Fabrication of a Promising Hierarchical Porous Surface on Titanium for Promoting Biocompatibility
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimens Preparation
2.2. Surface Property Analysis
2.3. In Vitro Biocompatibility Testing
3. Results and Discussion
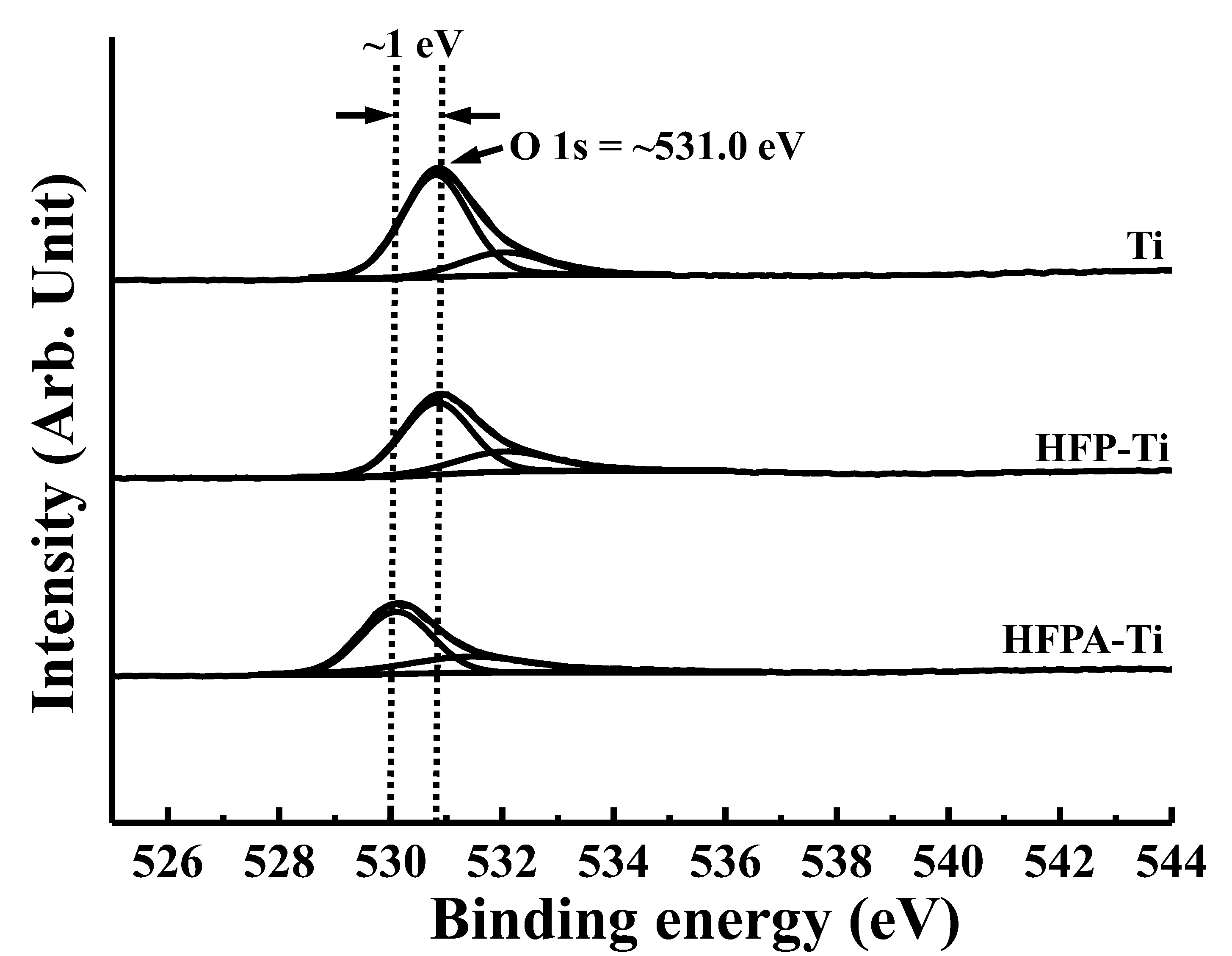
3.1. Surface Characterizations
3.2. Microstructural Variations
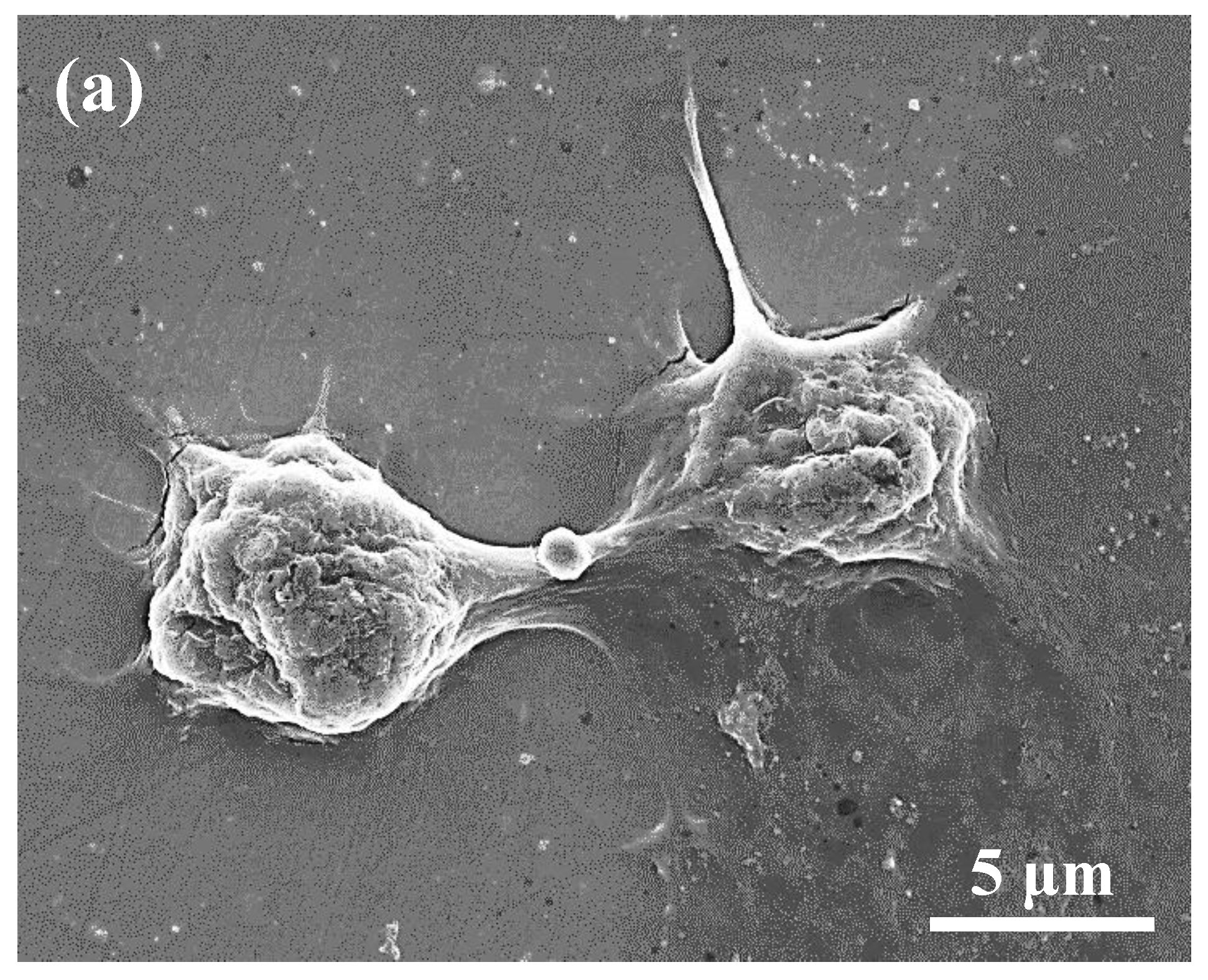
3.3. Cellular Behaviors
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Costa, B.C.; Tokuhara, C.K.; Rocha, L.A.; Oliveira, R.C.; Lisboa-Filho, P.N.; Pessoa, J.C. Vanadium ionic species from degradation of Ti-6Al-4V metallic implants: In vitro cytotoxicity and speciation evaluation. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 96, 730–739. [Google Scholar] [CrossRef] [PubMed]
- Gomes, C.C.; Moreira, L.M.; Santos, V.J.S.V.; Ramos, A.S.; Lyon, J.P.; Soares, C.P.; Santos, F.V. Assessment of the genetic risks of a metallic alloy used in medical implants. Genet. Mol. Biol. 2011, 34, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.B.; Hu, D.Y.; Wang, Q.; Hou, J.; Li, M. Diffuse polymorphic eosinophilic cellulitis in a patient with metallic alloy implants: A possible association? Int. J. Dermatol. 2011, 50, 1535–1537. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Qiu, X.; Niu, X.D.; Tian, Z.; Sun, W.; Liu, X.J.; Li, Y.D.; Li, W.R.; Meng, J. Microstructure, mechanical properties, in vitro degradation and cytotoxicity evaluations of Mg-1.5Y-1.2Zn-0.44Zr alloys for biodegradable metallic implants. Mater. Sci. Eng. C-Mater. 2013, 33, 2345–2352. [Google Scholar] [CrossRef] [PubMed]
- Bodelon, O.G.; Iglesias, C.; Garrido, J.; Clemente, C.; Garcia-Alonso, M.C.; Escudero, M.L. Analysis of metallic traces from the biodegradation of endomedullary AZ31 alloy temporary implants in rat organs after long implantation times. Biomed. Mater. 2015, 10, 045015. [Google Scholar] [CrossRef] [PubMed]
