Author Contributions
Conceptualization, A.C. and H.N.; methodology, M.K. and A.C.; validation, A.S.S., M.K. and A.C.; formal analysis, A.C.; investigation, S.M.; resources, A.C.; data curation, S.M.; writing—original draft preparation, A.C., M.K., S.M.; writing—review and editing, A.S.S.; visualization, S.M.; supervision, A.C.; project administration, M.K. All authors have read and agreed to the published version of the manuscript.
Figure 1.
Particle size distribution (PSD) of the used bentonite clay and the used sawdust (SD).
Figure 1.
Particle size distribution (PSD) of the used bentonite clay and the used sawdust (SD).
Figure 2.
Scanning electron microscopic of the (a) Bentonite and (b) Portland cement (PC).
Figure 2.
Scanning electron microscopic of the (a) Bentonite and (b) Portland cement (PC).
Figure 3.
Cured mixture (7 days) of the bentonite-slag-cement (a) 0%, (b) 3%, (c) 5%, (d) 10% and (e) 15% after sulphate attack.
Figure 3.
Cured mixture (7 days) of the bentonite-slag-cement (a) 0%, (b) 3%, (c) 5%, (d) 10% and (e) 15% after sulphate attack.
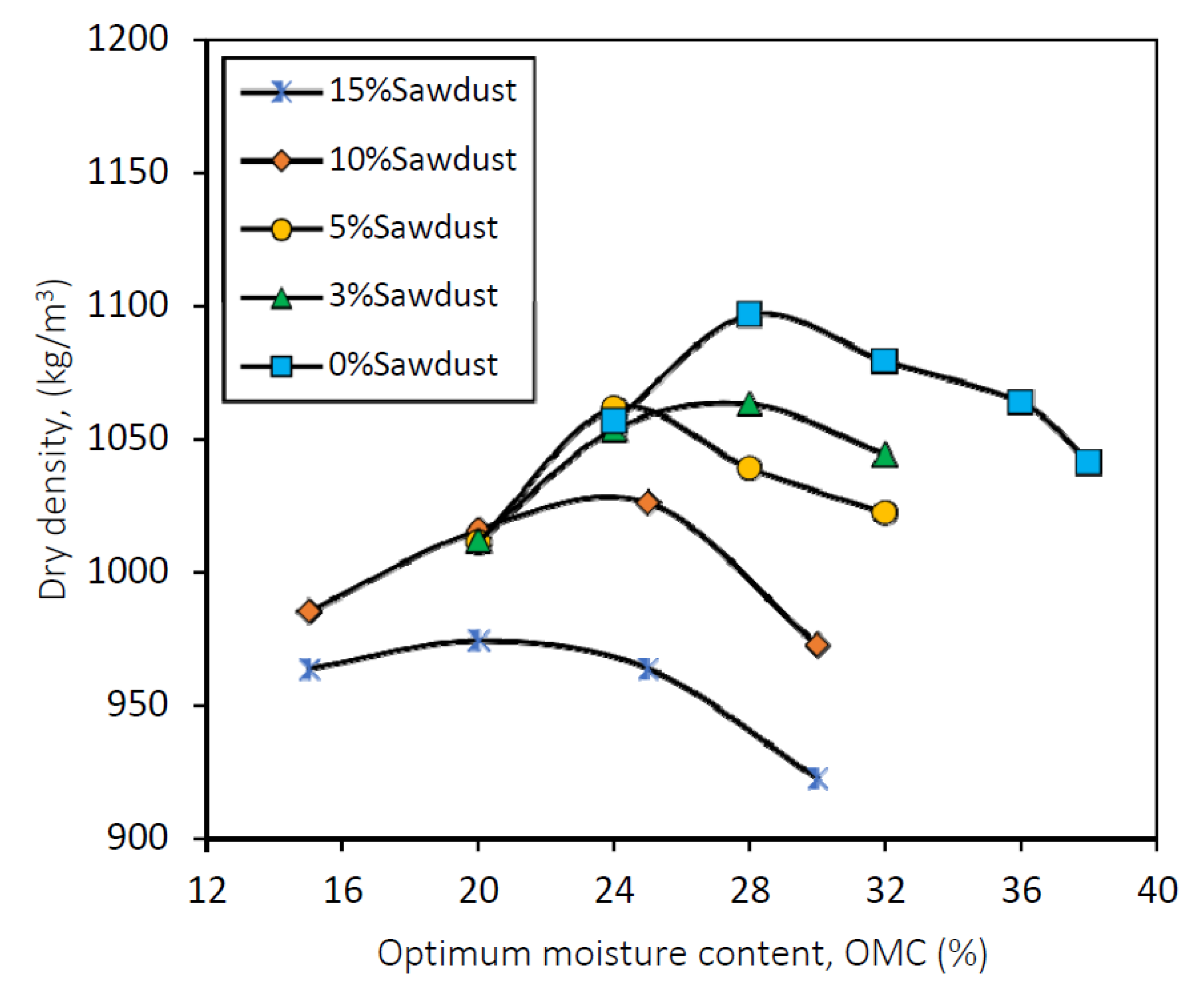
Figure 4.
