The Protective Effect of New Carnosine-Hyaluronic Acid Conjugate on the Inflammation and Cartilage Degradation in the Experimental Model of Osteoarthritis
Abstract
:1. Introduction
2. Material and Methods
2.1. Animals
2.2. Osteoarthritis Induction and Treatment
2.3. Experimental Groups
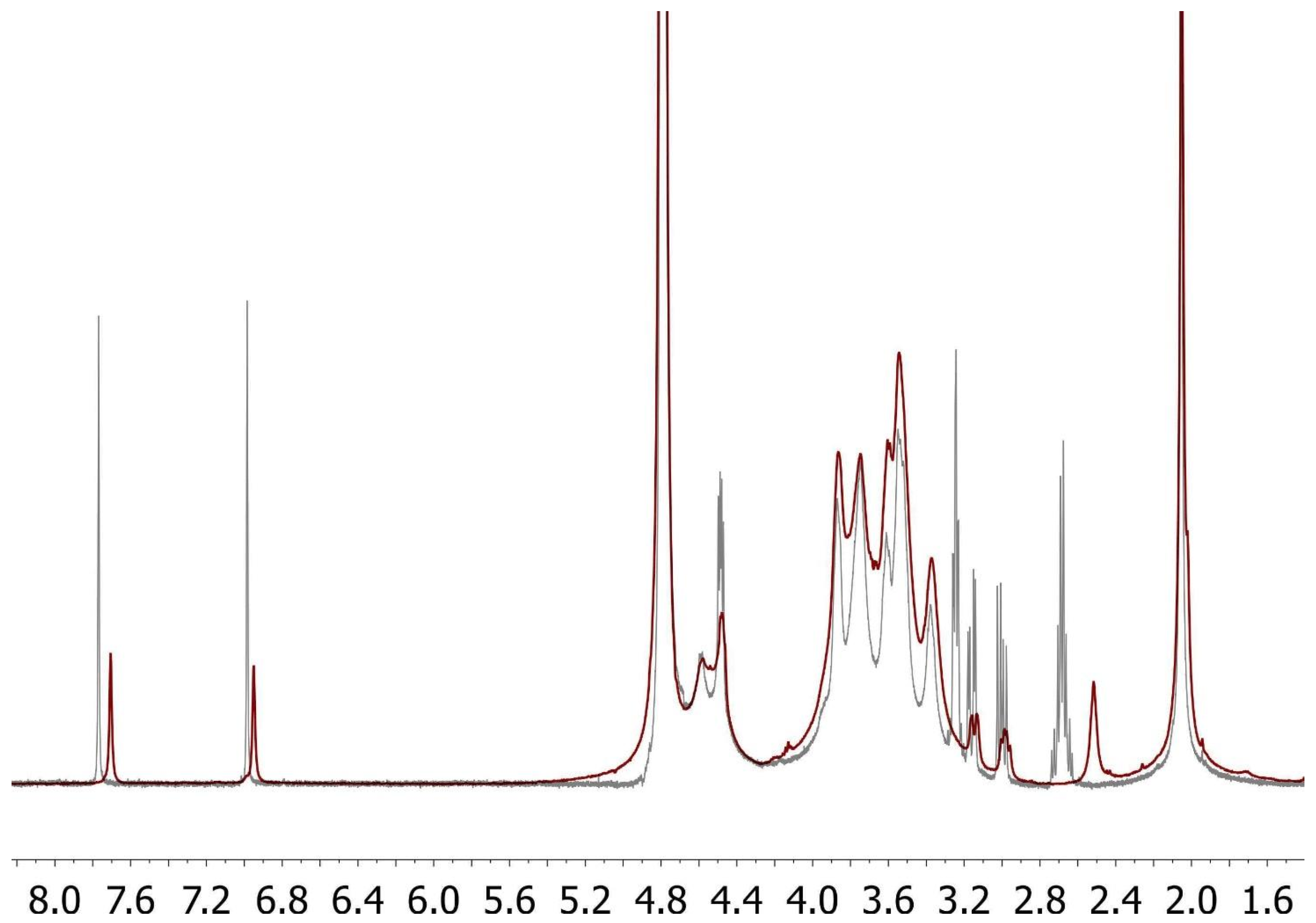
2.4. Synthesis of FidHycarn
3. Behavioral Analysis
3.1. Evaluation of Pain-Related Behavior
3.2. Macroscopic Examination of the MIA Induced OA Site
3.3. Radiographic Analysis
3.4. Histological Investigation
3.5. Immunohistochemistry
3.6. Measurement of Cytokines
3.7. Measurement of Chemokines
4. Materials
5. Data Evaluation
6. Results
6.1. Effects of FidHycarn on Thermal Hyperalgesia and Weight Distribution of the Hind Paw after OA Induction
6.2. Effects of FidHycarn on Macroscopic and Radiographic Analysis after MIA Injection
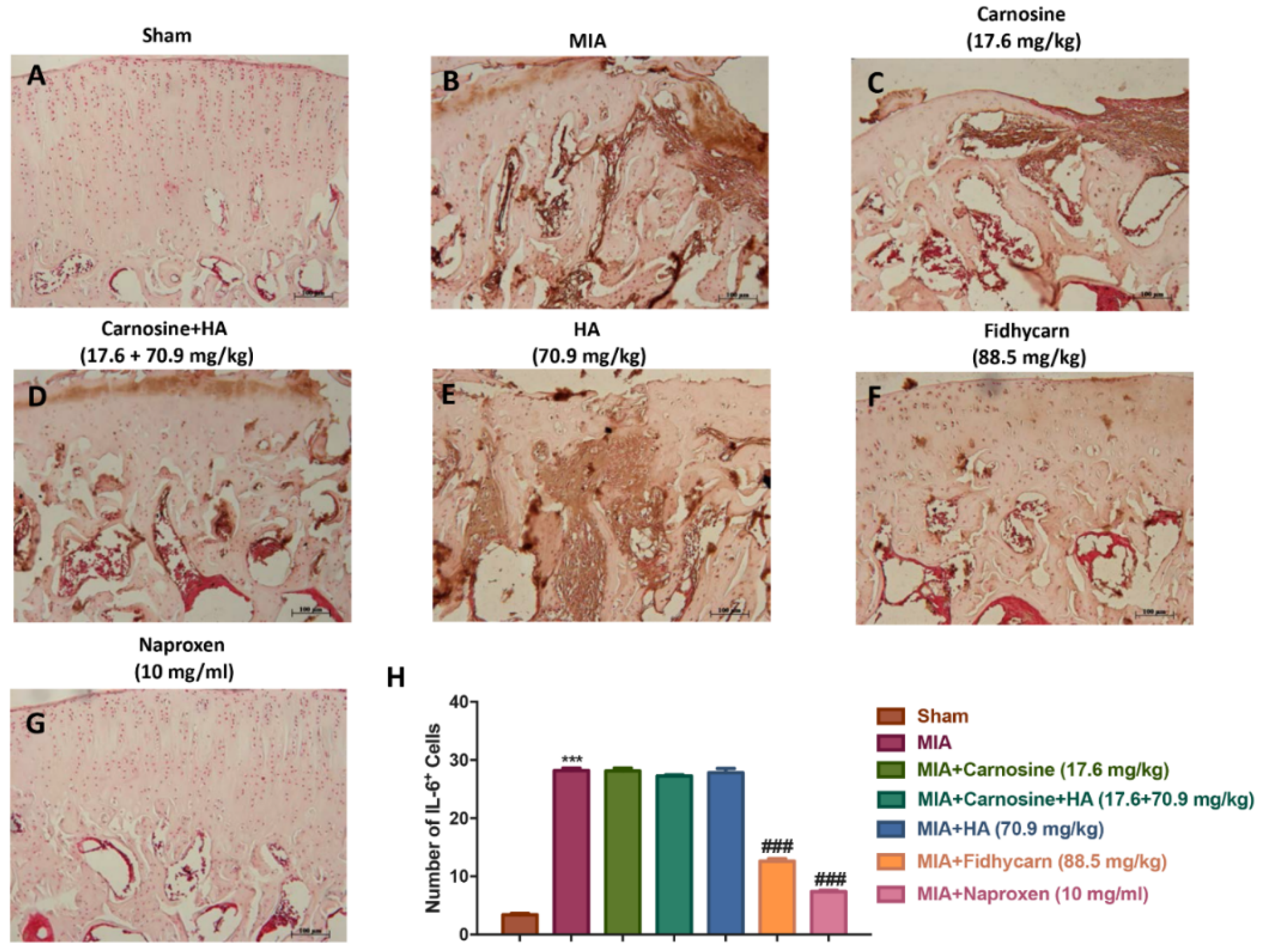
6.3. Effects of FidHycarn on Histological Damage and Cartilage Degeneration after MIA Injection
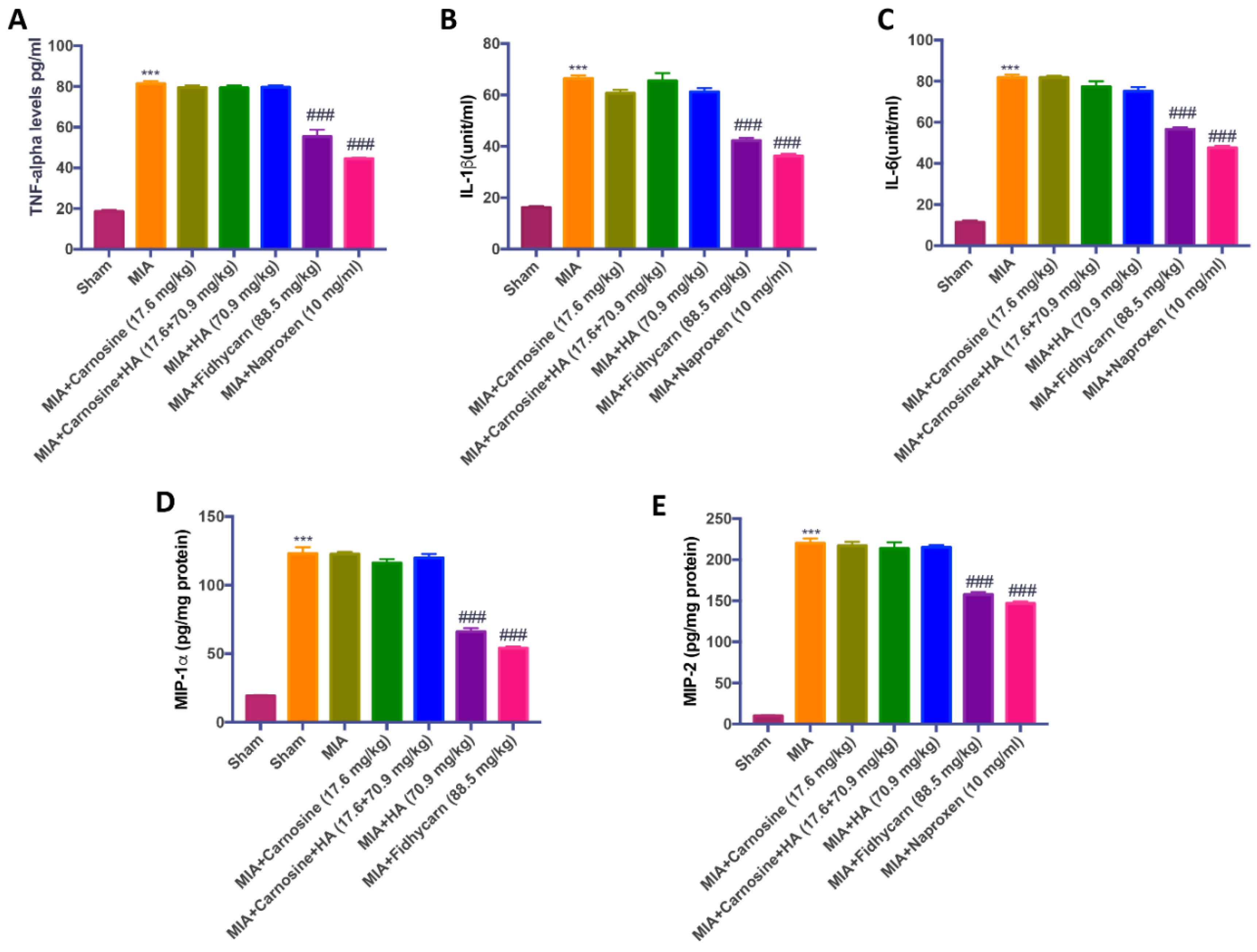
6.4. Effects of FidHycarn on Cytokine and Chemokine Levels after OA Induction
6.5. The Effects of FidHycarn on Nitrotyrosine, INOS, IL-1β, and IL-6 Expression after OA Induction
7. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bank, R.A.; Soudry, M.; Maroudas, A.; Mizrahi, J.; TeKoppele, J.M. The increased swelling and instantaneous deformation of osteoarthritic cartilage is highly correlated with collagen degradation. Arthritis Rheum. 2000, 43, 2202–2210. [Google Scholar] [CrossRef]
- Nelson, F.; Dahlberg, L.; Laverty, S.; Reiner, A.; Pidoux, I.; Ionescu, M.; Fraser, G.L.; Brooks, E.; Tanzer, M.; Rosenberg, L.C.; et al. Evidence for altered synthesis of type II collagen in patients with osteoarthritis. J. Clin. Investig. 1998, 102, 2115–2125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldring, M.B.; Marcu, K.B. Cartilage homeostasis in health and rheumatic diseases. Arthritis Res. Ther. 2009, 11, 224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abramson, S.B.; Attur, M. Developments in the scientific understanding of osteoarthritis. Arthritis Res. Ther. 2009, 11, 227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, R.; Sylvester, J.; Ahmad, M.; Zafarullah, M. Involvement of H-Ras and reactive oxygen species in proinflammatory cytokine-induced matrix metalloproteinase-13 expression in human articular chondrocytes. Arch. Biochem. Biophys. 