Antimicrobial Air Filter Coating with Plant Extracts Against Airborne Microbes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Antimicrobial Activity of Plant Extracts
2.3. Preparation of Plant Extract-Coated Filters and the Antimicrobial Test
2.4. Analytical Methods
3. Results and Discussion
3.1. Comparison of the Antimicrobial Activities of the Three Plant Extracts
3.2. Characteristics of Plant Extract-Coated Filters
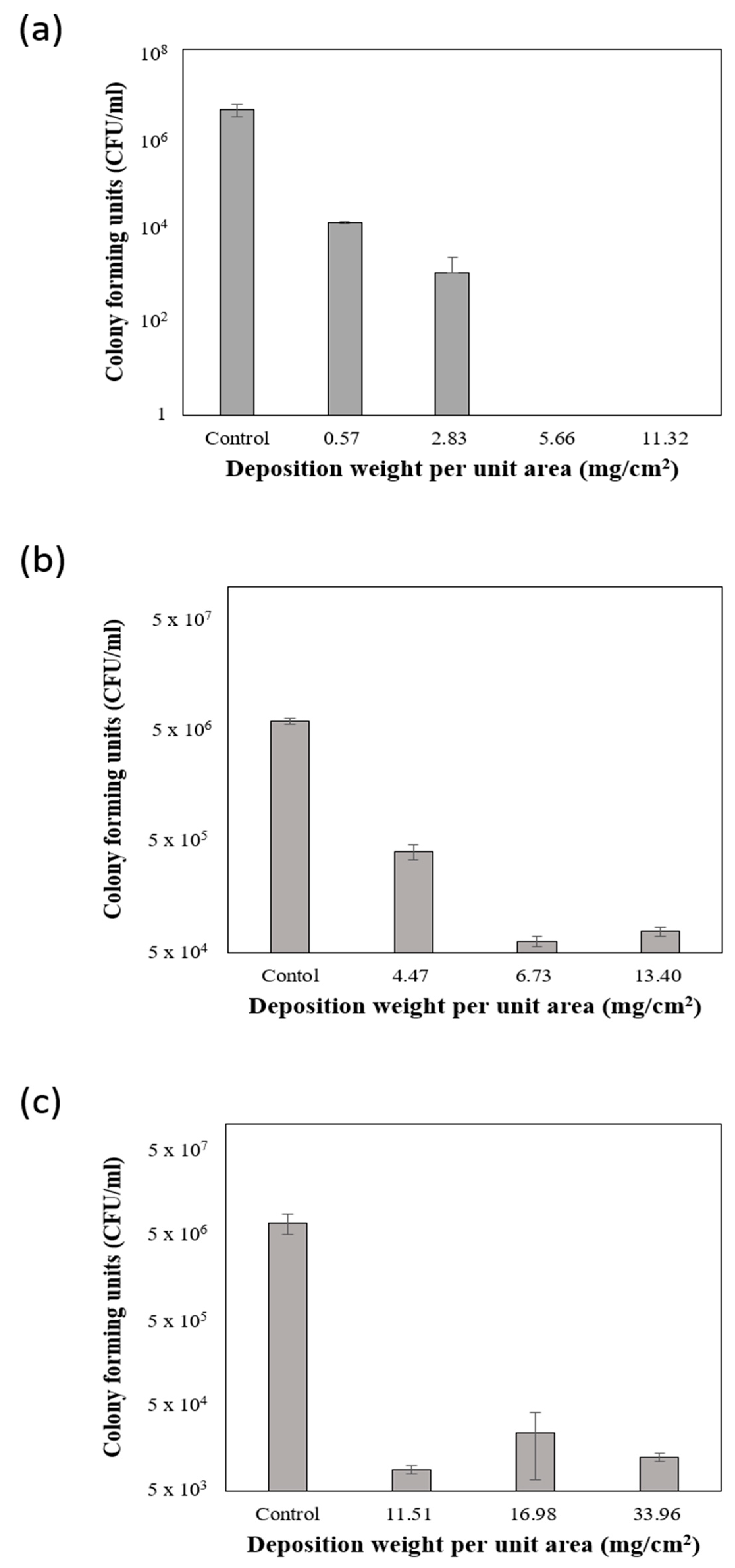
3.3. Inactivation of Microorganisms on Plant Extract-Coated Air Filters
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hwang, G.B.; Heo, K.J.; Yun, J.H.; Lee, J.E.; Lee, H.J.; Nho, C.W.; Bae, G.-N.; Jung, J.H. Antimicrobial air filters using natural Euscaphis japonica nanoparticles. PLoS ONE 2015, 10, 0126481. [Google Scholar] [CrossRef] [PubMed]
- Douwes, J.; Thorne, P.; Pearce, N.; Heederik, D. Bioaerosol health effects and exposure assessment: Progress and prospects. Ann. Occup. Hyg. 2003, 47, 187–200. [Google Scholar] [PubMed]
- Kalogerakis, N.; Paschali, D.; Lekaditis, V.; Pantidou, A.; Eleftheriadis, K.; Lazaridis, M. Indoor air quality—Bioaerosol measurements in domestic and office premises. J. Aerosol Sci. 2005, 36, 751–761. [Google Scholar] [CrossRef]
- Sim, K.M.; Kim, K.H.; Hwang, G.B.; Seo, S.; Bae, G.N.; Jung, J.H. Development and evaluation of antimicrobial activated carbon fiber filters using Sophora flavescens nanoparticles. Sci. Total Environ. 2014, 493, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Dales, R.E.; Zwanenburg, H.; Burnett, R.; Franklin, C.A. Respiratory health effects of home dampness and molds among Canadian children. Am. J. Epidemiol. 1991, 134, 196–203. [Google Scholar] [CrossRef]
- Siersted, H.C.; Gravesen, S. Extrinsic allergic alveolitis after exposure to the yeast Rhodotorula rubra. Allergy 1993, 48, 298–299. [Google Scholar] [CrossRef]
- Beaumont, F. Clinical manifestations of pulmonary Aspergillus infections. Mycoses 1988, 31, 15. [Google Scholar]
- Lin, C.Y.; Li, C.S. Control effectiveness of ultraviolet germicidal irradiation on bioaerosols. Aerosol Sci. Technol. 2002, 36, 474–478. [Google Scholar] [CrossRef]
- Chen, F.; Yang, X.; Mak, H.K.; Chan, D.W. Photocatalytic oxidation for antimicrobial control in built environment: A brief literature overview. Build. Environ. 2010, 45, 1747–1754. [Google Scholar] [CrossRef]
- Escombe, A.R.; Moore, D.A.; Gilman, R.H.; Navincopa, M.; Ticona, E.; Mitchell, B.; Noakes, C.; Martínez, C.; Sheen, P.; Ramirez, R.; et al. Upper-room ultraviolet light and negative air ionization to prevent tuberculosis transmission. PLoS Med. 2009, 6. [Google Scholar] [CrossRef]
