Two-Photon Excitation of Azobenzene Photoswitches for Synthetic Optogenetics
Abstract
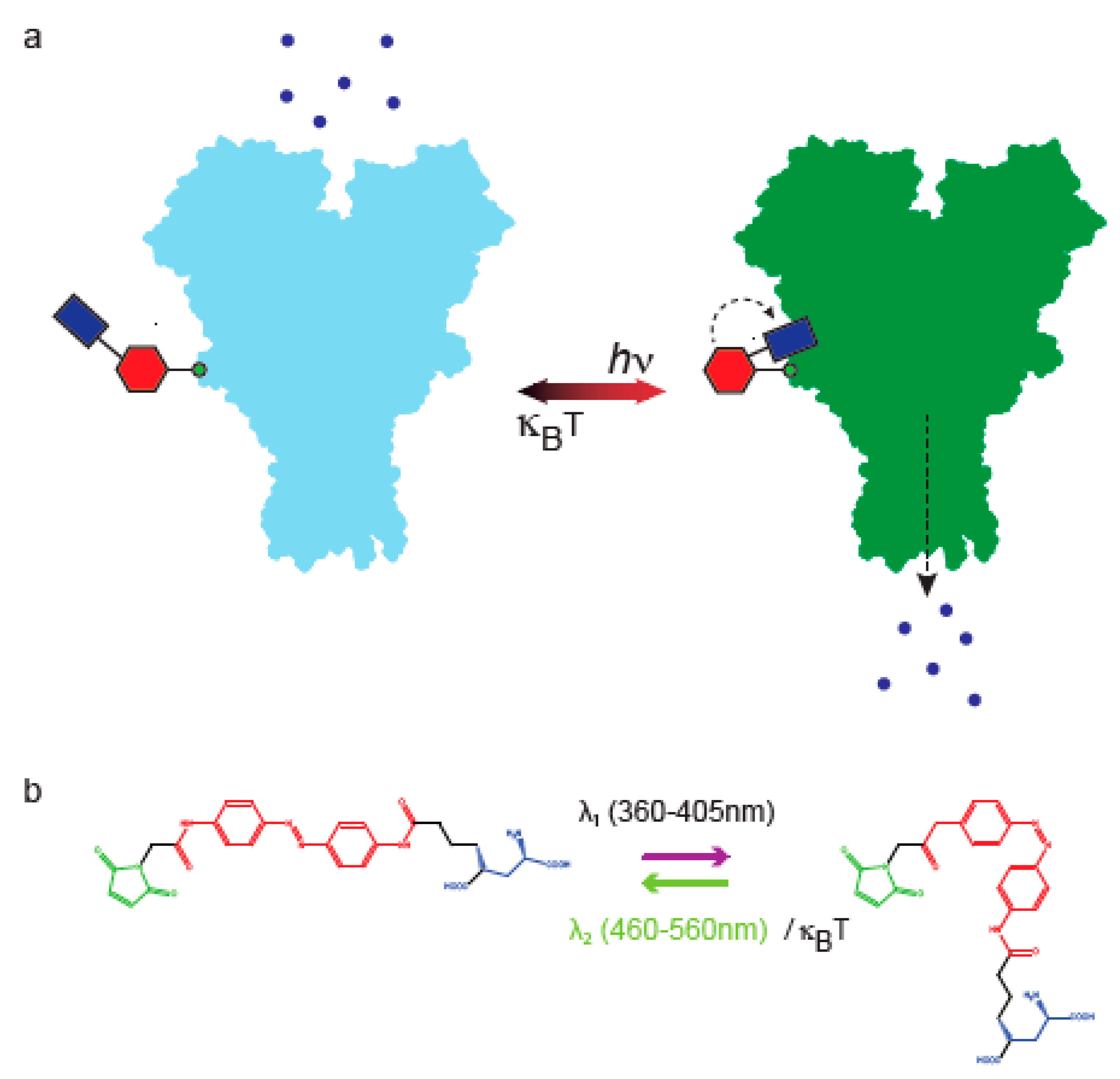
:1. Introduction
2. Photoswitches
3. Photomodulating Cellular Activity
4. Shifting from Visible to Near-Infrared
5. Two-Photon Compatible MAG-Based Photoswitches
6. Novel Photoswitches with 2PE-Potential
7. Future Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Kottke, T.; Xie, A.; Larsen, D.S.; Hoff, W.D. Photoreceptors take charge: Emerging principles for light sensing. Ann. Rev. Biophys. 2018, 47, 291–313. [Google Scholar] [CrossRef] [PubMed]
- Mathes, T.; Kennis, J.T.M. Editorial: Optogenetic tools in the molecular spotlight. Front. Mol. Biosci. 2016, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eleftheriou, C.; Cesca, F.; Maragliano, L.; Benfenati, F.; Maya-Vetencourt, J.F. Optogenetic modulation of intracellular signalling and transcription: Focus on neuronal plasticity. J. Exp. Neurosci. 2017, 11. [Google Scholar] [CrossRef] [PubMed]
- Deisseroth, K.; Feng, G.; Majewska, A.K.; Miesenböck, G.; Ting, A.; Schnitzer, M.J. Next-generation optical technologies for illuminating genetically targeted brain circuits. J. Neurosci. 2006, 26, 10380–10386. [Google Scholar] [CrossRef] [Green Version]
- Boyden, E.S.; Zhang, F.; Bamberg, E.; Nagel, G.; Deisseroth, K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 2005, 8, 1263–1268. [Google Scholar] [CrossRef]
- Nagel, G.; Ollig, D.; Fuhrmann, M.; Kateriya, S.; Musti, A.M.; Bamberg, E.; Hegemann, P. Channelrhodopsin-1: A light-gated proton channel in green algae. Science 2002, 296, 2395–2398. [Google Scholar] [CrossRef]
- Deisseroth, K.; Hegemann, P. The form and function of channelrhodopsin. Science 2017, 357, eaan5544. [Google Scholar] [CrossRef] [Green Version]
- Deisseroth, K. Optogenetics: 10 years of microbial opsins in neuroscience. Nat. Neurosci. 2015, 18, 1213–1225. [Google Scholar] [CrossRef] [Green Version]
- Berlin, S.; Isacoff, E.Y. Synapses in the spotlight with synthetic optogenetics. EMBO Rep. 2017, 18, 677–692. [Google Scholar] [CrossRef] [Green Version]
- Berlin, S.; Isacoff, E.Y. Optical Control of Glutamate Receptors of the NMDA-Kind in Mammalian Neurons, with the Use of Photoswitchable Ligands; Springer Science+Business Media LLC: Berlin, Germany, 2017; Volume 130. [Google Scholar]
- Ziegler, T.; Möglich, A. Photoreceptor engineering. Front. Mol. Biosci. 2015, 2. [Google Scholar] [CrossRef] [Green Version]
- Paoletti, P.; Ellis-Davies, G.C.R.; Mourot, A. Optical control of neuronal ion channels and receptors. Nat. Rev. Neurosci. 2019, 20, 514–532. [Google Scholar] [CrossRef]
- Folgering, J.H.A.; Kuiper, J.M.; de Vries, A.H.; Engberts, J.B.F.N.; Poolman, B. Lipid-mediated light activation of a mechanosensitive channel of large conductance. Langmuir 2004, 20, 6985–6987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerrero, L.; Smart, O.S.; Woolley, G.A.; Allemann, R.K. Photocontrol of DNA binding specificity of a miniature engrailed homeodomain. J. Am. Chem. Soc. 2005, 127, 15624–15629. [Google Scholar] [CrossRef] [PubMed]
- Frank, J.A.; Franquelim, H.G.; Schwille, P.; Trauner, D. Optical control of lipid rafts with photoswitchable ceramides. J. Am. Chem. Soc. 2016, 138, 12981–12986. [Google Scholar] [CrossRef] [PubMed]