2011, 507, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Kar, S.; Subbaram, S.; Carrico, P.M.; Melendez, J.A. Redox-control of matrix metalloproteinase-1: A critical link between free radicals, matrix remodeling and degenerative disease. Respir. Physiol. Neurobiol. 2010, 174, 299–306. [Google Scholar] [CrossRef] [Green Version]
- Tiku, M.L.; Shah, R.; Allison, G.T. Evidence linking chondrocyte lipid peroxidation to cartilage matrix protein degradation. Possible role in cartilage aging and the pathogenesis of osteoarthritis. J. Biol. Chem. 2000, 275, 20069–20076. [Google Scholar] [CrossRef] [Green Version]
- Seitz, M.; Loetscher, P.; Dewald, B.; Towbin, H.; Rordorf, C.; Gallati, H.; Baggiolini, M.; Gerber, N.J. Methotrexate action in rheumatoid arthritis: Stimulation of cytokine inhibitor and inhibition of chemokine production by peripheral blood mononuclear cells. Rheumatology 1995, 34, 602–609. [Google Scholar] [CrossRef] [Green Version]
- Koda, M.; Yoshino, S.; Nakamura, H.; Asano, G. Effects of disease modifying antirheumatic drugs (DMARDs) and DEX on IL-1 beta and IL-6 production by IL-1 beta stimulated synovial culture cells. Nihon Daigaku Zasshi 1996, 63, 419–423. [Google Scholar] [CrossRef] [Green Version]
- Fries, J.F.; Williams, C.A.; Ramey, D.R.; Bloch, D.A. The relative toxicity of alternative therapies for rheumatoid arthritis: Implications for the therapeutic progression. Semin. Arthritis Rheum. 1993, 23, 68–73. [Google Scholar] [CrossRef]
- Soltes, L.; Kogan, G. Catabolism of hyaluronan: Involvement of transition metals. Interdiscip. Toxicol. 2009, 2, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Boldyrev, A.A. Protection of proteins from oxidative stress: A new illusion or a novel strategy? Ann. N. Y. Acad. Sci. 2005, 1057, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Vonsy, J.L.; Ghandehari, J.; Dickenson, A.H. Differential analgesic effects of morphine and gabapentin on behavioural measures of pain and disability in a model of osteoarthritis pain in rats. Eur. J. Pain 2009, 13, 786–793. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Okun, A.; Ren, J.; Guo, R.C.; Ossipov, M.H.; Xie, J.; King, T.; Porreca, F. Ongoing pain in the MIA model of osteoarthritis. Neurosci. Lett. 2011, 493, 72–75. [Google Scholar] [CrossRef] [Green Version]