- Jung, J.H.; Lee, J.E.; Lee, C.H.; Kim, S.S.; Lee, B.U. Treatment of fungal bioaerosols by a high-temperature, short-time process in a continuous-flow system. Appl. Environ. Microbiol. 2009, 75, 2742–2749. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.L.; Lee, M.G.; Tai, J.H. Controlling indoor bioaerosols using a hybrid system of ozone and catalysts. Aerosol Air Qual. Res. 2012, 12, 73–82. [Google Scholar] [CrossRef]
- Li, C.S.; Wen, Y.M. Control effectiveness of electrostatic precipitation on airborne microorganisms. Aerosol Sci. Technol. 2003, 37, 933–938. [Google Scholar] [CrossRef]
- Han, B.; Kang, J.S.; Kim, H.J.; Woo, C.G.; Kim, Y.J. Investigation of antimicrobial activity of grapefruit seed extract and its application to air filters with comparison to propolis and shiitake. Aerosol Air Qual. Res. 2015, 15, 1035–1044. [Google Scholar] [CrossRef]
- Jin, W.J.; Jeon, H.J.; Kim, J.H.; Youk, J.H. A study on the preparation of poly (vinyl alcohol) nanofibers containing silver nanoparticles. Synth. Met. 2007, 157, 454–459. [Google Scholar] [CrossRef]
- Kang, S.; Pinault, M.; Pfefferle, L.D.; Elimelech, M. Single-walled carbon nanotubes exhibit strong antimicrobial activity. Langmuir 2007, 23, 8670–8673. [Google Scholar] [CrossRef]
- Lee, J.H.; Wu, C.Y.; Lee, C.N.; Anwar, D.; Wysocki, K.M.; Lundgren, D.A.; Farrah, S.; Wander, J.; Heimbuch, B.K. Assessment of iodine-treated filter media for removal and inactivation of MS2 bacteriophage aerosols. J. Appl. Microbiol. 2009, 107, 1912–1923. [Google Scholar] [CrossRef]
- Li, Y.; Leung, P.; Yao, L.; Song, Q.W.; Newton, E. Antimicrobial effect of surgical masks coated with nanoparticles. J. Hosp. Infect. 2006, 62, 58–63. [Google Scholar] [CrossRef]
- Ramyadevi, J.; Jeyasubramanian, K.; Marikani, A.; Rajakumar, G.; Rahuman, A.A. Synthesis and antimicrobial activity of copper nanoparticles. Mat. Lett. 2012, 71, 114–116. [Google Scholar] [CrossRef]
- Moore, M.N. Do nanoparticles present ecotoxicological risks for the health of the aquatic environment? Environ. Int. 2006, 32, 967–976. [Google Scholar] [CrossRef]
- Karlsson, H.L.; Cronholm, P.; Gustafsson, J.; Moller, L. Copper oxide nanoparticles are highly toxic: A comparison between metal oxide nanoparticles and carbon nanotubes. Chem. Res. Toxicol. 2008, 21, 1726–1732. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Schluesener, H.J. Nanosilver: A nanoproduct in medical application. Toxicol. Lett. 2008, 176, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Pulskamp, K.; Diabaté, S.; Krug, H.F. Carbon nanotubes show no sign of acute toxicity but induce intracellular reactive oxygen species in dependence on contaminants. Toxicol. Lett. 2007, 168, 58–74. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.H.; Jung, J.H.; Kim, S.S.; Yoon, J.U.; Park, J.D.; Choi, B.S.; Chung, Y.H.; Kwon, I.H.; Jeong, J.; Han, B.S. Twenty-eight-day inhalation toxicity study of silver nanoparticles in Sprague-Dawley rats. Inhal. Toxicol. 2007, 19, 857–871. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.H.; Hwang, G.B.; Park, S.Y.; Lee, J.E.; Nho, C.W.; Lee, B.U.; Bae, G.N. Antimicrobial air filtration using airborne Sophora flavescens natural-product nanoparticles. Aerosol Sci. Technol. 2011, 45, 1510–1518. [Google Scholar] [CrossRef][Green Version]
- Carson, C.F.; Hammer, K.A.; Riley, T.V. Melaleuca alternifolia (tea tree) oil: A review of antimicrobial and other medicinal properties. Clin. Microbiol. Rev. 2006, 19, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Rios, J.L.; Recio, M.C. Medicinal plants and antimicrobial activity. J. Ethnopharmacol. 2005, 100, 80–84. [Google Scholar] [CrossRef]
- Lee, C.J.; Chen, L.W.; Chen, L.G.; Chang, T.L.; Huang, C.W.; Huang, M.C.; Wang, C.C. Correlations of the components of tea tree oil with its antibacterial effects and skin irritation. J. Food Drug Anal. 2013, 21, 169–176. [Google Scholar] [CrossRef]
- Aloui, H.; Khwaldia, K.; Sánchez-González, L.; Muneret, L.; Jeandel, C.; Hamdi, M.; Desobry, S. Alginate coatings containing grapefruit essential oil or grapefruit seed extract for grapes preservation. Int. J. Food Sci. Technol. 2014, 49, 952–959. [Google Scholar] [CrossRef]
- Saito, M.; Hosoyama, H.; Ariga, T.; Kataoka, S.; Yamaji, N. Antiulcer activity of grape seed extract and procyanidins. J. Agric. Food Chem. 1998, 46, 1460–1464. [Google Scholar] [CrossRef]
- Schlüter, B.; Pflegel, P.; Lindequist, U.; Jülich, W.D. Aspects of the antimicrobial efficacy of grapefruit seed extract and its relation to preservative substances contained. Die Pharm. 1999, 54, 452–456. [Google Scholar]
- Hearst, R.; Nelson, D.; McCollum, G.; Millar, B.C.; Maeda, Y.; Goldsmith, C.E.; Rooney, P.J.; Loughrey, A.; Rao, J.R.; Moore, J.E. An examination of antibacterial and antifungal properties of constituents of Shiitake (Lentinula edodes) and Oyster (Pleurotus ostreatus) mushrooms. Complementary Ther. Clin. Pract. 2009, 15, 5–7. [Google Scholar] [CrossRef] [PubMed]
- May, J.; Chan, C.H.; King, A.; Williams, L.; French, G.L. Time–kill studies of tea tree oils on clinical isolates. J. Antimicrob. Chemother. 2000, 45, 639–643. [Google Scholar] [CrossRef]
