Analysis and Identification of Active Compounds from Salviae miltiorrhizae Radix Toxic to HCT-116 Human Colon Cancer Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Chemicals and Reagents
2.3. Preparation of 70% Ethanol SMR Extract
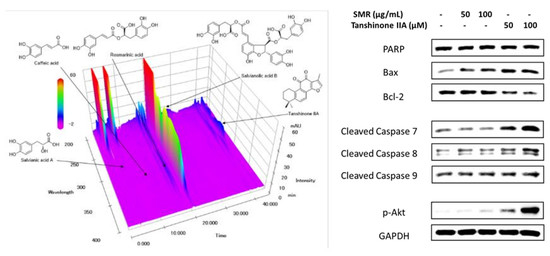
2.4. HPLC Analysis of Five Components in SMR
2.5. Cell Culture
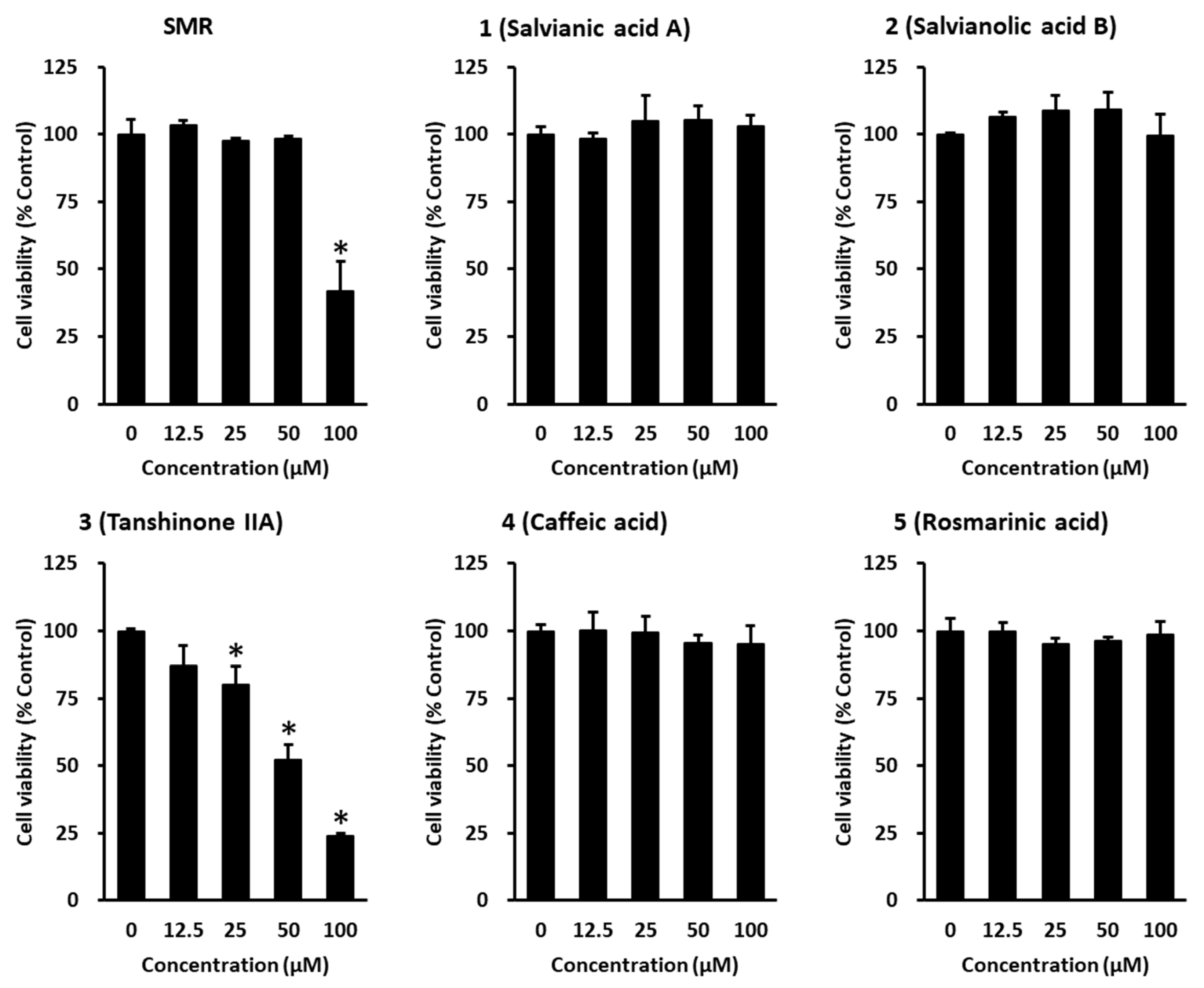
2.6. Cell Viability Assay
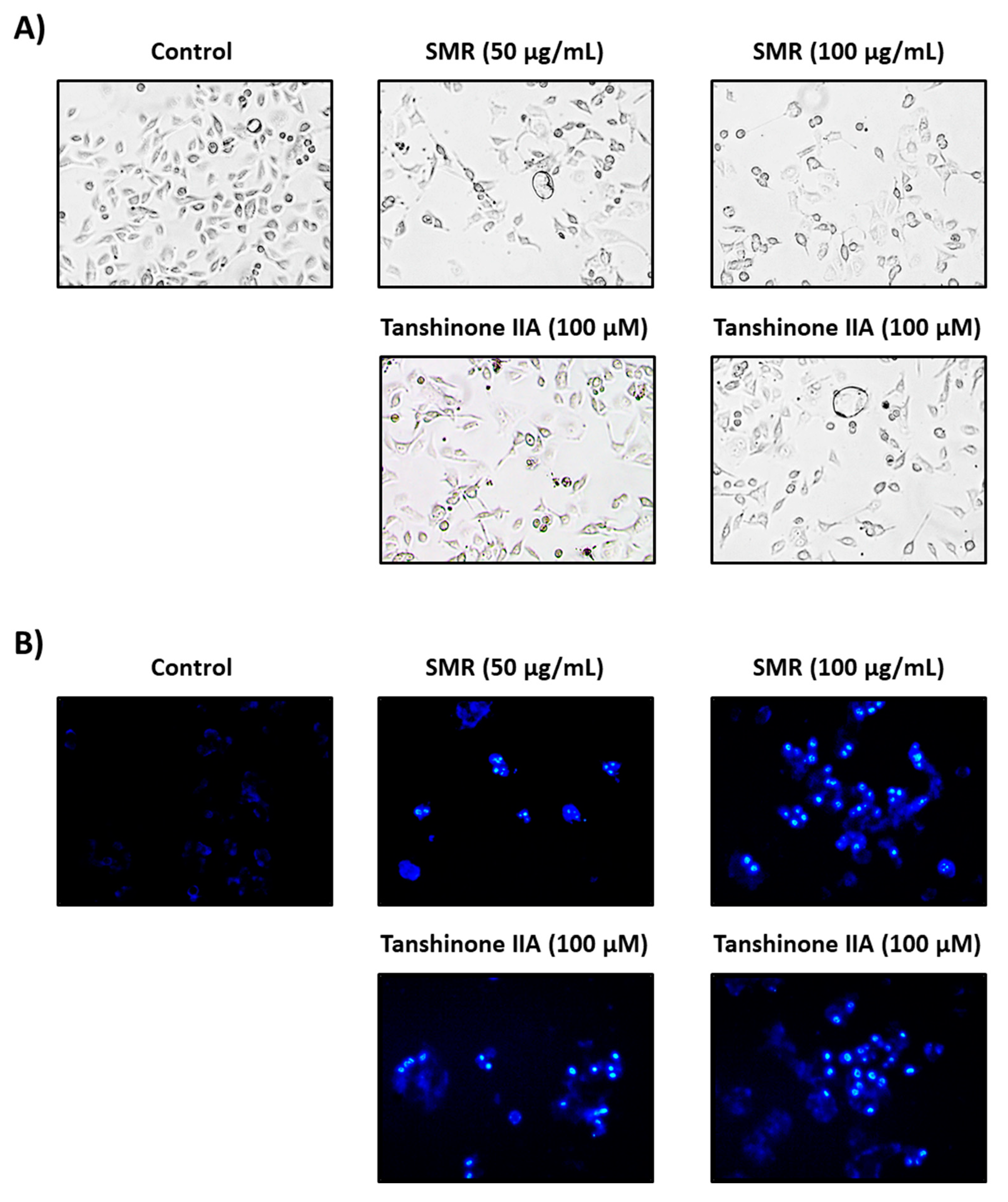
2.7. Hoechst 33342 Cell Staining
2.8. Western Blotting
2.9. Statistical Analysis
3. Results and Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheraghi, O.; Dehghan, G.; Mahdavi, M.; Rahbarghazi, R.; Rezabakhsh, A.; Charoudeh, H.N.; Iranshahi, M.; Montazersaheb, S. Potent anti-angiogenic and cytotoxic effect of conferone on human colorectal adenocarcinoma HT-29 cells. Phytomedicine 2016, 23, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Mignani, S.; Rodrigues, J.; Tomas, H.; Zablocka, M.; Shi, X.; Caminade, A.-M.; Majoral, J.-P. Dendrimers in combination with natural products and analogues as anti-cancer agents. Chem. Soc. Rev. 2018, 47, 514–532. [Google Scholar] [CrossRef]
- Yang, E.-J.; An, J.-H.; Son, Y.K.; Yeo, J.-H.; Song, K.-S. The cytotoxic constituents of Betula platyphylla and their effects on human lung A549 cancer cells. Nat. Prod. Sci. 2018, 24, 219–224. [Google Scholar] [CrossRef] [Green Version]
- Ahuja, A.; Kim, J.H.; Kim, J.H.; Yi, Y.S.; Cho, J.Y. Functional role of ginseng-derived compounds in cancer. J. Ginseng Res. 2018, 42, 248–254. [Google Scholar] [CrossRef]
- Meeran, S.M.; Ahmed, A.; Tollefsbol, T.O. Epigenetic targets of bioactive dietary components for cancer prevention and therapy. Clin. Epigenetics 2010, 1, 101–116. [Google Scholar] [CrossRef] [Green Version]