- Da Rocha, S.S.; Adabo, G.L.; Vaz, L.G.; Henriques, G.E.P. Effect of thermal treatments on tensile strength of commercially cast pure titanium and Ti-6Al-4V alloys. J. Mater. Sci.-Mater. Med. 2005, 16, 759–766. [Google Scholar] [CrossRef]
- Wu, C.M.; Peng, P.W.; Chou, H.H.; Ou, K.L.; Sugiatno, E.; Liu, C.M.; Huang, C.F. Microstructural, mechanical and biological characterizations of the promising titanium-tantalum alloy for biomedical applications. J. Alloys Compd. 2018, 735, 2604–2610. [Google Scholar] [CrossRef]
- Bahl, S.; Das, S.; Suwas, S.; Chatterjee, K. Engineering the next-generation tin containing beta titanium alloys with high strength and low modulus for orthopedic applications. J. Mech. Behav. Biomed. Mater. 2018, 78, 124–133. [Google Scholar] [CrossRef]
- Kopova, I.; Strasky, J.; Harcuba, P.; Landa, M.; Janecek, M.; Bacakova, L. Newly developed Ti-Nb-Zr-Ta-Si-Fe biomedical beta titanium alloys with increased strength and enhanced biocompatibility. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 60, 230–238. [Google Scholar] [CrossRef]
- Hou, P.J.; Ou, K.L.; Wang, C.C.; Huang, C.F.; Ruslin, M.; Sugiatno, E.; Yang, T.S.; Chou, H.H. Hybrid micro/nanostructural surface offering improved stress distribution and enhanced osseointegration properties of the biomedical titanium implant. J. Mech. Behav. Biomed. Mater. 2018, 79, 173–180. [Google Scholar] [CrossRef]
- Ou, K.L.; Weng, C.C.; Lin, Y.H.; Huang, M.S. A promising of alloying modified beta-type Titanium-Niobium implant for biomedical applications: Microstructural characteristics, in vitro biocompatibility and antibacterial performance. J. Alloys Compd. 2017, 697, 231–238. [Google Scholar] [CrossRef]
- Szewczenko, J.; Marciniak, J.; Kajzer, W.; Kajzer, A. Evaluation of Corrosion Resistance of Titanium Alloys Used for Medical Implants. Arch. Metall. Mater. 2016, 61, 695–699. [Google Scholar] [CrossRef]