- Moreno, S.; Scheyer, T.; Romano, C.S.; Vojnov, A.A. Antioxidant and antimicrobial activities of rosemary extracts linked to their polyphenol composition. Free Radic. Res. 2006, 40, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Ankri, S.; Mirelman, D. Antimicrobial properties of allicin from garlic. Microb. Infect. 1999, 1, 125–129. [Google Scholar] [CrossRef]
- Li, T.C.; Ambu, S.; Mohandas, K.; Wah, M.J.; Sulaiman, L.H.; Murgaivah, M. Bacterial constituents of indoor air in a high throughput building in the tropics. Trop. Biomed. 2014, 31, 540–556. [Google Scholar]
- US Environmental Protection Agency. Air Sanitizers—Efficacy Data Recommendations; Test Guideline No. OCSPP 810.2500-Air Sanitizers-2013-03-12 [EPA 730-C-11-003]; US Environmental Protection Agency: Washington, DC, USA, 2012.
- KACA (Korea Air Cleaning Association). Antibiotic Filter; SPS-KACA009-139; Korea Air Cleaning Association: Seoul, Korea, 2002. [Google Scholar]
- Korean Standards Association. Test Method for Antibacterial Activity of Textile Materials; KS K, 0693:2016; Korean Standards Association: Seoul, Korea, 2016. [Google Scholar]
- Silva, A.C.R.D.; Lopes, P.M.; Azevedo, M.M.B.D.; Costa, D.C.M.; Alviano, C.S.; Alviano, D.S. Biological activities of a-pinene and β-pinene enantiomers. Molecules 2012, 17, 6305–6316. [Google Scholar] [CrossRef]
- Andrade, B.F.M.T.; Barbosa, L.N.; Probst, I.S.; Fernandes Júnior, A. Antimicrobial activity of essential oils. J. Essent. Oil Res. 2014, 26, 34–40. [Google Scholar] [CrossRef]
- Leite, A.M.; Lima, E.D.O.; Souza, E.L.D.; Diniz, M.D.F.F.M.; Trajano, V.N.; Medeiros, I.A.D. Inhibitory effect of beta-pinene, alpha-pinene and eugenol on the growth of potential infectious endocarditis causing Gram-positive bacteria. Rev. Bras. Cienc. Farm. 2007, 43, 121–126. [Google Scholar] [CrossRef]
- İşcan, G.; Kırımer, N.; Demirci, F.; Demirci, B.; Noma, Y.; Başer, K.H.C. Biotransformation of ()-(R)-α-Phellandrene: Antimicrobial Activity of Its Major Metabolite. Chem. Biodivers. 2012, 9, 1525–1532. [Google Scholar] [CrossRef]
- Carson, C.F.; Riley, T.V. Antimicrobial activity of the major components of the essential oil of Melaleuca alternifolia. J. Appl. Bacterol. 1995, 78, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Kiskó, G.; Roller, S. Carvacrol and p-cymene inactivate Escherichia coli O157: H7 in apple juice. BMC Microbiol. 2005, 5, 36. [Google Scholar] [CrossRef] [PubMed]
- Zahi, M.R.; Liang, H.; Yuan, Q. Improving the antimicrobial activity of D-limonene using a novel organogel-based nanoemulsion. Food Control. 2015, 50, 554–559. [Google Scholar] [CrossRef]
- Safaei-Ghomi, J.; Ahd, A.A. Antimicrobial and antifungal properties of the essential oil and methanol extracts of Eucalyptus largiflorens and Eucalyptus intertexta. Pharmacogn. Mag. 2010, 6, 172. [Google Scholar] [CrossRef] [PubMed]
