Experimental Investigation and Optimal 3D Bioprinting Parameters of SA-Gel Porous Cartilage Scaffold
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of SA-GEL Composites Material
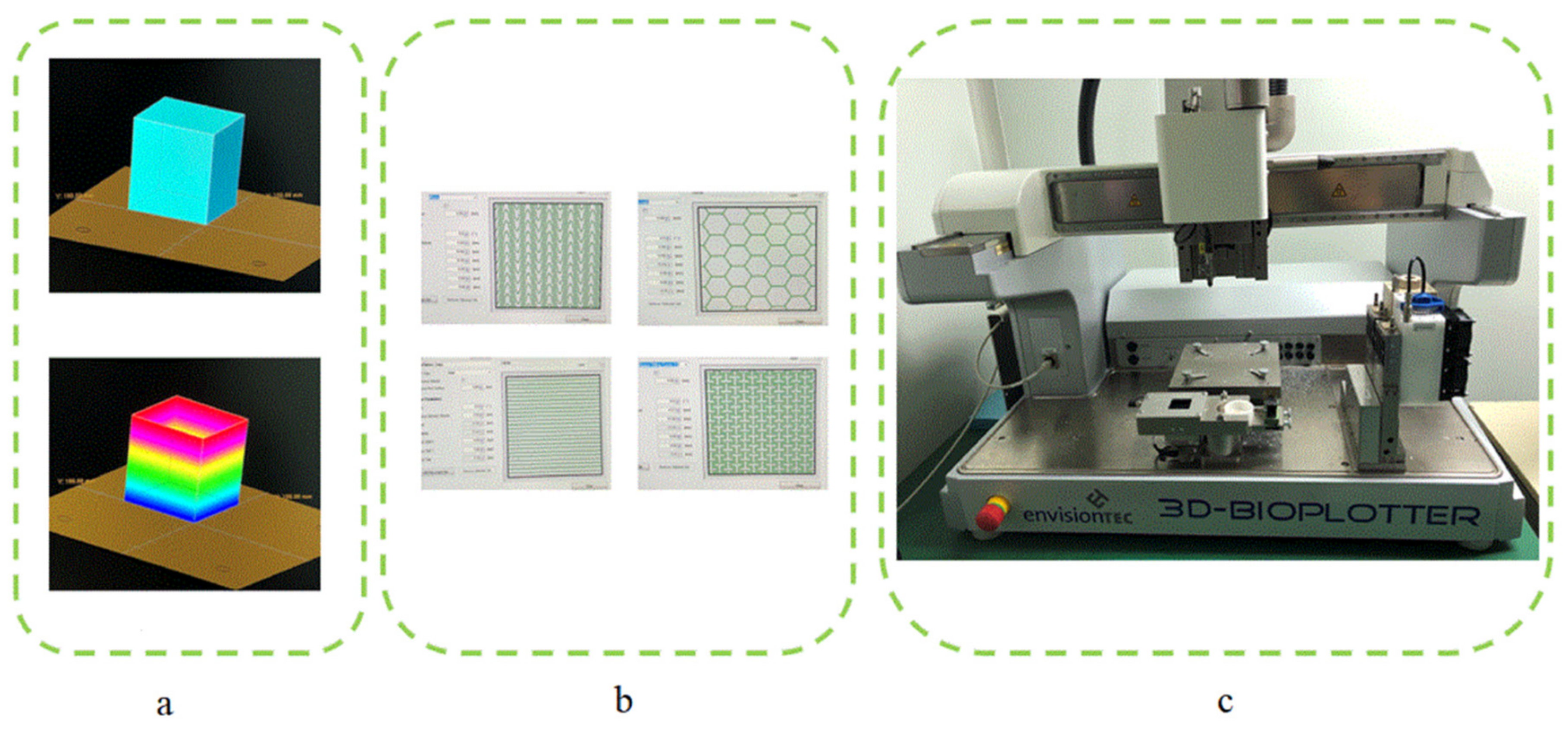
2.2. Design and Fabrication of Scaffolds
2.3. Extrusion Process Theory and Simulation Model of Printing Process
2.3.1. Extrusion Process Theory Model
- The composite solution was in-compressible.
- The flow of compound solution in needle was stable and fully developed.
- The slip between the composite solution and the needle wall was significant.
- The diameter of the barrel was much larger than that of the conical needle, so the pressure loss in the barrel could be neglected.
- The slight loss caused by the entrance effects and accessories could be neglected.
2.3.2. Extrusion Process Simulation Model
3. Results and Discussion
- Suppression bubbles of printing materials—the continuity of the extrusion line is very important for the accuracy and forming effects of the scaffold [22]. In the preparation of SA-GEL composite material, bubbles will be introduced due to mixing and other factors. The bubbles in the material will affect the continuity of extrusion, and the break point will affect the mechanical properties of scaffolds. Accordingly, bubbles in the barrel solution should be cleaned. In this paper, a static suppression method was used to solve the problem—the cylinder filled with composite solution was heated upside down until the bubbles in the material could not be observed by naked eyes.
- Selection of printing platforms—unlike Fused Deposition Molding (FDM) method, the SA-GEL composite materials used in this paper had a large amount of water, so it was indispensable to select appropriate deposition platforms [23]. Hydrophobicity is the main index for selecting deposition platforms. The platform made of hydrophobic materials, such as plastic containers, shrinks the complex gel deposited on the hydrophobic platform to form a globule, which seriously affects the forming of scaffolds. When the gel is deposited on the platform made of hydrophilic materials, problem of excessive deposition will occur. Therefore, after many experiments, PS film was chosen as the deposition platform [24]. Due to the differences between hydrophilic and hydrophobic materials, the deposition and diffusion of hydrogels on PS film are not obvious. In addition, adhesion of the gel on the PS film can overcome surface tension, so that the shrinkage does not occur.
