Wavelia Breast Imaging: The Optical Breast Contour Detection Subsystem
Abstract
:1. Introduction
- compute the volume of the patient’s breast, thus indirectly deriving the required volume of transition liquid such that the container of the MBI subsystem is optimally filled after immersion of the breast;
- compute the distance (vertical extent) from the lowest point of the pendulous breast to the lower plane of the examination table, in order to optimally define the vertical scan positions for the MBI scan of each breast; the number of vertical scan positions and thus the time duration of the MBI scan will vary accordingly to the size of each breast;
- reconstruct the external envelope of the breast, with millimetric precision; such information will further serve to quantify the level of deformation of the breast due to immersion in the transition liquid during the microwave imaging scan, as introduced in [8]; in the case of breasts minimally deformed when immersed in the transition liquid (younger patients with breasts not very large), the OBCD-reconstructed breast surface, after registration with the MBI scan, is also meant to improve the localization of the abnormalities detected with MBI in the breast, by means of revealing the exact orientation of the pendulous breast under scan, as well as details such as the nipple location, which cannot be defined using the MBI-derived reconstruction of the breast surface alone, due to its centimetric level of spatial resolution. With no access to such level of detail, the annotation of the breast quadrant and clock-position at which an abnormality has been detected with MBI cannot be very accurate, when using the MBI system stand-alone, thus inhibiting the comparison and ultimate validation of the microwave breast imaging modality against conventional reference breast imaging data (X-Ray mammography and Ultrasound breast scan).
- the requirement for integration of the OBCD system in a specific examination table, the layout of which is driven by the architecture of the MBI subsystem; this is the main reason why system-specific algorithms for denoising and extraction of the useful portion of the recorded 3D point clouds are required to be designed;
