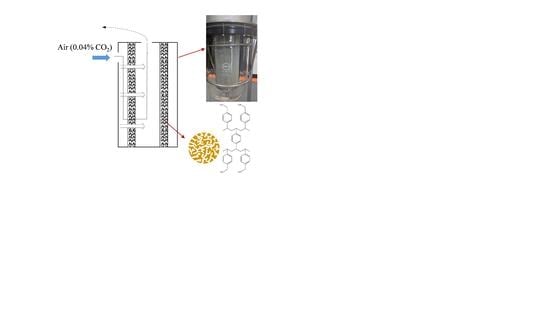
A Radial Flow Contactor for Ambient Air CO2 Capture
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
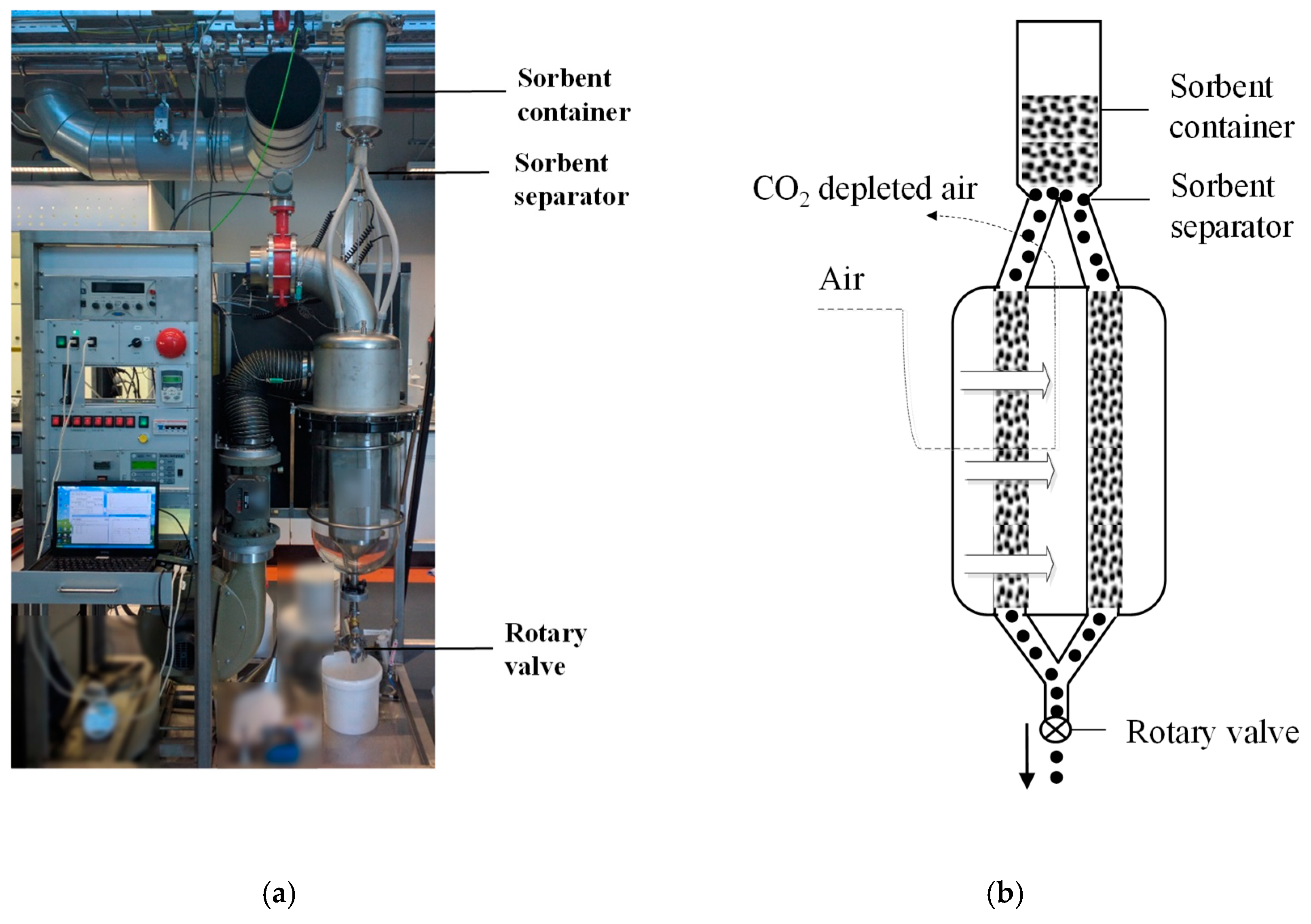
2.2. Radial Flow Reactor Design
3. Results and Discussion
3.1. Pressure Drop
3.2. Performance of the Fixed-Bed RFL
- CO2 adsorption performance at a larger scale (2 kg) in the RFR in comparison with the results obtained on small scale (1 g) in the FB on which the RFR design is based;
- CO2 breakthrough and temperature profiles during a typical CO2 adsorption test;
- the rates of CO2 and water adsorption.
