Association between Rapid Maxillary Expansion and Nocturnal Enuresis in Children: A Pilot Study for a Randomized Controlled Clinical Trial
Abstract
:1. Introduction
2. Materials and Methods
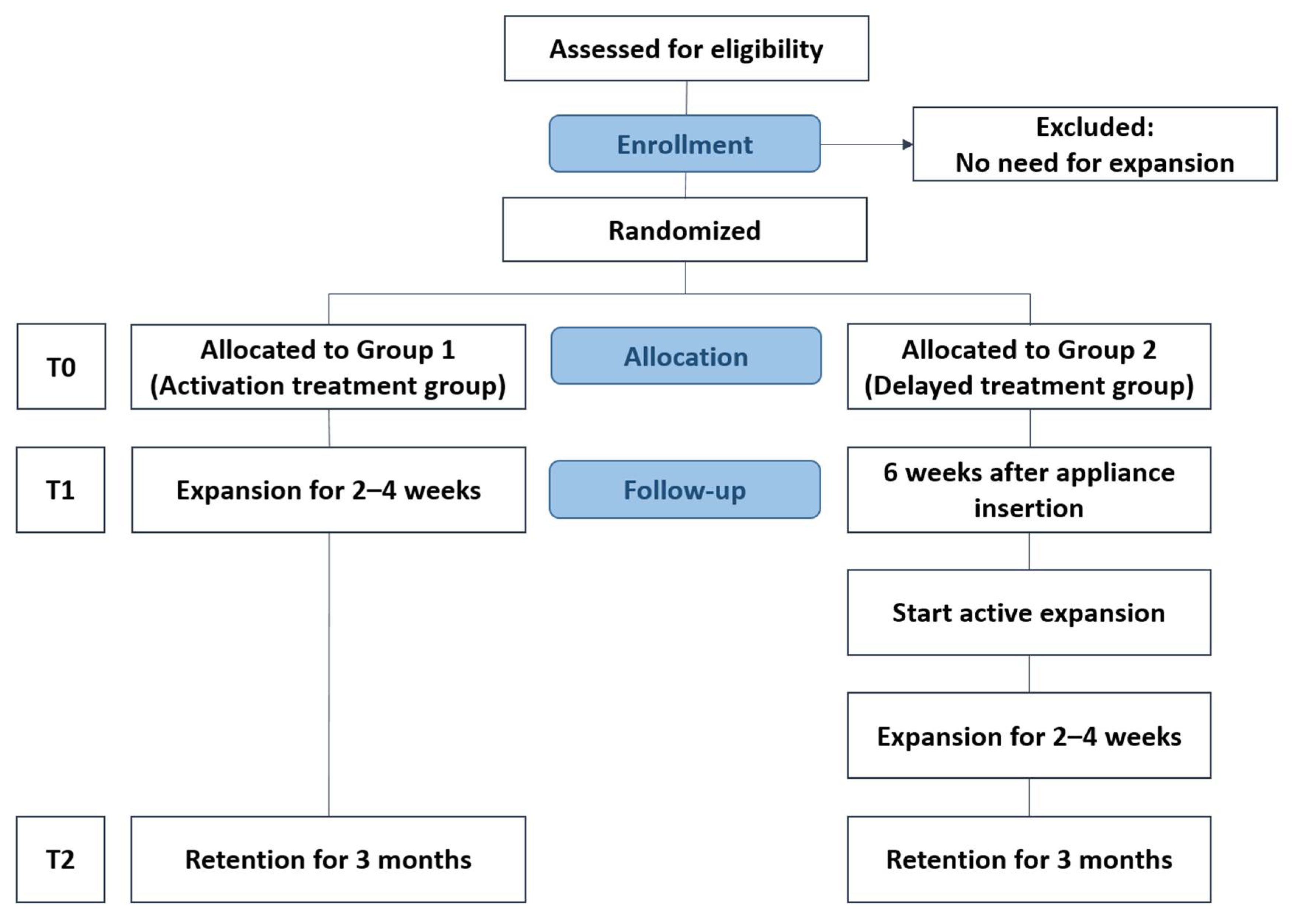
2.1. Trial Design
2.2. Participants, Eligibility Criteria, and Settings
2.3. Sample Size
2.4. Randomization
2.5. Blinding
2.6. Interventions
2.7. CBCT
2.8. Outcomes (Primary and Secondary)
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Butler, R.J.; Heron, J. The prevalence of infrequent bedwetting and nocturnal enuresis in childhood. A large British cohort. Scand. J. Urol. Nephrol. 2008, 42, 257–264. [Google Scholar] [CrossRef] [PubMed]
- von Gontard, A.; Heron, J.; Joinson, C. Family history of nocturnal enuresis and urinary incontinence: Results from a large epidemiological study. J. Urol. 2011, 185, 2303–2306. [Google Scholar] [CrossRef] [PubMed]
- Yeung, C.K.; Sihoe, J.D.; Sit, F.K.; Bower, W.; Sreedhar, B.; Lau, J. Characteristics of primary nocturnal enuresis in adults: An epidemiological study. BJU Int. 2004, 93, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Neveus, T.; Eggert, P.; Evans, J.; Macedo, A.; Rittig, S.; Tekgül, S.; Vande Walle, J.; Yeung, C.K.; Robson, L. Evaluation of and treatment for monosymptomatic enuresis: A standardization document from the International Children’s Continence Society. J. Urol. 2010, 183, 441–447. [Google Scholar] [CrossRef]
- Kamperis, K.; Rittig, S.; Jørgensen, K.A.; Djurhuus, J.C. Nocturnal polyuria in monosymptomatic nocturnal enuresis refractory to desmopressin treatment. Am. J. Physiol. Ren. Physiol. 2006, 291, F1232–F1240. [Google Scholar] [CrossRef]
- Vande Walle, J.; Vande Walle, C.; Van Sintjan, P.; De Guchtenaere, A.; Raes, A.; Donckerwolcke, R.; Van Laecke, E.; Mauel, R.; Dehoorne, J.; Van Hoyweghen, E.; et al. Nocturnal polyuria is related to 24-hour diuresis and osmotic excretion in an enuresis population referred to a tertiary center. J. Urol. 2007, 178, 2630–2634. [Google Scholar] [CrossRef]
