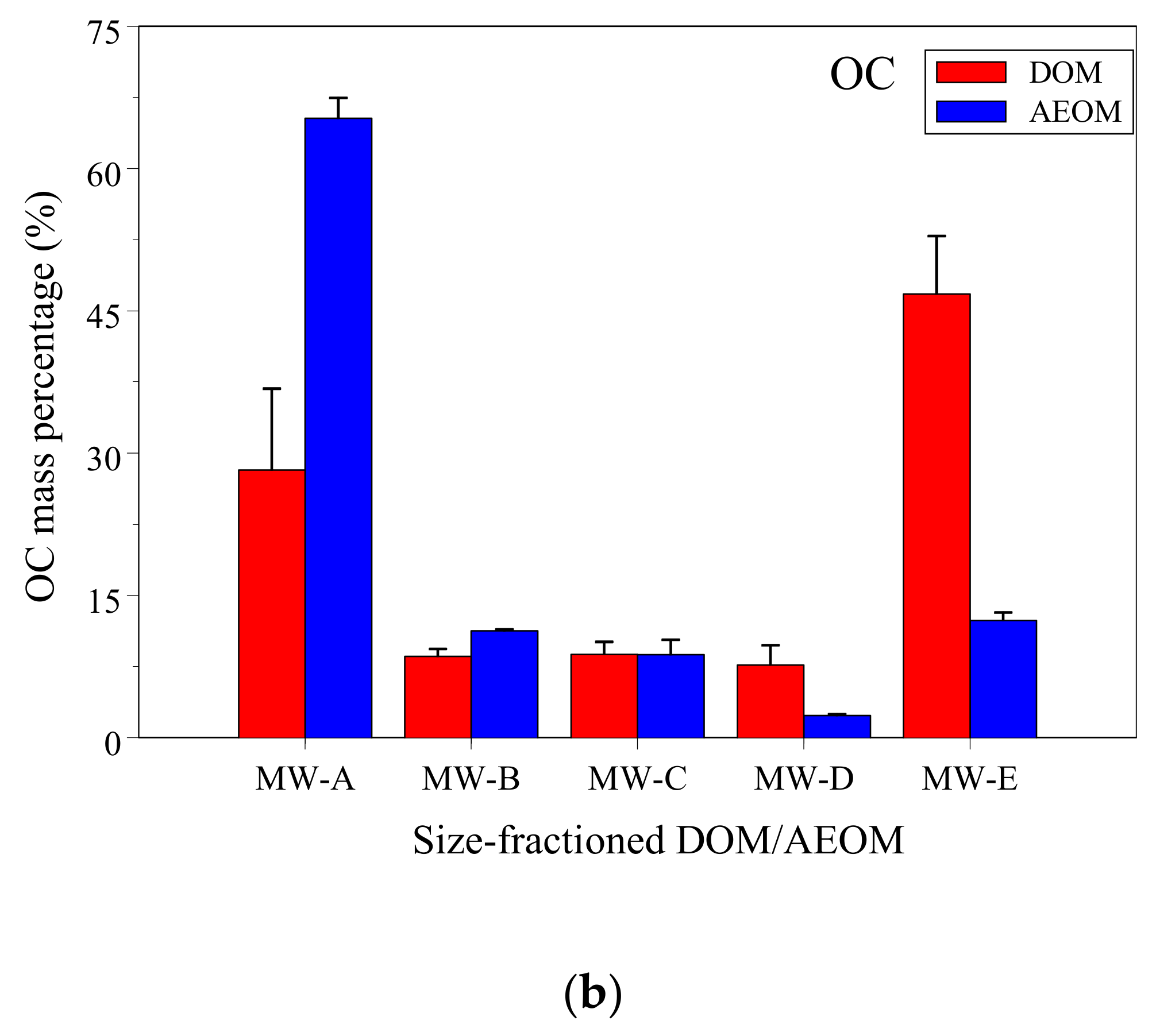
3.1. DOC and Ni Concentrations and Mass Fractions in DOM and AEOM
3.2. [Ni]/[DOC] Ratio
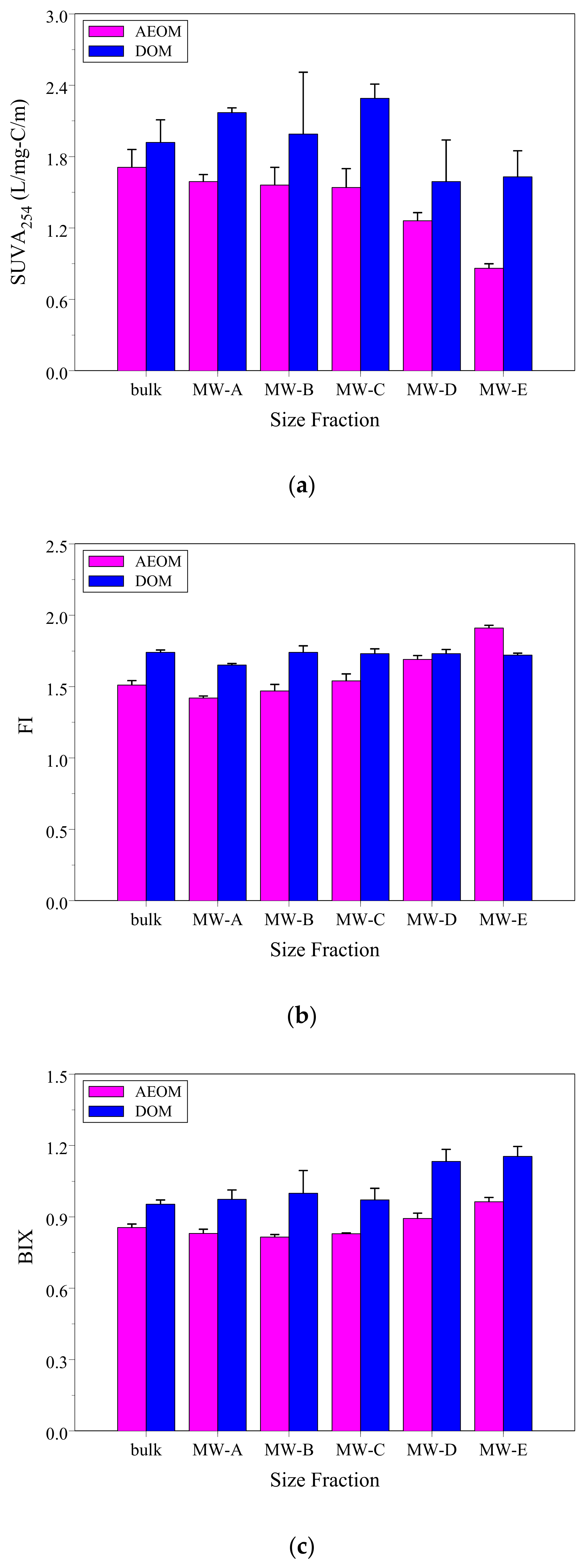
3.3. DOM and AEOM Optical Indicators
3.4. Correlation between [Ni]/[DOC] Ratios and Optical Indicators
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yu, Y.; Wan, Y.; Camara, A.Y.; Li, H. Effects of the addition and aging of humic acid-based amendments on the solubility of Cd in soil solution and its accumulation in rice. Chemosphere 2018, 196, 303–310. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Chen, M.; Schlautman, M.A.; Hur, J. Dynamic exchanges between DOM and POM pools in coastal and inland aquatic ecosystems: A review. Sci. Total Environ. 2016, 551–552, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Hur, J. Pre-treatments, characteristics, and biogeochemical dynamics of dissolved organic matter in sediments: A review. Water Res. 2015, 79, 10–25. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, D.; Shen, Z.; Chen, J.; Feng, C. Characterization and spacial distribution variability of chromophoric dissolved organic matter (CDOM) in the Yangtze Estuary. Chemosphere 2014, 95, 353–362. [Google Scholar] [CrossRef]
- Burdige, D.J.; Komada, T. Sediment pore waters. In Biogeochemistry of Marine Dissolved Organic Matter, 2nd ed.; Chapter 12; Academic Press: Burlington, VT, USA, 2015; pp. 535–577. [Google Scholar]
- Aiken, G.R.; Hsu-Kim, H.; Ryan, J.N. Influence of Dissolved Organic Matter on the Environmental Fate of Metals, Nanoparticles, and Colloids. Environ. Sci. Technol. 2011, 45, 3196–3201. [Google Scholar] [CrossRef]
- Feng, C.; Guo, X.; Yin, S.; Tian, C.; Li, Y.; Shen, Z. Heavy metal partitioning of suspended particulate matter–water and sediment–water in the Yangtze Estuary. Chemosphere 2017, 185, 717–725. [Google Scholar] [CrossRef]
- Ahmed, I.A.; Hamilton–Taylor, J.; Bieroza, M.; Zhang, H.; Davison, W. Improving and testing geochemical speciation predictions of metal ions in natural waters. Water Res. 2014, 67, 276–291. [Google Scholar] [CrossRef]
- Xu, H.; Houghton, E.M.; Houghton, C.J.; Guo, L. Variations in size and composition of colloidal organic matter in a negative freshwater estuary. Sci. Total Environ. 2018, 615, 931–941. [Google Scholar] [CrossRef]
- Hur, J.; Lee, B.-M.; Shin, K.-H. Spectroscopic characterization of dissolved organic matter isolates from sediments and the association with phenanthrene binding affinity. Chemosphere 2014, 111, 450–457. [Google Scholar] [CrossRef]