- Schiavinato, A.I.; Greco, V.I.; Messina, L.I.; Vaccaro, S.I.; Rizzarelli, E.I.; Sciuto, S. Pharmaceutical Composition Containing Hyaluronic Acid and Carnosine and Relative Use. Patent WO2019069258, 11 April 2019. [Google Scholar]
- Hargreaves, K.; Dubner, R.; Brown, F.; Flores, C.; Joris, J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 1988, 32, 77–88. [Google Scholar] [CrossRef]
- Izumi, M.; Ikeuchi, M.; Ji, Q.; Tani, T. Local ASIC3 modulates pain and disease progression in a rat model of osteoarthritis. J. Biomed. Sci. 2012, 19, 77. [Google Scholar] [CrossRef] [Green Version]
- Kuroda, R.; Usas, A.; Kubo, S.; Corsi, K.; Peng, H.R.; Rose, T.; Cummins, J.; Fu, F.H.; Huard, J. Cartilage repair using bone morphogenetic protein 4 and muscle-derived stem cells. Arthritis Rheum. 2006, 54, 433–442. [Google Scholar] [CrossRef]
- Ahmed, A.S.; Li, J.; Erlandsson-Harris, H.; Stark, A.; Bakalkin, G.; Ahmed, M. Suppression of pain and joint destruction by inhibition of the proteasome system in experimental osteoarthritis. Pain 2012, 153, 18–26. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, Q.; Liu, Z.L.; Lim, L.; Chen, W.H.; Lin, N. Treatment with SiMiaoFang, an anti-arthritis chinese herbal formula, inhibits cartilage matrix degradation in osteoarthritis rat model. Rejuvenation Res. 2013, 16, 364–376. [Google Scholar] [CrossRef] [Green Version]
- Di Paola, R.; Fusco, R.; Impellizzeri, D.; Cordaro, M.; Britti, D.; Morittu, V.M.; Evangelista, M.; Cuzzocrea, S. Adelmidrol, in combination with hyaluronic acid, displays increased anti-inflammatory and analgesic effects against monosodium iodoacetate-induced osteoarthritis in rats. Arthritis Res. Ther. 2016, 18, 291. [Google Scholar] [CrossRef] [Green Version]