- Kamperis, K.; Hagstroem, S.; Rittig, S.; Djurhuus, J.C. Urinary calcium excretion in healthy children and children with primary monosymptomatic nocturnal enuresis. J. Urol. 2006, 176, 770–773. [Google Scholar] [CrossRef]
- Umlauf, M.G.; Chasens, E.R. Sleep disordered breathing and nocturnal polyuria: Nocturia and enuresis. Sleep Med. Rev. 2003, 7, 403–411. [Google Scholar] [CrossRef]
- Alexopoulos, E.I.; Malakasioti, G.; Varlami, V.; Miligkos, M.; Gourgoulianis, K.; Kaditis, A.G. Nocturnal enuresis is associated with moderate-to-severe obstructive sleep apnea in children with snoring. Pediatric Res. 2014, 76, 555–559. [Google Scholar] [CrossRef]
- Alexopoulos, E.I.; Kostadima, E.; Pagonari, I.; Zintzaras, E.; Gourgoulianis, K.; Kaditis, A.G. Association between primary nocturnal enuresis and habitual snoring in children. Urology 2006, 68, 406–409. [Google Scholar] [CrossRef]
- Alexopoulos, E.I.; Kaditis, A.G.; Kostadima, E.; Gourgoulianis, K. Resolution of nocturnal enuresis in snoring children after treatment with nasal budesonide. Urology 2005, 66, 194. [Google Scholar] [CrossRef] [PubMed]
- Bazargani, F.; Jönson-Ring, I.; Nevéus, T. Rapid maxillary expansion in therapy-resistant enuretic children: An orthodontic perspective. Angle Orthod. 2016, 86, 481–486. [Google Scholar] [CrossRef]
- Kovacevic, L.; Lu, H.; Wolfe-Christensen, C.; Abdulhamid, I.; Thottam, P.J.; Lulgjuraj, M.; Madgy, D.N.; Lakshmanan, Y. Adenotonsillectomy Normalizes Hormones and Urinary Electrolytes in Children with Nocturnal Enuresis and Sleep-Disordered Breathing. Urology 2015, 86, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Al-Taai, N.; Alfatlawi, F.; Ransjö, M.; Fakhry, S. Effect of rapid maxillary expansion on monosymptomatic primary nocturnal enuresis. Angle Orthod. 2015, 85, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Katyal, V.; Pamula, Y.; Daynes, C.N.; Martin, J.; Dreyer, C.W.; Kennedy, D.; Sampson, W.J. Craniofacial and upper airway morphology in pediatric sleep-disordered breathing and changes in quality of life with rapid maxillary expansion. Am. J. Orthod. Dentofac. Orthop. 2013, 144, 860–871. [Google Scholar] [CrossRef]
