Neurotrophic Factors in Glaucoma and Innovative Delivery Systems
Abstract
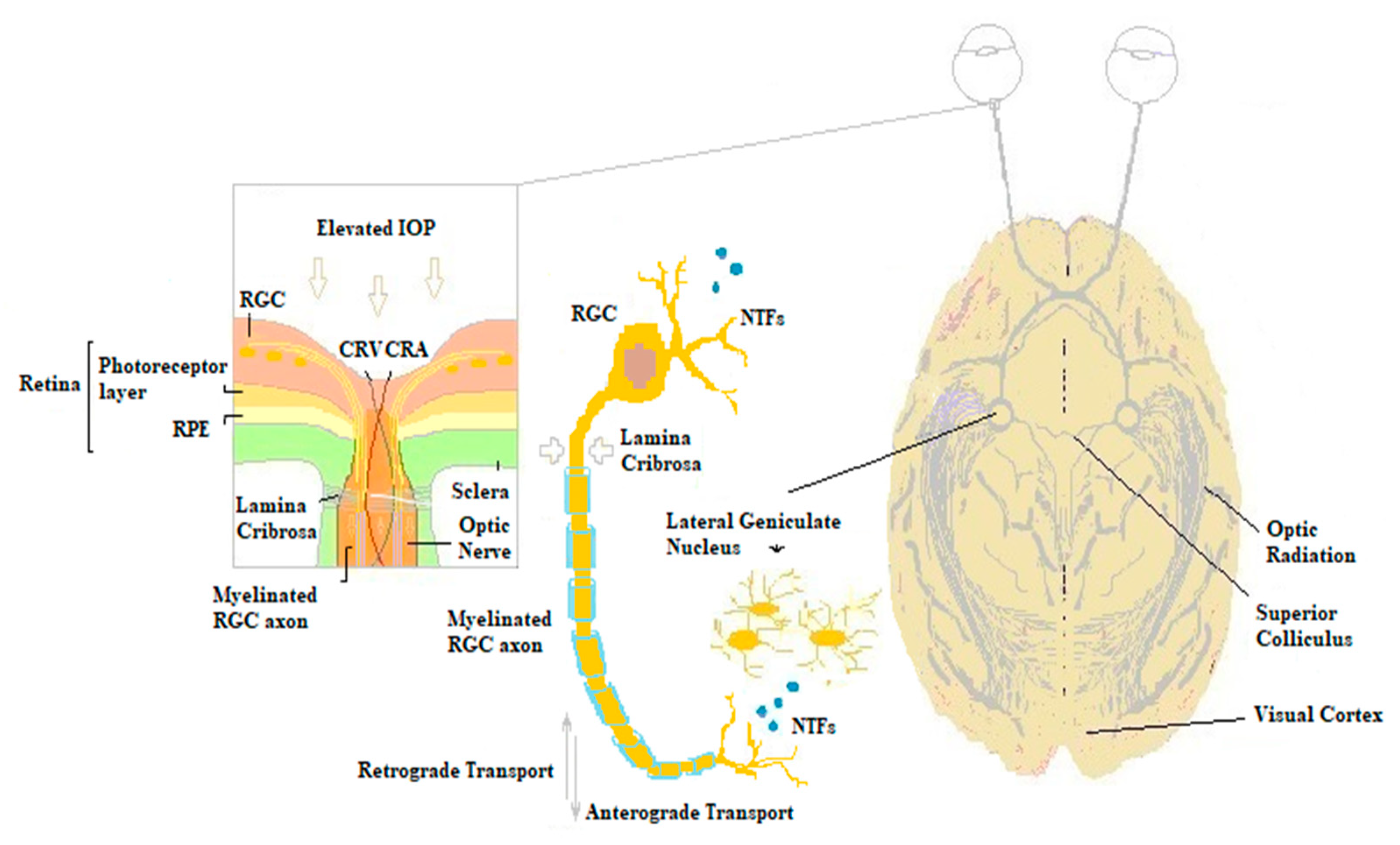
:1. Introduction
2. Neuroprotection: Insights on Biochemical Pathways and Treatment Opportunities
2.1. Neurotrophic Factors Rationale for Use in Glaucoma Treatment and Preliminary Results
2.1.1. Nerve Growth Factor
2.1.2. Brain Derived Neurotrophic Factor
2.1.3. Glial Cell-Derived Neurotrophic Factor and Ciliary Neurotrophic Factor
3. Advances in Drug and Gene Delivery Systems for Neuroprotection in Glaucoma
3.1. Routes of Administration and Devices
3.2. Neurotrophic Factors Delivery in Glaucoma
3.3. Gene Delivery for Neurotrophic Factors in Glaucoma
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Weinreb, R.N.; Kaufman, P.L. Glaucoma research community and FDA look to the future, II: NEI/FDA glaucoma clinical trial design and endpoints symposium: Measures of structural change and visual function. Investig. Ophthalmol. Vis. Sci. 2011, 52, 7842–7851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Glaucoma Society Terminology and Guidelines for Glaucoma, 4th Edition - Chapter 3: Treatment principles and options Supported by the EGS Foundation. Br. J. Ophthalmol. 2017, 101, 130–191. [CrossRef] [PubMed] [Green Version]
- Weinreb, R.N.; Liebmann, J.M.; Cioffi, G.A.; Goldberg, I.; Brandt, J.D.; Johnson, C.A.; Zangwill, L.M.; Schneider, S.; Badger, H.; Bejanian, M. Oral Memantine for the Treatment of Glaucoma: Design and Results of 2 Randomized, Placebo-Controlled, Phase 3 Studies. Ophthalmology 2018, 125, 1874–1885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krupin, T.; Liebmann, J.M.; Greenfield, D.S.; Ritch, R.; Gardiner, S. A randomized trial of brimonidine versus timolol in preserving visual function: Results from the low-pressure glaucoma treatment study. Am. J. Ophthalmol. 2011, 151, 671–681. [Google Scholar] [CrossRef]
- Ko, M.L.; Hu, D.N.; Ritch, R.; Sharma, S.C.; Chen, C.F. Patterns of retinal ganglion cell survival after brain-derived neurotrophic factor administration in hypertensive eyes of rats. Neurosci. Lett. 2001, 305, 139–142. [Google Scholar] [CrossRef]
- Ma, Y.T.; Hsieh, T.; Forbes, M.E.; Johnson, J.E.; Frost, D.O. BDNF injected into the superior colliculus reduces developmental retinal ganglion cell death. J. Neurosci. 1998, 18, 2097–2107. [Google Scholar] [CrossRef]
- Ji, J.Z.; Elyaman, W.; Yip, H.K.; Lee, V.W.H.; Yick, L.W.; Hugon, J.; So, K.F. CNTF promotes survival of retinal ganglion cells after induction of ocular hypertension in rats: The possible involvement of STAT3 pathway. Eur. J. Neurosci. 2004, 19, 265–272. [Google Scholar] [CrossRef]
- Lambiase, A.; Centofanti, M.; Micera, A.; Manni, G.L.; Mattei, E.; De Gregorio, A.; De Feo, G.; Bucci, M.G.; Aloe, L. Nerve growth factor (NGF) reduces and NGF antibody exacerbates retinal damage induced in rabbit by experimental ocular hypertension. Graefe’s Arch. Clin. Exp. Ophthalmol. 1997, 235, 780–785. [Google Scholar] [CrossRef]
- Lambiase, A.; Aloe, L.; Centofanti, M.; Parisi, V.; Mantelli, F.; Colafrancesco, V.; Manni, G.L.; Bucci, M.G.; Bonini, S.; Levi-Montalcini, R. Experimental and clinical evidence of neuroprotection by nerve growth factor eye drops: Implications for glaucoma. Proc. Natl. Acad. Sci. USA 2009, 106, 13469–13474. [Google Scholar] [CrossRef] [Green Version]
- Cosker, K.E.; Courchesne, S.L.; Segal, R.A. Action in the axon: Generation and transport of signaling endosomes. Curr. Opin. Neurobiol. 2008, 18, 270–275. [Google Scholar] [CrossRef] [Green Version]
- Pease, M.; McKinnon, S.J.; Quigley, H.A.; Kerrigan–Baumrind, L.A.; Zack, D.J. Obstructed axonal transport of BDNF and its receptor TrkB in experimental glaucoma. Investig. Ophthalmol. Vis. Sci. 2000, 41, 764–774. [Google Scholar]
- Weltman, J.K. The 1986 Nobel Prize for Physiology or Medicine awarded for discovery of growth factors: Rita Levi-Montalcini, M.D., and Stanley Cohen, Ph.D. N. Engl. Reg. Allergy Proc. 1987, 8, 47–48. [Google Scholar] [CrossRef] [PubMed]
- Levi-Montalcini, R.; Skaper, S.D.; Dal Toso, R.; Petrelli, L.; Leon, A. Nerve growth factor: From neurotrophin to neurokine. Trends Neurosci. 1996, 19, 514–520. [Google Scholar] [CrossRef]
- Frade, J.M.; Barde, Y.A. Nerve growth factor: Two receptors, multiple functions. BioEssays 1998, 20, 137–145. [Google Scholar] [CrossRef]
- Deister, C.; Schmidt, C.E. Optimizing neurotrophic factor combinations for neurite outgrowth. J. Neural Eng. 2006, 3, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Blanco, A.A.; Bagnasco, L.; Bagnis, A.; Barton, K.; Baudouin, C.; Bengtsson, B. European glaucoma society terminology and guidelines for glaucoma, 4th edition—Chapter 2: Classification and terminology Supported by the EGS Foundation: Part 1: Foreword; Introduction; Glossary; Chapter 2 classification and terminology. Br. J. Ophthalmol. 2017, 101, 73–127. [Google Scholar] [CrossRef] [Green Version]