- Zhou, S.X.; Wang, J.; Cai, P. Corrosion Resistance Investigation of Titanium Alloy as Tissue Engineered Bone Implant. Int. J. Electrochem. Sci. 2017, 12, 7174–7182. [Google Scholar] [CrossRef]
- Mohan, L.; Raja, M.D.; Uma, T.S.; Rajendran, N.; Anandan, C. In-Vitro Biocompatibility Studies of Plasma-Nitrided Titanium Alloy beta-21S Using Fibroblast Cells. J. Mater. Eng. Perform. 2016, 25, 1508–1514. [Google Scholar] [CrossRef]
- Markhoff, J.; Krogull, M.; Schulze, C.; Rotsch, C.; Hunger, S.; Bader, R. Biocompatibility and Inflammatory Potential of Titanium Alloys Cultivated with Human Osteoblasts, Fibroblasts and Macrophages. Materials 2017, 10, 52. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Singh, K. Review on titanium and titanium based alloys as biomaterials for orthopaedic applications. Mat. Sci. Eng. C-Mater. 2019, 102, 844–862. [Google Scholar] [CrossRef]
- Lopez, M.F.; Jimenez, J.A.; Gutierrez, A. XPS characterization of surface modified titanium alloys for use as biomaterials. Vacuum 2011, 85, 1076–1079. [Google Scholar] [CrossRef]
- Vasilescu, M.; Dobrescu, M. Titanium and Titanium Alloys as Biomaterials. Properties, Processing and Dental Applications. Metal. Int. 2010, 15, 69–73. [Google Scholar]
- Cheng, H.C.; Lee, S.Y.; Chen, C.C.; Shyng, Y.C.; Ou, K.L. Influence of hydrogen charging on the formation of nanostructural titania by anodizing with cathodic pretreatment. J. Electrochem. Soc. 2007, 154, E13–E18. [Google Scholar] [CrossRef]
- Eliaz, N. Corrosion of Metallic Biomaterials: A Review. Materials 2019, 12, 16. [Google Scholar] [CrossRef]
- Shin, Y.C.; Pang, K.M.; Han, D.W.; Lee, K.H.; Ha, Y.C.; Park, J.W.; Kim, B.; Kim, D.; Lee, J.H. Enhanced osteogenic differentiation of human mesenchymal stem cells on Ti surfaces with electrochemical nanopattern formation. Mat. Sci. Eng. C-Mater. 2019, 99, 1174–1181. [Google Scholar] [CrossRef] [PubMed]