- Sawant, S.; Gokulan, R.; Dongre, H.; Vaidya, M.; Chaukar, D.; Prabhash, K.; Ingle, A.; Joshi, S.; Dange, P.; Joshi, S.; et al. Prognostic role of Oct4, CD44 and c-Myc in radio-chemo-resistant oral cancer patients and their tumourigenic potential in immunodeficient mice. Clin. Oral Investig. 2016, 20, 43–56. [Google Scholar] [CrossRef]
- Varghese, F.; Bukhari, A.B.; Malhotra, R.; De, A. IHC Profiler: An open source plugin for the quantitative evaluation and automated scoring of immunohistochemistry images of human tissue samples. PLoS ONE 2014, 9, e96801. [Google Scholar] [CrossRef] [Green Version]
- Seed, S.M.; Dunican, K.C.; Lynch, A.M.; Desilets, A.R. An update in options for the treatment of pain: A review of new opioid formulations. Hosp. Pract. 2012, 40, 166–175. [Google Scholar] [CrossRef]
- Lawrence, R.C.; Felson, D.T.; Helmick, C.G.; Arnold, L.M.; Choi, H.; Deyo, R.A.; Gabriel, S.; Hirsch, R.; Hochberg, M.C.; Hunder, G.G.; et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008, 58, 26–35. [Google Scholar] [CrossRef] [Green Version]
- Lane, N.E.; Brandt, K.; Hawker, G.; Peeva, E.; Schreyer, E.; Tsuji, W.; Hochberg, M.C. OARSI-FDA initiative: Defining the disease state of osteoarthritis. Osteoarthr. Cartil. 2011, 19, 478–482. [Google Scholar] [CrossRef] [Green Version]
- Sinusas, K. Osteoarthritis: Diagnosis and treatment. Am. Fam. Physician 2012, 85, 49–56. [Google Scholar]
- Egan, B.A.; Mentes, J.C. Benefits of physical activity for knee osteoarthritis: A brief review. J. Gerontol. Nurs. 2010, 36, 9–14. [Google Scholar] [CrossRef]
- Lim, A.Y.; Doherty, M. What of guidelines for osteoarthritis? Int. J. Rheum. Dis. 2011, 14, 136–144. [Google Scholar] [CrossRef]
- Woodcock, J. A difficult balance--pain management, drug safety, and the FDA. N. Engl. J. Med. 2009, 361, 2105–2107. [Google Scholar] [CrossRef] [Green Version]
- Scheiman, J.M.; Sidote, D. Which NSAID for your patient with osteoarthritis? J. Fam. Pract. 2010, 59, E1–E6. [Google Scholar]
- Howes, F.; Buchbinder, R.; Winzenberg, T.B. Opioids for osteoarthritis? Weighing benefits and risks: A Cochrane Musculoskeletal Group review. J. Fam. Pract. 2011, 60, 206–212. [Google Scholar] [PubMed]