- Habermacher, C.; Martz, A.; Calimet, N.; Lemoine, D.; Peverini, L.; Specht, A.; Cecchini, M.; Grutter, T. Photo-switchable tweezers illuminate pore-opening motions of an ATP-gated P2X ion channel. eLife 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Kienzler, M.A.; Isacoff, E.Y. Precise modulation of neuronal activity with synthetic photoswitchable ligands. Curr. Opin. Neurobiol. 2017, 45, 202–209. [Google Scholar] [CrossRef]
- Kramer, R.H.; Mourot, A.; Adesnik, H. Optogenetic pharmacology for control of native neuronal signaling proteins. Nat. Neurosci. 2013, 16, 816–823. [Google Scholar] [CrossRef]
- Kaufman, H.; Vratsanos, S.M.; Erlanger, B.F. Photoregulation of an enzymic process by means of a light-sensitive ligand. Science 1968, 162, 1487–1489. [Google Scholar] [CrossRef]
- Szymański, W.; Beierle, J.M.; Kistemaker, H.A.V.; Velema, W.A.; Feringa, B.L. Reversible photocontrol of biological systems by the incorporation of molecular photoswitches. Chem. Rev. 2013, 113, 6114–6178. [Google Scholar] [CrossRef] [Green Version]
- Beharry, A.A.; Woolley, G.A. Azobenzene photoswitches for biomolecules. Chem. Soc. Rev. 2011, 40, 4422. [Google Scholar] [CrossRef]
- Zhu, M.; Zhou, H. Azobenzene-based small molecular photoswitches for protein modulation. Org. Biomol. Chem. 2018, 16, 8434–8445. [Google Scholar] [CrossRef] [PubMed]
- Hüll, K.; Morstein, J.; Trauner, D. In vivo photopharmacology. Chem. Rev. 2018, 118, 10710–10747. [Google Scholar] [CrossRef] [PubMed]
- Fehrentz, T.; Schönberger, M.; Trauner, D. Optochemical Genetics. Angew. Chem. Int. Ed. 2011, 50, 12156–12182. [Google Scholar] [CrossRef] [PubMed]
- Reiner, A.; Isacoff, E.Y. Photoswitching of cell surface receptors using tethered ligands. In Photoswitching Protein; Cambridge, S., Ed.; Springer: New York, NY, USA, 2014; Volume 1148, pp. 45–68. [Google Scholar] [CrossRef]
- Berlin, S.; Szobota, S.; Reiner, A.; Carroll, E.C.; Kienzler, M.A.; Guyon, A.; Xiao, T.; Trauner, D.; Isacoff, E.Y. A family of photoswitchable NMDA receptors. elife 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Broichhagen, J.; Damijonaitis, A.; Levitz, J.; Sokol, K.R.; Leippe, P.; Konrad, D.; Isacoff, E.Y.; Trauner, D. Orthogonal optical control of a G protein-coupled receptor with a SNAP-Tethered photochromic ligand. ACS Cent. Sci. 2015, 1, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo-Serra, M.; Bautista-Barrufet, A.; Trapero, A.; Garrido-Charles, A.; Díaz-Tahoces, A.; Camarero, N.; Pittolo, S.; Valbuena, S.; Pérez-Jiménez, A.; Gay, M.; et al. Optical control of endogenous receptors and cellular excitability using targeted covalent photoswitches. Nat. Commun. 2016, 7. [Google Scholar] [CrossRef]
- Donthamsetti, P.C.; Broichhagen, J.; Vyklicky, V.; Stanley, C.; Fu, Z.; Visel, M.; Levitz, J.L.; Javitch, J.A.; Trauner, D.; Isacoff, E.Y. Genetically Targeted Optical Control of an Endogenous G Protein-Coupled Receptor. J. Am. Chem. Soc. 2019, 141, 11522–11530. [Google Scholar] [CrossRef]
- Banghart, M.; Borges, K.; Isacoff, E.; Trauner, D.; Kramer, R.H. Light-activated ion channels for remote control of neuronal firing. Nat. Neurosci. 2004, 7, 1381–1386. [Google Scholar] [CrossRef] [Green Version]
- Fodje, M.N.; Al-Karadaghi, S. Occurrence, conformational features and amino acid propensities for the pi-helix. Protein Eng. 2002, 15, 353–358. [Google Scholar] [CrossRef]
- Lester, H.A.; Krouse, M.E.; Nass, M.M.; Wassermann, N.H.; Erlanger, B.F. Light-activated drug confirms a mechanism of ion channel blockade. Nature 1979, 280, 509–510. [Google Scholar] [CrossRef]