Compaction curves for the used mixtures (i.e., 0%, 3%, 5%, 10% and 15% sawdust).
Figure 4.
Compaction curves for the used mixtures (i.e., 0%, 3%, 5%, 10% and 15% sawdust).
Figure 5.
Trend of variation of compaction results for 0%, 3%, 5%, 10% and 15% sawdust.
Figure 5.
Trend of variation of compaction results for 0%, 3%, 5%, 10% and 15% sawdust.
Figure 6.
Effect of sawdust addition on Unconfined Compressive Strength (UCS) peak values (3 days curing) (a) 3% PC; (b) 5% PC; (c) 7% PC.
Figure 6.
Effect of sawdust addition on Unconfined Compressive Strength (UCS) peak values (3 days curing) (a) 3% PC; (b) 5% PC; (c) 7% PC.
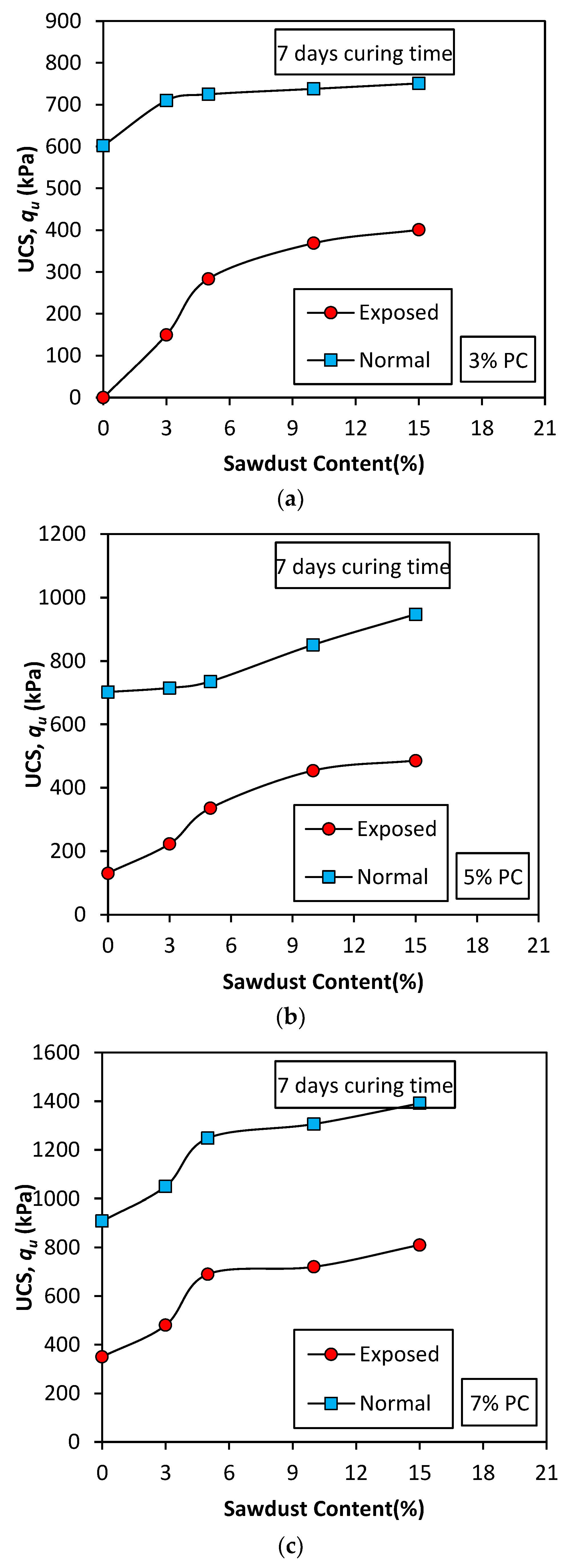
Figure 7.