- Calcium chloride assisted stereotyping—in the process of printing, the calcium chloride solution was used to semi-crosslink atomically with the deposited composite SA-GEL. As a result, not only the curing process, but also the shaping effects of scaffolds could be improved [25].
- Selection of the diameter of conical needle—as the diameter of conical needle increased, the required air pressure decreased, and the needle speed needed to be accelerated. At the same time, when the pressure was low, cells in the composite materials received less damage due to extrusion. The acceleration of the needle movement reduced the printing time of the scaffold and improves preparation efficiency. With the increase of conical needle diameter, the porosity of the printing scaffold decreased gradually [26]. Additionally, considering that the diameter of tissue cells varies from more than ten microns to tens of microns, the outlet diameter was 0.4 mm to print the scaffold.
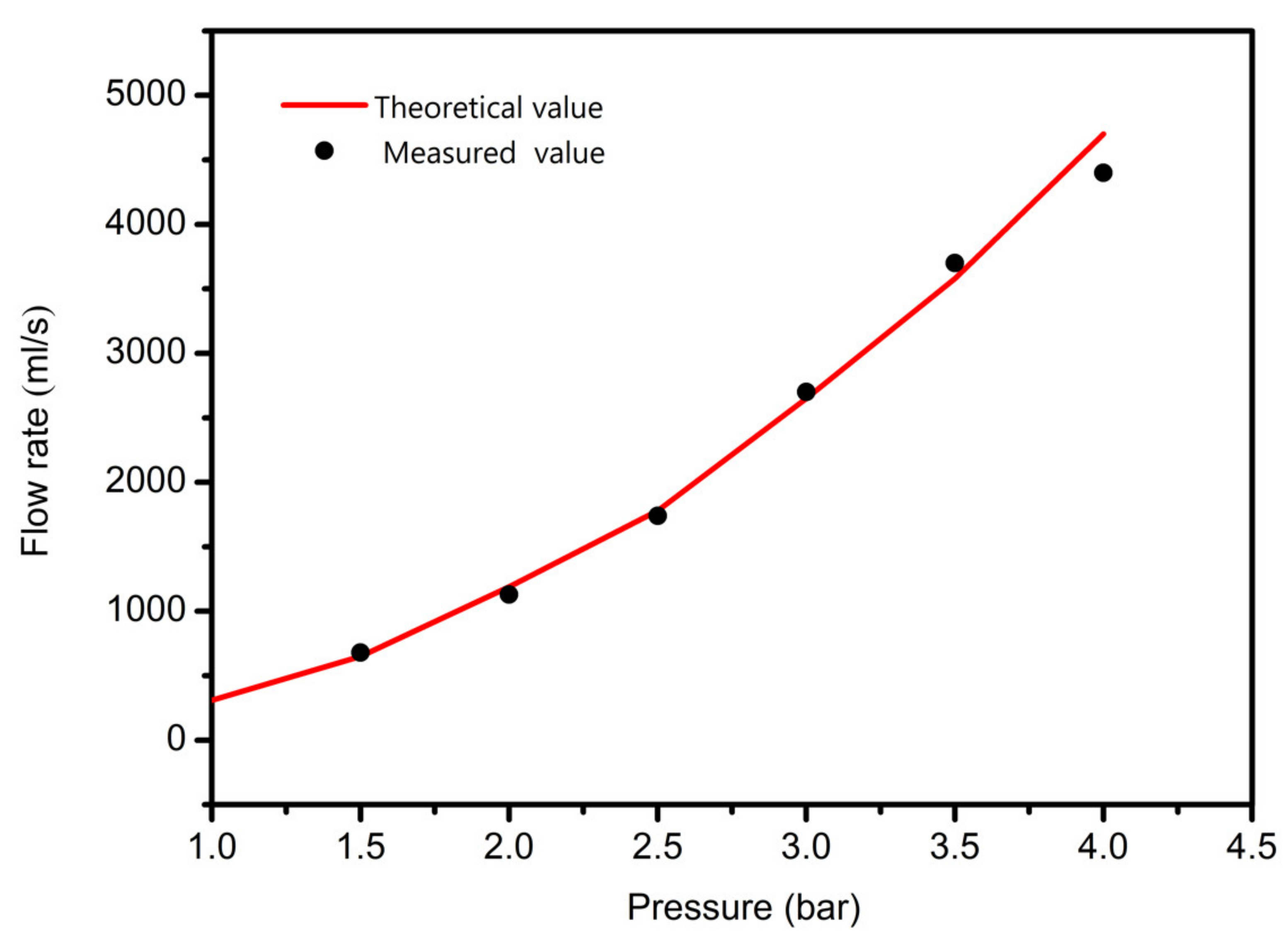
3.1. Pressure Affective Analysis
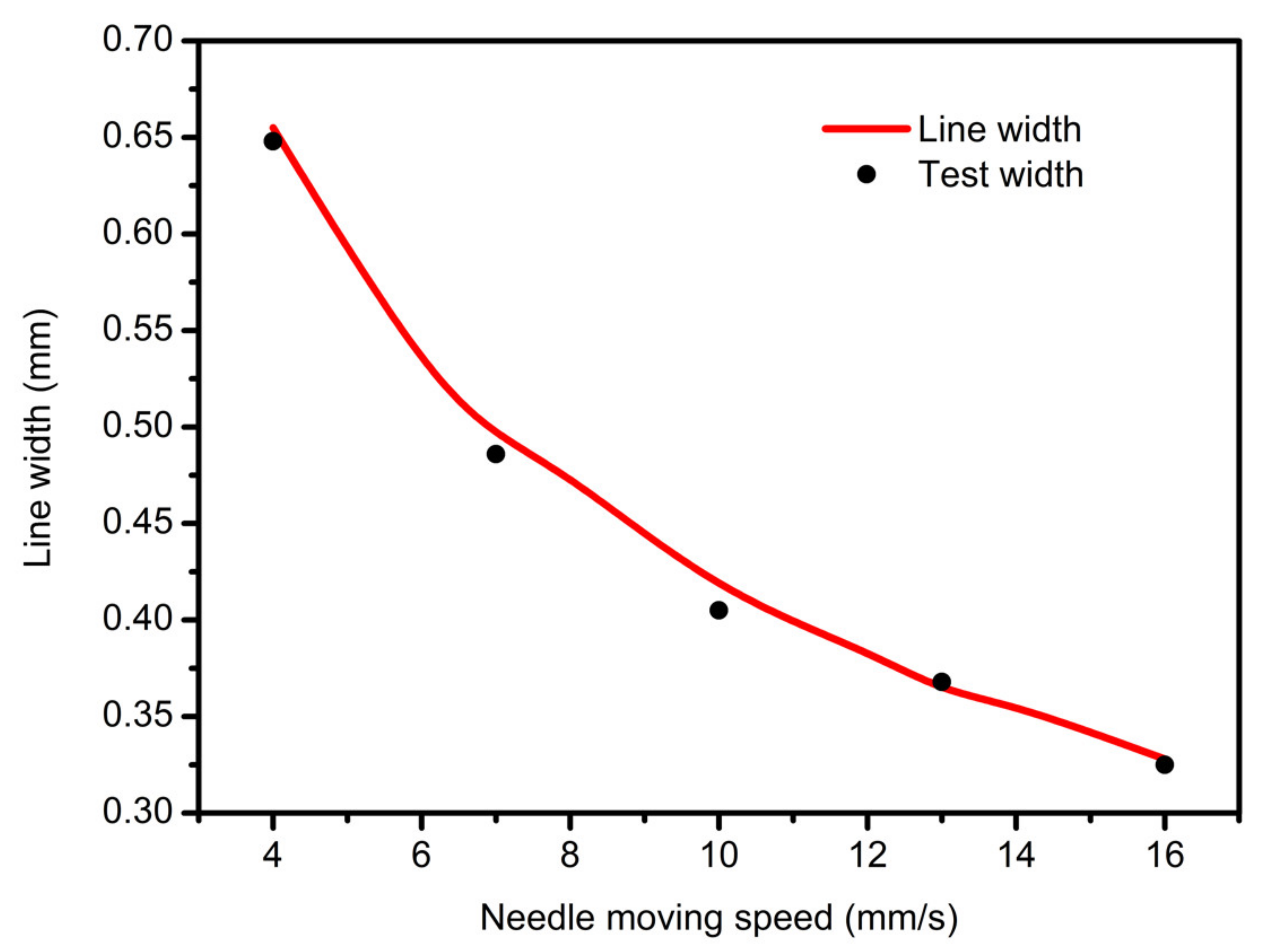
3.2. Needle Moving Speed Affective Analysis
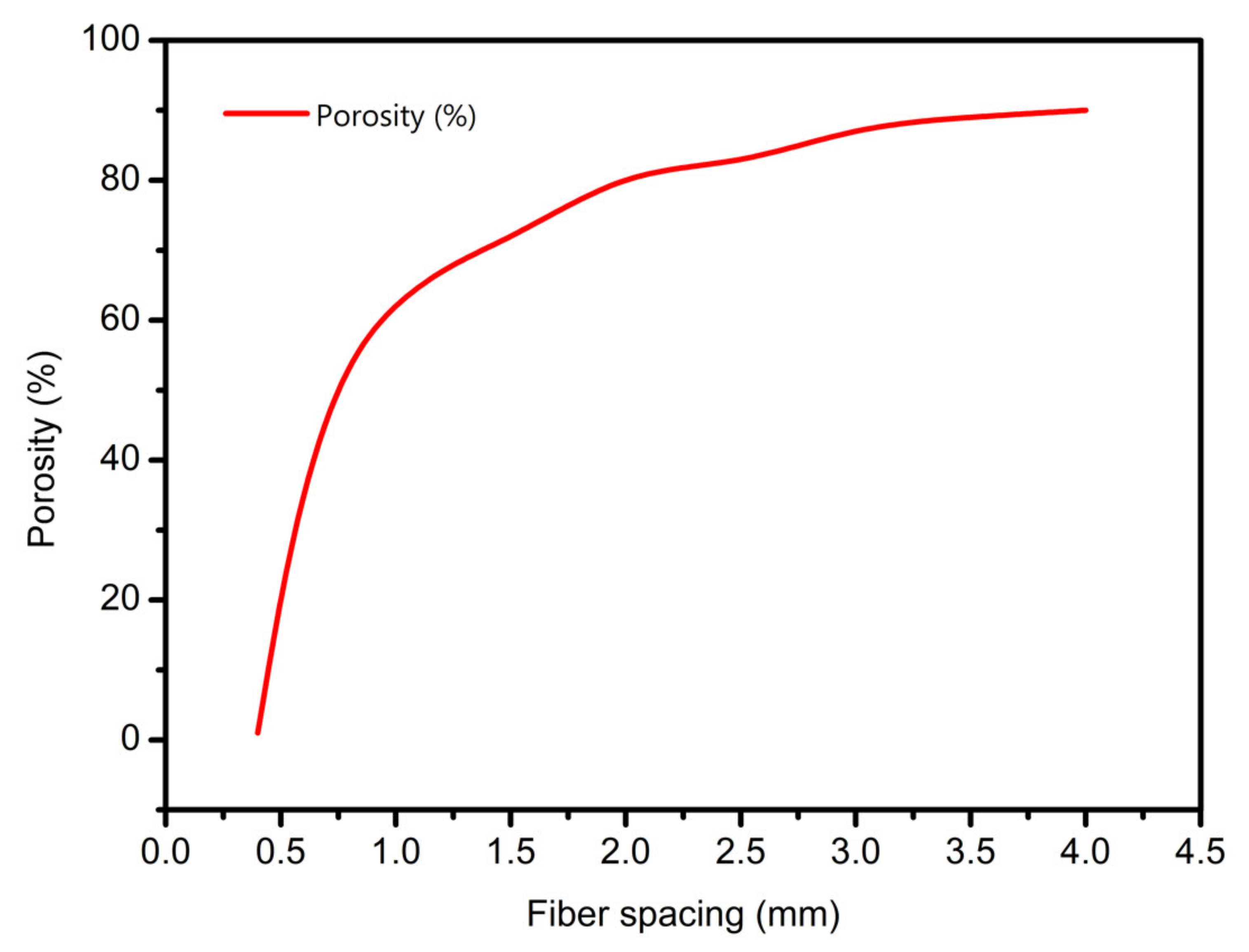
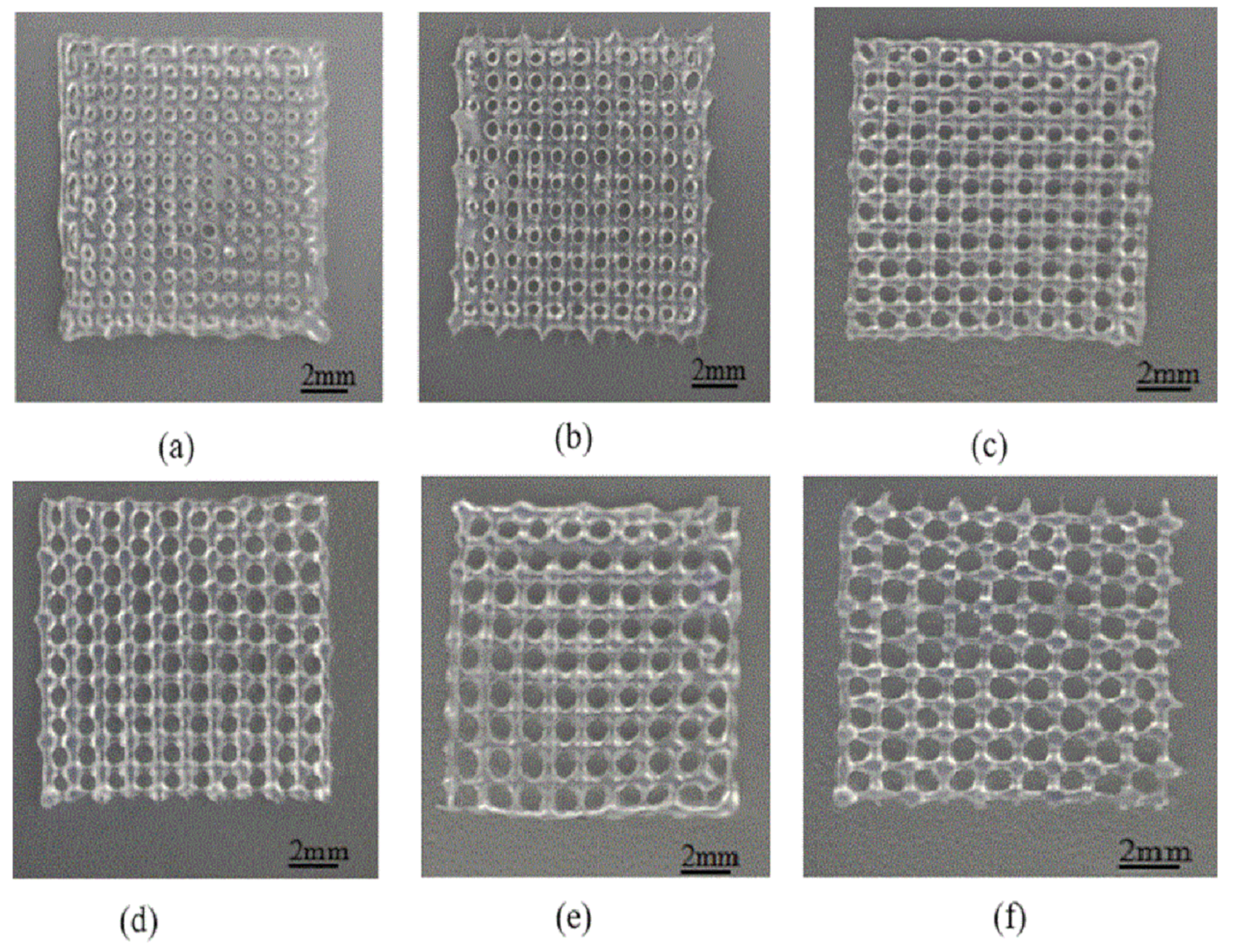
3.3. Scaffolds Porosity Affective Analysis
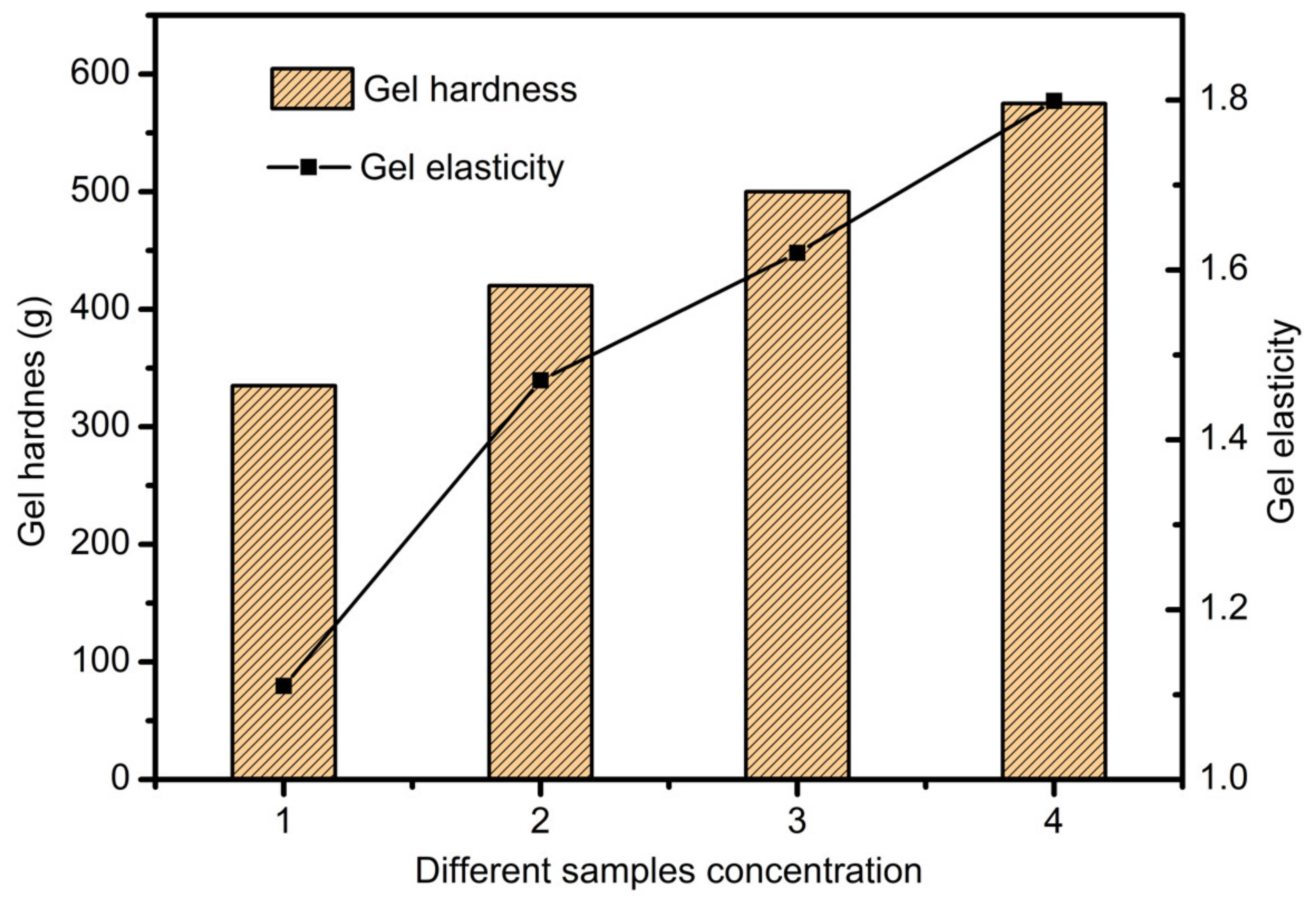
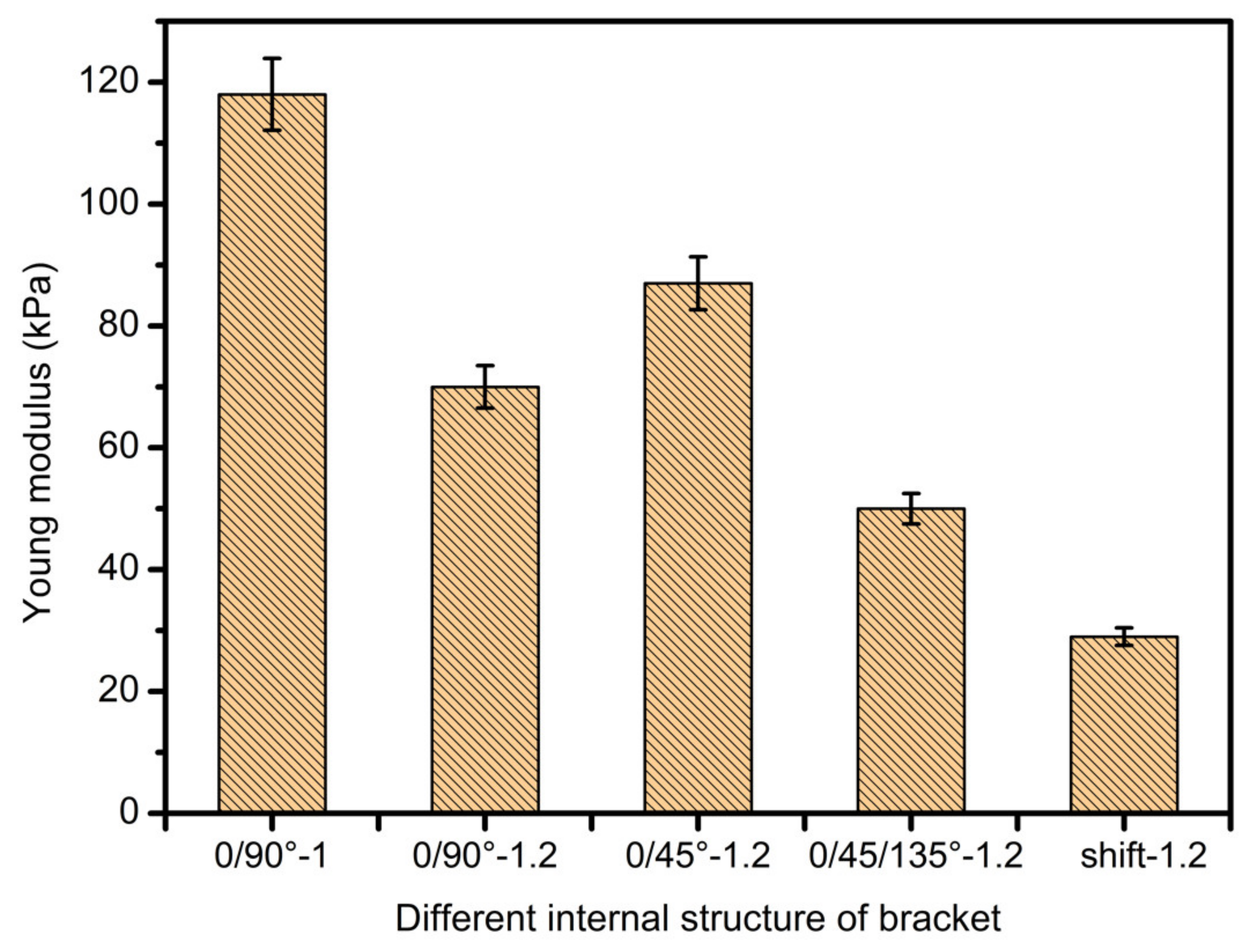
3.4. Scaffolds Mechanical Properties Analysis
3.5. Scaffold Biocompatibility Test
4. Conclusions
- The SA-GEL printing material was prepared. The properties of SA-GEL composite material under various ratios were analyzed from viscosity tests and mechanical performance. The optimum material ratio (sodium alginate:gelatin = 3:8) was determined by comprehensive evaluation.