- the need to operate in an obscure, closed environment, which justifies the specific choice of a stereoscopic 3D camera to scan the breast. The procedure that led to the selection of the specific 3D camera is presented in Appendix A.
2. Materials and Methods
2.1. Wavelia Breast Examination: OBCD Subsystem Architecture
- The circular array of microwave sensors moves along an opaque cylindrical container filled with a creamy opaque transition liquid. These opaque materials have dielectric properties appropriately selected to favor the penetration of the electromagnetic waves in the breast, while being biocompatible and acceptable in the clinical setting. Their replacement by semi-transparent counterparts with the desired properties is feasible, but not evident or straightforward;
- An appropriate motorization system needs to be designed, such that the MBI and OBCD scans can be performed sequentially, while the sensors (optical and microwave) are not in close vicinity to each other during the respective scans.
2.2. Optical Breast Contour Detection (OBCD) Subsystem: The Calibration Procedure
2.2.1. The Calibration Procedure: Step A
2.2.2. The Calibration Procedure: Step B
- upper section extraction conditions:
- lower section extraction conditions:
- left section extraction conditions:
- right section extraction conditions:
2.3. Optical Breast Contour Detection (OBCD) Subsystem: The 3D Breast Surface Reconstruction Procedure
- linear stretching procedure;
- denoising procedure;
- smoothing procedure;
- meshing procedure.
2.3.1. Linear Stretching Procedure
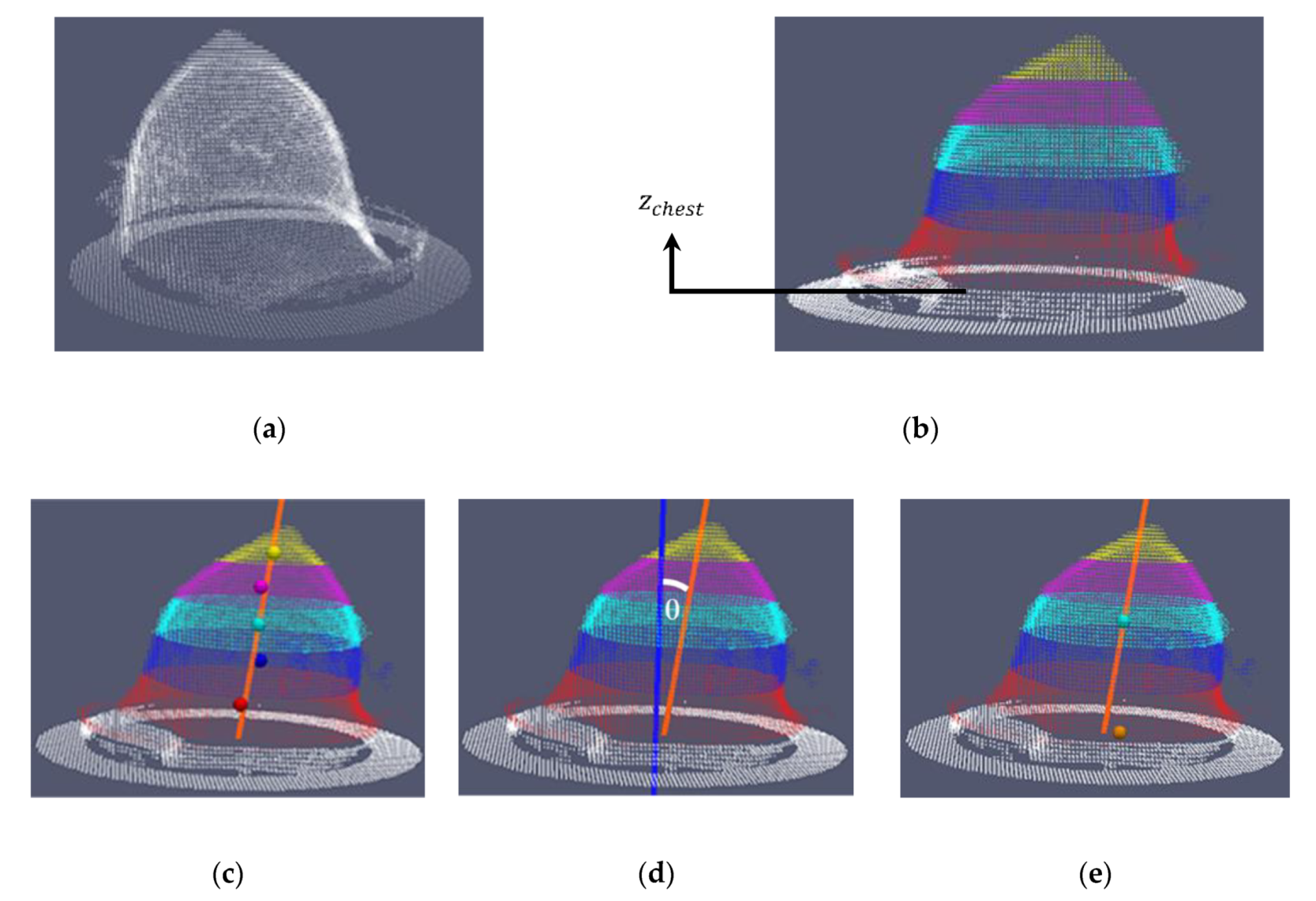
- the number of points of the point cloud is reduced by voxelization (Figure 14a).
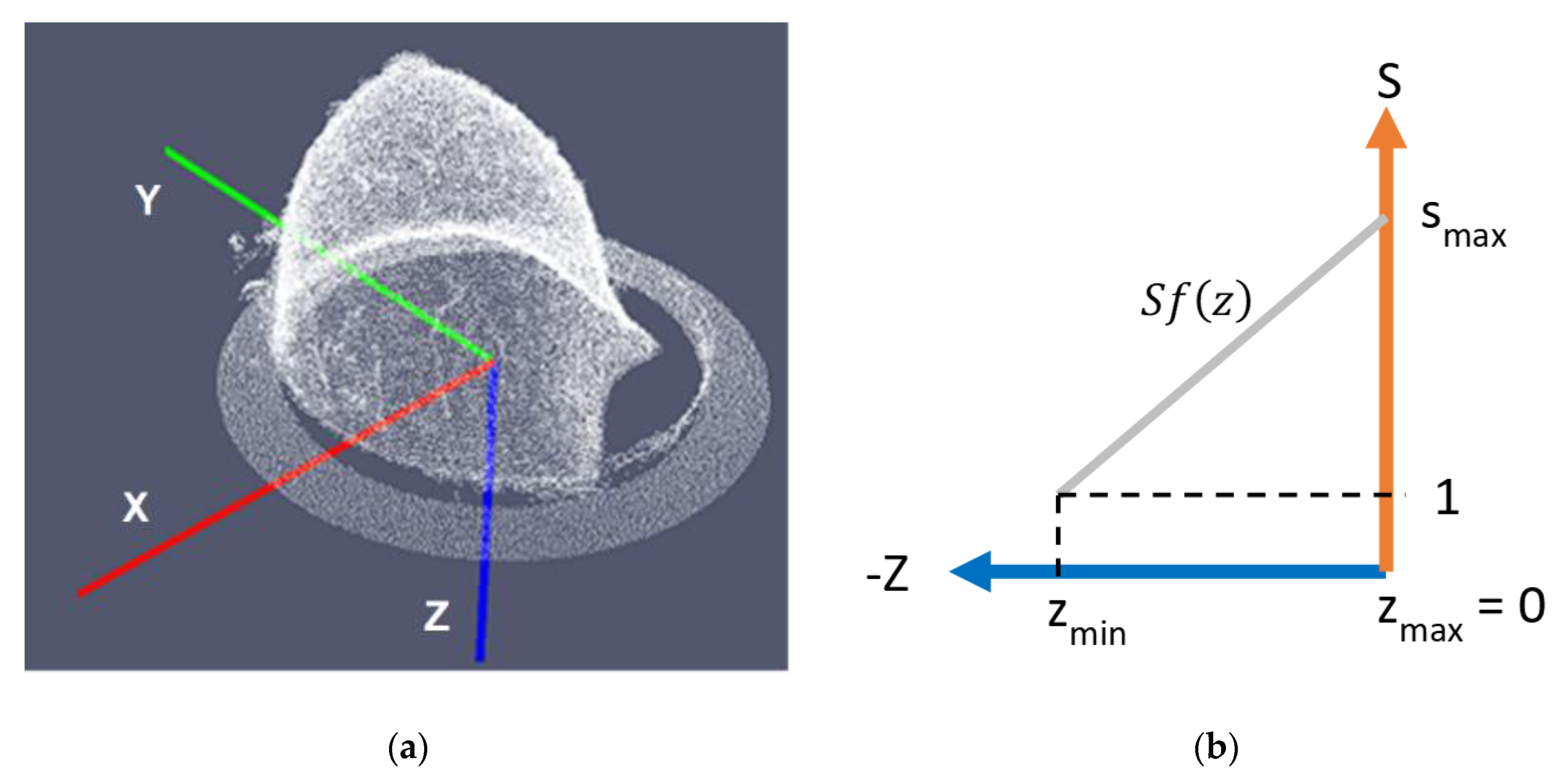
- the points associated to the chest wall are removed from the voxelized point cloud, by removing the points where the z-coordinate is in range from 0 to (white points in Figure 14b); has been empirically determined, considering a series of tests on both human and phantom breasts. The aim of this step is to preserve the points corresponding to the pendulous breast.