- Gezici, S.; Şekeroğlu, N. Current perspectives in the application of medicinal plants against cancer: Novel therapeutic agents. Anticancer Agents Med. Chem. 2019, 19, 101–111. [Google Scholar] [CrossRef]
- Wang, Z.J.; Cui, L.J.; Chen, C.X.; Liu, J.; Yan, Y.P.; Wang, Z.Z. Down regulation of cinnamoyl CoA reductase affects lignin and phenolic acids biosynthesis in Salvia miltiorrhiza Bunge plant. Plant Mol. Biol. Rep. 2012, 30, 1229–1236. [Google Scholar] [CrossRef]
- Guon, T.-E.; Chung, H.S. Induction of apoptosis with Moringa oleifera fruits in HCT116 human colon cancer cells via intrinsic pathway. Nat. Prod. Sci. 2017, 23, 227–234. [Google Scholar] [CrossRef] [Green Version]
- Rahman, I.; Chung, S. Dietary polyphenols, deacetylases and chromatin remodeling in inflammation. J. Nutrigenet Nutrigenomics 2010, 3, 220–230. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.H.; Jang, M.; Nah, S.Y.; Oh, S.; Cho, I.H. Multitarget effects of Korean Red Ginseng in animal model of Parkinson’s disease: Antiapoptosis, antioxidant, antiinflammation, and maintenance of blood-brain barrier integrity. J. Ginseng Res. 2018, 42, 379–388. [Google Scholar] [CrossRef]
- Zhao, W.; Yuan, Y.; Zhao, H.; Han, Y.; Chen, X. Aqueous extract of Salvia miltiorrhiza Bunge-Radix Puerariae herb pair ameliorates diabetic vascular injury by inhibiting oxidative stress in streptozotocin-induced diabetic rats. Food Chem. Toxicol. 2019, 129, 97–107. [Google Scholar] [CrossRef]
- Li, W.; Jiang, Y.H.; Wang, Y.; Zhao, M.; Hou, G.J.; Hu, H.Z.; Zhou, L. Protective Effects of Combination of Radix Astragali and Radix Salviae Miltiorrhizae on Kidney of Spontaneously Hypertensive Rats and Renal Intrinsic Cells. Chin. J. Integr. Med. 2019. (Epub ahead of print). [Google Scholar] [CrossRef]