- Shafaghat, A. Comparison of the antimicrobial activity and chemical constituents of the essential oil and hexanic extract from Chaerophyllum macropodum. J. Essent. Oil Bear. Plants 2017, 20, 835–843. [Google Scholar] [CrossRef]
- Giweli, A.; Džamić, A.M.; Soković, M.; Ristić, M.S.; Marin, P.D. Antimicrobial and antioxidant activities of essential oils of Satureja thymbra growing wild in Libya. Molecules 2012, 17, 4836–4850. [Google Scholar] [CrossRef]
- Lee, D.H.; Jung, J.H.; Lee, B.U. Effect of treatment with a natural extract of Mukdenia Rossii (Oliv) Koidz and unipolar ion emission on the antibacterial performance of air filters. Aerosol Air Qual. Res. 2013, 13, 771–776. [Google Scholar] [CrossRef]
- Park, S.N.; Lim, Y.K.; Freire, M.O.; Cho, E.; Jin, D.; Kook, J.K. Antimicrobial effect of linalool and α-terpineol against periodontopathic and cariogenic bacteria. Anaerobe 2012, 18, 369–372. [Google Scholar] [CrossRef]
- Alma, M.H.; Nitz, S.; Kollmannsberger, H.; Digrak, M.; Efe, F.T.; Yilmaz, N. Chemical composition and antimicrobial activity of the essential oils from the gum of Turkish pistachio (Pistacia vera L.). J. Agric. Food Chem. 2004, 52, 3911–3914. [Google Scholar] [CrossRef]
- Oyedemi, S.O.; Okoh, A.I.; Mabinya, L.V.; Pirochenva, G.; Afolayan, A.J. The proposed mechanism of bactericidal action of eugenol, α-terpineol and g-terpinene against Listeria monocytogenes, Streptococcus pyogenes, Proteus vulgaris and Escherichia coli. Afr. J. Biotechnol. 2009, 8, 1280–1286. [Google Scholar]
- George, V.; Mathew, J.; Sabulal, B.; Dan, M.; Shiburaj, S. Chemical composition and antimicrobial activity of essential oil from the rhizomes of Amomum cannicarpum. Fitoterapia 2006, 77, 392–394. [Google Scholar] [CrossRef] [PubMed]
- Stojanović, G.; Palić, I.; Irsić-Janković, J. Composition and antimicrobial activity of the essential oil of Micromeria cristata and Micromeria juliana. Flavour Fragr. J. 2006, 21, 77–79. [Google Scholar] [CrossRef]
- Carson, C.F.; Mee, B.J.; Riley, T.V. Mechanisms of action of Melaleuca alternifolia (tea tree) oil on Staphylococcus aureus determined by time-kill, lysis, leakage, and salt tolerance assays and electron microscopy. Antimicrob. Agents Chemother. 2002, 46, 1914–1920. [Google Scholar] [CrossRef] [PubMed]
- Kumar Tyagi, A.; Bukvicki, D.; Gottardi, D.; Veljic, M.; Guerzoni, M.E.; Malik, A.; Marin, P.D. Antimicrobial potential and chemical characterization of Serbian liverwort (Porella arboris-vitae): SEM and TEM observations. J. Evid. Based Complementary Altern. Med. 2013, 2013, 382927. [Google Scholar] [CrossRef] [PubMed]