- the point cloud obtained in the previous step is divided in five vertical sections (red, blue, cyan, magenta and yellow in Figure 14b).
- the orientation axis of the breast is computed, by applying a LS method on the centroids of the five vertical sections (see Figure 14c).
- the θ angle between this axis and the z axis of the Wavelia reference coordinate system is computed (see Figure 14d).
2.3.2. Denoising Procedure
- ,, and : these values correspond to the minimum and maximum x and y coordinates of the bounding box of ;
- mesh resolution (Dxy): it corresponds to the desired resolution on the xy plane for the 3D breast reconstructed mesh, before the linear stretching transformation (see definition also in Appendix B.2);
- : this is the maximal stretching factor, as computed in the previous step (see Section 2.3.1);
- and : these are integer values. They correspond to the size of the rectangular grid along the x and y axes after the linear stretching transformation. These values are computed by using Equations (14) and (15), where corresponds to the stretched mesh resolution, after the stretching transformation. To preserve small parts like the nipple, after the stretching transformation, it was decided to increase the stretched mesh resolution by a factor of 2, as indicated in Equations (14) and (15).
- and : these values correspond to the minimum and maximum z coordinates of the bounding box of .
- : this parameter concerns the z limit of the nipple section (see Figure 18). Its value is computed by using Equation (16), where is an empirically defined value within the range 0 to 1 based on tests performed on human and phantom breasts.