- Anderson, D.R.; Drance, S.M.; Schulzer, M. Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Am. J. Ophthalmol. 1998, 126, 487–497. [Google Scholar] [CrossRef]
- Cho, H.K.; Kee, C. Population-based glaucoma prevalence studies in Asians. Surv. Ophthalmol. 2014, 59, 434–447. [Google Scholar] [CrossRef]
- Rotchford, A.P.; Johnson, G.J. Glaucoma in Zulus: A population-based cross-sectional survey in a rural district in South Africa. Arch. Ophthalmol. 2002, 120, 471–478. [Google Scholar] [CrossRef] [Green Version]
- Bonomi, L.; Marchini, G.; Marraffa, M.; Bernardi, P.; De Franco, I.; Perfetri, S.; Varotto, A.; Tenna, V. Prevalence of glaucoma and intraocular pressure distribution in a defined population: The Egna-Neumarkt study. Ophthalmology 1998, 105, 209–215. [Google Scholar] [CrossRef]
- Klein, B.E.K.; Klein, R.; Sponsel, W.E.; Franke, T.; Cantor, L.B.; Martone, J.; Menage, M.J. Prevalence of Glaucoma: The Beaver Dam Eye Study. Ophthalmology 1992, 99, 1499–1504. [Google Scholar] [CrossRef]
- Hartwick, A.T.E. Beyond intraocular pressure: Neuroprotective strategies for future glaucoma therapy. Optom. Vis. Sci. 2001, 78, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.; London, A. Glaucoma as a neuropathy amenable to neuroprotection and immune manipulation. Prog. Brain Res. 2008, 173, 375–384. [Google Scholar] [PubMed]
- Sena, D.F.; Lindsley, K. Neuroprotection for treatment of glaucoma in adults. Cochrane Database Syst. Rev. 2017, 2017. [Google Scholar] [CrossRef]
- Höltje, M.; Boato, F. Neuroprotection and Regeneration in the Central Nervous System. Klin. Monbl. Augenheilkd. 2020, 237, 128–132. [Google Scholar] [PubMed]
- Moramarco, A.; Sacchetti, M.; Franzone, F.; Segatto, M.; Cecchetti, D.; Miraglia, E.; Roberti, V.; Iacovino, C.; Giustini, S. Ocular surface involvement in patients with neurofibromatosis type 1 syndrome. Graefe’s Arch. Clin. Exp. Ophthalmol. 2020, 258, 1757–1762. [Google Scholar] [CrossRef]
- HA, Q.; RW, N.; LA, K.; ME, P.; DJ, T.; DJ, Z. Retinal ganglion cell death in experimental glaucoma and after axotomy occurs by apoptosis. Invest. Ophthalmol. Vis. Sci. 1995, 36. [Google Scholar]
- Raju, T.R.; Rao, M.S.; Nagaraja, T.N.; Meti, B.L.; Schulz, M. Retinal ganglion cell survival and neurite regeneration in vitro after cell death period are dependent upon target derived trophic factor and retinal glial factor(s). Brain Res. 1994, 664, 247–251. [Google Scholar] [CrossRef]
- Lambiase, A.; Bonini, S.; Manni, L.; Ghinelli, E.; Tirassa, P.; Rama, P.; Aloe, L. Intraocular production and release of nerve growth factor after iridectomy. Investig. Ophthalmol. Vis. Sci. 2002, 43, 2334–2340. [Google Scholar]
- Oddone, F.; Roberti, G.; Micera, A.; Busanello, A.; Bonini, S.; Quaranta, L.; Agnifili, L.; Manni, G. Exploring serum levels of Brain Derived Neurotrophic Factor and Nerve Growth Factor across glaucoma stages. PLoS ONE 2017, 12, e0168565. [Google Scholar] [CrossRef] [Green Version]
- Levi-Montalcini, R.; Angeletti, P.U. Nerve growth factor. Physiol. Rev. 1968, 48, 534–569. [Google Scholar] [CrossRef] [PubMed]
- Levi-Montalcini, R. The nerve growth factor 35 years later. Science 1987, 237, 1154–1162. [Google Scholar] [CrossRef] [PubMed]
- Hefti, F. Nerve growth factor promotes survival of septal cholinergic neurons after fimbrial transections. J. Neurosci. 1986, 6, 2155–2162. [Google Scholar] [CrossRef] [Green Version]
- Gage, F.H.; Batchelor, P.; Chen, K.S.; Chin, D.; Higgins, G.A.; Koh, S.; Deputy, S.; Rosenberg, M.B.; Fischer, W.; Bjorklund, A. NGF receptor reexpression and NGF-mediated cholinergic neuronal hypertrophy in the damaged adult neostriatum. Neuron 1989, 2, 1177–1184. [Google Scholar] [CrossRef]
- Aloe, L.; Alleva, E.; De Simone, R. Changes of NGF level in mouse hypothalamus following intermale aggressive behaviour: Biological and immunohistochemical evidence. Behav. Brain Res. 1990, 39, 53–61. [Google Scholar] [CrossRef]
- Fischer, W.; Wictorin, K.; Björklund, A.; Williams, L.R.; Varon, S.; Gage, F.H. Amelioration of cholinergic neuron atrophy and spatial memory impairment in aged rats by nerve growth factor. Nature 1987, 329, 65–68. [Google Scholar] [CrossRef]
- Kromer, L.F. Nerve growth factor treatment after brain injury prevents neuronal death. Science 1987, 235, 214–216. [Google Scholar] [CrossRef]
- Tuszynski, M.H.; Sang U, H.; Amaral, D.G.; Gage, F.H. Nerve growth factor infusion in the primate brain reduces lesion-induced cholinergic neuronal degeneration. J. Neurosci. 1990, 10, 3604–3614. [Google Scholar] [CrossRef] [Green Version]
- Tuszynski, M.H.; Sang U, H.; Yoshida, K.; Gage, F.H. Recombinant human nerve growth factor infusions prevent cholinergic neuronal degeneration in the adult primate brain. Ann. Neurol. 1991, 30, 625–636. [Google Scholar] [CrossRef]