Effect of sawdust addition on USC peak values (7 days curing) (a) 3% PC; (b) 5% PC; (c) 7% PC.
Figure 7.
Effect of sawdust addition on USC peak values (7 days curing) (a) 3% PC; (b) 5% PC; (c) 7% PC.
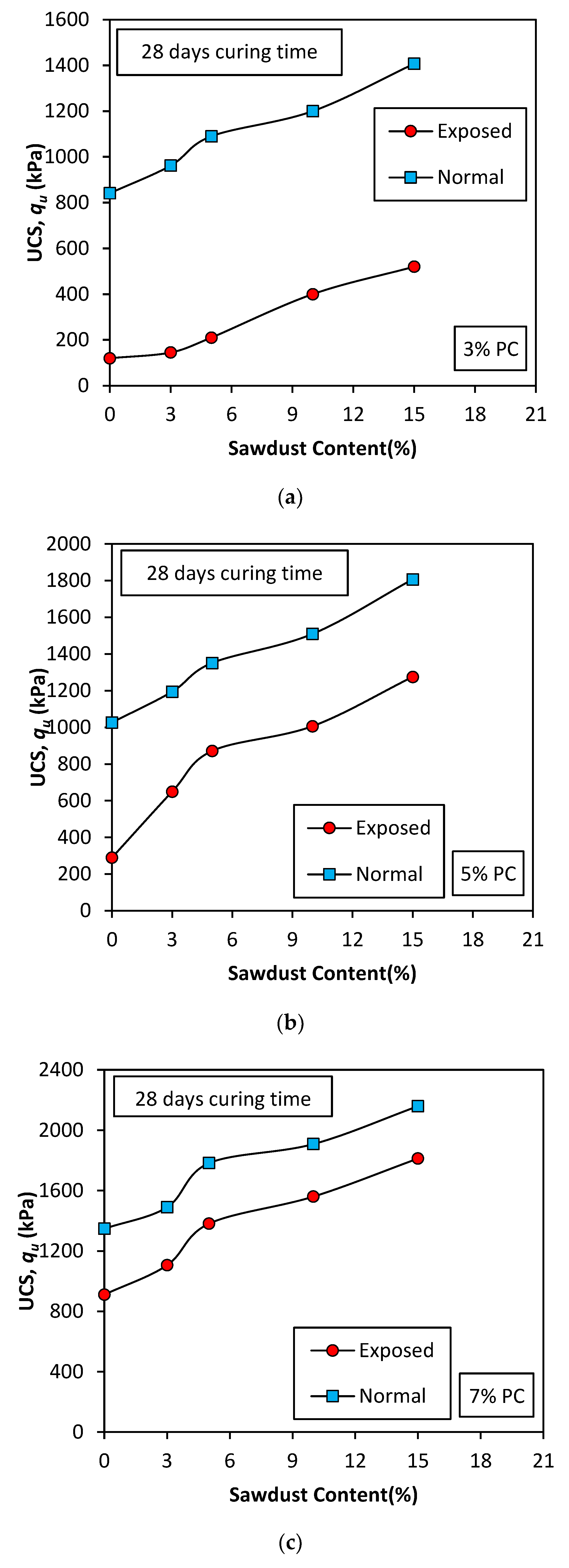
Figure 8.
Effect of sawdust addition on USC peak values (28 days curing): (a) 3% PC; (b) 5% PC; (c) 7% PC.
Figure 8.
Effect of sawdust addition on USC peak values (28 days curing): (a) 3% PC; (b) 5% PC; (c) 7% PC.
Figure 9.
Effect of sawdust addition on USC peak values (90 days curing): (a) 3% PC; (b) 5% PC; (c) 7% PC.
Figure 9.
Effect of sawdust addition on USC peak values (90 days curing): (a) 3% PC; (b) 5% PC; (c) 7% PC.
Figure 10.
Effect of sawdust addition on USC peak values before exposure to sulphate: (a) 3 days curing; (b) 7 days curing; (c) 28 days curing; (d) 90 days curing.
Figure 10.