- Schauseil, M.; Ludwig, B.; Zorkun, B.; Hellak, A.; Korbmacher-Steiner, H. Density of the midpalatal suture after RME treatment—A retrospective comparative low-dose CT-study. Head Face Med. 2014, 10, 18. [Google Scholar] [CrossRef]
- Liu, S.; Xu, T.; Zou, W. Effects of rapid maxillary expansion on the midpalatal suture: A systematic review. Eur. J. Orthod. 2015, 37, 651–655. [Google Scholar] [CrossRef]
- Baratieri, C.; Alves, M., Jr.; de Souza, M.M.; de Souza Araújo, M.T.; Maia, L.C. Does rapid maxillary expansion have long-term effects on airway dimensions and breathing? Am. J. Orthod. Dentofac. Orthop. 2011, 140, 146–156. [Google Scholar] [CrossRef]
- Usumez, S.; Işeri, H.; Orhan, M.; Basciftci, F.A. Effect of rapid maxillary expansion on nocturnal enuresis. Angle Orthod. 2003, 73, 532–538. [Google Scholar]
- Kurol, J.; Modin, H.; Bjerkhoel, A. Orthodontic maxillary expansion and its effect on nocturnal enuresis. Angle Orthod. 1998, 68, 225–232. [Google Scholar]
- Poorsattar-Bejeh Mir, K.; Poorsattar-Bejeh Mir, A.; Poorsattar-Bejeh Mir, M.; Moradi-Lakeh, M.; Balmeh, P.; Nosrati, K. Rapid Palatal Expansion to Treat Nocturnal Enuretic Children: A Systematic Review and Meta-Analysis. J. Dent. 2015, 16, 138–148. [Google Scholar]
- Nevéus, T.; Läckgren, G.; Tuvemo, T.; Hetta, J.; Hjälmås, K.; Stenberg, A. Enuresis—Background and treatment. Scand. J. Urol. Nephrol. 2000, 1–44. [Google Scholar] [CrossRef]
- Graf, S.; Cornelis, M.A.; Hauber Gameiro, G.; Cattaneo, P.M. Computer-aided design and manufacture of hyrax devices: Can we really go digital? Am. J. Orthod. Dentofac. Orthop. 2017, 152, 870–874. [Google Scholar] [CrossRef] [PubMed]