- Lambiase, A.; Manteli, F.; Sacheti, M.; Rosi, S.; Aloe, L.; Bonini, S. Clinical applications of NGF in ocular diseases. Arch. Ital. Biol. 2011, 149, 283–292. [Google Scholar] [CrossRef]
- Carmignoto, G.; Comelli, M.C.; Candeo, P.; Cavicchioli, L.; Yan, Q.; Merighi, A.; Maffei, L. Expression of NGF receptor and NGF receptor mRNA in the developing and adult rat retina. Exp. Neurol. 1991, 111, 302–311. [Google Scholar] [CrossRef]
- Ayer-Lelievre, C.S.; Ebendal, T.; Olson, L.; Seiger, A. Localization of nerve growth factor-like immunoreactivity in rat nervous tissue. Med. Biol. 1983, 61, 296–304. [Google Scholar]
- Maffei, L.; Berardi, N.; Domenici, L.; Parisi, V.; Pizzorusso, T. Nerve growth factor (NGF) prevents the shift in ocular dominance distribution of visual cortical neurons in monocularly deprived rats. J. Neurosci. 1992, 12, 4651–4662. [Google Scholar] [CrossRef] [Green Version]
- Yip, H.K.; Johnson, E.M. Retrograde transport of nerve growth factor in lesioned goldfish retinal ganglion cells. J. Neurosci. 1983, 3, 2172–2182. [Google Scholar] [CrossRef] [Green Version]
- Micera, A.; Lambiase, A.; Aloe, L.; Bonini, S.; Levi-Schaffer, F.; Bonini, S. Nerve growth factor involvement in the visual system: Implications in allergic and neurodegenerative diseases. Cytokine Growth Factor Rev. 2004, 15, 411–417. [Google Scholar] [CrossRef]
- Miraglia, E.; Moramarco, A.; Bianchini, D.; Iacovino, C.; Calvieri, S.; Giustini, S. Retinitis pigmentosa: An unusual ocular manifestation in a patient with neurofibromatosis type 1. G. Ital. Dermatol. Venereol. 2017, 152, 543–544. [Google Scholar]
- Carmignoto, G.; Maffei, L.; Candeo, P.; Canella, R.; Comelli, C. Effect of NGF on the survival of rat retinal ganglion cells following optic nerve section. J. Neurosci. 1989, 9, 1263–1272. [Google Scholar] [CrossRef] [Green Version]
- Siliprandi, R.; Canella, R.; Carmignoto, G. Nerve growth factor promotes functional recovery of retinal ganglion cells after ischemia. Investig. Ophthalmol. Vis. Sci. 1993, 34, 3232–3245. [Google Scholar]
- Lambiase, A.; Aloe, L. Nerve Growth Factor delays retinal degeneration in C3H mice. Graefe’s Arch. Clin. Exp. Ophthalmol. 1996, 234. [Google Scholar] [CrossRef]
- Coassin, M.; Lambiase, A.; Sposato, V.; Micera, A.; Bonini, S.; Aloe, L. Retinal p75 and bax overexpression is associated with retinal ganglion cells apoptosis in a rat model of glaucoma. Graefe’s Arch. Clin. Exp. Ophthalmol. 2008, 246, 1743–1749. [Google Scholar] [CrossRef]
- Lambiase, A.; Tirassa, P.; Micera, A.; Aloe, L.; Bonini, S. Pharmacokinetics of conjunctivally applied nerve growth factor in the retina and optic nerve of adult rats. Investig. Ophthalmol. Vis. Sci. 2005, 46, 3800–3806. [Google Scholar] [CrossRef] [Green Version]
- Bonini, S.; Lambiase, A.; Rama, P.; Caprioglio, G.; Aloe, L. Tropical treatment with nerve growth factor for neurotrophic keratitis. Ophthalmology 2000, 107, 1347–1351. [Google Scholar] [CrossRef]
- Bonini, S.; Lambiase, A.; Rama, P.; Filatori, I.; Allegretti, M.; Chao, W.; Mantelli, F.; Bonini, S.; Lambiase, A.; Rama, P.; et al. Phase I Trial of Recombinant Human Nerve Growth Factor for Neurotrophic Keratitis. Ophthalmology 2018, 125, 1468–1471. [Google Scholar] [CrossRef] [Green Version]
- Bonini, S.; Lambiase, A.; Rama, P.; Sinigaglia, F.; Allegretti, M.; Chao, W.; Mantelli, F.; Bonini, S.; Lambiase, A.; Rama, P.; et al. Phase II Randomized, Double-Masked, Vehicle-Controlled Trial of Recombinant Human Nerve Growth Factor for Neurotrophic Keratitis. Ophthalmology 2018, 125, 1332–1343. [Google Scholar] [CrossRef] [Green Version]
- Sacchetti, M.; Lambiase, A.; Schmidl, D.; Schmetterer, L.; Ferrari, M.; Mantelli, F.; Allegretti, M.; Garhoefer, G. Effect of recombinant human nerve growth factor eye drops in patients with dry eye: A phase IIa, open label, multiple-dose study. Br. J. Ophthalmol. 2020, 104, 127–135. [Google Scholar] [CrossRef] [Green Version]
- Lambiase, A.; Rama, P.; Bonini, S.; Caprioglio, G.; Aloe, L. Topical treatment with nerve growth factor for corneal neurotrophic ulcers. N. Engl. J. Med. 1998, 338, 1174–1180. [Google Scholar] [CrossRef]
- Sacchetti, M.; Bruscolini, A.; Lambiase, A. Cenegermin for the treatment of neurotrophic keratitis. Drugs of Today 2017, 53, 585–595. [Google Scholar] [CrossRef]
- Sacchetti, M.; Mantelli, F.; Rocco, M.L.; Micera, A.; Brandolini, L.; Focareta, L.; Pisano, C.; Aloe, L.; Lambiase, A. Recombinant Human Nerve Growth Factor Treatment Promotes Photoreceptor Survival in the Retinas of Rats with Retinitis Pigmentosa. Curr. Eye Res. 2017, 42, 1064–1068. [Google Scholar] [CrossRef]
- Study to Evaluate Safety and Efficacy of rhNGF Eye Drops Solution Versus Vehicle in Patients With Glaucoma—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT02855450 (accessed on 28 September 2020).