Effect of sawdust addition on USC peak values before exposure to sulphate: (a) 3 days curing; (b) 7 days curing; (c) 28 days curing; (d) 90 days curing.
Figure 11.
Effect of sawdust addition on IMP values after exposure to sulphate: (a) 90 days curing; (b) 28 days curing; (c) 7 days curing.
Figure 11.
Effect of sawdust addition on IMP values after exposure to sulphate: (a) 90 days curing; (b) 28 days curing; (c) 7 days curing.
Figure 12.
Effect of cement addition on IMP values after exposure to sulphate: (a) 7% cement; (b) 5% cement; (c) 3% cement.
Figure 12.
Effect of cement addition on IMP values after exposure to sulphate: (a) 7% cement; (b) 5% cement; (c) 3% cement.
Figure 13.
Scanning electron microscopic (SEM) analysis of the specimen 7PC-10SD: (a) before and (b) after sulphate attack.
Figure 13.
Scanning electron microscopic (SEM) analysis of the specimen 7PC-10SD: (a) before and (b) after sulphate attack.
Table 1.
Characteristics of the used Type II PC [
21].
Table 1.
Characteristics of the used Type II PC [
21].
| Characteristics | Quantities |
|---|
| specific gravity | 2.5–3.2 |
| pH | 12 |
| Gypsum | 3% to 8% |
| Limestone | 0% to 5% |
| granulated blast furnace slag | 0% to 5% |
Table 2.
Chemical properties of bentonite [
22].
Table 2.
Chemical properties of bentonite [
22].
| Ingredients | (%) |
|---|
| (SiO2) | 63.6 |
| (Al2O3) | 14.6 |
| (Fe2O3) | 2.8 |
| (Na2O) | 1.3 |
| (MgO) | 2 |
Table 3.
Testing plan and mixtures.
Table 3.
Testing plan and mixtures.
| No. | Sample ID | Portland Cement, PC (%) | Sawdust, SD (%) | Curing Time |
|---|
| 1 | B | - | - | - |
| 1 | B-3PC | 3 | - | 7, 14, 28, 90 |
| 2 | B-5PC | 5 | - | 7, 14, 28, 90 |
| 3 | B-7PC | 7 | - | 7, 14, 28, 90 |
| 4 | B-3SD | 3, 5, 7 | 3 | 7, 14, 28, 90 |
| 5 | B-5SD | 3, 5, 7 | 5 | 7, 14, 28, 90 |
| 6 | B-10SD | 3, 5, 7 | 10 | 7, 14, 28, 90 |
| 7 | B-15SD | 3, 5, 7 | 15 | 7, 14, 28, 90 |
Table 4.
UCS results before sulphate exposure in presence of SD (3 days curing).
Table 4.
UCS results before sulphate exposure in presence of SD (3 days curing).
| SD Content | Unconfined Compressive Strength, UCS (kPa) |
|---|
| 3% PC = 180 | 5% PC = 296 | 7% PC = 352 |
|---|
| UCS (kPa) | UCS (kPa) | UCS (kPa) |
|---|
| 3% | 241 ± 2 | 322.3 ± 2 | 391 ± 4 |
| 5% | 302 ± 2 | 360.8 ± 4 | 458 ± 2 |
| 10% | 349 ± 4 | 399.9 ± 2 | 502 ± 3 |
| 15% | 401 ± 2 | 472.6 ± 3 | 574 ± 2 |
Table 5.
UCS results after sulphate exposure in presence of SD (3 days curing).
Table 5.
UCS results after sulphate exposure in presence of SD (3 days curing).
| SD Content | Unconfined Compressive Strength, UCS (kPa) |
|---|
| 3% PC = Vanished | 5% PC = Vanished | 7% PC = Vanished |
|---|
| UCS (kPa) | Improvement (%) | UCS (kPa) | Improvement (%) | UCS (kPa) | Improvement (%) |
|---|
| 3% | 20 ± 1 | - | 72.8 ± 1 | - | 102 ± 2 | - |
| 5% | 61 ± 2 | - | 96.7 ± 2 | - | 164 ± 1 | - |
| 10% | 106 ± 2 | - | 165.4 ± 3 | - | 250 ± 2 | - |
| 15% | 157 ± 3 | - | 223.5 ± 2 | - | 340 ± 3 | - |
Table 6.