- valid distance: this parameter is applied to the points outside the nipple section. It corresponds to the maximal signed vertical distance to the reference rectangular grid for the denoising operation to preserve points corresponding to the breast surface.
- valid distance for nipple section: this parameter is applied to the points inside the nipple section, where the nipple points are above the breast surface. It corresponds to the maximal signed vertical distance to the reference rectangular grid for the denoising operation to preserve points corresponding to the nipple and areola vicinity on the breast surface.
- Type-A hole: the holes bordered by valid points the z coordinates of which are located inside the first interval (bright green points in Figure 22). This type of hole is composed of at least two consecutive empty points along the x axis or along the y axis (see yellow delimited zones in Figure 22). These holes correspond to the zones located between the internal vertical wall of the ring and the border of the breast surface. The z coordinate value of these empty points is set to 0.
- Type-B hole: all the holes not completely bordered by valid points (see Figure 23). These holes correspond to the zones located beyond the ring, where no section of the breast surface is present. The z coordinate value of these empty points is set to 0.
- Type-C hole: the holes bordered by valid points the z coordinates of which are outside the first interval (holes located inside the dark green section).
2.3.3. Smoothing Step
- a (3 × 3) kernel size for the nipple section (this kernel size allows to smooth this section while sufficiently preserving the breast surface details around the nipple);
- a (5 × 5) kernel size for the second breast section;
- a (7 × 7) kernel size for the third breast section;
- a (9 × 9) kernel size for the fourth breast section.
2.3.4. Meshing Procedure
2.3.5. Breast Volume Computation
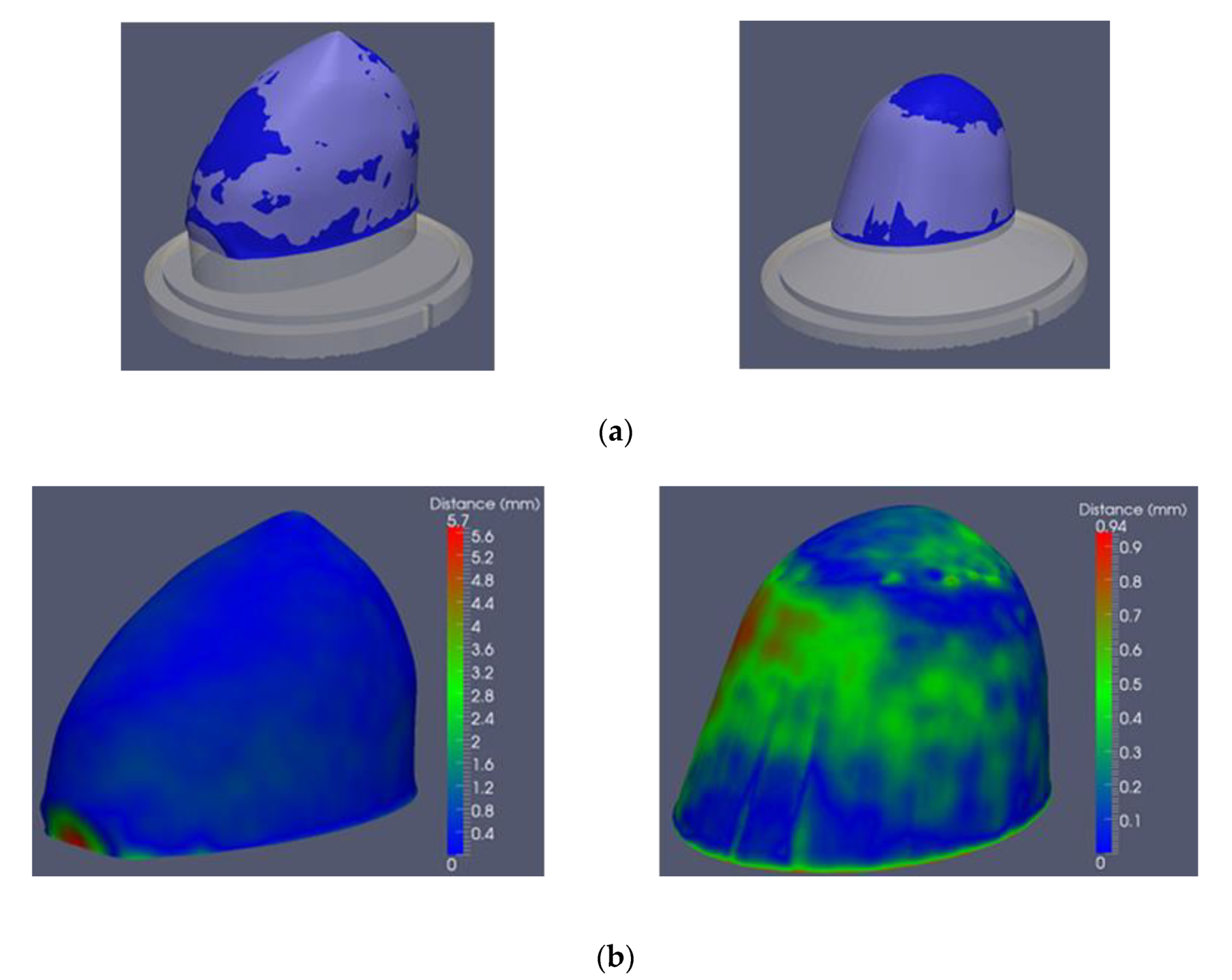
2.4. Description of the Method to Quantify the 3D Breast Surface Reconstruction Accuracy
2.4.1. Generation of 3D Breast Reference Surfaces
2.4.2. Procedure to Quantify the Accuracy of the 3D Breast Surface Reconstruction
- percentage of points with distance error less or equal to 0.5 mm;
- percentage of points with distance error less or equal to 1.0 mm;
- percentage of points with distance error less or equal to 1.5 mm;
- Root Mean Square (RMS) distance error in mm.
- Breast vertical extent error [mm]: this metric is defined as: .
- Breast volume error [mL]: this metric is defined as: .
- Breast volume error in percentage: this metric is defined as: .
3. Results and Discussion
3.1. Calibration Evaluation
3.2. D Breast Surface Reconstruction: Quantitative Evaluation Results
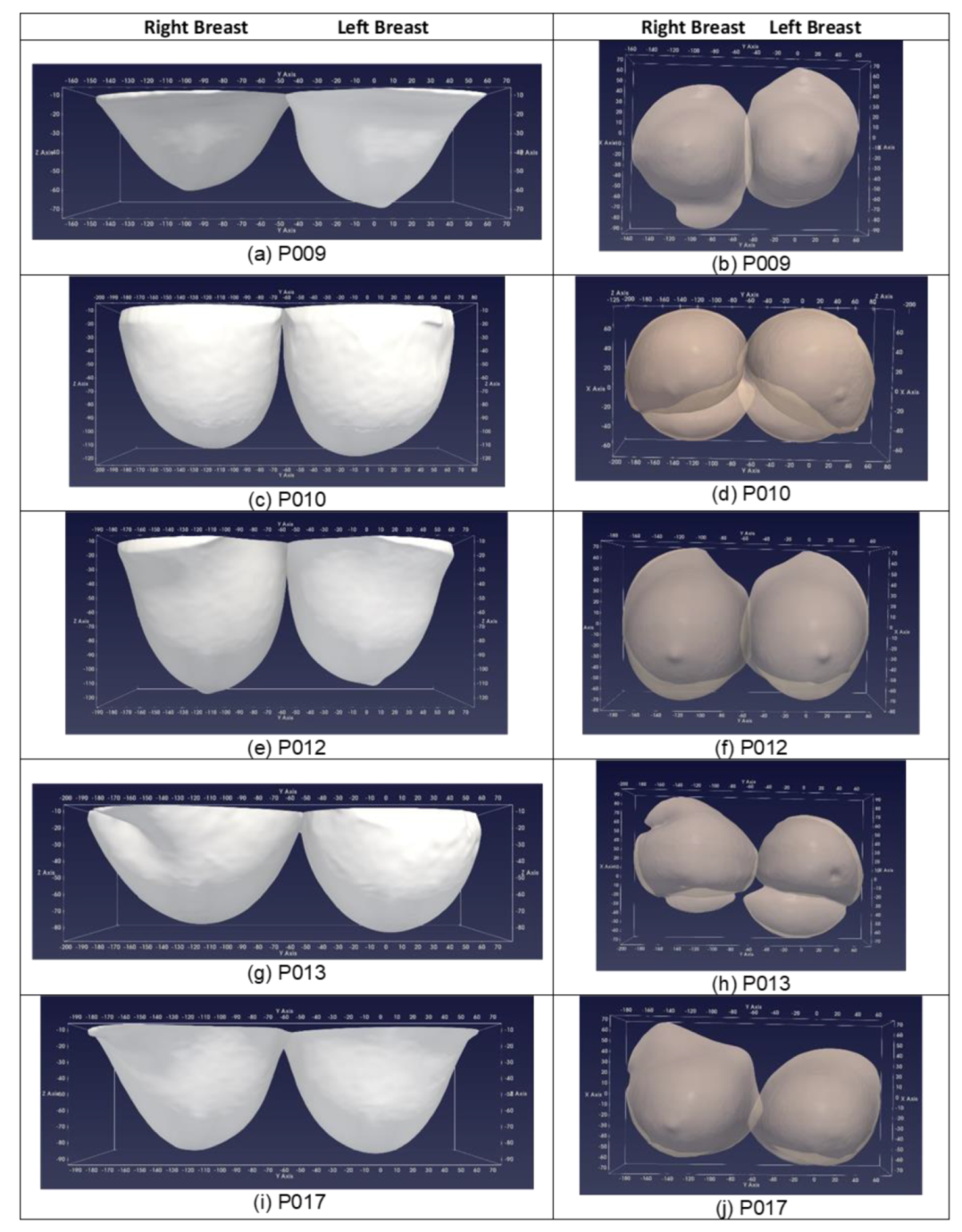
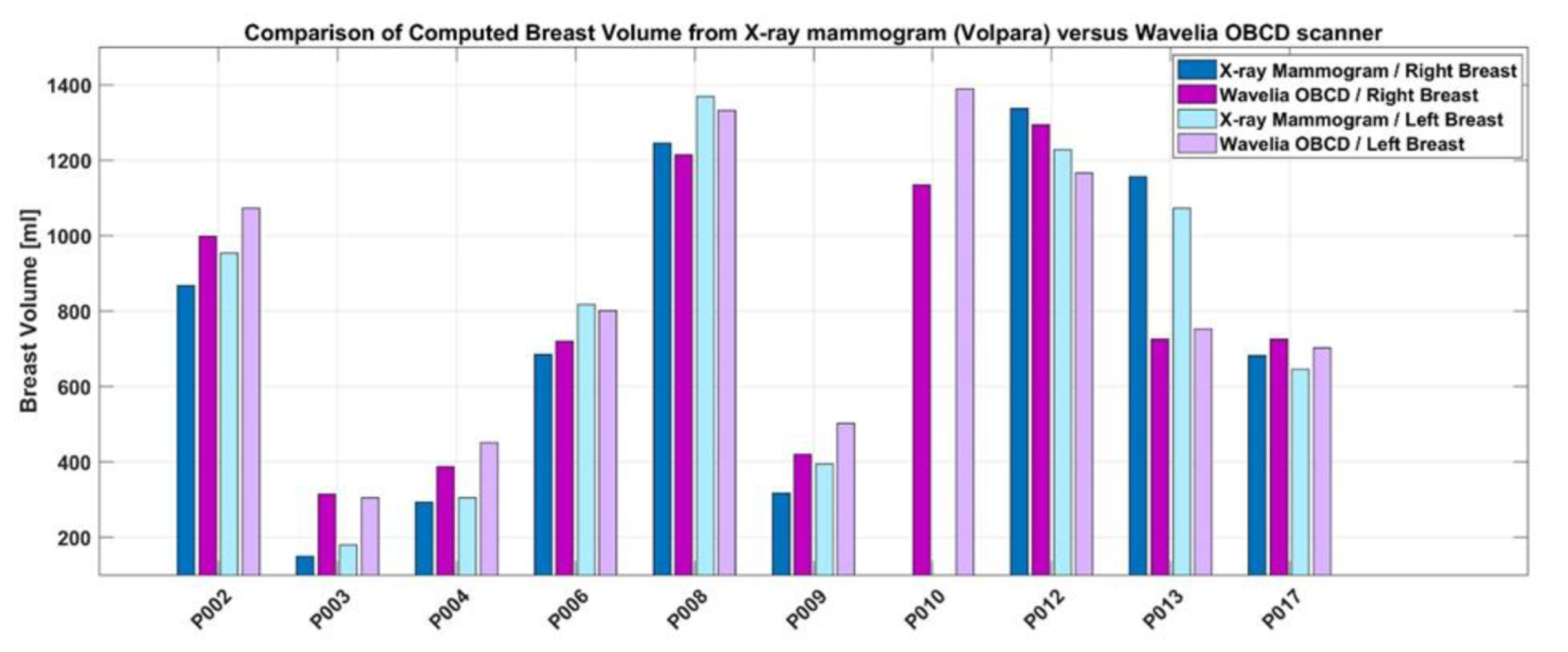
3.3. D Breast Surface Reconstruction: Indicative Results from the First Ten Patient Scans
- the Wavelia OBCD 3D scan data, or
- the 2D X-ray mammogram (Volpara software package, computation on ‘For processing’ DICOM files of the two standard mammographic views – Cranio-Caudal and Medio-Lateral Oblique).