- Askanas, V. Neurotrophic factors and amyotrophic lateral sclerosis. Adv. Neurol. 1995, 68, 241–244. [Google Scholar]
- Siegel, G.J.; Chauhan, N.B. Neurotrophic factors in Alzheimer’s and Parkinson’s disease brain. Brain Res. Rev. 2000, 33, 199–227. [Google Scholar] [CrossRef]
- Mysona, B.A.; Zhao, J.; Bollinger, K.E. Role of BDNF/TrkB pathway in the visual system: Therapeutic implications for glaucoma. Expert Rev. Ophthalmol. 2017, 12, 69–81. [Google Scholar] [CrossRef] [Green Version]
- Gupta, V.; You, Y.; Li, J.; Gupta, V.; Golzan, M.; Klistorner, A.; van den Buuse, M.; Graham, S. BDNF impairment is associated with age-related changes in the inner retina and exacerbates experimental glaucoma. Biochim. Biophys. Acta-Mol. Basis Dis. 2014, 1842, 1567–1578. [Google Scholar] [CrossRef]
- Shen, S.; Wiemelt, A.P.; McMorris, F.A.; Barres, B.A. Retinal ganglion cells lose trophic responsiveness after axotomy. Neuron 1999, 23, 285–295. [Google Scholar] [CrossRef] [Green Version]
- Ghaffariyeh, A.; Honarpisheh, N.; Shakiba, Y.; Puyan, S.; Chamacham, T.; Zahedi, F.; Zarrineghbal, M. Brain-derived neurotrophic factor in patients with normal-tension glaucoma. Optometry 2009, 80, 635–638. [Google Scholar] [CrossRef]
- Ghaffariyeh, A.; Honarpisheh, N.; Heidari, M.H.; Puyan, S.; Abasov, F. Brain-derived neurotrophic factor as a biomarker in primary open-angle glaucoma. Optom. Vis. Sci. 2011, 88, 80–85. [Google Scholar] [CrossRef]
- Domenici, L.; Origlia, N.; Falsini, B.; Cerri, E.; Barloscio, D.; Fabiani, C.; Sansò, M.; Giovannini, L. Rescue of retinal function by BDNF in a mouse model of glaucoma. PLoS ONE 2014, 9, e115579. [Google Scholar] [CrossRef] [Green Version]
- Frank, L. BDNF down-regulates neurotrophin responsiveness, TrkB protein and TrkB mRNA levels in cultured rat hippocampal neurons. Eur. J. Neurosci. 1996, 8, 1220–1230. [Google Scholar] [CrossRef]
- Chen, H.; Weber, A.J. Brain-derived neurotrophic factor reduces TrkB protein and mRNA in the normal retina and following optic nerve crush in adult rats. Brain Res. 2004, 1011, 99–106. [Google Scholar] [CrossRef]
- Küst, B.M.; Copray, J.C.V.M.; Brouwer, N.; Troost, D.; Boddeke, H.W.G.M. Elevated levels of neurotrophins in human biceps brachii tissue of amyotrophic lateral sclerosis. Exp. Neurol. 2002, 177, 419–427. [Google Scholar] [CrossRef]
- Maki, T.; Arishima, K.; Yamamoto, M.; Sakaue, M. TrkB is involved in the mechanism by which BDNF accelerates the glutamate-induced death of rat neuroblastoma B35 cells. Neurol. Res. 2015, 37, 30–34. [Google Scholar] [CrossRef]
- Cellerino, A.; Carroll, P.; Thoenen, H.; Barde, Y.A. Reduced size of retinal ganglion cell axons and hypomyelination in mice lacking brain-derived neurotrophic factor. Mol. Cell. Neurosci. 1997, 9, 397–408. [Google Scholar] [CrossRef]
- Meyer-Franke, A.; Kaplan, M.; Pfieger, F.; Barres, B.A. Characterization of the signaling interactions that promote the survival and growth of developing retinal ganglion cells in culture. Neuron 1995, 15, 805–819. [Google Scholar] [CrossRef] [Green Version]
- Bradley, W.G. A controlled trial of recombinant methionyl human BDNF in ALS. Neurology 1999, 52, 1427–1433. [Google Scholar] [CrossRef]
- Ochs, G.; Penn, R.D.; York, M.; Giess, R.; Beck, M.; Tonn, J.; Haigh, J.; Malta, E.; Traub, M.; Sendtner, M.; et al. A phase I/II trial of recombinant methionyl human brain derived neurotrophic factor administered by intrathecal infusion to patients with amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Other Motor Neuron Disord. 2000, 1, 201–206. [Google Scholar] [CrossRef]
- Jang, S.W.; Liu, X.; Yepes, M.; Shepherd, K.R.; Miller, G.W.; Liu, Y.; Wilson, W.D.; Xiao, G.; Blanchi, B.; Sun, Y.E.; et al. A selective TrkB agonist with potent neurotrophic activities by 7,8-dihydroxyflavone. Proc. Natl. Acad. Sci. USA 2010, 107, 2687–2692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, V.K.; You, Y.; Li, J.C.; Klistorner, A.; Graham, S.L. Protective effects of 7,8-dihydroxyflavone on retinal ganglion and rgc-5 cells against excitotoxic and oxidative stress. J. Mol. Neurosci. 2013, 49, 96–104. [Google Scholar] [CrossRef]
- Hu, Y.; Cho, S.; Goldberg, J.L. Neurotrophic effect of a novel TrkB agonist on retinal ganglion cells. Investig. Ophthalmol. Vis. Sci. 2010, 51, 1747–1754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, Y.; Xu, J.; Brahimi, F.; Zhuo, Y.; Sarunic, M.V.; Uri Saragovi, H. An agonistic TrKb mAb causes sustained TrkB activation, delays RGC death, and protects the retinal structure in optic nerve axotomy and in glaucoma. Investig. Ophthalmol. Vis. Sci. 2010, 51, 4722–4731. [Google Scholar] [CrossRef]
- Almasieh, M.; Lieven, C.J.; Levin, L.A.; Di Polo, A. A cell-permeable phosphine-borane complex delays retinal ganglion cell death after axonal injury through activation of the pro-survival extracellular signal-regulated kinases 1/2 pathway. J. Neurochem. 2011, 118, 1075–1086. [Google Scholar] [CrossRef]
- Gill, S.S.; Patel, N.K.; Hotton, G.R.; O’Sullivan, K.; McCarter, R.; Bunnage, M.; Brooks, D.J.; Svendsen, C.N.; Heywood, P. Direct brain infusion of glial cell line-derived neurotrophic factor in Parkinson disease. Nat. Med. 2003, 9, 589–595. [Google Scholar] [CrossRef]
- Pascual, A.; Hidalgo-Figueroa, M.; Piruat, J.I.; Pintado, C.O.; Gómez-Díaz, R.; López-Barneo, J. Absolute requirement of GDNF for adult catecholaminergic neuron survival. Nat. Neurosci. 2008, 11, 755–761. [Google Scholar] [CrossRef] [Green Version]
- Paratcha, G.; Ledda, F. GDNF and GFRα: A versatile molecular complex for developing neurons. Trends Neurosci. 2008, 31, 384–391. [Google Scholar] [CrossRef]
- Koeberle, P.D.; Ball, A.K. Effects of GDNF on retinal ganglion cell survival following axotomy. Vis. Res. 1998, 38, 1505–1515. [Google Scholar] [CrossRef] [Green Version]
- Yan, Q.; Wang, J.; Matheson, C.R.; Urich, J.L. Glial cell line-derived neurotrophic factor (GDNF) promotes the survival of axotomized retinal ganglion cells in adult rats: Comparison to and combination with brain-derived neurotrophic factor (BDNF). J. Neurobiol. 1999, 38, 382–390. [Google Scholar] [CrossRef]