- Modeling and simulation analysis of gel material extrusion process. First of all, the rheological properties of gel materials were analyzed, and then the cone needle model was simplified. Considering the slip effects, the mathematical model of extrusion flow was established from two facets of shear flow and slip flow. Finally, the printing line width and porosity prediction model was obtained, and the pressure and needle movement were analyzed by fluent simulation software. The variation law of flow rate, line width and porosity of support is affected by velocity and other factors.
- In study of pressure, the flow model and fluent expansion simulation were validated by the measurement of extrusion flow rate and the experiment of extrusion swell. Then the needle speed of 10.7 mm/s was selected from the influence of needle speed on stent size and line width. In the analysis of fiber spacing, it was found that the mechanical strength of scaffolds was not enough when the spacing was too large, and the cells could not grow well when the spacing was too small, and the interval of porosity and mechanical strength was 1–1.4 mm. In the analysis of influence factors about different internal scaffold structure, by adjusting the proper internal structures, mechanical properties of scaffolds can be improved, when the internal structure of the scaffold was 0/45-1.2, the Young’s and dynamic modulus were 86.6 ± 10 KPa and 671.4 ± 31 KPa respectively, which has good mechanical property.
- C5.18 osteoblasts cells were implanted on the scaffold surface and cultured; the results indicate that the cells would survive well.
- Finally, the performance of the printed composite SA-GEL scaffolds was tested and analyzed. The mechanical properties of the bracket show that the mechanical performance of the bracket is mainly affected by the porosity, the contact area between the extruded fiber lines and the relative position of the extruded lines. These experimental results support the original simulation that, under the control of printing parameters such as a temperature of 37 C°, pressure of 1.8 bar, moving speed of 10.7 mm/s and an interval of 1.2 mm, better scaffold printing results can be obtained for newly prepared sodium alginate–gelatin composite bio-printing materials.