- Jiang, T.; Bravo, A.G.; Skyllberg, U.; Björn, E.; Wang, D.; Yan, H.; Green, N.W. Influence of dissolved organic matter (DOM) characteristics on dissolved mercury (Hg) species composition in sediment porewater of lakes from southwest China. Water Res. 2018, 146, 146–158. [Google Scholar] [CrossRef]
- Baken, S.; Degryse, F.; Verheyen, L.; Merckx, R.; Smolders, E. Metal complexation properties of freshwater dissolved organic matter are explained by its aromaticity and by anthropogenic ligands. Environ. Sci. Technol. 2011, 45, 2584–2590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.H.; Lee, J.H.; Kang, S.Y.; Kim, S.Y. Hydroclimatic controls on dissolved organic matter (DOM) characteristics and implications for trace metal transport in Hwangryong River Watershed, Korea, during a summer monsoon period. Hydrol. Process. Int. J. 2007, 21, 3025–3034. [Google Scholar] [CrossRef]
- Yan, M.; Ma, J.; Zhang, C.; Zhou, Y.; Liu, F.; Han, X.; Li, M.; Ni, J. Optical property of dissolved organic matters (DOMs) and its link to the presence of metal ions in surface freshwaters in China. Chemosphere 2017, 188, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Amery, F.; Degryse, F.; Degeling, W.; Smolders, E.; Merckx, R. The copper-mobilizing-potential of dissolved organic matter in soils varies 10-fold depending on soil incubation and extraction procedures. Environ. Sci. Technol. 2007, 41, 2277–2281. [Google Scholar] [CrossRef]
- Amery, F.; Degryse, F.; Cheyns, K.; De Troyer, I.; Mertens, J.; Merckx, R.; Smolders, E. The UV-absorbance of dissolved organic matter predicts the fivefold variation in its affinity for mobilizing Cu in an agricultural soil horizon. Eur. J. Soil Sci. 2008, 59, 1087–1095. [Google Scholar] [CrossRef]
- Kikuchi, T.; Fujii, M.; Terao, K.; Jiwei, R.; Lee, Y.P.; Yoshimura, C. Correlations between aromaticity of dissolved organic matter and trace metal concentrations in natural and effluent waters: A case study in the Sagami River Basin, Japan. Sci. Total Environ. 2017, 576, 36–45. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Yue, D.; Liu, J.; Nie, Y. Size fractionation of organic matter and heavy metals in raw and treated leachate. Waste Manag. 2009, 29, 2527–2533. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, S.; Wu, W.; Liu, H. Metal sorption on soils as affected by the dissolved organic matter in sewage sludge and the relative calculation of sewage sludge application. J. Hazard. Mater. 2007, 149, 399–407. [Google Scholar] [CrossRef]