- Read, S.P.; Cashman, S.M.; Kumar-Singh, R. POD nanoparticles expressing GDNF provide structural and functional rescue of light-induced retinal degeneration in an adult mouse. Mol. Ther. 2010, 18, 1917–1926. [Google Scholar] [CrossRef]
- Osanai, M.; Nishikiori, N.; Lee, G.-H.; Sawada, N. Emerging Novel Treatment Strategies for Diabetic Eye Diseases. Curr. Diabetes Rev. 2010, 6, 35–41. [Google Scholar] [CrossRef]
- Hudgins, S.N.; Levison, S.W. Ciliary neurotrophic factor stimulates astroglial hypertrophy in vivo and in vitro. Exp. Neurol. 1998, 150, 171–182. [Google Scholar] [CrossRef]
- ALS CNTF Treatment Study Group. A double-blind placebo-controlled clinical trial of subcutaneous recombinant human ciliary neurotrophic factor (rHCNTF) in amyotrophic lateral sclerosis. Neurology 1996, 46, 1244. [Google Scholar] [CrossRef] [PubMed]
- Penn, R.; Kroin, J.; York, M.; Cedarbaum, J.M. Intrathecal ciliary neurotrophic factor delivery for treatment of amyotrophic lateral sclerosis (phase I trial). Neurosurgery 1997, 40, 94–100. [Google Scholar] [PubMed]
- Beltran, W.A.; Zhang, Q.; Kijas, J.W.; Gu, D.; Rohrer, H.; Jordan, J.A.; Aguirre, G.D. Cloning, mapping, and retinal expression of the canine ciliary neurotrophic factor receptor α (CNTFRα). Investig. Ophthalmol. Vis. Sci. 2003, 44, 3642–3649. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Clark, A.; Wordinger, R.J. Expression of ciliary neurotrophic factor (CNTF) and its tripartite receptor complex by cells of the human optic nerve head. Mol. Vis. 2007, 13, 758. [Google Scholar]
- Valter, K.; Bisti, S.; Gargini, C.; Di Loreto, S.; Maccarone, R.; Cervetto, L.; Stone, J. Time course of neurotrophic factor upregulation and retinal protection against light-induced damage after optic nerve section. Investig. Ophthalmol. Vis. Sci. 2005, 46, 1748–1754. [Google Scholar] [CrossRef]
- Yu, S.; Tanabe, T.; Yoshimura, N. A rat model of glaucoma induced by episcleral vein ligation. Exp. Eye Res. 2006, 83, 758–770. [Google Scholar] [CrossRef] [Green Version]
- Mey, J.; Thanos, S. Intravitreal injections of neurotrophic factors support the survival of axotomized retinal ganglion cells in adult rats in vivo. Brain Res. 1993, 602, 304–317. [Google Scholar] [CrossRef]
- Lu, Q.; Cui, Q.; Yip, H.; So, K.F. c-Jun expression in surviving and regenerating retinal ganglion cells: Effects of intravitreal neurotrophic supply. Investig. Ophthalmol. Vis. Sci. 2003, 44, 5342–5348. [Google Scholar] [CrossRef] [Green Version]
- Shpak, A.; Guekht, A.; Druzhkova, T.A.; Kozlova, K.I.; Gulyaeva, N.V. Ciliary neurotrophic factor in patients with primary open-angle glaucoma and age-related cataract. Mol. Vis. 2017, 23, 799. [Google Scholar]
- Yadav, K.; Rajpurohit, R.; Sharma, S. Glaucoma: Current treatment and impact of advanced drug delivery systems. Life Sci. 2019, 221, 362–376. [Google Scholar] [CrossRef]
- Castro-Balado, A.; Mondelo-García, C.; Zarra-Ferro, I.; Fernández-Ferreiro, A. New ophthalmic drug delivery systems. Farm. Hosp. 2020, 44, 149–157. [Google Scholar] [CrossRef]
- Nayak, K.; Misra, M. A review on recent drug delivery systems for posterior segment of eye. Biomed. Pharmacother. 2018, 107, 1564–1582. [Google Scholar] [CrossRef]
- Sobrado-Calvo, P.; Vidal-Sanz, M.; Villegas-Pérez, M.P. Rat retinal microglial cells under normal conditions, after optic nerve section, and after optic nerve section and intravitreal injection of trophic factors or macrophage inhibitory factor. J. Comp. Neurol. 2007, 501, 866–878. [Google Scholar] [CrossRef]
- Emami-Naeini, P.; Yiu, G. Medical and surgical applications for the suprachoroidal space. Int. Ophthalmol. Clin. 2019, 59, 195–207. [Google Scholar] [CrossRef]
- Hosoya, K.I.; Yamamoto, A.; Akanuma, S.I.; Tachikawa, M. Lipophilicity And Transporter Influence On Blood-Retinal Barrier Permeability: A comparison with blood-brain barrier permeability. Pharm. Res. 2010, 27, 2715–2724. [Google Scholar] [CrossRef]
- Herrero-Vanrell, R.; Bravo-Osuna, I.; Andrés-Guerrero, V.; Vicario-De-La-Torre, M.; Molina-Martínez, I.T. The potential of using biodegradable microspheres in retinal diseases and other intraocular pathologies. Prog. Retin. Eye Res. 2014, 42, 27–43. [Google Scholar] [CrossRef]
- Kompella, U.B.; Kadam, R.S.; Lee, V.H.L. Recent advances in ophthalmic drug delivery. Ther. Deliv. 2010, 1, 435–456. [Google Scholar] [CrossRef] [Green Version]
- Kuno, N.; Fujii, S. Biodegradable intraocular therapies for retinal disorders: Progress to date. Drugs Aging 2010, 27, 117–134. [Google Scholar] [CrossRef]
- Herrero-Vanrell, R.; Cardillo, J.A.; Kuppermann, B.D. Clinical applications of the sustained-release dexamethasone implant for treatment of macular edema. Clin. Ophthalmol. 2011, 5, 139–146. [Google Scholar]
- Andrieu-Soler, C.; Aubert-Pouëssel, A.; Doat, M.; Picaud, S.; Halhal, M.; Simonutti, M.; Venier-Julienne, M.; Benoit, J.; Behar-Cohen, F. Intravitreous injection of PLGA microspheres encapsulating GDNF promotes the survival of photoreceptors in the rd1/rd1 mouse. Mol. Vis. 2005, 11, 1002–1011. [Google Scholar]
- García-Caballero, C.; Lieppman, B.; Arranz-Romera, A.; Molina-Martínez, I.T.; Bravo-Osuna, I.; Young, M.; Baranov, P.; Herrero-Vanrell, R. Photoreceptor preservation induced by intravitreal controlled delivery of GDNF and GDNF/melatonin in rhodopsin knockout mice. Mol. Vis. 2018, 24, 733. [Google Scholar]
- Mezu-Ndubuisi, O.J.; Wang, Y.; Schoephoerster, J.; Falero-Perez, J.; Zaitoun, I.S.; Sheibani, N.; Gong, S. Intravitreal Delivery of VEGF-A 165 -loaded PLGA Microparticles Reduces Retinal Vaso-Obliteration in an In Vivo Mouse Model of Retinopathy of Prematurity. Curr. Eye Res. 2019, 44, 275–286. [Google Scholar] [CrossRef] [Green Version]
- Jiang, C.; Moore, M.J.; Zhang, X.; Klassen, H.; Langer, R.; Young, M. Intravitreal injections of GDNF-loaded biodegradable microspheres are neuroprotective in a rat model of glaucoma. Mol. Vis. 2007, 13, 1783–1792. [Google Scholar]
- Ward, M.S.; Khoobehi, A.; Lavik, E.B.; Langer, R.; Young, M.J. Neuroprotection of retinal ganglion cells in DBA/2J mice with GDNF-loaded biodegradable microspheres. J. Pharm. Sci. 2007, 96, 558–568. [Google Scholar] [CrossRef] [PubMed]