- McKenzie, C.K.; Sanchez-Romero, I.; Janovjak, H. Flipping the photoswitch: Ion channels under light control. In Novel Chemical Tools to Study Ion Channel Biology; Ahern, C., Pless, S., Eds.; Springer: New York, NY, USA, 2015; Volume 869, pp. 101–117. [Google Scholar] [CrossRef]
- Kamei, T.; Akiyama, H.; Morii, H.; Tamaoki, N.; Uyeda, T.Q.P. Visible-Light photocontrol of (E)/(Z) isomerization of the 4-(Dimethylamino)azobenzene pseudo-nucleotide unit incorporated into an oligonucleotide and DNA hybridization in aqueous media. Nucleosides Nucleotides Nucleic Acids 2009, 28, 12–28. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, P.; Maclean, M.; MacGregor, S.J.; Anderson, J.G.; Grant, M.H. Cytotoxic responses to 405nm light exposure in mammalian and bacterial cells: Involvement of reactive oxygen species. Toxicol. In Vitro 2016, 33, 54–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trigo, F.F.; Corrie, J.E.T.; Ogden, D. Laser photolysis of caged compounds at 405 nm: Photochemical advantages, localisation, phototoxicity and methods for calibration. J. Neurosci. Methods 2009, 180, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Salo, D.; Kim, D.M.; Komarov, S.; Tai, Y.-C.; Berezin, M.Y. Penetration depth of photons in biological tissues from hyperspectral imaging in shortwave infrared in transmission and reflection geometries. J. Biomed. Opt. 2016, 21. [Google Scholar] [CrossRef] [PubMed]
- Weissleder, R. A clearer vision for in vivo imaging. Nat. Biotechnol. 2001, 19, 316–317. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, M.; Ai, X.; Zhang, Z.; Xing, B. Near-infrared manipulation of membrane ion channels via upconversion optogenetics. Adv. Biosyst. 2019, 3, 1800233. [Google Scholar] [CrossRef] [Green Version]
- Ellis-Davies, G.C.R. Two-photon microscopy for chemical neuroscience. ACS Chem. Neurosci. 2011, 2, 185–197. [Google Scholar] [CrossRef]
- Carroll, E.C.; Berlin, S.; Levitz, J.; Kienzler, M.A.; Yuan, Z.; Madsen, D.; Larsen, D.S.; Isacoff, E.Y. Two-photon brightness of azobenzene photoswitches designed for glutamate receptor optogenetics. Proc. Natl. Acad. Sci. USA 2015, 112, E776–E785. [Google Scholar] [CrossRef] [Green Version]
- Hartley, G.S. The Cis-form of Azobenzene. Nature 1937, 140, 281. [Google Scholar] [CrossRef]
- Shao, J.; Lei, Y.; Wen, Z.; Dou, Y.; Wang, Z. Nonadiabatic simulation study of photoisomerization of azobenzene: Detailed mechanism and load-resisting capacity. J. Chem. Phys. 2008, 129, 164111. [Google Scholar] [CrossRef] [Green Version]
- Bandara, H.M.D.; Burdette, S.C. Photoisomerization in different classes of azobenzene. Chem. Soc. Rev. 2012, 41, 1809–1825. [Google Scholar] [CrossRef] [PubMed]
- Hamm, P.; Ohline, S.M.; Zinth, W. Vibrational cooling after ultrafast photoisomerization of azobenzene measured by femtosecond infrared spectroscopy. J. Chem. Phys. 1997, 106, 519–529. [Google Scholar] [CrossRef]
- Lednev, I.K.; Ye, T.Q.; Matousek, P.; Towrie, M.; Foggi, P.; Neuwahl, F.V.; Umapathy, S.; Hester, R.E.; Moore, J.N. Femtosecond time-resolved UV-visible absorption spectroscopy of trans-azobenzene: Dependence on excitation wavelength. Chem. Phys. Lett. 1998, 290, 68–74. [Google Scholar] [CrossRef]
- Drobizhev, M.; Makarov, N.S.; Tillo, S.E.; Hughes, T.E.; Rebane, A. Two-photon absorption properties of fluorescent proteins. Nat. Methods 2011, 8, 393–399. [Google Scholar] [CrossRef]