- Chon, K.; Chon, K.; Cho, J. Characterization of size fractionated dissolved organic matter from river water and wastewater effluent using preparative high performance size exclusion chromatography. Org. Geochem. 2017, 103, 105–112. [Google Scholar] [CrossRef]
- Hargreaves, A.J.; Vale, P.; Whelan, J.; Constantino, C.; Dotro, G.; Campo, P.; Cartmell, E. Distribution of trace metals (Cu, Pb, Ni, Zn) between particulate, colloidal and truly dissolved fractions in wastewater treatment. Chemosphere 2017, 175, 239–246. [Google Scholar] [CrossRef]
- Hsieh, S.-H.; Chiu, T.-P.; Huang, W.-S.; Chen, T.-C.; Yeh, Y.-L. Cadmium (Cd) and Nickel (Ni) Distribution on Size-Fractioned Soil Humic Substance (SHS). Int. J. Environ. Res. Public Health 2019, 16, 3398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yi, Y.; Xiao, M.; Mostofa, K.M.; Xu, S.; Wang, Z. Spatial Variations of Trace Metals and Their Complexation Behavior with DOM in the Water of Dianchi Lake, China. Int. J. Environ. Res. Public Health 2019, 16, 4919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansen, A.M.; Kraus, T.E.; Pellerin, B.A.; Fleck, J.A.; Downing, B.D.; Bergamaschi, B.A. Optical properties of dissolved organic matter (DOM): Effects of biological and photolytic degradation. Limnol. Oceanogr. 2016, 61, 1015–1032. [Google Scholar] [CrossRef] [Green Version]
- Derrien, M.; Yang, L.; Hur, J. Lipid biomarkers and spectroscopic indices for identifying organic matter sources in aquatic environments: A review. Water Res. 2017, 112, 58–71. [Google Scholar] [CrossRef]
- Li, P.; Hur, J. Utilization of UV-Vis spectroscopy and related data analyses for dissolved organic matter (DOM) studies: A review. Crit. Rev. Environ. Sci. Technol. 2017, 47, 131–154. [Google Scholar] [CrossRef]
- Matilainen, A.; Gjessing, E.T.; Lahtinen, T.; Hed, L.; Bhatnagar, A.; Sillanpää, M. An overview of the methods used in the characterisation of natural organic matter (NOM) in relation to drinking water treatment. Chemosphere 2011, 83, 1431–1442. [Google Scholar] [CrossRef]
- Birdwell, J.E.; Engel, A.S. Characterization of dissolved organic matter in cave and spring waters using UV–Vis absorbance and fluorescence spectroscopy. Org. Geochem. 2010, 41, 270–280. [Google Scholar] [CrossRef]
- Huguet, A.; Vacher, L.; Relexans, S.; Saubusse, S.; Froidefond, J.-M.; Parlanti, E. Properties of fluorescent dissolved organic matter in the Gironde Estuary. Org. Geochem. 2009, 40, 706–719. [Google Scholar] [CrossRef]
- Aderibigbe, D.O.; Giwa, A.-R.A.; Bello, I.A. Characterization and treatment of wastewater from food processing industry: A review. Imam J. Appl. Sci. 2017, 2, 27. [Google Scholar]