- Kyhn, M.V.; Klassen, H.; Johansson, U.E.; Warfvinge, K.; Lavik, E.; Kiilgaard, J.F.; Prause, J.U.; Scherfig, E.; Young, M.; la Cour, M. Delayed administration of glial cell line-derived neurotrophic factor (GDNF) protects retinal ganglion cells in a pig model of acute retinal ischemia. Exp. Eye Res. 2009, 89, 1012–1020. [Google Scholar] [CrossRef] [PubMed]
- Checa-Casalengua, P.; Jiang, C.; Bravo-Osuna, I.; Tucker, B.A.; Molina-Martínez, I.T.; Young, M.J.; Herrero-Vanrell, R. Retinal ganglion cells survival in a glaucoma model by GDNF/Vit e PLGA microspheres prepared according to a novel microencapsulation procedure. J. Control. Release 2011, 156, 92–100. [Google Scholar] [CrossRef]
- Checa-Casalengua, P.; Jiang, C.; Bravo-Osuna, I.; Tucker, B.A.; Molina-Martínez, I.T.; Young, M.J.; Herrero-Vanrell, R. Preservation of biological activity of glial cell line-derived neurotrophic factor (GDNF) after microencapsulation and sterilization by gamma irradiation. Int. J. Pharm. 2012, 436, 545–554. [Google Scholar] [CrossRef]
- García-Caballero, C.; Prieto-Calvo, E.; Checa-Casalengua, P.; García-Martín, E.; Polo-Llorens, V.; García-Feijoo, J.; Molina-Martínez, I.T.; Bravo-Osuna, I.; Herrero-Vanrell, R. Six month delivery of GDNF from PLGA/vitamin E biodegradable microspheres after intravitreal injection in rabbits. Eur. J. Pharm. Sci. 2017, 103, 19–26. [Google Scholar] [CrossRef]
- Seiler, M.J.; Thomas, B.B.; Chen, Z.; Arai, S.; Chadalavada, S.; Mahoney, M.J.; Sadda, S.R.; Aramant, R.B. BDNF-treated retinal progenitor sheets transplanted to degenerate rats: Improved restoration of visual function. Exp. Eye Res. 2008, 86, 92–104. [Google Scholar] [CrossRef] [Green Version]
- Grozdanic, S.D.; Lazic, T.; Kuehn, M.H.; Harper, M.M.; Kardon, R.H.; Kwon, Y.H.; Lavik, E.B.; Sakaguchi, D.S. Exogenous modulation of intrinsic optic nerve neuroprotective activity. Graefe’s Arch. Clin. Exp. Ophthalmol. 2010, 248, 1105–1116. [Google Scholar] [CrossRef] [Green Version]
- Gu, H.; Song, C.; Long, D.; Mei, L.; Sun, H. Controlled release of recombinant human nerve growth factor (rhNGF) from poly[(lactic acid)-co-(glycolic acid)] microspheres for the treatment of neurodegenerative disorders. Polym. Int. 2007, 56, 1272–1280. [Google Scholar] [CrossRef]
- Gu, H.; Long, D.; Song, C.; Li, X. Recombinant human NGF-loaded microspheres promote survival of basal forebrain cholinergic neurons and improve memory impairments of spatial learning in the rat model of Alzheimer’s disease with fimbria-fornix lesion. Neurosci. Lett. 2009, 453, 204–209. [Google Scholar] [CrossRef]
- Harvey, A.R.; Hu, Y.; Leaver, S.G.; Mellough, C.B.; Park, K.; Verhaagen, J.; Plant, G.W.; Cui, Q. Gene therapy and transplantation in CNS repair: The visual system. Prog. Retin. Eye Res. 2006, 25, 449–489. [Google Scholar] [CrossRef]
- Lebrun-Julien, F.; Di Polo, A. Molecular and Cell-Based Approaches for Neuroprotection in Glaucoma. Optom. Vis. Sci. 2008, 85, E417–E424. [Google Scholar] [CrossRef]
- Borrás, T. Advances in glaucoma treatment and management: Gene therapy. Investig. Ophthalmol. Vis. Sci. 2012, 53, 2506–2510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thanos, C.G.; Bell, W.J.; O’Rourke, P.; Kauper, K.; Sherman, S.; Stabila, P.; Tao, W. Sustained secretion of ciliary neurotrophic factor to the vitreous, using the encapsulated cell therapy-based NT-501 intraocular device. Tissue Eng. 2004, 10, 1617–1622. [Google Scholar] [CrossRef]
- Sieving, P.A.; Caruso, R.C.; Tao, W.; Coleman, H.R.; Thompson, D.J.S.; Fullmer, K.R.; Bush, R.A. Ciliary neurotrophic factor (CNTF) for human retinal degeneration: Phase I trial of CNTF delivered by encapsulated cell intraocular implants. Proc. Natl. Acad. Sci. USA 2006, 103, 3896–3901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, W. Application of encapsulated cell technology for retinal degenerative diseases. Expert Opin. Biol. Ther. 2006, 6, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Birch, D.G.; Weleber, R.G.; Duncan, J.L.; Jaffe, G.J.; Tao, W. Randomized trial of ciliary neurotrophic factor delivered by encapsulated cell intraocular implants for retinitis pigmentosa. Am. J. Ophthalmol. 2013, 156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, K.; Hopkins, J.J.; Heier, J.S.; Birch, D.G.; Halperin, L.S.; Albini, T.A.; Brown, D.M.; Jaffe, G.J.; Taoj, W.; Williams, G.A. Ciliary neurotrophic factor delivered by encapsulated cell intraocular implants for treatment of geographic atrophy in age-related macular degeneration. Proc. Natl. Acad. Sci. USA 2011, 108, 6241–6245. [Google Scholar] [CrossRef] [Green Version]
- Kauper, K.; McGovern, C.; Sherman, S.; Heatherton, P.; Rapoza, R.; Stabila, P.; Dean, B.; Lee, A.; Borges, S.; Bouchard, B.; et al. Two-year intraocular delivery of ciliary neurotrophic factor by encapsulated cell technology implants in patients with chronic retinal degenerative diseases. Investig. Ophthalmol. Vis. Sci. 2012, 53, 7484–7491. [Google Scholar] [CrossRef]
- Retinal Imaging of Subjects Implanted with Ciliary Neurotrophic Factor (CNTF)-Releasing Encapsulated Cell Implant for Early-stage Retinitis Pigmentosa—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT01530659 (accessed on 29 September 2020).
- Extension Study of NT-501 Ciliary Neurotrophic Factor (CNTF) Implant for Macular Telangiectasia (MacTel)—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03071965?term=NCT03071965.&draw=2&rank=1 (accessed on 29 September 2020).
- NT-501 CNTF Implant for Ischemic Optic Neuropathy: Safety, Neuroprotection and Neuroenhancement—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT01411657?term=NT-501+CNTF+Implant+for+Ischemic+Optic+Neuropathy%3A+Safety%2C+Neuroprotection+and+Neuroenhancement&draw=2&rank=1 (accessed on 29 September 2020).
- NT-501 CNTF Implant for Glaucoma: Safety, Neuroprotection and Neuroenhancement—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT01408472?term=NT-501+CNTF+Implant+for+Glaucoma%3A+Safety%2C+Neuroprotection+and+Neuroenhancement.&draw=2&rank=1 (accessed on 29 September 2020).