- Vista, E.S.; Lau, C.S. What about supplements for osteoarthritis? A critical and evidenced-based review. Int. J. Rheum. Dis. 2011, 14, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Busija, L.; Bridgett, L.; Williams, S.R.; Osborne, R.H.; Buchbinder, R.; March, L.; Fransen, M. Osteoarthritis. Best Pract. Res. Clin. Rheumatol. 2010, 24, 757–768. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, M.; Martel-Pelletier, J.; Lajeunesse, D.; Pelletier, J.P.; Fahmi, H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 33–42. [Google Scholar] [CrossRef]
- Poulet, B.; Beier, F. Targeting oxidative stress to reduce osteoarthritis. Arthritis Res. Ther. 2016, 18, 32. [Google Scholar] [CrossRef] [Green Version]
- Palmieri, B.; Lodi, D.; Capone, S. Osteoarthritis and degenerative joint disease: Local treatment options update. Acta Biomed 2010, 81, 94–100. [Google Scholar]
- Iannitti, T.; Lodi, D.; Palmieri, B. Intra-articular injections for the treatment of osteoarthritis: Focus on the clinical use of hyaluronic acid. Drugs R D 2011, 11, 13–27. [Google Scholar] [CrossRef]
- Huang, S.L.; Ling, P.X.; Zhang, T.M. Oral absorption of hyaluronic acid and phospholipids complexes in rats. World J. Gastroenterol. 2007, 13, 945–949. [Google Scholar] [CrossRef] [Green Version]
- Bannuru, R.R.; Natov, N.S.; Dasi, U.R.; Schmid, C.H.; McAlindon, T.E. Therapeutic trajectory following intra-articular hyaluronic acid injection in knee osteoarthritis--meta-analysis. Osteoarthr. Cartil. 2011, 19, 611–619. [Google Scholar] [CrossRef] [Green Version]
- Cooper, C.; Rannou, F.; Richette, P.; Bruyere, O.; Al-Daghri, N.; Altman, R.D.; Brandi, M.L.; Basset, S.C.; Herrero-Beaumont, G.; Migliore, A.; et al. Use of Intraarticular Hyaluronic Acid in the Management of Knee Osteoarthritis in Clinical Practice. Arthritis Care Res. 2017, 69, 1287–1296. [Google Scholar] [CrossRef]
- Bowman, S.; Awad, M.E.; Hamrick, M.W.; Hunter, M.; Fulzele, S. Recent advances in hyaluronic acid based therapy for osteoarthritis. Clin. Transl. Med. 2018, 7, s40169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ponist, S.; Drafi, F.; Kuncirova, V.; Mihalova, D.; Rackova, L.; Danisovic, L.; Ondrejickova, O.; Tumova, I.; Trunova, O.; Fedorova, T.; et al. Effect of Carnosine in Experimental Arthritis and on Primary Culture Chondrocytes. Oxidative Med. Cell. Longev. 