- Chervin, R.D.; Hedger, K.; Dillon, J.E.; Pituch, K.J. Pediatric sleep questionnaire (PSQ): Validity and reliability of scales for sleep-disordered breathing, snoring, sleepiness, and behavioral problems. Sleep Med. 2000, 1, 21–32. [Google Scholar] [CrossRef]
- Niu, X.; Madhan, S.; Cornelis, M.A.; Cattaneo, P.M. Novel three-dimensional methods to analyze the morphology of the nasal cavity and pharyngeal airway. Angle Orthod. 2020, in press. [Google Scholar]
- Robertson, C.J. Treatment of long-standing nocturnal enuresis by mandibular advancement. Sleep Breath. 2004, 8, 57–60. [Google Scholar] [CrossRef]
- Weider, D.J.; Sateia, M.J.; West, R.P. Nocturnal enuresis in children with upper airway obstruction. Otolaryngol. Head Neck Surg. 1991, 105, 427–432. [Google Scholar] [CrossRef]
- Brooks, L.J.; Topol, H.I. Enuresis in children with sleep apnea. J. Pediatrics 2003, 142, 515–518. [Google Scholar] [CrossRef]
- Abtahi, S.; Witmans, M.; Alsufyani, N.A.; Major, M.P.; Major, P.W. Pediatric sleep-disordered breathing in the orthodontic population: Prevalence of positive risk and associations. Am. J. Orthod. Dentofac. Orthop. 2020, 157, 466–473.e1. [Google Scholar] [CrossRef]
- Schütz-Fransson, U.; Kurol, J. Rapid maxillary expansion effects on nocturnal enuresis in children: A follow-up study. Angle Orthod. 2008, 78, 201–208. [Google Scholar] [CrossRef]
- Nevéus, T.; Leissner, L.; Rudblad, S.; Bazargani, F. Orthodontic widening of the palate may provide a cure for selected children with therapy-resistant enuresis. Acta Paediatr. 2014, 103, 1187–1191. [Google Scholar] [CrossRef] [PubMed]
- Oshagh, M.; Bahramnia, F.; Aminsharifi, A.R.; Fallahzadeh, M.H.; Ghodrati, P. Effects of maxillary expansion and placebo effect of appliances on nocturnal enuresis—Preliminary results. Cent. Eur. J. Urol. 2014, 67, 51–55. [Google Scholar]
- Hyla-Klekot, L.; Truszel, M.; Paradysz, A.; Postek-Stefańska, L.; Życzkowski, M. Influence of Orthodontic Rapid Maxillary Expansion on Nocturnal Enuresis in Children. BioMed Res. Int. 2015, 2015, 201039. [Google Scholar] [CrossRef] [PubMed]