- Study of NT-501 Encapsulated Cell Therapy for Glaucoma Neuroprotection and Vision Restoration—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT02862938?term=Study+of+NT-501+Encapsulated+Cell+Therapy+for+Glaucoma+Neuroprotection+and+Vision+Restoration.&draw=2&rank=1 (accessed on 29 September 2020).
- Nafissi, N.; Foldvari, M. Neuroprotective therapies in glaucoma: I. Neurotrophic factor delivery. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016, 8, 240–254. [Google Scholar] [CrossRef]
- Martin, K.R.G.; Quigley, H.A.; Zack, D.J.; Levkovitch-Verbin, H.; Kielczewski, J.; Valenta, D.; Baumrind, L.; Pease, M.E.; Klein, R.L.; Hauswirth, W.W. Gene therapy with brain-derived neurotrophic factor as a protection: Retinal ganglion cells in a rat glaucoma model. Investig. Ophthalmol. Vis. Sci. 2003, 44, 4357–4365. [Google Scholar] [CrossRef]
- Van Adel, B.A.; Kostic, C.; Déglon, N.; Ball, A.K.; Arsenijevic, Y. Delivery of ciliary neurotrophic factor via lentiviral-mediated transfer protects axotomized retinal ganglion cells for an extended period of time. Hum. Gene Ther. 2003, 14, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Leaver, S.G.; Cui, Q.; Plant, G.W.; Arulpragasam, A.; Hisheh, S.; Verhaagen, J.; Harvey, A.R. AAV-mediated expression of CNTF promotes long-term survival and regeneration of adult rat retinal ganglion cells. Gene Ther. 2006, 13, 1328–1341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, F.Q.; Aleman, T.S.; Dejneka, N.S.; Dudus, L.; Fisher, K.J.; Maguire, A.M.; Jacobson, S.G.; Bennett, J. Long-term protection of retinal structure but not function using rAAV.CNTF in animal models of retinitis pigmentosa. Mol. Ther. 2001, 4, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Bok, D.; Yasumura, D.; Matthes, M.T.; Ruiz, A.; Duncan, J.L.; Chappelow, A.V.; Zolutukhin, S.; Hauswirth, W.; LaVail, M.M. Effects of adeno-associated virus-vectored ciliary neurotrophic factor on retinal structure and function in mice with a P216L rds/peripherin mutation. Exp. Eye Res. 2002, 74, 719–735. [Google Scholar] [CrossRef]
- Adamus, G.; Sugden, B.; Shiraga, S.; Timmers, A.M.; Hauswirth, W.W. Anti-apoptotic effects of CNTF gene transfer on photoreceptor degeneration in experimental antibody-induced retinopathy. J. Autoimmun. 2003, 21, 121–129. [Google Scholar] [CrossRef]
- Cheng, L.; Sapieha, P.; Kittlerová, P.; Hauswirth, W.W.; Di Polo, A. TrkB Gene Transfer Protects Retinal Ganglion Cells from Axotomy-Induced Death in Vivo. J. Neurosci. 2002, 22, 3977–3986. [Google Scholar] [CrossRef] [Green Version]
- Schmeer, C.; Straten, G.; Kügler, S.; Gravel, C.; Bähr, M.; Isenmann, S. Dose-dependent rescue of axotomized rat retinal ganglion cells by adenovirus-mediated expression of glial cell-line derived neurotrophic factor in vivo. Eur. J. Neurosci. 2002, 15, 637–643. [Google Scholar] [CrossRef]
- Straten, G.; Schmeer, C.; Kretz, A.; Gerhardt, E.; Kügler, S.; Schulz, J.B.; Gravel, C.; Bähr, M.; Isenmann, S. Potential synergistic protection of retinal ganglion cells from axotomy-induced apoptosis by adenoviral administration of glial cell line-derived neurotrophic factor and X-chromosome-linked inhibitor of apoptosis. Neurobiol. Dis. 2002, 11, 123–133. [Google Scholar] [CrossRef] [Green Version]
- McKinnon, S.J.; Lehman, D.M.; Tahzib, N.G.; Ransom, N.L.; Reitsamer, H.A.; Liston, P.; LaCasse, E.; Li, Q.; Korneluk, R.G.; Hauswirth, W.W. Baculoviral IAP repeat-containing-4 protects optic nerve axons in a rat glaucoma model. Mol. Ther. 2002, 5, 780–787. [Google Scholar] [CrossRef]
- Weleber, R.G.; Pennesi, M.E.; Wilson, D.J.; Kaushal, S.; Erker, L.R.; Jensen, L.; McBride, M.T.; Flotte, T.R.; Humphries, M.; Calcedo, R.; et al. Results at 2 Years after Gene Therapy for RPE65-Deficient Leber Congenital Amaurosis and Severe Early-Childhood-Onset Retinal Dystrophy. Ophthalmology 2016, 123, 1606–1620. [Google Scholar] [CrossRef] [Green Version]
- Bennett, J.; Wellman, J.; Marshall, K.A.; McCague, S.; Ashtari, M.; DiStefano-Pappas, J.; Elci, O.U.; Chung, D.C.; Sun, J.; Wright, J.F.; et al. Safety and durability of effect of contralateral-eye administration of AAV2 gene therapy in patients with childhood-onset blindness caused by RPE65 mutations: A follow-on phase 1 trial. Lancet 2016, 388, 661–672. [Google Scholar] [CrossRef] [Green Version]
- Petrs-Silva, H.; Dinculescu, A.; Li, Q.; Min, S.H.; Chiodo, V.; Pang, J.J.; Zhong, L.; Zolotukhin, S.; Srivastava, A.; Lewin, A.S.; et al. High-efficiency transduction of the mouse retina by tyrosine-mutant AAV serotype vectors. Mol. Ther. 2009, 17, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Lebherz, C.; Maguire, A.; Tang, W.; Bennett, J.; Wilson, J.M. Novel AAV serotypes for improved ocular gene transfer. J. Gene Med. 2008, 10, 375–382. [Google Scholar] [CrossRef]
- Shiozawa, A.L.; Igarashi, T.; Kobayashi, M.; Nakamoto, K.; Kameya, S.; Fujishita, S.; Takahashi, H.; Okada, T. Tyrosine triple mutated AAV2-BDNF gene therapy in an inner retinal injury model induced by intravitreal injection of N–methyl-D-aspartate (NMDA). Mol. Vis. 2020, 26, 409–422. [Google Scholar]
- Pease, M.E.; Zack, D.J.; Berlinicke, C.; Bloom, K.; Cone, F.; Wang, Y.; Klein, R.L.; Hauswirth, W.W.; Quigley, H.A. Effect of cntf on retinal ganglion cell survival in experimental glaucoma. Investig. Ophthalmol. Vis. Sci. 2009, 50, 2194–2200. [Google Scholar] [CrossRef] [Green Version]