3.4. Wavelia OBCD Breast Surface Reconstruction: Towards Development of a Real-Time Support Tool for the Wavelia MBI Examination and Breast Cancer Diagnosis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Ethical Approval
Informed Consent
Appendix A. Selection Procedure for the 3D Camera to be Integrated in the Wavelia OBCD Subsystem
| IFM O3D302 | Ensenso N10-804-18 | |
|---|---|---|
| Image resolution [pixels] | 176 × 132 | 752 × 480 |
| Max. reading rate [Hz] | 25 | 30 |
| Operating distance [mm] | 300…8000 | 450…1600 |
| View field size X at 500 mm [mm] | 500 | 158.49 |
| View field size Y at 500 mm [mm] | 370 | 158.13 |
| Z-Accuracy [mm] | --- | 0.452 |
| Baseline (Pupillary Distance) [mm] | --- | 100 |
| Illumination | 850 nm, infrared | 850 nm, infrared |



Appendix B. Generation of the Input Point Cloud for the 3D Breast Surface Reconstruction Method
- extraction of the working point cloud;
- ring removal and verification.
Appendix B.1. Extraction of the Working Point Cloud


Appendix B.2. Ring Removal and Verification
- inner radius (InRad): this parameter is defined as the physical radius of the ring minus an estimate of the expected variation due to reflections on the vertical wall of the ring during the OBCD scan of the breast.

- ring height: it is the physical height of the ring, including an estimate of the noise along the z axis on the flat ring section (see Figure A6b) during the OBCD scan;
- mesh resolution (Dxy): it corresponds to the desired resolution on the xy plane for the 3D breast reconstructed mesh;
- remaining reference factor: it is a factor in the range [0–1], defining whether processing to remove remaining ring points is required, or not. This factor has been determined empirically, based on observations from a series of available scan data, involving both human and phantom breasts.

Appendix C. Final Denoising
- removal of the artifacts and/or removal of ring residuals;
- removal of remaining artifacts.
Appendix C.1. Removal of the Artifacts And/or Removal of Ring Residuals

Appendix C.2. Removal of Remaining Artifacts

- a (1 × 1) kernel size for the nipple section (this kernel size allows to best preserve the breast surface details around the nipple);
- a (5 × 5) kernel size for the second breast section;
- a (7 × 7) kernel size for the third breast section;
- a (9 × 9) kernel size for the fourth breast section;

References
- Fear, E.C.; Meaney, P.M.; Stuchly, M.A. Microwaves for Breast Cancer Detection? IEEE Potentials 2003. [Google Scholar] [CrossRef]
- O’Loughlin, D.; O’Halloran, M.; Moloney, B.M.; Glavin, M.; Jones, E.; Elahi, M.A. Microwave breast imaging: Clinical advances and remaining challenges. IEEE Trans. Biomed. Eng. 2018, 65, 2580–2590. [Google Scholar] [CrossRef] [PubMed]
- Fasoula, A.; Duchesne, L.; Cano, J.G.; Lawrence, P.; Robin, G.; Bernard, J.-G. On-Site Validation of a Microwave Breast Imaging System, before First Patient Study. Diagnostics 2018, 8, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iversen, P.O.; Garreau, P.; Burrell, D. Real-time spherical near-field handset antenna measurements. IEEE Antennas Propag. Mag. 2001. [Google Scholar] [CrossRef]
- Fasoula, A.; Anwar, S.; Toutain, Y.; Duchesne, L. Microwave vision: From RF safety to medical imaging. In Proceedings of the 2017 11th European Conference on Antennas and Propagation, EUCAP, Paris, France, 19–24 March 2017. [Google Scholar] [CrossRef]
- Fasoula, A.; Bernard, J.; Robin, G.; Duchesne, L. Elaborated breast phantoms and experimental benchmarking of a microwave breast imaging system before first clinical studyTitle. In Proceedings of the 12th European Conference on Antennas and Propagation, London, UK, 9–13 April 2018. [Google Scholar]
- Registered Cinical Trial Protocol. Available online: https://clinicaltrials.gov/ct2/show/NCT03475992 (accessed on 11 February 2020).