- Ring, I.J.; Nevéus, T.; Markström, A.; Magnuson, A.; Bazargani, F. Rapid maxillary expansion in children with nocturnal enuresis: A randomized placebo-controlled trial. Angle Orthod. 2020, 90, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Timms, D.J. Rapid maxillary expansion in the treatment of nocturnal enuresis. Angle Orthod. 1990, 60, 229–233. [Google Scholar]
- Pedersen, M.J.; Rittig, S.; Jennum, P.J.; Kamperis, K. The role of sleep in the pathophysiology of nocturnal enuresis. Sleep Med. Rev. 2020, 49, 101228. [Google Scholar] [CrossRef]

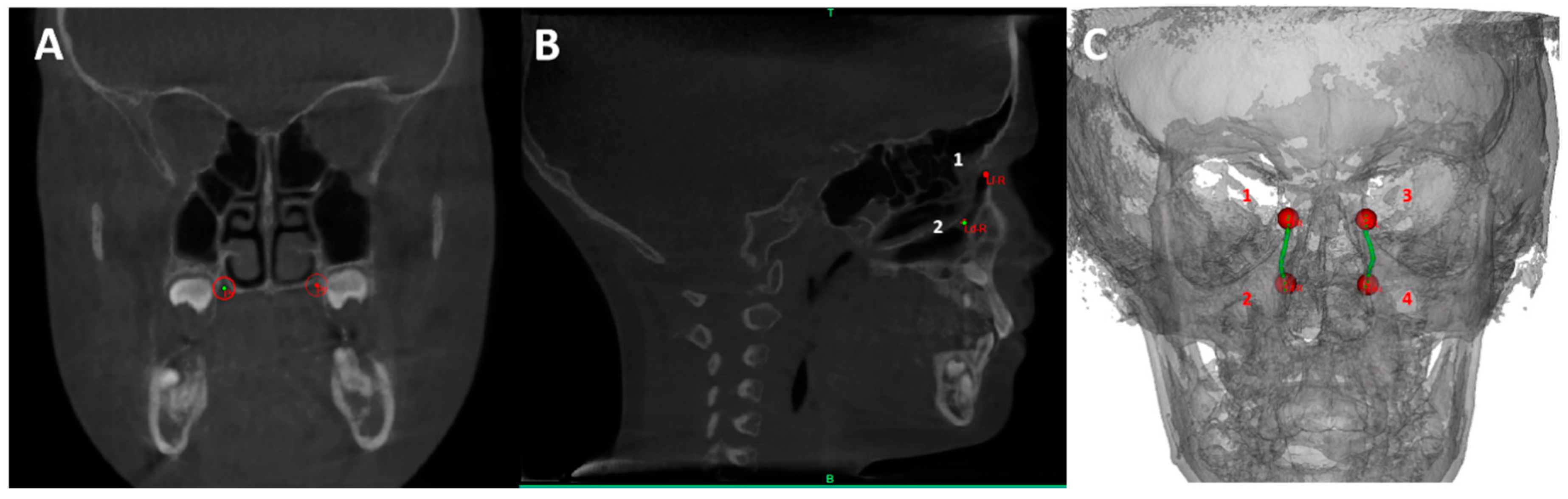
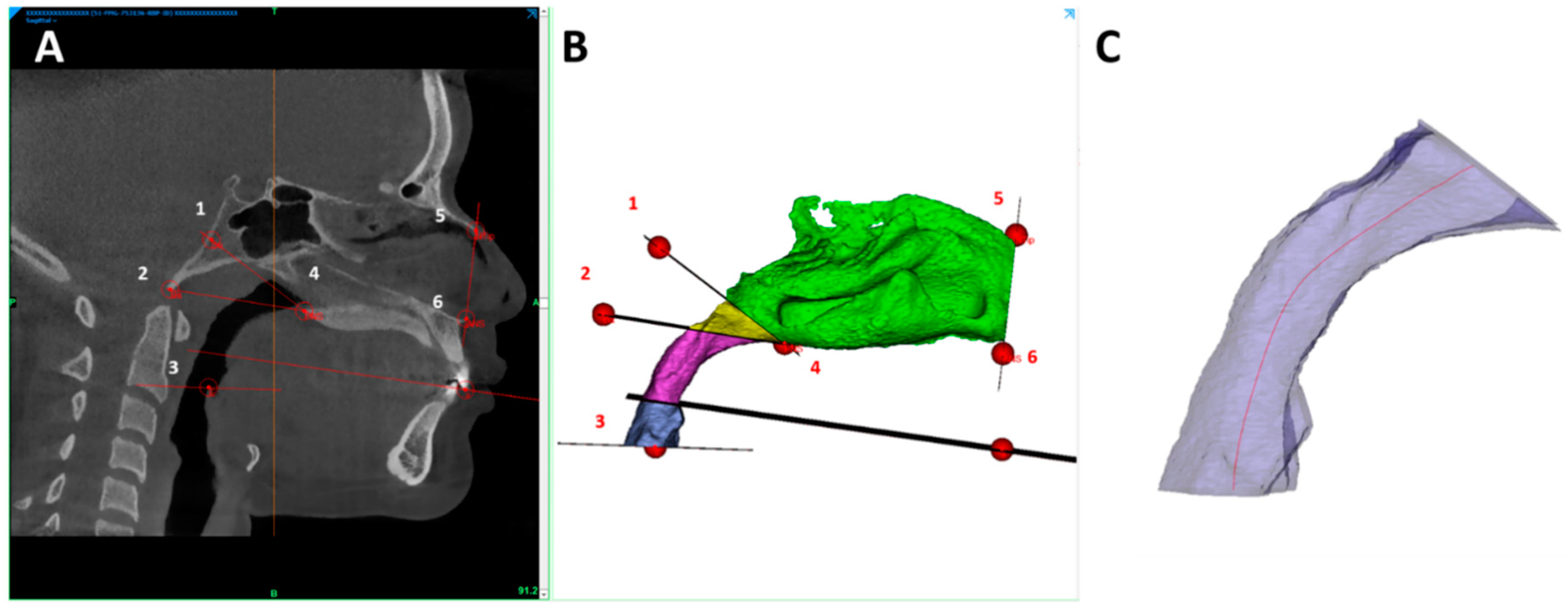

| Points | Description |
| ANS | The most anterior point on the nasal spine |
| Ba | The most posteroinferior point on the clivus |
| E | Most superior point of the epiglottis |
| LD-L | The most inferior point of the left lacrimal duct |
| LD-R | The most inferior point of the right lacrimal duct |
| LF-L | Centroid of the Lacrimal foramen left |
| LF-R | Centroid of the Lacrimal foramen right |
| MoL | The distal-palatal tip of the first left molar in the upper jaw |
| MoR | The distal-palatal tip of the first right molar in the upper jaw |
| N | The intersection of the internasal and frontonasal sutures in the midsagittal plane |
| Ntip | The tip of the nasal bone |
| OrL | Orbital left, the most inferior anterior point on left orbit’s margin |
| OrR | Orbital right, the most inferior anterior point on right orbit’s margin |
| PNS | The most posterior point on the nasal spine |
| Pl | Centroid of the greater palatine foramen left |
| Pr | Centroid of the greater palatine foramen right |
| PoL | Porion Left: the most upper point on the left bony external auditory meatus |
| PoR | Porion Left: the most upper point on Right bony external auditory meatus |
| S | The midpoint of the sella turcica |
| So | The midpoint of the sella-basion line |