- Zhang, L.J.; Chen, L.; Lu, Y.; Wu, J.M.; Xu, B.; Sun, Z.G.; Zheng, S.Z.; Wang, A.Y. Danshensu has anti-tumor activity in B16F10 melanoma by inhibiting angiogenesis and tumor cell invasion. Eur. J. Pharmacol. 2010, 643, 195–201. [Google Scholar] [CrossRef]
- Gu, M.; Zhang, G.; Su, Z.; Ouyang, F. Identification of major active constituents in the fingerprint of Salvia miltiorrhiza Bunge developed by high-speed counter-current chromatography. J. Chromatogr. A 2004, 1041, 239–243. [Google Scholar] [CrossRef]
- Li, Y.G.; Song, L.; Liu, M.; Hu, Z.B.; Wang, Z.T. Advancement in analysis of Salviae miltiorrhizae Radix et Rhizoma (Danshen). J. Chromatogr. A 2009, 1216, 1941–1953. [Google Scholar] [CrossRef]
- Bae, W.J.; Choi, J.B.; Kim, K.S.; Syn, H.U.; Hong, S.H.; Lee, J.Y.; Hwang, T.K.; Hwang, S.Y.; Wang, Z.P.; Kim, S.W. Inhibition of proliferation of prostate cancer cell line DU-145 in vitro and in vivo using Salvia miltiorrhiza Bunge. Chin. J. Integr. Med. 2017, 1, 1. [Google Scholar] [CrossRef]
- Luo, Y.; Feng, Y.; Song, L.; He, G.Q.; Li, S.; Bai, S.S.; Huang, Y.J.; Li, S.Y.; Almutairi, M.M.; Shi, H.L.; et al. A network pharmacology-based study on the anti-hepatoma effect of Radix Salviae Miltiorrhizae. Chin. Med. 2019, 14, 27. [Google Scholar] [CrossRef] [Green Version]
- Trinh, T.A.; Park, E.-J.; Lee, D.; Song, J.H.; Lee, H.L.; Kim, K.H.; Kim, Y.; Jung, K.; Kang, K.S.; Yoo, J.-E. Estrogenic activity of Sanguiin H-6 through activation of estrogen receptor α Coactivator-binding Site. Nat. Prod. Sci. 2019, 25, 28–33. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, D.W.; Jung, B.H.; Lee, J.H.; Lee, H.; Hwang, G.S.; Kang, K.S.; Lee, J.W. Ginsenoside Rb2 suppresses the glutamate-mediated oxidative stress and neuronal cell death in HT22 cells. J Ginseng Res 2019, 43, 326–334. [Google Scholar] [CrossRef]