- Bishop, K.M.; Hofer, E.K.; Mehta, A.; Ramirez, A.; Sun, L.; Tuszynski, M.; Bartus, R.T. Therapeutic potential of CERE-110 (AAV2-NGF): Targeted, stable, and sustained NGF delivery and trophic activity on rodent basal forebrain cholinergic neurons. Exp. Neurol. 2008, 211, 574–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rafii, M.S.; Baumann, T.L.; Bakay, R.A.E.; Ostrove, J.M.; Siffert, J.; Fleisher, A.S.; Herzog, C.D.; Barba, D.; Pay, M.; Salmon, D.P.; et al. A phase1 study of stereotactic gene delivery of AAV2-NGF for Alzheimer’s disease. Alzheimers. Dement. 2014, 10, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Castle, M.J.; Baltanás, F.C.; Kovacs, I.; Nagahara, A.H.; Barba, D.; Tuszynski, M.H. Postmortem analysis in a clinical trial of AAV2-NGF gene therapy for alzheimer’s disease identifies a need for improved vector delivery. Hum. Gene Ther. 2020, 31, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Rafii, M.S.; Tuszynski, M.H.; Thomas, R.G.; Barba, D.; Brewer, J.B.; Rissman, R.A.; Siffert, J.; Aisen, P.S.; Mintzer, J.; Lerner, A.; et al. Adeno-associated viral vector (serotype 2)-nerve growth factor for patients with Alzheimer disease a randomized clinical trial. JAMA Neurol. 2018, 75, 834–841. [Google Scholar] [CrossRef]
- Liaw, J.; Chang, S.F.; Hsiao, F.C. In vivo gene delivery into ocular tissues by eye drops of poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) (PEO-PPO-PEO) polymeric micelles. Gene Ther. 2001, 8, 999–1004. [Google Scholar] [CrossRef] [Green Version]
- Shakhbazau, A.; Shcharbin, D.; Seviaryn, I.; Goncharova, N.; Kosmacheva, S.; Potapnev, M.; Bryszewska, M.; Kumar, R.; Biernaskie, J.; Midha, R. Dendrimer-driven neurotrophin expression differs in temporal patterns between rodent and human stem cells. Mol. Pharm. 2012, 9, 1521–1528. [Google Scholar] [CrossRef] [PubMed]
- Mo, X.; Yokoyama, A.; Oshitari, T.; Negishi, H.; Dezawa, M.; Mizota, A.; Adachi-Usami, E. Rescue of axotomized retinal ganglion cells by BDNF gene electroporation in adult rats. Investig. Ophthalmol. Vis. Sci. 2002, 43, 2401–2405. [Google Scholar]
- Negishi, H.; Dezawa, M.; Oshitari, T.; Adachi-Usami, E. Optic nerve regeneration within artificial Schwann cell graft in the adult rat. Brain Res. Bull. 2001, 55, 409–419. [Google Scholar] [CrossRef]

| Neurotrophic Factors (NTFs) | Delivery Systems | Main Preclinical Studies | Main Clinical Studies | Clinical Trials Identifier |
|---|---|---|---|---|
| NGF | IVT PLGA microspheres-NGF IVT AAV2-NGF (CERE-110) Artificial graft transplantation-NGF | Gu et al. 2007, in vitro and in vivo (AD) Gu et al. 2009, in vitro and in vivo (AD) Bishop et al. 2008, in vivo (AD) Negishi et al. 2001, in vivo (ON transection) | Rafii et al.2014 (AD) Rafii et al.2018 (AD) | |
| BDNF | Mimetic ligands binding TrkB
IVT AAV-BDNF IVT AAV-TrkB+BDNF IVT AAV2-BDNF IVT AAV2-BDNF Polyamidoamine dendrimer-BDNF IVT BDNF+electroporation Artificial graft transplantation-BDNF | Jang et al. 2010, in vivo (PD) Gupta et al. 2013, in vitro (excitotoxic and oxidative stress induced RGC apoptosis) Hu et al. 2010, in vitro and in vivo (ON transection) Bai et al. 2010, in vivo (ON transection and POAG) Almasieh et al. 2011, in vivo (ON transection and POAG) Seiler et al. 2008, in vivo (photoreceptor degeneration) Grozdanic et al. 2010, in vivo (retinal ischemia) Martin et al. 2003, in vivo (POAG) Pease et al. 2009, in vivo (POAG) Cheng et al. 2002, in vivo (ON transection) Leaver et al. 2006, in vivo (ON transection) Shiozawa et al.2020 in vivo (inner retinal injury model) Shakhbazau et al. 2012, in vitro (rodent and human stem cells) Mo et al. 2002, in vivo (ON transection) Negishi et al. 2001, in vivo (ON transection) | ||
| GDNF | IVT PLGA microspheres-GDNF IVT Ad. GDNF IVT Ad. GDNF+Ad.XIAP IVT AAV-GDNF Subretinal PEG-POD-GDNF | Ward et al. 2007, in vivo (POAG) Jiang et al. 2007, in vivo (POAG) Kyhn et al. 2009, in vivo (retinal ischemia) Checa-Casalengua et al. 2011, in vitro and in vivo (POAG) Checa-Casalengua et al. 2012, in vitro (retinal cells) Garcia-Caballero et al. 2017, in vitro and in vivo (wild-type) Grozdanic et al. 2010, in vivo (retinal ischemia) Schmeer et al. 2002, in vivo (ON transection) Straten et al. 2002, in vivo (ON transection) Pease et al. 2009, in vivo (POAG) Read et al. 2010, in vitro and in vivo (photoreceptor degeneration) | ||
| CNTF | IVT PLGA microspheres-CNTF IVT NT 501 CET-based implants IVT AAV-CNTF Subretinal AAV-CNTF IVT LV-CNTF IVT AAV2-CNTF | Grozdanic et al. 2010, in vivo (retinal ischemia) Thanos et al. 2004, in vitro and in vivo (wild-type) Liang et al., 2001, in vivo (RP) Bok et al.2002, in vivo (photoreceptor degeneration) Adamus et al.2003, in vivo (photoreceptor degeneration) van Adel et al., 2003 in vivo (ON transection) Leaver et al., 2006, in vivo (ON transection) Pease et al. 2009, in vivo (POAG) | Sieving et al. 2006, (RP) Birch et al. 2013, (RP) Zhang et al. 2011 (AMD) Kauper et al. 2012 (RP/AMD) | Phase I NCT01408472 (POAG) Phase II NCT02862938 (POAG) Phase II NCT01530659 (RP/Usher synd.) Phase II NCT03071965 (MacTel) Phase I NCT01411657(ION) |
| NT-3 | Polyamidoamine dendrimer-NT-3 | Shakhbazau et al. 2012, in vitro (rodent and human stem cells) | ||
| NT-4/5 | Artificial graft transplantation-NT-4/5 | Negishi et al. 2001, in vivo (ON transection) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mallone, F.; Sacchetti, M.; Bruscolini, A.; Scuderi, L.; Marenco, M.; Lambiase, A. Neurotrophic Factors in Glaucoma and Innovative Delivery Systems. Appl. Sci. 2020, 10, 9015. https://doi.org/10.3390/app10249015
Mallone F, Sacchetti M, Bruscolini A, Scuderi L, Marenco M, Lambiase A. Neurotrophic Factors in Glaucoma and Innovative Delivery Systems. Applied Sciences. 2020; 10(24):9015. https://doi.org/10.3390/app10249015
Chicago/Turabian StyleMallone, Fabiana, Marta Sacchetti, Alice Bruscolini, Luca Scuderi, Marco Marenco, and Alessandro Lambiase. 2020. "Neurotrophic Factors in Glaucoma and Innovative Delivery Systems" Applied Sciences 10, no. 24: 9015. https://doi.org/10.3390/app10249015
APA StyleMallone, F., Sacchetti, M., Bruscolini, A., Scuderi, L., Marenco, M., & Lambiase, A. (2020). Neurotrophic Factors in Glaucoma and Innovative Delivery Systems. Applied Sciences, 10(24), 9015. https://doi.org/10.3390/app10249015