- Lawrence, P.; Fasoula, A.; Duchesne, L. RF-based Breast Surface Estimation—Registration with Reference Imaging Modality. In Proceedings of the 2018 IEEE International Symposium on Antennas and Propagation & USNC/URSI National Radio Science Meeting, Boston, MA, USA, 8–13 July 2018. [Google Scholar]
- Winters, D.W.; Shea, J.D.; Madsen, E.L.; Frank, G.R.; van Veen, B.D.; Hagness, S.C. Estimating the breast surface using UWB microwave monostatic backscatter measurements. IEEE Trans. Biomed. Eng. 2007. [Google Scholar] [CrossRef]
- Sakamotov, T.; Sato, T. A target shape estimation algorithm for pulse radar systems based on boundary scattering transform. IEICE Trans. Commun. 2004. [Google Scholar] [CrossRef]
- Endo, F.; Kidera, S. Accuracy enhanced beamforming method based on envelope surface extraction for non-contact UWB breast cancer radar. In Proceedings of the ISAP 2016—International Symposium on Antennas and Propagation, Okinawa, Japan, 24–28 October 2016. [Google Scholar]
- Sarafianou, M.; Preece, A.W.; Craddock, I.J.; Klemm, M.; Leendertz, J.A. Evaluation of Two Approaches for Breast Surface Measurement Applied to a Radar-Based Imaging System. IEEE Trans. Antennas Propag. 2016. [Google Scholar] [CrossRef] [Green Version]
- Williams, T.C.; Bourqui, J.; Cameron, T.R.; Okoniewski, M.; Fear, E.C. Laser surface estimation for microwave breast imaging systems. IEEE Trans. Biomed. Eng. 2011. [Google Scholar] [CrossRef]
- Kurrant, D.; Bourqui, J.; Fear, E. Surface estimation for microwave imaging. Sensors 2017, 7, 1658. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.Y.; Kim, M.J.; Lew, D.H.; Song, S.Y.; Lee, D.W. Three-dimensional surface imaging is an effective tool for measuring breast volume: A validation study. Arch. Plast. Surg. 2016. [Google Scholar] [CrossRef] [Green Version]
- Chae, M.P.; Rozen, W.M.; Spychal, R.T.; Hunter-Smith, D.J. Breast volumetric analysis for aesthetic planning in breast reconstruction: A literature review of techniques. Gland. Surg. 2016. [Google Scholar] [CrossRef]
- Choppin, S.B.; Wheat, J.S.; Gee, M.; Goyal, A. The accuracy of breast volume measurement methods: A systematic review. Breast 2016. [Google Scholar] [CrossRef]
- O’Connell, R.L.; Khabra, K.; Bamber, J.C.; deSouza, N.; Meybodi, F.; Barry, P.A.; Rusby, J.E. Validation of the Vectra XT three-dimensional imaging system for measuring breast volume and symmetry following oncological reconstruction. Breast Cancer Res. Treat. 2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khawaldeh, S.; Aleef, T.A.; Pervaiz, U.; Minh, V.H.; Hagos, Y.B. Complete End-to-End Low Cost Solution to a 3D Scanning System with Integrated Turntable. Int. J. Comput. Sci. Inf. Technol. 2017. [Google Scholar] [CrossRef]
- Song, T.; Zhou, L.; Ding, X.; Yi, W. 3D Surface Reconstruction Based on Kinect Sensor. Int. J. Comput. Theory Eng. 2013. [Google Scholar] [CrossRef] [Green Version]
- Ye, Y.; Song, Z. An accurate 3D point cloud registration approach for the turntable-based 3D scanning system. In Proceedings of the 2015 IEEE International Conference on Information and Automation, ICIA, Lijiang, China, 8–10 August 2015. [Google Scholar] [CrossRef]
- Xiao, W.; Deng, M.; Li, C. A surface reconstruction algorithm based on 3D point cloud stratified sliced. Sens. Transducers 2014, 173, 197. [Google Scholar]
- Rankin, J.R. Computer Graphics Software Construction; Prentice Hall of Australia: Sydney, Australia, 1989; ISBN 0-7248-0194-9. [Google Scholar]
- Tomasi, C.; Manduchi, R. Bilateral filtering for gray and color images. In Proceedings of the IEEE International Conference on Computer Vision, Bombay, India, 7 January 1998. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, T. Efficient feature extraction for 2D/3D objects in mesh representation. In Proceedings of the IEEE International Conference on Image Processing, Thessaloniki, Greece, 7–10 October 2001. [Google Scholar] [CrossRef]
- Klamkin, M.S. On the Volume of a Class of Truncated Prisms and Some Related Centroid Problems. Math. Mag. 1968. [Google Scholar] [CrossRef]
- Zastrow, E.; Davis, S.K.; Lazebnik, M.; Kelcz, F.; van Veen, B.D.; Hagness, S.C. Development of anatomically realistic numerical breast phantoms with accurate dielectric properties for modeling microwave interactions with the human breast. IEEE Trans. Biomed. Eng. 2008. [Google Scholar] [CrossRef] [Green Version]
- Cignoni, P.; Callieri, M.; Corsini, M.; Dellepiane, M.; Ganovelli, F.; Ranzuglia, G. MeshLab: An open-source mesh processing tool. In Proceedings of the 6th Eurographics Italian Chapter Conference, Salerno, Italy, 2–4 July 2008. [Google Scholar]
- Ahrens, J.; Geveci, B.; Law, C. ParaView: An end-user tool for large-data visualization. In Visualization Handbook; Elsevier: Amsterdam, The Netherlands, 2005; ISBN 978-0123875822. [Google Scholar]
- Highnam, R.; Brady, M.; Yaffe, M.J.; Karssemeijer, N.; Harvey, J. Robust breast composition measurement—VolparaTM. In International Workshop on Digital Mammography; Springer: Berlin, Germany, 2010. [Google Scholar] [CrossRef]
- Brandt, K.R.; Scott, C.G.; Ma, L.; Mahmoudzadeh, A.P.; Jensen, M.R.; Whaley, D.H.; Wu, F.F.; Malkov, S.; Hruska, C.B.; Norman, A.D.; et al. Comparison of Clinical and Automated Breast Density Measurements: Implications for Risk Prediction and Supplemental Screening. Radiology 2016. [Google Scholar] [CrossRef] [Green Version]
- Ko, S.Y.; Kim, E.-K.; Kim, M.J.; Moon, H.J. Mammographic density estimation with automated volumetric breast density measurement. Korean J. Radiol. 2014. [Google Scholar] [CrossRef] [Green Version]
- Gubern-Mérida, A.; Kallenberg, M.; Platel, B.; Mann, R.M.; Martí, R.; Karssemeijer, N. Volumetric breast density estimation from full-field digital mammograms: A validation study. PLoS ONE 2014. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Azziz, A.; Fan, B.; Malkov, S.; Klifa, C.; Newitt, D.; Yitta, S.; Hylton, N.; Kerlikowske, K.; Shepherd, J.A. Agreement of mammographic measures of volumetric breast density to MRI. PLoS ONE 2013. [Google Scholar] [CrossRef] [PubMed]
- Fasoula, A.; Moloney, B.M.; Duchesne, L.; Cano, J.G.; Oliveira, B.L.; Bernard, J.G.; Kerin, M.J. Super-resolution radar imaging for breast cancer detection with microwaves: The integrated information selection criteria. In Proceedings of the 41st Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Berlin, Germany, 23–27 July 2019. [Google Scholar]
- O3D Camera. Available online: https://www.ifm.com/fr/en/product/O3D302 (accessed on 11 February 2020).