- Lee, D.; Lee, D.S.; Jung, K.; Hwang, G.S.; Lee, H.L.; Yamabe, N.; Lee, H.J.; Eom, D.W.; Kim, K.H.; Kang, K.S. Protective effect of ginsenoside Rb1 against tacrolimus-induced apoptosis in renal proximal tubular LLC-PK1 cells. J. Ginseng Res. 2018, 42, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Park, H.-J.; Jung, H.A.; Choi, J.S. Estragole exhibits anti-inflammatory activity with the regulation of NF-κB and Nrf-2 signaling pathways in LPS-induced RAW 264.7 cells. Nat. Prod. Sci. 2018, 24, 13–20. [Google Scholar] [CrossRef] [Green Version]
- Debatin, K.-M. Activation of apoptosis pathways by anticancer treatment. Toxicol. Lett. 2000, 112, 41–48. [Google Scholar] [CrossRef]
- Bai, R.; Li, W.; Li, Y.; Ma, M.; Wang, Y.; Zhang, J.; Hu, F. Cytotoxicity of two water-soluble polysaccharides from Codonopsis pilosula Nannf. var. modesta (Nannf.) LT Shen against human hepatocellular carcinoma HepG2 cells and its mechanism. Int. J. Biol. Macromol. 2018, 120, 1544–1550. [Google Scholar] [CrossRef]
- Ghasemian, M.; Mahdavi, M.; Zare, P.; Feizi, M.A.H. Spiroquinazolinone-induced cytotoxicity and apoptosis in K562 human leukemia cells: Alteration in expression levels of Bcl-2 and Bax. J. Toxicol. Sci. 2015, 40, 115–126. [Google Scholar] [CrossRef] [Green Version]
- Wong, R.S.Y. Apoptosis in cancer: From pathogenesis to treatment. JECCR 2011, 30, 87. [Google Scholar] [CrossRef] [Green Version]
- Adams, J.M.; Cory, S. The BCL-2 arbiters of apoptosis and their growing role as cancer targets. Cell Death Differ 2018, 25, 27. [Google Scholar] [CrossRef] [Green Version]
- Waziri, P.M.; Abdullah, R.; Rosli, R.; Omar, A.R.; Abdul, A.B.; Kassim, N.K.; Malami, I.; Etti, I.C.; Sani, J.A.M.; Lila, M.A.M. Clausenidin induces caspase 8-dependent apoptosis and suppresses production of VEGF in liver cancer cells. Asian Pac. J. Cancer Prev. 2018, 19, 917. [Google Scholar]