- Stoltzfus, C.R.; Barnett, L.M.; Drobizhev, M.; Wicks, G.; Mikhaylov, A.; Hughes, T.E.; Rebane, A. Two-photon directed evolution of green fluorescent proteins. Sci. Rep. 2015, 5, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Quick, M.; Dobryakov, A.L.; Gerecke, M.; Richter, C.; Berndt, F.; Ioffe, I.N.; Granovsky, A.A.; Mahrwald, R.; Ernsting, N.P.; Kovalenko, S.A. Photoisomerization dynamics and pathways of trans- and cis-azobenzene in solution from broadband femtosecond spectroscopies and calculations. J. Phys. Chem. B 2014, 118, 8756–8771. [Google Scholar] [CrossRef]
- Moreno, J.; Gerecke, M.; Dobryakov, A.L.; Ioffe, I.N.; Granovsky, A.A.; Bleger, D.; Hecht, S.; Kovalenko, S.A. Two-photon-induced versus one-photon-induced isomerization dynamics of a bistable azobenzene derivative in solution. J. Phys. Chem. B 2015, 119, 12281–12288. [Google Scholar] [CrossRef]
- Denk, W.; Strickler, J.; Webb, W. Two-photon laser scanning fluorescence microscopy. Science 1990, 248, 73–76. [Google Scholar] [CrossRef] [Green Version]
- Benninger, R.K.P.; Piston, D.W. Two-photon excitation microscopy for the study of living cells and tissues. Curr. Protoc. Cell. Biol. 2013, 49, 4–11. [Google Scholar] [CrossRef] [Green Version]
- Lee, G.H.; Moon, H.; Kim, H.; Lee, G.H.; Kwon, W.; Yoo, S.; Myung, D.; Yun, S.H.; Bao, Z.; Hahn, S.K. Multifunctional materials for implantable and wearable photonic healthcare devices. Nat. Rev. Mater. 2020, 1–7. [Google Scholar] [CrossRef]
- Rost, B.R.; Schneider-Warme, F.; Schmitz, D.; Hegemann, P. Optogenetic tools for subcellular applications in neuroscience. Neuron 2017, 96, 572–603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oron, D.; Papagiakoumou, E.; Anselmi, F.; Emiliani, V. Chapter 7-Two-Photon Optogenetics. Prog. Brain Res. 2012, 196, 119–143. [Google Scholar] [CrossRef] [Green Version]
- Chaigneau, E.; Ronzitti, E.; Gajowa, M.A.; Soler-Llavina, G.J.; Tanese, D.; Brureau, A.Y.; Papagiakoumou, E.; Zeng, H.; Emiliani, V. Two-photon holographic stimulation of ReaChR. Front. Cell. Neurosci. 2016, 10. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Carrillo-Reid, L.; Bando, Y.; Peterka, D.S.; Yuste, R. Simultaneous two-photon imaging and two-photon optogenetics of cortical circuits in three dimensions. eLife 2018, 7, e32671. [Google Scholar] [CrossRef] [PubMed]
- Yizhar, O.; Fenno, L.E.; Prigge, M.; Schneider, F.; Davidson, T.J.; O’shea, D.J.; Sohal, V.S.; Goshen, I.; Finkelstein, J.; Paz, J.T.; et al. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature 2011, 477, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Ronzitti, E.; Ventalon, C.; Canepari, M.; Forget, B.C.; Papagiakoumou, E.; Emiliani, V. Recent advances in patterned photostimulation for optogenetics. J. Opt. 2017, 19, 113001. [Google Scholar] [CrossRef]
- Forli, A.; Vecchia, D.; Binini, N.; Succol, F.; Bovetti, S.; Moretti, C.; Nespoli, F.; Mahn, M.; Baker, C.A.; Bolton, M.M.; et al. Two-photon bidirectional control and imaging of neuronal excitability with high spatial resolution in vivo. Cell Rep. 2018, 22, 3087–3098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohanty, S.K.; Reinscheid, R.K.; Liu, X.; Okamura, N.; Krasieva, T.B.; Berns, M.W. In-depth activation of channelrhodopsin 2-sensitized excitable cells with high spatial resolution using two-photon excitation with a near-infrared laser microbeam. Biophys. J. 2008, 95, 3916–3926. [Google Scholar] [CrossRef] [Green Version]