2016, 2016, 8470589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fatih Aydin, A.; Kucukgergin, C.; Bingul, I.; Dogan-Ekici, I.; Dogru-Abbasoglu, S.; Uysal, M. Effect of Carnosine on Renal Function, Oxidation and Glycation Products in the Kidneys of High-Fat Diet/Streptozotocin-Induced Diabetic Rats. Exp. Clin. Endocrinol. Diabetes 2017, 125, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Hipkiss, A.R. On the enigma of carnosine’s anti-ageing actions. Exp. Gerontol. 2009, 44, 237–242. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Wang, Y.; Kong, Y.; Zhang, X.; Zhang, H.; Gang, Y.; Bai, L. Carnosine Prevents Type 2 Diabetes-Induced Osteoarthritis Through the ROS/NF-kappaB Pathway. Front. Pharmacol. 2018, 9, 598. [Google Scholar] [CrossRef] [Green Version]
- Suantawee, T.; Tantavisut, S.; Adisakwattana, S.; Tanavalee, A.; Yuktanandana, P.; Anomasiri, W.; Deepaisarnsakul, B.; Honsawek, S. Oxidative stress, vitamin e, and antioxidant capacity in knee osteoarthritis. J. Clin. Diagn. Res. JCDR 2013, 7, 1855–1859. [Google Scholar] [CrossRef]
- Chevalier, X.; Eymard, F.; Richette, P. Biologic agents in osteoarthritis: Hopes and disappointments. Nat. Rev. Rheumatol. 2013, 9, 400–410. [Google Scholar] [CrossRef]
- Scanzello, C.R. Chemokines and inflammation in osteoarthritis: Insights from patients and animal models. J. Orthop. Res. 2017, 35, 735–739. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.K.; Ke, Y.; Wang, B.; Lin, J.H. The role of MCP-1-CCR2 ligand-receptor axis in chondrocyte degradation and disease progress in knee osteoarthritis. Biol. Res. 2015, 48, 64. [Google Scholar] [CrossRef] [Green Version]
- Campo, G.M.; Avenoso, A.; Campo, S.; D’Ascola, A.; Ferlazzo, A.M.; Calatroni, A. The antioxidant and antifibrogenic effects of the glycosaminoglycans hyaluronic acid and chondroitin-4-sulphate in a subchronic rat model of carbon tetrachloride-induced liver fibrogenesis. Chem. Biol. Interact. 2004, 148, 125–138. [Google Scholar] [CrossRef]
- Braga, P.C.; Dal Sasso, M.; Lattuada, N.; Greco, V.; Sibilia, V.; Zucca, E.; Stucchi, L.; Ferro, E.; Ferrucci, F. Antioxidant activity of hyaluronic acid investigated by means of chemiluminescence of equine neutrophil bursts and electron paramagnetic resonance spectroscopy. J. Vet. Pharmacol. Ther. 2015, 38, 48–54. [Google Scholar] [CrossRef]