- Ensenso N10 Camera. Available online: https://en.ids-imaging.com/ensenso-n10.html (accessed on 11 February 2020).































| Test ID | Calibration Transform Error (mm) |
|---|---|
| 1 | 1.40 |
| 2 | 0.58 |
| 3 | 0.70 |
| 4 | 0.97 |
| 5 | 0.88 |
| 6 | 0.80 |
| B1 Test 1 | B1 Test 2 | B1 Test 3 | B1 Test 4 | B2 Test 1 | B2 Test 2 | B2 Test 3 | B2 Test 4 | |
|---|---|---|---|---|---|---|---|---|
| Total number of points on the extracted breast surface | 177,228 | 178,570 | 178,562 | 179,896 | 134,808 | 137,000 | 119,758 | 119,748 |
| Percentage of points with distance error <= 0.5 mm (%) | 76.80 | 90.20 | 89.01 | 87.28 | 82.18 | 93.77 | 83.88 | 79.88 |
| Percentage of points with distance error <= 1.0 mm (%) | 96.26 | 95.19 | 94.58 | 97.45 | 100 | 99.14 | 98.12 | 99.77 |
| Percentage of points with distance error <= 1.5 mm (%) | 97.55 | 97.42 | 97.37 | 98.94 | 100 | 100 | 99.86 | 99.93 |
| Percentage of points with distance error >1.5 mm (%) | 2.45 | 2.58 | 2.63 | 1.06 | 0.00 | 0.00 | 0.14 | 0.07 |
| RMS distance error (mm) | 0.74 | 0.65 | 0.69 | 0.50 | 0.40 | 0.30 | 0.44 | 0.42 |
| Reference Vertical Extent of the Breast (mm) | 110.19 | 110.96 | 110.96 | 111.16 | 84.67 | 84.97 | 67.88 | 67.59 |
| Computed Vertical Extent of the Breast (mm) | 109.36 | 109.55 | 109.60 | 109.85 | 84.71 | 84.79 | 67.73 | 67.50 |
| Error in the Vertical Extent of the Breast (mm) | −0.83 | −1.41 | −1.36 | −1.31 | 0.04 | −0.19 | −0.15 | −0.09 |
| Reference Volume of the Breast (mL) | 915.57 | 924.57 | 924.57 | 928.28 | 498.92 | 502.51 | 349.48 | 350.01 |
| Computed Volume of the Breast (mL) | 909.81 | 921.25 | 921.25 | 921.97 | 494.27 | 502.21 | 349.51 | 347.48 |
| Breast Volume Error (mL) | −5.75 | −3.31 | −3.31 | −6.31 | −4.64 | −0.31 | 0.03 | −2.52 |
| Breast Volume Error (%) | −0.63 | −0.36 | −0.36 | −0.68 | −0.93 | −0.06 | 0.01 | −0.72 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gil Cano, J.D.; Fasoula, A.; Duchesne, L.; Bernard, J.-G. Wavelia Breast Imaging: The Optical Breast Contour Detection Subsystem. Appl. Sci. 2020, 10, 1234. https://doi.org/10.3390/app10041234
Gil Cano JD, Fasoula A, Duchesne L, Bernard J-G. Wavelia Breast Imaging: The Optical Breast Contour Detection Subsystem. Applied Sciences. 2020; 10(4):1234. https://doi.org/10.3390/app10041234
Chicago/Turabian StyleGil Cano, Julio Daniel, Angie Fasoula, Luc Duchesne, and Jean-Gael Bernard. 2020. "Wavelia Breast Imaging: The Optical Breast Contour Detection Subsystem" Applied Sciences 10, no. 4: 1234. https://doi.org/10.3390/app10041234