| ii | The point midway between the incisal edges of the maxillary central incisors |
| References Planes | Description |
| Frankfurt plane | A plane passing through the inferior borders of the bony orbits, encompassed by OrR and OrL, and the upper margin of the auditory meatus encompassed by PoL |
| Sagittal SN plane | Plane perpendicular to Frankfurt plane passing through S and N points |
| NTip-ANS plane | Plane through NTip and ANS points, perpendicular to Sagittal SN plane |
| PNS-So plane | Plane through PNS and So points, perpendicular to Sagittal SN plane |
| PNS-Ba plane | Plane through PNS and Ba points, perpendicular to Sagittal SN plane |
| Occlusion plane | Plane through MoL, MoR, and ii points |
| E plane | Plane through E point, parallel to Frankfurt plane |
| Description | |
|---|---|
| NC measurment | |
| NCV | Bounded anteriorly by NTip-ANS plane and posterior by PNS-So plane |
| PA measurments | |
| TPAV | Bounded superiorly by PNS-So plane and inferiorly by E1-E2 plane |
| Miminal CS | The minimal cross-sectional area in Total PA |
| Minimal DH | The minimal hydraulic diameter in Total PA |
| Width | Description |
| Inter lacrimal duct distanct (LD) | Distance between Ld-L and Ld-R |
| Inter lacrimal foramen distance (LF) | Distance between Lf-L and Lf-R |
| Inter-molar distance | Distance between MoL and MoR |
| Palatal width | Distance between Pl and Pr |
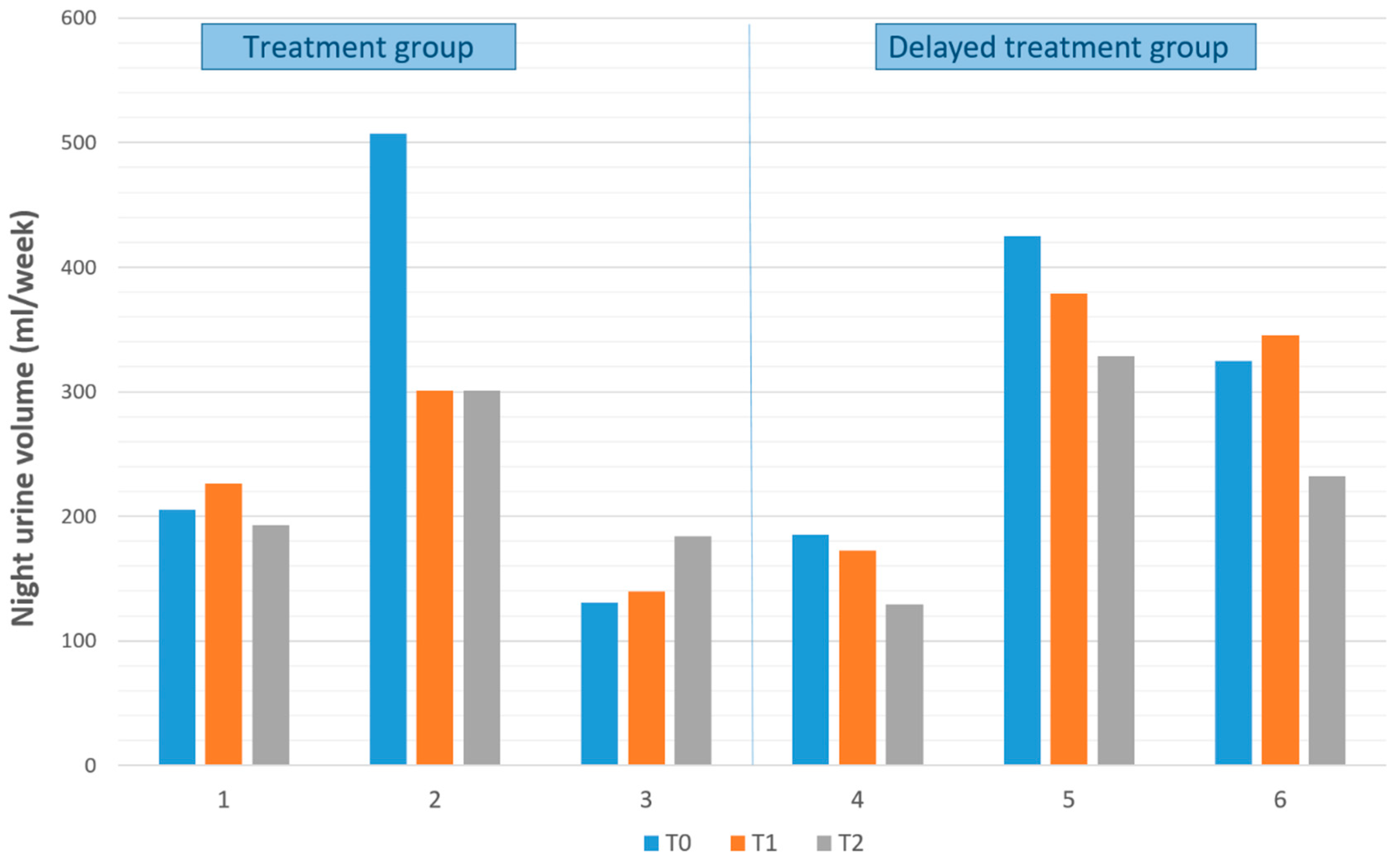
| Patient | Age, y | Gender a | Groups b | Frequency of Bedwetting per Night, No | Previous Treatment for Bedwetting | PSQ Score |
|---|---|---|---|---|---|---|
| 1 | 7.34 | M | 1 | 7 | Medicine | 0.24 |
| 2 | 12.66 | M | 1 | 7 | Alarm | 0.10 |
| 3 | 8.56 | M | 1 | 7 | Medicine | 0.62 |
| 4 | 9.08 | M | 2 | 7 | Medicine | 0.57 |
| 5 | 12.28 | M | 2 | 7 | No treatment | 0.36 |
| 6 | 13.28 | F | 2 | 3 | Medicine and Alarm | 0.25 |