- Treister, R.; Honigman, L.; Lawal, O.D.; Lanier, R.K.; Katz, N.P. A deeper look at pain variability and its relationship with the placebo response: Results from a randomized, double-blind, placebo-controlled clinical trial of naproxen in osteoarthritis of the knee. Pain 2019, 160, 1522–1528. [Google Scholar] [CrossRef]








© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siracusa, R.; Impellizzeri, D.; Cordaro, M.; Peritore, A.F.; Gugliandolo, E.; D’Amico, R.; Fusco, R.; Crupi, R.; Rizzarelli, E.; Cuzzocrea, S.; et al. The Protective Effect of New Carnosine-Hyaluronic Acid Conjugate on the Inflammation and Cartilage Degradation in the Experimental Model of Osteoarthritis. Appl. Sci. 2020, 10, 1324. https://doi.org/10.3390/app10041324
Siracusa R, Impellizzeri D, Cordaro M, Peritore AF, Gugliandolo E, D’Amico R, Fusco R, Crupi R, Rizzarelli E, Cuzzocrea S, et al. The Protective Effect of New Carnosine-Hyaluronic Acid Conjugate on the Inflammation and Cartilage Degradation in the Experimental Model of Osteoarthritis. Applied Sciences. 2020; 10(4):1324. https://doi.org/10.3390/app10041324
Chicago/Turabian StyleSiracusa, Rosalba, Daniela Impellizzeri, Marika Cordaro, Alessio F. Peritore, Enrico Gugliandolo, Ramona D’Amico, Roberta Fusco, Rosalia Crupi, Enrico Rizzarelli, Salvatore Cuzzocrea, and et al. 2020. "The Protective Effect of New Carnosine-Hyaluronic Acid Conjugate on the Inflammation and Cartilage Degradation in the Experimental Model of Osteoarthritis" Applied Sciences 10, no. 4: 1324. https://doi.org/10.3390/app10041324
APA StyleSiracusa, R., Impellizzeri, D., Cordaro, M., Peritore, A. F., Gugliandolo, E., D’Amico, R., Fusco, R., Crupi, R., Rizzarelli, E., Cuzzocrea, S., Vaccaro, S., Pulicetta, M., Greco, V., Sciuto, S., Schiavinato, A., Messina, L., & Di Paola, R. (2020). The Protective Effect of New Carnosine-Hyaluronic Acid Conjugate on the Inflammation and Cartilage Degradation in the Experimental Model of Osteoarthritis. Applied Sciences, 10(4), 1324. https://doi.org/10.3390/app10041324