- Ellis-Davies, G.C.R. Two-photon uncaging of glutamate. Front. Synaptic Neurosci. 2019, 10. [Google Scholar] [CrossRef]
- Volgraf, M.; Gorostiza, P.; Numano, R.; Kramer, R.H.; Isacoff, E.Y.; Trauner, D. Allosteric control of an ionotropic glutamate receptor with an optical switch. Nat. Chem. Biol. 2006, 2, 47–52. [Google Scholar] [CrossRef]
- Levitz, J.; Pantoja, C.; Gaub, B.; Janovjak, H.; Reiner, A.; Hoagland, A.; Schoppik, D.; Kane, B.; Stawski, P.; Schier, A.F.; et al. Optical control of metabotropic glutamate receptors. Nat. Neurosci. 2013, 16, 507–516. [Google Scholar] [CrossRef]
- Izquierdo-Serra, M.; Gascón-Moya, M.; Hirtz, J.J.; Pittolo, S.; Poskanzer, K.E.; Ferrer, E.; Alibés, R.; Busqué, F.; Yuste, R.; Hernando, J.; et al. Two-photon neuronal and astrocytic stimulation with azobenzene-based photoswitches. J. Am. Chem. Soc. 2014, 136, 8693–8701. [Google Scholar] [CrossRef] [PubMed]
- Gascón-Moya, M.; Pejoan, A.; Izquierdo-Serra, M.; Pittolo, S.; Cabré, G.; Hernando, J.; Alibés, R.; Gorostiza, P.; Busqué, F. An optimized glutamate receptor photoswitch with sensitized azobenzene isomerization. J. Org. Chem. 2015, 80, 9915–9925. [Google Scholar] [CrossRef] [PubMed]
- Cabré, G.; Garrido-Charles, A.; Moreno, M.; Bosch, M.; Porta-de-la-Riva, M.; Krieg, M.; Gascón-Moya, M.; Camarero, N.; Gelabert, R.; Lluch, J.M.; et al. Rationally designed azobenzene photoswitches for efficient two-photon neuronal excitation. Nat. Commun. 2019, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Rullo, A.; Reiner, A.; Reiter, A.; Trauner, D.; Isacoff, E.Y.; Woolley, G.A. Long wavelength optical control of glutamate receptor ion channels using a tetra-ortho-substituted azobenzene derivative. Chem. Commun. 2014, 50, 14613–14615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laprell, L.; Repak, E.; Franckevicius, V.; Hartrampf, F.; Terhag, J.; Hollmann, M.; Sumser, M.; Rebola, N.; DiGregorio, D.A.; Trauner, D. Optical control of NMDA receptors with a diffusible photoswitch. Nat. Commun. 2015, 6. [Google Scholar] [CrossRef] [Green Version]
- Riefolo, F.; Matera, C.; Garrido-Charles, A.; MJGomila, A.; Sortino, R.; Agnetta, L.; Claro, E.; Masgrau, R.; Holzgrabe, U.; Batlle, M.; et al. Optical control of cardiac function with a photoswitchable muscarinic agonist. J. Am. Chem. Soc. 2019, 141, 7628–7636. [Google Scholar] [CrossRef]
- Pittolo, S.; Lee, H.; Lladó, A.; Tosi, S.; Bosch, M.; Bardia, L.; Gómez-Santacana, X.; Llebaria, A.; Soriano, E.; Colombelli, J.; et al. Reversible silencing of endogenous receptors in intact brain tissue using 2-photon pharmacology. Proc. Natl. Acad. Sci. USA 2019, 116, 13680–13689. [Google Scholar] [CrossRef] [Green Version]
- Cabré, G.; Garrido-Charles, A.; González-Lafont, A.; Moormann, W.; Langbehn, D.; Egea, D.; Lluch, J.M.; Herges, R.; Alibés, R.; Busqué, F.; et al. Synthetic photoswitchable neurotransmitters based on bridged azobenzenes. Org. Lett. 2019, 21, 3780–3784. [Google Scholar] [CrossRef]
- Passlick, S.; Richers, M.T.; Ellis-Davies, G.C.R. Thermodynamically stable, photoreversible pharmacology in neurons with one- and two-photon excitation. Angew. Chem. Int. Ed. 2018, 57, 12554–12557. [Google Scholar] [CrossRef]