3.3. Checking the Feasibility of a Moving-Bed RFR
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
Selection of the Contacting Method







References
- Lackner, K.S.; Brennan, S.; Matter, J.M.; Park, A.H.A.; Wright, A.; van der Zwaan, B. The urgency of the development of CO2 capture from ambient air. Proc. Natl. Acad. Sci. USA 2012, 109, 13156–13162. [Google Scholar] [CrossRef] [PubMed]
- Goeppert, A.; Czaun, M.; Prakash, G.K.S.; Olah, G.A. Air as the renewable carbon source of the future: An overview of CO2 capture from the atmosphere. Energy Environ. Sci 2012, 5, 7833–7853. [Google Scholar] [CrossRef]
- Graves, C.; Ebbesen, S.D.; Mogensen, M.; Lackner, K.S. Sustainable hydrocarbon fuels by recycling CO2 and H2O with renewable or nuclear energy. Renew. Sust. Energ. Rev 2011, 15, 1–23. [Google Scholar] [CrossRef]
- Martens, J.A.; Bogaerts, A.; De Kimpe, N.; Jacobs, P.A.; Marin, G.B.; Rabaey, K.; Saeys, M.; Verhelst, S. The Chemical Route to a Carbon Dioxide Neutral World. ChemSusChem 2017, 10, 1039–1055. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Pérez, E.S.; Murdock, C.R.; Didas, S.A.; Jones, C.W. Direct Capture of CO2 from Ambient Air. Chem. Rev. 2016, 116, 11840–11876. [Google Scholar] [CrossRef] [PubMed]
- Lackner, K.S.; Ziock, H.-J.; Grimes, P. Carbon capture from air, is it an option? In Proceedings of the 24th Annual Technical Conference on Coal Utilisation and Fuel Systems, Clearwater, FL, USA, 8–11 March 1999. [Google Scholar]
- Baciocchi, R.; Storti, G.; Mazzotti, M. Process design and energy requirements for the capture of carbon dioxide from air. Chem. Eng. Process. Process Intensif. 2006, 45, 1047–1058. [Google Scholar] [CrossRef]
- Zeman, F. Energy and material balance of CO2 capture from ambient air. Environ. Sci. Technol. 2007, 41, 7558–7563. [Google Scholar] [CrossRef]
- Keith, D.W.; Holmes, G.; St. Angelo, D.; Heidel, K. A Process for Capturing CO2 from the Atmosphere. Joule 2018, 2, 1573–1594. [Google Scholar] [CrossRef]
- Tollefson, J. Price of sucking CO2 from air plunges. Nature 2018, 558, 173. [Google Scholar] [CrossRef]
- Belmabkhout, Y.; Serna-Guerrero, R.; Sayari, A. Amine-bearing mesoporous silica for CO2 removal from dry and humid air. Chem. Eng. Sci. 2010, 65, 3695–3698. [Google Scholar] [CrossRef]
- Breault, R.W.; Spenik, J.L.; Shadle, L.J.; Hoffman, J.S.; Gray, M.L.; Panday, R.; Stehle, R.C. Carbon capture test unit design and development using amine-based solid sorbent. Chem. Eng. Res. Des. 2016, 112, 251–262. [Google Scholar] [CrossRef]
- Brilman, D.W.F.; Veneman, R. Capturing atmospheric CO2 using supported amine sorbents. Energy Procedia 2013, 37, 6070–6078. [Google Scholar] [CrossRef]
- Wang, T.; Lackner, K.S.; Wright, A. Moisture Swing Sorbent for Carbon Dioxide Capture from Ambient Air. Environ. Sci. Technol. 2011, 45, 6670–6675. [Google Scholar] [CrossRef]
- Wang, T.; Hou, C.; Ge, K.; Lackner, K.S.; Shi, X.; Liu, J.; Fang, M.; Luo, Z. Spontaneous Cooling Absorption of CO2 by a Polymeric Ionic Liquid for Direct Air Capture. J. Phys. Chem. Lett 2017, 8, 3986–3990. [Google Scholar] [CrossRef]
- Wurzbacher, J.A.; Gebald, C.; Brunner, S.; Steinfeld, A. Heat and mass transfer of temperature–vacuum swing desorption for CO2 capture from air. Chem. Eng. J. 2016, 283, 1329–1338. [Google Scholar] [CrossRef]