- Rajagopal, R.; Saady, N.M.C.; Torrijos, M.; Thanikal, J.V.; Hung, Y.-T. Sustainable agro-food industrial wastewater treatment using high rate anaerobic process. Water 2013, 5, 292–311. [Google Scholar] [CrossRef] [Green Version]
- Hur, J.; Lee, D.-H.; Shin, H.-S. Comparison of the structure, spectroscopic and phenanthrene binding characteristics of humic acids from soils and lake sediments. Org. Geochem. 2009, 40, 1091–1099. [Google Scholar] [CrossRef]
- Helms, J.R.; Stubbins, A.; Ritchie, J.D.; Minor, E.C.; Kieber, D.J.; Mopper, K. Absorption spectral slopes and slope ratios as indicators of molecular weight, source, and photobleaching of chromophoric dissolved organic matter. Limnol. Oceanogr. 2008, 53, 955–969. [Google Scholar] [CrossRef] [Green Version]
- Weishaar, J.L.; Aiken, G.R.; Bergamaschi, B.A.; Fram, M.S.; Fujii, R.; Mopper, K. Evaluation of specific ultraviolet absorbance as an indicator of the chemical composition and reactivity of dissolved organic carbon. Environ. Sci. Technol. 2003, 37, 4702–4708. [Google Scholar] [CrossRef] [PubMed]
- Lapworth, D.J.; Kinniburgh, D. An R script for visualising and analysing fluorescence excitation–emission matrices (EEMs). Comput. Geosci. 2009, 35, 2160–2163. [Google Scholar] [CrossRef] [Green Version]
- Her, N.; Amy, G.; McKnight, D.; Sohn, J.; Yoon, Y. Characterization of DOM as a function of MW by fluorescence EEM and HPLC-SEC using UVA, DOC, and fluorescence detection. Water Res. 2003, 37, 4295–4303. [Google Scholar] [CrossRef]
- Dabrin, A.; Roulier, J.-L.; Coquery, M. Colloidal and truly dissolved metal (oid) fractionation in sediment pore waters using tangential flow filtration. Appl. Geochem. 2013, 31, 25–34. [Google Scholar] [CrossRef]
- Xu, H.; Zou, L.; Guan, D.; Li, W.; Jiang, H. Molecular weight-dependent spectral and metal binding properties of sediment dissolved organic matter from different origins. Sci. Total Environ. 2019, 665, 828–835. [Google Scholar] [CrossRef]
- Shi, M.-S.; Huang, W.-S.; Hsu, L.-F.; Yeh, Y.-L.; Chen, T.-C. Fluorescence of Size-Fractioned Humic Substance Extracted from Sediment and Its Effect on the Sorption of Phenanthrene. Int. J. Environ. Res. Public Health 2019, 16, 5087. [Google Scholar] [CrossRef] [Green Version]
- Duc, T.A.; Loi, V.D.; Thao, T.T. Partition of heavy metals in a tropical river system impacted by municipal waste. Environ. Monit. Assess. 2013, 185, 1907–1925. [Google Scholar] [CrossRef]
- Wang, W.; Wen, B.; Zhang, S.; Shan, X.-Q. Distribution of heavy metals in water and soil solutions based on colloid-size fractionation. Int. J. Environ. Anal. Chem. 2003, 83, 357–365. [Google Scholar] [CrossRef]