- Dong, M.; Babalhavaeji, A.; Samanta, S.; Beharry, A.A.; Woolley, G.A. Red-shifting azobenzene photoswitches for in vivo use. Acc. Chem. Res. 2015, 48, 2662–2670. [Google Scholar] [CrossRef]
- Kienzler, M.A.; Reiner, A.; Trautman, E.; Yoo, S.; Trauner, D.; Isacoff, E.Y. A red-shifted, fast-relaxing azobenzene photoswitch for visible light control of an ionotropic glutamate receptor. J. Am. Chem. Soc. 2013, 135, 17683–17686. [Google Scholar] [CrossRef] [Green Version]
- Hansen, M.J.; Lerch, M.M.; Szymanski, W.; Feringa, B.L. Direct and versatile synthesis of red-shifted azobenzenes. Angew. Chem. Int. Ed. 2016, 55, 13514–13518. [Google Scholar] [CrossRef]
- Mourot, A.; Kienzler, M.A.; Banghart, M.R.; Fehrentz, T.; Huber, F.M.; Stein, M.; Kramer, R.H.; Trauner, D. Tuning photochromic ion channel blockers. ACS Chem. Neurosci. 2011, 2, 536–543. [Google Scholar] [CrossRef] [Green Version]
- Carmi, I.; De Battista, M.; Maddalena, L.; Carroll, E.C.; Kienzler, M.A.; Berlin, S. Holographic two-photon activation for synthetic optogenetics. Nat. Protoc. 2018, 14, 864, under final review. [Google Scholar] [CrossRef]
- Kim, H.M.; Cho, B.R. Two-photon probes for intracellular free metal ions, acidic vesicles, and lipid rafts in live tissues. Acc. Chem. Res. 2009, 42, 863–872. [Google Scholar] [CrossRef]
- Dong, M.; Babalhavaeji, A.; Hansen, M.J.; Kálmán, L.; Woolley, G.A. Red, far-red, and near infrared photoswitches based on azonium ions. Chem. Commun. 2015, 51, 12981–12984. [Google Scholar] [CrossRef] [Green Version]
- Sell, H.; Näther, C.; Herges, R. Amino-substituted diazocines as pincer-type photochromic switches. Beilstein J. Org. Chem. 2013, 9, 1–7. [Google Scholar] [CrossRef]
- Thapaliya, E.R.; Zhao, J.; Ellis-Davies, G.C.R. Locked-azobenzene: testing the scope of a unique photoswitchable scaffold for cell physiology. ACS Chem. Neurosci. 2019, 10, 2481–2488. [Google Scholar] [CrossRef]
- Hartrampf, F.W.W.; Barber, D.M.; Gottschling, K.; Leippe, P.; Hollmann, M.; Trauner, D. Development of a photoswitchable antagonist of NMDA receptors. Tetrahedron 2017, 73, 4905–4912. [Google Scholar] [CrossRef]
- GÓmez-Santacana, X.; Pittolo, S.; Rovira, X.; Lopez, M.; Zussy, C.; Dalton, J.A.; Faucherre, A.; Jopling, C.; Pin, J.P.; Ciruela, F.; et al. Illuminating phenylazopyridines to photoswitch metabotropic glutamate receptors: from the flask to the animals. ACS Cent. Sci. 2017, 3, 81–91. [Google Scholar] [CrossRef]
- Volgraf, M.; Gorostiza, P.; Szobota, S.; Helix, M.R.; Isacoff, E.Y.; Trauner, D. Reversibly caged glutamate: a photochromic agonist of ionotropic glutamate receptors. J. Am. Chem. Soc. 2007, 129, 260–261. [Google Scholar] [CrossRef]
- Jiang, C.; Li, H.T.; Zhou, Y.M.; Wang, X.; Wang, L.; Liu, Z.Q. Cardiac optogenetics: A novel approach to cardiovascular disease therapy. EP Eur. 2017, 20, 1741–1749. [Google Scholar] [CrossRef]
- Gepstein, L.; Gruber, A. Optogenetic neuromodulation of the heart. J. Am. Coll. Cardiol. 2017, 70, 2791–2794. [Google Scholar] [CrossRef]
- Boyle, P.M.; Karathanos, T.V.; Trayanova, N.A. Cardiac optogenetics: 2018. JACC Clin. Electrophys. 2018, 4, 155–167. [Google Scholar] [CrossRef]
- Tochitsky, I.; Kienzler, M.A.; Isacoff, E.; Kramer, R.H. Restoring vision to the blind with chemical photoswitches. Chem. Rev. 2018, 118, 10748–10773. [Google Scholar] [CrossRef]