- Choi, S.; Drese, J.H.; Eisenberger, P.M.; Jones, C.W. Application of amine-tethered solid sorbents for direct CO2 capture from the ambient air. Environ. Sci. Technol. 2011, 45, 2420–2427. [Google Scholar] [CrossRef]
- Darunte, L.A.; Oetomo, A.D.; Walton, K.S.; Sholl, D.S.; Jones, C.W. Direct Air Capture of CO2 Using Amine Functionalized MIL-101(Cr). ACS Sustain. Chem. Eng. 2016, 4, 5761–5768. [Google Scholar] [CrossRef]
- Elfving, J.; Bajamundi, C.; Kauppinen, J.; Sainio, T. Modelling of equilibrium working capacity of PSA, TSA and TVSA processes for CO2 adsorption under direct air capture conditions. J CO2 Util. 2017, 22, 270–277. [Google Scholar] [CrossRef]
- Gebald, C.; A. Wurzbacher, J.; Borgschulte, A.; Zimmermann, T.; Steinfeld, A. Single-Component and Binary CO2 and H2O Adsorption of Amine-Functionalized Cellulose. Environ. Sci. Technol. 2014, 48, 2497–2504. [Google Scholar] [CrossRef]
- Lackner, K.S.; Grimes, P.; Ziock, H.J. Capturing carbon dioxide from air. Carbon Capture Storage CO2 Manag. Technol. 2014, 364–376. [Google Scholar]
- Mutyala, S.; Jonnalagadda, M.; Mitta, H.; Gundeboyina, R. CO2 capture and adsorption kinetic study of amine-modified MIL-101 (Cr). Chem. Eng. Res. Des. 2019, 143, 241–248. [Google Scholar] [CrossRef]
- Sakwa-Novak, M.A.; Jones, C.W. Steam Induced Structural Changes of a Poly(ethylenimine) Impregnated gamma-Alumina Sorbent for CO2 Extraction from Ambient Air. ACS Appl. Mater. Interfaces 2014, 6, 9245–9255. [Google Scholar] [CrossRef]
- Sehaqui, H.; Gálvez, M.E.; Becatinni, V.; Cheng Ng, Y.; Steinfeld, A.; Zimmermann, T.; Tingaut, P. Fast and reversible direct CO2 capture from air onto all-polymer nanofibrillated cellulose-polyethylenimine foams. Environ. Sci. Technol. 2015, 49. [Google Scholar] [CrossRef]
- Goeppert, A.; Czaun, M.; May, R.B.; Prakash, G.K.S.; Olah, G.A.; Narayanan, S.R. Carbon dioxide capture from the air using a polyamine based regenerable solid adsorbent. J. Am. Chem. Soc. 2011, 133, 20164–20167. [Google Scholar] [CrossRef]
- Lu, W.; Sculley, J.P.; Yuan, D.; Krishna, R.; Zhou, H.-C. Carbon Dioxide Capture from Air Using Amine-Grafted Porous Polymer Networks. J. Phys. Chem. C 2013, 117, 4057–4061. [Google Scholar] [CrossRef]
- McDonald, T.M.; Lee, W.R.; Mason, J.A.; Wiers, B.M.; Hong, C.S.; Long, J.R. Capture of Carbon Dioxide from Air and Flue Gas in the Alkylamine-Appended Metal–Organic Framework mmen-Mg2(dobpdc). J. Am. Chem. Soc. 2012, 134, 7056–7065. [Google Scholar] [CrossRef]
- Sculley, J.P.; Zhou, H.-C. Enhancing Amine-Supported Materials for Ambient Air Capture. Angew. Chem. Int. Ed. 2012, 51, 12660–12661. [Google Scholar] [CrossRef]
- Yu, Q.; Brilman, D.W.F. Design Strategy for CO2 Adsorption from Ambient Air Using a Supported Amine Based Sorbent in a Fixed Bed Reactor. Energy Procedia 2017, 114, 6102–6114. [Google Scholar] [CrossRef]
- Li, J.C.H. Radial-Flow Packed-Bed Reactors. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Hoboken, NJ, USA, 2007. [Google Scholar]
- Kareeri, A.A.; Zughbi, H.D.; Al-Ali, H.H. Simulation of Flow Distribution in Radial Flow Reactors. Ind. Eng. Chem. Res. 2006, 45, 2862–2874. [Google Scholar] [CrossRef]
- Mu, Z.; Wang, J.; Wang, T.; Jin, Y. Optimum design of radial flow moving-bed reactors based on a mathematical hydrodynamic model. Chem. Eng. Process. Process Intensif. 2003, 42, 409–417. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H. Discrete method for design of flow distribution in manifolds. Appl. Therm. Eng. 2015, 89, 927–945. [Google Scholar] [CrossRef]