- Mulyaningsih, S.; Sporer, F.; Reichling, J.; Wink, M. Antimicrobial activity of essential oils from Eucalyptus and of selected components against multidrug-resistant bacterial pathogens. Pharm. Biol. 2011, 49, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.L.; Wang, E.I.C.; Tseng, Y.H.; Liao, P.C.; Lin, C.N.; Chou, J.C.; Su, Y.C. Composition and antimicrobial activity of the leaf and twig oils of Litsea mushaensis and L. linii from Taiwan. Nat. Prod. Commun. 2010, 5, 1823–1828. [Google Scholar] [CrossRef]
- Tan, M.; Zhou, L.; Huang, Y.; Wang, Y.; Hao, X.; Wang, J. Antimicrobial activity of globulol isolated from the fruits of Eucalyptus globulus Labill. Nat. Prod. Res. 2008, 22, 569–575. [Google Scholar] [CrossRef]
- Cunico, M.M.; Lopes, A.R.; Côcco, L.C.; Yamamoto, C.I.; Plocharski, R.C.; Miguel, M.D.; Miguel, O.G. Phytochemical and antibacterial evaluation of essential oils from Ottonia mariana Miq. (Piperaceae). J. Braz. Chem. Soc. 2007, 18, 184–188. [Google Scholar] [CrossRef]
- Ertürk, Ö.; Çíl, E.; Yoloğlu, N.; Yavuz, C. An in vitro study on antimicrobial and antioxidant activity of propolis from rize province of Turkey. Mellifera 2016, 16, 4–18. [Google Scholar]



| E. coli | M. luteus | |
|---|---|---|
| Tea-tree oil | 1.62 | 2.06 |
| Rosemary | 224 | 181 |
| Garlic | 407 | 419 |
| Plant Extract | Phytochemical | Formula | MW 1 | Ref 2 | Amount (wt%) |
|---|---|---|---|---|---|
| Tea-tree oil | α-Pinene | C10H16 | 136 | [40] | 1.93 |
| Sabinene | C10H16 | 136 | [41] | 0.21 | |
| β-Pinene | C10H16 | 136 | [42] | 0.32 | |
| α-Phellandrene | C10H16 | 136 | [43] | 0.17 | |
| α-Terpinene | C10H16 | 136 | [44] | 4.99 | |
| Cymene | C10H14 | 134 | [45] | 9.79 | |
| Dipentene | C10H16 | 136 | [46] | 15.00 | |
| Eucalyptol | C10H18O | 154 | [47] | 3.11 | |
| Trans-β-Ocimene | C10H16 | 136 | [48] | 1.18 | |
| γ-Terpinene | C10H16 | 136 | [49] | 24.30 | |
| Bicyclo[3.1.0]hexan-2-ol, 2-methyl-5-(1-methylethyl)-, (1α, 2α, 5α)- | C10H18O | 154 | - | 0.14 | |
| 2-Furanmethanol, 5-ethenyltertrahydro- α, α, 5-trimethyl-, cis- | C10H18O2 | 170 | - | 0.49 | |
| Terpinolene | C10H16 | 136 | [50] | 3.32 | |
| Linalool | C10H18O | 154 | [51] | 0.09 | |
| (-)-trans-Pinocarveol | C10H16O | 152 | [52] | 0.43 | |
| α-Terpineol | C10H18O | 154 | [53] | 0.16 | |
| β-Terpineol | C10H18O | 154 | [54] | 0.08 | |
| Isoborneol | C10H18O | 154 | [55] | 0.12 | |
| (-)-Terpinen-4-ol | C10H18O | 154 | [56] | 24.86 | |
| L-α-Terpineol | C10H18O | 154 | - | 4.68 | |
| Cyclohexene, 4-ethenyl-4-methyl-3-(1-methylethenyl)-1-(1-methylethyl)-, (3R-trans)- | C15H24 | 204 | - | 0.35 | |
| 7-Ethylnonan-4-one | C11H22O | 170 | - | 0.34 | |