Author Contributions
Funding
Conflicts of Interest
References
- Simon, T.M.; Jackson, D.W. Articular cartilage: Injury pathways and treatment options. Sports Med. Arthrosc. Rev. 2018, 26, 31–39. [Google Scholar] [CrossRef]
- Brophy, R.H.; Zeltser, D.; Wright, R.W.; Flanigan, D. Anterior Cruciate Ligament Reconstruction and Concomitant Articular Cartil. Injury: Incidence and Treatment. Arthrosc. J. Arthrosc. Relat. Surg. 2010, 26, 112–120. [Google Scholar] [CrossRef]
- Jones, H.R.; Crawford, D.C. An autologous tissue implant, NeoCart, for treatment of hyaline cartilage injury in the knee. Oper. Tech. Orthop. 2014, 24, 264–270. [Google Scholar] [CrossRef]
- Shi, W.; Sun, M.; Hu, X.; Ren, B.; Cheng, J.; Li, C.; Duan, X.; Fu, X.; Zhang, J.; Chen, H. Structurally and functionally optimized silk-fibroin–gelatin scaffold using 3D printing to repair cartilage injury in vitro and in vivo. Adv. Mater. 2017, 29, 1701089. [Google Scholar] [CrossRef] [PubMed]
- Jung, A.; Makkar, P.; Amirian, J.; Lee, B.-T. A novel hybrid multichannel biphasic calcium phosphate granule-based composite scaffold for cartilage tissue regeneration. J. Biomater. Appl. 2018, 32, 775–787. [Google Scholar] [CrossRef] [PubMed]
- Uz, U.; Gunhan, K.; Vatansever, S.; Kivanc, M.; Yuceturk, A.V. Novel Simple Strategy for Cartilage Tissue Engineering Using Stem Cells and Synthetic Polymer Scaffold. J. Craniofacial Surg. 2019, 30, 940–943. [Google Scholar] [CrossRef] [PubMed]
- Bryant, S.J.; Anseth, K.S. The effects of scaffold thickness on tissue engineered cartilage in photocrosslinked poly (ethylene oxide) hydrogels. Biomaterials 2001, 22, 619–626. [Google Scholar] [CrossRef]
- Filardo, G.; Kon, E.; Roffi, A.; Di Martino, A.; Marcacci, M. Scaffold-based repair for cartilage healing: A systematic review and technical note. Arthrosc. J. Arthrosc. Relat. Surg. 2013, 29, 174–186. [Google Scholar] [CrossRef]
- Nettles, D.L.; Elder, S.H.; Gilbert, J.A. Potential use of chitosan as a cell scaffold material for cartilage tissue engineering. Tissue Eng. 2002, 8, 1009–1016. [Google Scholar] [CrossRef]
- Zhu, W.; Cui, J.; Duan, L.; Chen, J.; Zeng, Y.; Wang, D. Research progress of scaffold materials in cartilage tissue engineering. J. Biomater. Tissue Eng. 2015, 5, 673–679. [Google Scholar] [CrossRef]
- Balakrishnan, B.; Joshi, N.; Jayakrishnan, A.; Banerjee, R. Self-crosslinked oxidized alginate/gelatin hydrogel as injectable, adhesive biomimetic scaffolds for cartilage regeneration. Acta Biomater. 2014, 10, 3650–3663. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Xu, Z.; Liang, Q.; Liu, B.; Li, H.; Wu, Y.; Zhang, Y.; Lin, Z.; Wu, M.; Ruan, C. Direct 3D printing of high strength biohybrid gradient hydrogel scaffolds for efficient repair of osteochondral defect. Adv. Funct. Mater. 2018, 28, 1706644. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, J.; Zhang, Q.; Gao, Y.; Yan, J.; Zhao, X.; Yang, Y.; Kong, D.; Zhao, J.; Shi, Y. Evaluation of bone matrix gelatin/fibrin glue and chitosan/gelatin composite scaffolds for cartilage tissue engineering. Genet. Mol. Res. 2016, 15, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Mase, A.; Takizawa, Y.; Shinkai, M.; Honda, H.; Hata, K.-I.; Ueda, M.; Kobayashi, T. Transglutaminase-mediated gelatin matrices incorporating cell adhesion factors as a biomaterial for tissue engineering. J. Biosci. Bioeng. 2003, 95, 196–199. [Google Scholar] [CrossRef]
- Fassina, L.; Saino, E.; Visai, L.; Avanzini, M.A.; De Angelis, M.G.C.; Benazzo, F.; Van Vlierberghe, S.; Dubruel, P.; Magenes, G. Use of a Gelatin Cryogel as Biomaterial Scaffold in the Differentiation Process of Human Bone Marrow Stromal Cells. In Proceedings of the 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology, Buenos Aires, Argentina, 31 August–4 September 2010; pp. 247–250. [Google Scholar]
- Loth, T.; Hötzel, R.; Kascholke, C.; Anderegg, U.; Schulz-Siegmund, M.; Hacker, M.C. Gelatin-based biomaterial engineering with anhydride-containing oligomeric cross-linkers. Biomacromolecules 2014, 15, 2104–2118. [Google Scholar] [CrossRef]
- Chang, C.H.; Liu, H.C.; Lin, C.C.; Chou, C.H.; Lin, F.H. Gelatin–chondroitin–hyaluronan tri-copolymer scaffold for cartilage tissue engineering. Biomaterials 2003, 24, 4853–4858. [Google Scholar] [CrossRef]
- Chen, C.-H.; Chen, J.-P.; Lee, M.-Y. Effects of gelatin modification on rapid prototyping PCL scaffolds for cartilage engineering. J. Mech. Med. Biol. 2011, 11, 993–1002. [Google Scholar] [CrossRef]