- Shamas-Din, A.; Kale, J.; Leber, B.; Andrews, D.W. Mechanisms of action of Bcl-2 family proteins. Cold Spring Harb. Perspect. Biol. 2013, 5, a008714. [Google Scholar] [CrossRef] [Green Version]
- Brentnall, M.; Rodriguez-Menocal, L.; De Guevara, R.L.; Cepero, E.; Boise, L.H. Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biol. 2013, 14, 32. [Google Scholar] [CrossRef] [Green Version]
- Keskin-Aktan, A.; Akbulut, K.G.; Yazici-Mutlu, Ç.; Sonugur, G.; Ocal, M.; Akbulut, H. The effects of melatonin and curcumin on the expression of SIRT2, Bcl-2 and Bax in the hippocampus of adult rats. Brain Res. Bull 2018, 137, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Papadatos-Pastos, D.; Rabbie, R.; Ross, P.; Sarker, D. The role of the PI3K pathway in colorectal cancer. Crit. Rev. Oncol/Hematol. 2015, 94, 18–30. [Google Scholar] [CrossRef] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, B.; Lee, S.; Seo, C.-S.; Kang, K.S.; Choi, Y.-K. Analysis and Identification of Active Compounds from Salviae miltiorrhizae Radix Toxic to HCT-116 Human Colon Cancer Cells. Appl. Sci. 2020, 10, 1304. https://doi.org/10.3390/app10041304
Kang B, Lee S, Seo C-S, Kang KS, Choi Y-K. Analysis and Identification of Active Compounds from Salviae miltiorrhizae Radix Toxic to HCT-116 Human Colon Cancer Cells. Applied Sciences. 2020; 10(4):1304. https://doi.org/10.3390/app10041304
Chicago/Turabian StyleKang, Bohyung, Sullim Lee, Chang-Seob Seo, Ki Sung Kang, and You-Kyung Choi. 2020. "Analysis and Identification of Active Compounds from Salviae miltiorrhizae Radix Toxic to HCT-116 Human Colon Cancer Cells" Applied Sciences 10, no. 4: 1304. https://doi.org/10.3390/app10041304