| Patient | Group | Frequency of Bedwetting per Week | PSQ Score | CBCT Measurements: Differences T2–T0 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T0 | T1 | T2 | T0 | T1 | T2 | NCV | TPAV | Minimal CS | Minimal DH | Inter-Molar Distance | Palatal Width | Inter-LD Distance | Inter-LF Distance | ||
| 1 | 1 | 7 | 7 | 7 | 0.24 | 0.17 | 0.45 | −54 | −911 | −15.92 | 0.04 | 0.5 | 0.22 | −0.12 | 0.19 |
| 2 | 1 | 7 | 3 | 1 | 0.1 | 0.41 | 0.36 | 2939 | 3766 | 22.85 | 0.32 | 1.15 | 0.35 | 0.65 | 0.12 |
| 3 | 1 | 7 | 7 | 7 | 0.62 | 0.59 | 0.64 | 312 | 7496 | 104.45 | 2.73 | 1.27 | 0.46 | 0.57 | 0.59 |
| 4 | 2 | 7 | 7 | 7 | 0.57 | 0.5 | 0.38 | 519 | −107 | 0.32 | −0.09 | 4.63 | 1.16 | 0.38 | −0.21 |
| 5 | 2 | 7 | 7 | 4 | 0.36 | 0.45 | 0.45 | 456 | −151 | −3.12 | 0.10 | 0.48 | 0.00 | 0.11 | 0.94 |
| 6 | 2 | 3 | 1 | 1 | 0.25 | 0.21 | 0.1 | 2513 | 3670 | 52.46 | 1.77 | 3.35 | 0.62 | 0.2 | 0.44 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, X.; Cornelis, M.A.; Kamperis, K.; Cattaneo, P.M. Association between Rapid Maxillary Expansion and Nocturnal Enuresis in Children: A Pilot Study for a Randomized Controlled Clinical Trial. Appl. Sci. 2020, 10, 9025. https://doi.org/10.3390/app10249025
Niu X, Cornelis MA, Kamperis K, Cattaneo PM. Association between Rapid Maxillary Expansion and Nocturnal Enuresis in Children: A Pilot Study for a Randomized Controlled Clinical Trial. Applied Sciences. 2020; 10(24):9025. https://doi.org/10.3390/app10249025
Chicago/Turabian StyleNiu, Xiaowen, Marie A. Cornelis, Konstantinos Kamperis, and Paolo M. Cattaneo. 2020. "Association between Rapid Maxillary Expansion and Nocturnal Enuresis in Children: A Pilot Study for a Randomized Controlled Clinical Trial" Applied Sciences 10, no. 24: 9025. https://doi.org/10.3390/app10249025
APA StyleNiu, X., Cornelis, M. A., Kamperis, K., & Cattaneo, P. M. (2020). Association between Rapid Maxillary Expansion and Nocturnal Enuresis in Children: A Pilot Study for a Randomized Controlled Clinical Trial. Applied Sciences, 10(24), 9025. https://doi.org/10.3390/app10249025