- Iranshahi, D.; Rahimpour, M.R.; Asgari, A. A novel dynamic radial-flow, spherical-bed reactor concept for naphtha reforming in the presence of catalyst deactivation. Int. J. Hydrogen Energy 2010, 35, 6261–6275. [Google Scholar] [CrossRef]
- Rahimpour, M.R.; Iranshahi, D.; Pourazadi, E.; Paymooni, K. Evaluation of Optimum Design Parameters and Operating Conditions of Axial- and Radial-Flow Tubular Naphtha Reforming Reactors, Using the Differential Evolution Method, Considering Catalyst Deactivation. Energy Fuels 2011, 25, 762–772. [Google Scholar] [CrossRef]
- Hamedi, N.; Tohidian, T.; Rahimpour, M.R.; Iranshahi, D.; Raeissi, S. Conversion enhancement of heavy reformates into xylenes by optimal design of a novel radial flow packed bed reactor, applying a detailed kinetic model. Chem. Eng. Res. Des. 2015, 95, 317–336. [Google Scholar] [CrossRef]
- Parvasi, P.; Jokar, S.M. A novel reactor configuration for industrial methanol production from the synthesis gas. J. Energy Resour. Technol. Trans. ASME 2019, 141. [Google Scholar] [CrossRef]
- Palma, V.; Palo, E.; Ciambelli, P. Structured catalytic substrates with radial configurations for the intensification of the WGS stage in H2 production. Catal. Today 2009, 147, S107–S112. [Google Scholar] [CrossRef]
- Tian, Q.; He, G.; Wang, Z.; Cai, D.; Chen, L. A Novel Radial Adsorber with Parallel Layered Beds for Prepurification of Large-Scale Air Separation Units. Ind. Eng. Chem. Res. 2015, 54, 7502–7515. [Google Scholar] [CrossRef]
- Kulkarni, A.R.; Sholl, D.S. Analysis of equilibrium-based TSA processes for direct capture of CO2 from Air. Ind. Eng. Chem. Res. 2012, 51, 8631–8645. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, H.; Sun, C.; Drage, T.C.; Snape, C.E. Capturing CO2 from ambient air using a polyethyleneimine-silica adsorbent in fluidized beds. Chem. Eng. Sci. 2014, 116, 306–316. [Google Scholar] [CrossRef]
- Alesi, W.R.; Kitchin, J.R. Evaluation of a primary amine-functionalized ion-exchange resin for CO2 capture. Ind. Eng. Chem. Res. 2012, 51, 6907–6915. [Google Scholar] [CrossRef]
- Yu, Q.; Delgado, J.D.L.P.; Veneman, R.; Brilman, D.W.F. Stability of a Benzyl Amine Based CO2 Capture Adsorbent in View of Regeneration Strategies. Ind. Eng. Chem. Res. 2017, 56, 3259–3269. [Google Scholar] [CrossRef]
- Chaikittisilp, W.; Kim, H.J.; Jones, C.W. Mesoporous alumina-supported amines as potential steam-stable adsorbents for capturing CO2 from simulated flue gas and ambient air. Energy Fuels 2011, 25, 5528–5537. [Google Scholar] [CrossRef]
- Chaikittisilp, W.; Khunsupat, R.; Chen, T.T.; Jones, C.W. Poly(allylamine)–Mesoporous Silica Composite Materials for CO2 Capture from Simulated Flue Gas or Ambient Air. Ind. Eng. Chem. Res. 2011, 50, 14203–14210. [Google Scholar] [CrossRef]
- Veneman, R.; Hilbers, T.; Brilman, D.W.F.; Kersten, S.R.A. CO2 capture in a continuous gas–solid trickle flow reactor. Chem. Eng. J. 2016, 289, 191–202. [Google Scholar] [CrossRef]
- Gebald, C.; Wurzbacher, J.A.; Tingaut, P.; Zimmermann, T.; Steinfeld, A. Amine-Based Nanofibrillated Cellulose As Adsorbent for CO2 Capture from Air. Environ. Sci. Technol. 2011, 45, 9101–9108. [Google Scholar] [CrossRef]