- Gaub, B.M.; Berry, M.H.; Visel, M.; Holt, A.; Isacoff, E.Y.; Flannery, J.G. Optogenetic retinal gene therapy with the light gated GPCR vertebrate rhodopsin. In Retinal Gene Therapy; Boon, C.J.F., Wijnholds, J., Eds.; Springer: New York, NY, USA, 2018; Volume 1715, pp. 177–189. [Google Scholar] [CrossRef]
- Berry, M.H.; Holt, A.; Levitz, J.; Broichhagen, J.; Gaub, B.M.; Visel, M.; Stanley, C.; Aghi, K.; Kim, Y.J.; Cao, K.; et al. Restoration of patterned vision with an engineered photoactivatable G protein-coupled receptor. Nat. Commun. 2017, 8. [Google Scholar] [CrossRef]
- Hüll, K.; Benster, T.; Manookin, M.B.; Trauner, D.; Van Gelder, R.N.; Laprell, L. Photopharmacologic vision restoration reduces pathological rhythmic field potentials in blind mouse retina. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Samia, S.; Berlin, S. Bringing synthetic optogenetics to the clinic. J. Cell Signal. 2017, 2. [Google Scholar] [CrossRef]
- Donthamsetti, P.C.; Winter, N.; Schönberger, M.; Levitz, J.; Stanley, C.; Javitch, J.A.; Isacoff, E.Y.; Trauner, D. Optical control of dopamine receptors using a photoswitchable tethered inverse agonist. J. Am. Chem. Soc. 2017, 139, 18522–18535. [Google Scholar] [CrossRef] [Green Version]
- Borowiak, M.; Nahaboo, W.; Reynders, M.; Nekolla, K.; Jalinot, P.; Hasserodt, J.; Rehberg, M.; Delattre, M.; Zahler, S.; Vollmar, A.; et al. Photoswitchable inhibitors of microtubule dynamics optically control mitosis and cell death. Cell 2015, 162, 403–411. [Google Scholar] [CrossRef] [Green Version]
- Cela, E.; Sjöström, P.J. Novel optogenetic approaches in epilepsy research. Front. Neurosci. 2019, 13. [Google Scholar] [CrossRef] [PubMed]
- Gradinaru, V.; Zhang, F.; Ramakrishnan, C.; Mattis, J.; Prakash, R.; Diester, I.; Goshen, I.; Thompson, K.R.; Deisseroth, K. Molecular and cellular approaches for diversifying and extending optogenetics. Cell 2010, 141, 154–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fenno, L.; Yizhar, O.; Deisseroth, K. The development and application of optogenetics. Annu. Rev. Neurosci. 2011, 34, 389–412. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.W.; Ronzitti, E.; Lee, B.R.; Daigle, T.L.; Dalkara, D.; Zeng, H.; Emiliani, V.; Papagiakoumou, E. In vivo submillisecond two-photon optogenetics with temporally focused patterned light. J. Neurosci. 2019, 39, 3484–3497. [Google Scholar] [CrossRef] [Green Version]


| Photoswitch | Type | 1P and 2P Peak Absorption (nm) | 2P-Absorption Cross-Section (GM) | Two Photon Imaging Method | Cell Type | Application | Ref |
|---|---|---|---|---|---|---|---|
| L-MAG0 | PTL | 1P 376 nm 2P maximal responses at 820 nm | σ2 = 10 GM at 820 nm a and σ2 = 300 GM at 630 nm (-trans) a | 2P-laser scanning at 820 nm | HEK293 | 2PE elicited optimal photocurrents of Imax~120 pA in GluK2-L439C-(LiGluR) expressing HEK293 cells. Responses were 10–20% of those obtained by 1P. | [65] |
| MAG2p | PTL | 1P ~420 nm 2P maximal response at 900 nm | σ2 = 56 GM at 780 nm b | 2P-laser scanning at 900 nm | HEK293 and dissociated hippocampal neurons | Cells expressing LiGluR, in voltage clamp 2PE elicited photocurrents recordings of ~50 and ~20 pA in HEK and neurons, respectively. These were 10–20% of those obtained by 1P illumination. | [65] |
| MAGA2p | PTL | 1P ~420 nm 2P maximal response at 880 nm | Naphthalene (antenna), σ2 ≈ 200 GM at 780 nm c | 2P-laser scanning at 900 nm | HEK293 | Cells expressing LiGluR, in voltage clamp 2PE elicited photocurrents recordings of 6 pA. | [65] |