| 2,2-Dimethyl-4,5-di(1-propenyl)-1,3-dioxolane | C11H18O2 | 182 | - | 0.06 | |
| (-)-α-gurjunene | C15H24 | 204 | [57] | 0.22 | |
| (+)-Aromadendrene | C15H24 | 204 | [58] | 1.84 | |
| β-selinene | C15H24 | 204 | [59] | 0.17 | |
| α-Guaiene | C15H24 | 204 | - | 0.15 | |
| (+)-Ledene | C15H24 | 204 | - | 1.02 | |
| (−)-Globulol | C15H26O | 222 | [60] | 0.38 | |
| Viridiflorol | C15H26O | 222 | [61] | 0.09 | |
| Rosemary | 3,6-Dioxa-2,7-disilaoctane, 2,2,4,7,7-pentamethyl- | C9H24O2Si2 | 220 | - | 15.04 |
| Isobutoxytrimethylsilane | C7H18OSi | 146 | - | 19.35 | |
| [(1-Methyl-1,3-propanediyl)bis(oxy)]bis(trimethylsilane) | C10H26O2Si2 | 234 | - | 17.18 | |
| Trimethyl(1-methylpropoxy)silane | C7H18OSi | 146 | - | 0.42 | |
| Trimethylsilyl-Glycerol | C12H32O3Si3 | 308 | [62] | 31.89 | |
| Butane, 1,2,3-tris(trimethylsiloxy)- | C13H34O3Si3 | 322 | - | 12.18 | |
| Garlic | 3,6-Dioxa-2,7-disilaoctane, 2,2,4,7,7-pentamethyl- | C9H24O2Si2 | 220 | - | 22.99 |
| [(1-Methyl-1,3-propanediyl)bis(oxy)]bis(trimethylsilane) | C10H26O2Si2 | 234 | - | 11.21 | |
| Trimethylsilyl-Glycerol | C12H32O3Si3 | 308 | [62] | 0.62 | |
| Butane, 1,2,3-tris(trimethylsiloxy)- | C13H34O3Si3 | 322 | - | 0.79 | |
| 2,2,4,4,6,6,-hexamethyl-2,4,6-trisila-heptane | C10H28Si3 | 232 | - | 64.14 | |
| 2-O,3-O,5-O,6-O,7-O-Pentakis(trimethylsilyl)-D-glycero-L-manno-heptonic acid 1,4-lactone | C22H52O7Si5 | 568 | - | 0.07 |
| Control | Tea-tree oil | Rosemary | Garlic | |
|---|---|---|---|---|
| Pressure drop (mmAq) | 1.97 ± 0.06 | 2.17 ± 0.17 | 2.13 ± 0.22 | 2.16 ± 0.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Byun, H.R.; Park, S.Y.; Hwang, E.T.; Sang, B.I.; Min, J.; Sung, D.; Choi, W.I.; Kim, S.; Lee, J.H. Antimicrobial Air Filter Coating with Plant Extracts Against Airborne Microbes. Appl. Sci. 2020, 10, 9120. https://doi.org/10.3390/app10249120
Byun HR, Park SY, Hwang ET, Sang BI, Min J, Sung D, Choi WI, Kim S, Lee JH. Antimicrobial Air Filter Coating with Plant Extracts Against Airborne Microbes. Applied Sciences. 2020; 10(24):9120. https://doi.org/10.3390/app10249120
Chicago/Turabian StyleByun, Ha Ram, Seon Young Park, Ee Taek Hwang, Byoung In Sang, Jiho Min, Daekyung Sung, Won Il Choi, Sunghyun Kim, and Jin Hyung Lee. 2020. "Antimicrobial Air Filter Coating with Plant Extracts Against Airborne Microbes" Applied Sciences 10, no. 24: 9120. https://doi.org/10.3390/app10249120
APA StyleByun, H. R., Park, S. Y., Hwang, E. T., Sang, B. I., Min, J., Sung, D., Choi, W. I., Kim, S., & Lee, J. H. (2020). Antimicrobial Air Filter Coating with Plant Extracts Against Airborne Microbes. Applied Sciences, 10(24), 9120. https://doi.org/10.3390/app10249120