- Moulijn, J.A.; Makkee, M.; van Diepen, A. Chemical Process Technology; John Wiley & Sons: Hoboken, NJ, USA, 2001; ISBN 9780471630098. [Google Scholar]
- Veneman, R.; Frigka, N.; Zhao, W.; Li, Z.; Kersten, S.; Brilman, W. Adsorption of H2O and CO2 on supported amine sorbents. Int. J. Greenh. Gas Control 2015, 41, 268–275. [Google Scholar] [CrossRef]
- Long, W.; Xu, J.; Fan, Y.; Lu, C. Pinning and cavity in two types of cross-flow moving beds. Powder Technol. 2015, 269, 66–74. [Google Scholar] [CrossRef]
- Song, X.; Jin, Y.; Yu, Z. Influence of outward radial gas flow on particle movement in an annular moving bed. Powder Technol. 1994, 79, 247–256. [Google Scholar] [CrossRef]
- Choi, S.; Gray, M.L.; Jones, C.W. Amine-tethered solid adsorbents coupling high adsorption capacity and regenerability for CO2 capture from ambient air. ChemSusChem 2011, 4, 628–635. [Google Scholar] [CrossRef]
- Sinha, A.; Darunte, L.A.; Jones, C.W.; Realff, M.J.; Kawajiri, Y. Systems Design and Economic Analysis of Direct Air Capture of CO2 through Temperature Vacuum Swing Adsorption Using MIL-101(Cr)-PEI-800 and mmen-Mg2(dobpdc) MOF Adsorbents. Ind. Eng. Chem. Res. 2017, 56, 750–764. [Google Scholar] [CrossRef]







| Adsorption | |
|---|---|
| Sorbent mass (kg) | 1.7 |
| Flow rate (m3/h) | 41–313 |
| CO2 concentration (ppm) | 429–464 |
| Relative humidity (%) | 40–65 |
| Temperature (°C) | 19–22 |
| Desorption | |
| Nitrogen purge (m3/h) | 3 |
| Temperature (°C) | 120 |
| Duration (h) | 16 |
| Parameter | Value |
|---|---|
| Sorbent mass in buffer (kg) | 2.4 |
| Air flow rate (m3/h) | 188 |
| CO2 concentration (ppm) | 436 |
| Parameter | Units | Fixed Bed | Radial Flow |
|---|---|---|---|
| Air flow rate | m3/h | 0.072 | 188 |
| Contacting area | m2 | 2.0·× 10−4 | 0.23 |
| Aspect ratio (thickness/area) | m/m2 | 49.7 | 0.065 |
| Temperature | °C | 25 | 20 |
| Mass of the sorbent | g | 1 | 1720 |
| CO2 inlet concentration | ppm | 400 | 452 |
| Stoichiometric time, tsto | min | 43 | 43 |
| Relative humidity, RH | % | 0 | 60 |
| Superficial velocity | m/s | 0.1 | 0.23 |
| Max. working capacity | mol/kg | 0.9 | 1.5 |
| Reference | [41] | [40] | [53] | (This Work) |
|---|---|---|---|---|
| Sorbent | PEI-silica | TRI-PE-MCM-41 | MIL-101(Cr)-PEI800/mmen-Mg2(dobpdc) | Lewatit VP OC 1065 |
| Sorbent type | impregnated | grafted | MOF | Polymeric |
| Capture system | Circ. Fluid Bed | monolith | monolith | RFR |
| ΔP adsorber (Pa) | 1592 | 100 | n.m. | 348-681 (a) |
| tsto (min) | 14 | 101 | 31/88 | 24–43 |
| Selected tads (min) | 15 | 101 | 19/60 | 24–43 |
| Contact energy (GJ/tCO2) | 3.4 (b) | 0.3 (c) | 2.3/2.1 | 0.7–1.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Q.; Brilman, W. A Radial Flow Contactor for Ambient Air CO2 Capture. Appl. Sci. 2020, 10, 1080. https://doi.org/10.3390/app10031080
Yu Q, Brilman W. A Radial Flow Contactor for Ambient Air CO2 Capture. Applied Sciences. 2020; 10(3):1080. https://doi.org/10.3390/app10031080
Chicago/Turabian StyleYu, Qian, and Wim Brilman. 2020. "A Radial Flow Contactor for Ambient Air CO2 Capture" Applied Sciences 10, no. 3: 1080. https://doi.org/10.3390/app10031080
APA StyleYu, Q., & Brilman, W. (2020). A Radial Flow Contactor for Ambient Air CO2 Capture. Applied Sciences, 10(3), 1080. https://doi.org/10.3390/app10031080