- Wenming, X.; Zhang, S.; Lin, R.; Mingyue, Y.; Weiming, S.; Zhang, H.; Weihua, L. Evaluating soil dissolved organic matter extraction using three-dimensional excitation-emission matrix fluorescence spectroscopy. Pedosphere 2017, 27, 968–973. [Google Scholar]
- Ilina, S.M.; Lapitskiy, S.A.; Alekhin, Y.V.; Viers, J.; Benedetti, M.; Pokrovsky, O.S. Speciation, size fractionation and transport of trace elements in the continuum soil water–mire–humic lake–river–large oligotrophic lake of a Subarctic watershed. Aquat. Geochem. 2016, 22, 65–95. [Google Scholar] [CrossRef]
- Xu, H.; Yu, G.; Yang, L.; Jiang, H. Combination of two-dimensional correlation spectroscopy and parallel factor analysis to characterize the binding of heavy metals with DOM in lake sediments. J. Hazard. Mater. 2013, 263, 412–421. [Google Scholar] [CrossRef] [PubMed]

| Samples | DOM | AEOM | ||
|---|---|---|---|---|
| DOC (mg/L) | Ni (μg/L) | DOC (mg/L) | Ni (µg/L) | |
| Bulk | 8.3 ± 0.4 a | 5.56 ± 2.16 | 739 ± 33 | 85 ± 7 |
| MW-A | 24.8 ± 6.9 | 4.49 ± 3.77 | 5040 ± 231 | 520 ± 82 |
| MW-B | 8.4 ± 0.8 | 7.56 ± 1.75 | 966 ± 3 | 137 ± 12 |
| MW-C | 9.6 ± 1.6 | 6.23 ± 1.49 | 834 ± 141 | 95 ± 19 |
| MW-D | 9.3 ± 2.4 | 3.46 ± 3.44 | 247 ± 16 | 17 ± 3 |
| MW-E | 6.3 ± 1.0 | 6.71 ± 3.96 | 145 ± 9 | 6.0 ± 0.0 |
| SUVA254 | FI | BIX | |
|---|---|---|---|
| SUVA254 | −0.93 *** | −0.85 *** | |
| FI | −0.41 | 0.92 *** | |
| BIX | −0.31 | −0.03 |
| Samples | SUVA254 | FI | BIX |
|---|---|---|---|
| [Ni]/[DOC]AEOM | 0.87 *** | −0.87 *** | −0.92 *** |
| [Ni]/[DOC]DOM | −0.13 | 0.40 | 0.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng-Wen, C.; Liang-Fong, H.; Hsiang-Chun, T.; Yung-Yu, L.; Wei-Shiang, H.; Ting-Chien, C. Nickel Binding Affinity with Size-Fractioned Sediment Dissolved and Particulate Organic Matter and Correlation with Optical Indicators. Appl. Sci. 2020, 10, 8995. https://doi.org/10.3390/app10248995
Cheng-Wen C, Liang-Fong H, Hsiang-Chun T, Yung-Yu L, Wei-Shiang H, Ting-Chien C. Nickel Binding Affinity with Size-Fractioned Sediment Dissolved and Particulate Organic Matter and Correlation with Optical Indicators. Applied Sciences. 2020; 10(24):8995. https://doi.org/10.3390/app10248995
Chicago/Turabian StyleCheng-Wen, Chuang, Hsu Liang-Fong, Tsai Hsiang-Chun, Liu Yung-Yu, Huang Wei-Shiang, and Chen Ting-Chien. 2020. "Nickel Binding Affinity with Size-Fractioned Sediment Dissolved and Particulate Organic Matter and Correlation with Optical Indicators" Applied Sciences 10, no. 24: 8995. https://doi.org/10.3390/app10248995