| MAGA ligand-2 | PTL | 1P ~380 nm | Pyrene (antenna), σ2 = 55 GM | Not tested under 2PE | N.A. | N.A. | [66] |
| L-MAG460 | PTL | 1P ~460 nm | σ2 = 80 GM at 850 nm (-trans) | 2P digital holography (2P-DH) at 850 nm | HeLa cells and dissociated hippocampal neurons | Photocurrents elicited by 2P-DH were ~80 pA in size, ~39% of those obtained by 1P photocurrents from the same cell. HeLA cells expressing R-GECO showed reliable fluorescent responses when imaged at 561 nm and excited at 840 nm. | [41] |
| D-MAG460 | PTL | Similar properties as L-MAG460 | 2P digital holography (2P-DH) At 850 nm | HEK293 cells | Cells expressing mGluR3-Q306C (LimGluR3) illuminated by 2P-DH exhibited mean photocurrents of ~100 pA, namely ~85% of those obtained by 1P. | [41] | |
| PTL | 1P ~360 nm | σ2 = 69 GM d | 2P-laser scanning at 780 nm | HEK293 cells, rat hippocampal organotypic slices and C. elegans’ TRN neurons | Cells expressing LiGluR and R-GECO or RCaMP showed that 2PE elicited photoresponses (ΔF/F) similar to those obtained by 1P illumination (at 405 nm). Similar photoresponses from 1P and 2P were seen in vivo, in TRN neurons in C. elegans expressing GCaMP6s. | [67] | |
| toCl-MAG1 | PTL | 1P ~470 nm | N.A. | Not tested under 2PE | N.A. | N.A. | [68] |
| ATG | PCL | 1P ~330 nm | N.A. | 2P-laser scanning at 740 nm | Hippocampal neurons | cis-ATG-mediated synaptic currents of ~50 pA in size following 2P-photolysis. | [69] |
| PAI | PCL | 1P ~320 nm | N.A. | 2P-laser scanning at 840 nm | HEK293 cells | Cells co-expressing mAChR (m2R) and Gαq(TOP) loaded with the calcium indicator Oregon-green (OGB-AM) displayed reliable fluorescent responses (ΔF/F) to trans-PAI by 2PE. | [70] |
| Alloswitch | PCL | 1P and 2P absorbance N.A. 2P maximal response obtained by 760 nm | N.A. | 2P-laser scanning | HEK cells and organotypic hippocampal slices. | HEK cells expressing mGluR5-eYFP loaded with Fura-2 AM or neurons in organotypic hippocampal slices expressing GCaMP6s and DsRed were photostimulated by 2PE. trans-alloswitch released local inhibition of mGlu5 and enabled DHPG (mGluR5-agonist)-dependent activation of the receptor. Responses were monitored using Ca2+-indicators. 760 nm elicited highest increase in the frequency of calcium-oscillations. | [71] |
| Glu_brAzo2 | PCL | 1P -cis ~395 nm-trans ~480 nm | N.A. | Not tested under 2PE | N.A. | N.A. | [72] |
| 4FAB-QA | PCL | 1P ~415 nm | N.A. | 2P-laser scanning at 780 and 1000 nm | CA1 neurons from hippocampal organotypic slices | 2PE elicited ~32% inhibition of voltage gated Na+ and K+ channels than that obtained by 1P. | [73] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kellner, S.; Berlin, S. Two-Photon Excitation of Azobenzene Photoswitches for Synthetic Optogenetics. Appl. Sci. 2020, 10, 805. https://doi.org/10.3390/app10030805
Kellner S, Berlin S. Two-Photon Excitation of Azobenzene Photoswitches for Synthetic Optogenetics. Applied Sciences. 2020; 10(3):805. https://doi.org/10.3390/app10030805
Chicago/Turabian StyleKellner, Shai, and Shai Berlin. 2020. "Two-Photon Excitation of Azobenzene Photoswitches for Synthetic Optogenetics" Applied Sciences 10, no. 3: 805. https://doi.org/10.3390/app10030805
APA StyleKellner, S., & Berlin, S. (2020). Two-Photon Excitation of Azobenzene Photoswitches for Synthetic Optogenetics. Applied Sciences, 10(3), 805. https://doi.org/10.3390/app10030805