UCS results before sulphate exposure in presence of SD (7 days curing).
Table 6.
UCS results before sulphate exposure in presence of SD (7 days curing).
| SD Content | Unconfined Compressive Strength, UCS (kPa) |
|---|
| 3% PC = 602 | 5% PC = 702 | 7% PC = 908 |
|---|
| UCS (kPa) | UCS (kPa) | UCS (kPa) |
|---|
| 3% | 710 | 715 | 1050 |
| 5% | 725 | 735.56 | 1248 |
| 10% | 738 | 850.71 | 1306 |
| 15% | 751 | 947.16 | 1391 |
Table 7.
UCS results after sulphate exposure in presence of SD (7 days curing).
Table 7.
UCS results after sulphate exposure in presence of SD (7 days curing).
| SD Content | Unconfined Compressive Strength, UCS (kPa) |
|---|
| 3% PC = Vanished | 5% PC = 131.37 | 7% PC = 204 |
|---|
| UCS (kPa) | Improvement (%) | UCS (kPa) | Improvement (%) | UCS (kPa) | Improvement (%) |
|---|
| 3% | 150 | - | 223.37 | 70 | 480 | 135 |
| 5% | 284 | - | 336.29 | 156 | 685 | 235 |
| 10% | 396 | - | 454.25 | 245.8 | 720 | 252 |
| 15% | 401 | - | 485.96 | 270 | 810 | 297 |
Table 8.
UCS results before sulphate exposure in presence of SD (28 days curing).
Table 8.
UCS results before sulphate exposure in presence of SD (28 days curing).
| SD Content | Unconfined Compressive Strength, UCS (kPa) |
|---|
| 3% PC = 841 | 5% PC = 1026 | 7% PC = 1347 |
|---|
| UCS (kPa) | UCS (kPa) | UCS (kPa) |
|---|
| 3% | 962 | 1194 | 1489 |
| 5% | 1090 | 1351 | 1781 |
| 10% | 1200 | 1509 | 1908 |
| 15% | 1407 | 1807 | 2159 |
Table 9.
UCS results after sulphate exposure in presence of SD (28 days curing).
Table 9.
UCS results after sulphate exposure in presence of SD (28 days curing).
| SD Content | Unconfined Compressive Strength, UCS (kPa) |
|---|
| 3% PC = 120 | 5% PC = 289 | 7% PC = 409 |
|---|
| UCS (kPa) | Improvement (%) | UCS (kPa) | Improvement (%) | UCS (kPa) | Improvement (%) |
|---|
| 3% | 145 | 20.8 | 649 | 124.56 | 1105 | 170 |
| 5% | 210 | 75 | 871 | 201.38 | 1380 | 237 |
| 10% | 400 | 233 | 1006 | 248.1 | 1560 | 281 |
| 15% | 520 | 333 | 1275 | 341 | 1812 | 343 |
Table 10.
UCS results before sulphate exposure in presence of SD (90 days curing).
Table 10.
UCS results before sulphate exposure in presence of SD (90 days curing).
| SD Content | Unconfined Compressive Strength, UCS (kPa) |
|---|
| 3% PC = 1028 | 5% PC = 1405 | 7% PC = 1648 |
|---|
| UCS (kPa) | UCS (kPa) | UCS (kPa) |
|---|
| 3% | 1297 | 1680 | 1906 |
| 5% | 1468 | 1946 | 2192 |
| 10% | 1702 | 2019 | 2358 |
| 15% | 1925 | 2240 | 2450 |
Table 11.
UCS results after sulphate exposure in presence of SD (90 days curing).
Table 11.
UCS results after sulphate exposure in presence of SD (90 days curing).
| SD Content | Unconfined Compressive Strength, UCS (kPa) |
|---|
| 3% PC = 201 | 5% PC = 450 | 7% PC = 537 |
|---|
| UCS (kPa) | Improvement (%) | UCS (kPa) | Improvement (%) | UCS (kPa) | Improvement (%) |
|---|
| 3% | 250 | 24.3 | 1240 | 175.5 | 1700 | 216.5 |
| 5% | 407 | 102.4 | 1640 | 264 | 1985 | 269.6 |
| 10% | 690 | 243.2 | 1840 | 308.8 | 2201 | 309.8 |
| 15% | 885 | 340 | 1990 | 342 | 2400 | 346.9 |