- Suganya, P.; Venkadesh, A.; Mathiyarasu, J.; Radhakrishnan, S. MOF assisted synthesis of new porous nickel phosphate nanorods as an advanced electrode material for energy storage application. J. Solid. State Electr. 2019, 23, 3429–3435. [Google Scholar] [CrossRef]
- Yetim, A.F. Investigation of wear behavior of titanium oxide films, produced by anodic oxidation, on commercially pure titanium in vacuum conditions. Surf. Coat. Technol. 2010, 205, 1757–1763. [Google Scholar] [CrossRef]
- Velten, D.; Biehl, V.; Aubertin, F.; Valeske, B.; Possart, W.; Breme, J. Preparation of TiO2 layers on cp-Ti and Ti6Al4V by thermal and anodic oxidation and by sol-gel coating techniques and their characterization. J. Biomed. Mater. Res. 2002, 59, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.H.; Haung, C.F.; Shyu, S.S.; Chou, Y.R.; Lin, M.H.; Peng, P.W.; Ou, K.L.; Yu, C.H. Surface modification induced phase transformation and structure variation on the rapidly solidified recast layer of titanium. Mater. Charact. 2015, 106, 463–469. [Google Scholar] [CrossRef]
- Tanaka, S.; Aonuma, M.; Hirose, N.; Tanaki, T. The preparation of porous TiO2 by immersing Ti in NaOH solution. J. Electrochem. Soc. 2002, 149, D167–D171. [Google Scholar] [CrossRef]
- Kim, K.; Lee, B.A.; Piao, X.H.; Chung, H.J.; Kim, Y.J. Surface characteristics and bioactivity of an anodized titanium surface. J. Periodontal. Implant Sci. 2013, 43, 198–205. [Google Scholar] [CrossRef]
- HChiang, J.; Chou, H.H.; Ou, K.L.; Sugiatno, E.; Ruslin, M.; Waris, R.A.; Huang, C.F.; Liu, C.M.; Peng, P.W. Evaluation of Surface Characteristics and Hemocompatibility on the Oxygen Plasma-Modified Biomedical Titanium. Metals 2018, 8, 513. [Google Scholar] [CrossRef]
- Tanaka, S.; Iwatani, T.; Hirose, N.; Tanaki, T. Effect of hydrogen on the formation of porous TiO2 in alkaline solution. J. Electrochem. Soc. 2002, 149, F186–F190. [Google Scholar] [CrossRef]