- Ramadoss, P.; Arul, K.T.; Ramya, J.R.; Begam, M.R.; Chandra, V.S.; Manikandan, E. Enhanced mechanical strength and sustained drug release of gelatin/keratin scaffolds. Mater. Lett. 2017, 186, 109–112. [Google Scholar] [CrossRef]
- Li, Y.; Jia, H.; Cheng, Q.; Pan, F.; Jiang, Z. Sodium alginate–gelatin polyelectrolyte complex membranes with both high water vapor permeance and high permselectivity. J. Membr. Sci. 2011, 375, 304–312. [Google Scholar] [CrossRef]
- Sarker, M.; Chen, X. Modeling the flow behavior and flow rate of medium viscosity alginate for scaffold fabrication with a three-dimensional bioplotter. J. Manuf. Sci. Eng. 2017, 139, 081002. [Google Scholar] [CrossRef]
- Tai, H.; Mather, M.L.; Howard, D.; Wang, W.; White, L.J.; Crowe, J.A.; Morgan, S.P.; Chandra, A.; Williams, D.J.; Howdle, S.M. Control of pore size and structure of tissue engineering scaffolds produced by supercritical fluid processing. Eur. Cell Mater. 2007, 14, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.G.; Kim, S.Y.; Lee, Y.M. Novel porous gelatin scaffolds by overrun/particle leaching process for tissue engineering applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2006, 79, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Gummalla, M.; Tsapatsis, M.; Watkins, J.; Vlachos, D. Multiscale hybrid modeling of film deposition within porous substrates. AIChE J. 2004, 50, 684–695. [Google Scholar] [CrossRef]
- Yunoki, S.; Ikoma, T.; Tanaka, J. Development of collagen condensation method to improve mechanical strength of tissue engineering scaffolds. Mater. Charact. 2010, 61, 907–911. [Google Scholar] [CrossRef]
- Erisken, C.; Zhang, X.; Moffat, K.L.; Levine, W.N.; Lu, H.H. Scaffold fiber diameter regulates human tendon fibroblast growth and differentiation. Tissue Eng. Part A 2012, 19, 519–528. [Google Scholar] [CrossRef] [Green Version]
- Arul, K.A.; Rashia, B.S.; Arumaikkannu, G. Investigation of Porosity Relationship in Additive Manufactured Novel Bone Scaffold. In Applied Mechanics and Materials; Trans Tech Publications: Zurich, Switzerland, 2014; Volume 592, pp. 836–841. [Google Scholar]
- Li, J.; Wang, Q.; Gu, Y.; Zhu, Y.; Chen, L.; Chen, Y. Production of composite scaffold containing silk fibroin, chitosan, and gelatin for 3D cell culture and bone tissue regeneration. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2017, 23, 5311. [Google Scholar] [CrossRef] [Green Version]













| Gelatin | 1.5% | 2% | 2.5% | 3% | 3.5% | 4% | 4.5% | |
|---|---|---|---|---|---|---|---|---|
| Algin | Viscosity (cp) | |||||||
| 2% | 5.15 | 7.42 | 8.72 | 8.93 | 9.44 | 10.34 | 10.96 | |
| 4% | 5.96 | 8.08 | 9.01 | 10.3 | 11.5 | 12.65 | 14.01 | |
| 6% | 6.44 | 8.45 | 9.40 | 11.3 | 12.9 | 15.16 | 18.16 | |
| 8% | 7.36 | 8.98 | 9.63 | 13.5 | 15.01 | 18.27 | 23.24 | |
| 10% | 7.68 | 9.88 | 12.58 | 15.88 | 18.23 | 24.23 | 29.1 | |
| Number | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Algin (g/mL) | 0.015 | 0.02 | 0.025 | 0.03 |
| Gelatin (g/mL) | 0.08 | 0.08 | 0.08 | 0.08 |
| Fiber Distance (mm) | Theoretical Porosity | Experimental Porosity |
|---|---|---|
| 0.6 | 32.3 | 31.8 |
| 0.8 | 49.6 | 48.9 |
| 1 | 60.3 | 60.7 |
| 1.2 | 66.4 | 66.5 |
| 1.4 | 71.0 | 68.8 |
| 1.6 | 75.5 | 72.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, Y.; Wang, F.; Al-Furjan, M.S.H.; Shan, L.; He, J.; Bian, X.; Bi, Z.; Liu, H.; Li, W.; Shao, H.; et al. Experimental Investigation and Optimal 3D Bioprinting Parameters of SA-Gel Porous Cartilage Scaffold. Appl. Sci. 2020, 10, 768. https://doi.org/10.3390/app10030768
Gong Y, Wang F, Al-Furjan MSH, Shan L, He J, Bian X, Bi Z, Liu H, Li W, Shao H, et al. Experimental Investigation and Optimal 3D Bioprinting Parameters of SA-Gel Porous Cartilage Scaffold. Applied Sciences. 2020; 10(3):768. https://doi.org/10.3390/app10030768
Chicago/Turabian StyleGong, Youping, Fei Wang, M. S. H. Al-Furjan, Lijun Shan, Jingyang He, Xiangjuan Bian, Zhikai Bi, Haiqiang Liu, Wenxin Li, Huifeng Shao, and et al. 2020. "Experimental Investigation and Optimal 3D Bioprinting Parameters of SA-Gel Porous Cartilage Scaffold" Applied Sciences 10, no. 3: 768. https://doi.org/10.3390/app10030768
APA StyleGong, Y., Wang, F., Al-Furjan, M. S. H., Shan, L., He, J., Bian, X., Bi, Z., Liu, H., Li, W., Shao, H., Chen, G., & Sulong, A. B. (2020). Experimental Investigation and Optimal 3D Bioprinting Parameters of SA-Gel Porous Cartilage Scaffold. Applied Sciences, 10(3), 768. https://doi.org/10.3390/app10030768