- Taborelli, M.; Jobin, M.; Francois, P.; Vaudaux, P.; Tonetti, M.; Szmukler-Moncler, S.; Simpson, J.P.; Descouts, P. Influence of surface treatments developed for oral implants on the physical and biological properties of titanium. (I) Surface characterization. Clin. Oral. Implants Res. 1997, 8, 208–216. [Google Scholar] [CrossRef]
- Sul, Y.T.; Johansson, C.B.; Jeong, Y.; Albrektsson, T. The electrochemical oxide growth behaviour on titanium in acid and alkaline electrolytes. Med. Eng. Phys. 2001, 23, 329–346. [Google Scholar] [CrossRef]
- Sunny, M.C.; Sharma, C.P. Titanium-protein interaction: Changes with oxide layer thickness. J. Biomater. Appl. 1991, 6, 89–98. [Google Scholar] [CrossRef] [PubMed]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lan, W.-C.; Wang, C.-H.; Huang, B.-H.; Cho, Y.-C.; Saito, T.; Huang, C.-C.; Huang, M.-S. Fabrication of a Promising Hierarchical Porous Surface on Titanium for Promoting Biocompatibility. Appl. Sci. 2020, 10, 1363. https://doi.org/10.3390/app10041363
Lan W-C, Wang C-H, Huang B-H, Cho Y-C, Saito T, Huang C-C, Huang M-S. Fabrication of a Promising Hierarchical Porous Surface on Titanium for Promoting Biocompatibility. Applied Sciences. 2020; 10(4):1363. https://doi.org/10.3390/app10041363
Chicago/Turabian StyleLan, Wen-Chien, Chia-Hsien Wang, Bai-Hung Huang, Yen-Chun Cho, Takashi Saito, Chien-Chia Huang, and Mao-Suan Huang. 2020. "Fabrication of a Promising Hierarchical Porous Surface on Titanium for Promoting Biocompatibility" Applied Sciences 10, no. 4: 1363. https://doi.org/10.3390/app10041363
APA StyleLan, W.-C., Wang, C.-H., Huang, B.-H., Cho, Y.-C., Saito, T., Huang, C.-C., & Huang, M.-S. (2020). Fabrication of a Promising Hierarchical Porous Surface on Titanium for Promoting Biocompatibility. Applied Sciences, 10(4), 1363. https://doi.org/10.3390/app10041363




