Front-Face Fluorescence of Honey of Different Botanic Origin: A Case Study from Tuscany (Italy)
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Honey Samples
2.2. Chemical Standards
2.3. Spectroscopic Investigations
2.3.1. UV–Vis Absorption Spectroscopy
2.3.2. Front-Face Fluorescence Spectroscopy
2.3.3. Data Analysis
3. Results
3.1. UV–Vis Absorption of Honey of Different Botanic Origin
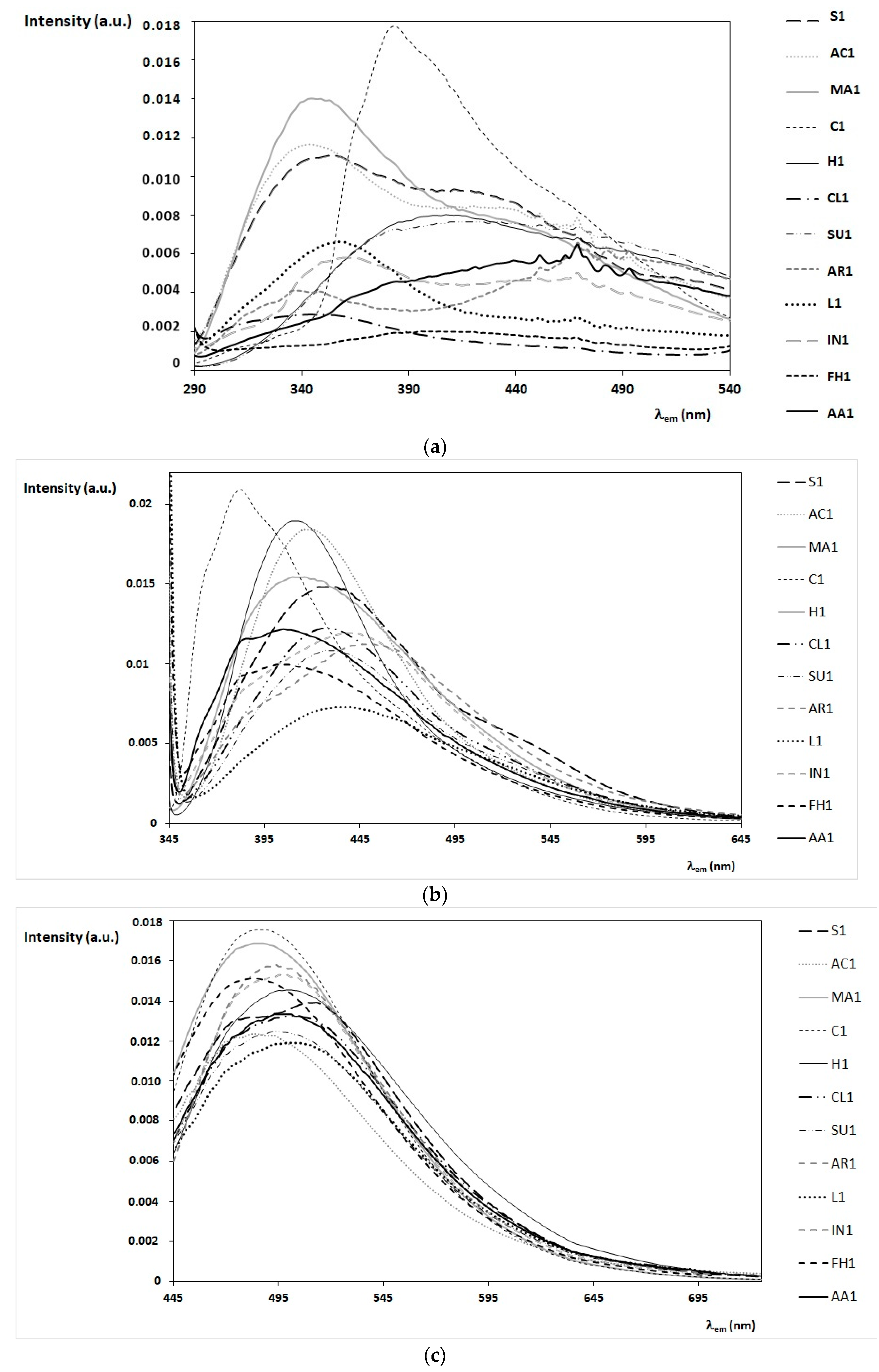
3.2. FFF Spectral Profiles of Honey of Different Botanic Origin
- -
- the first group (lavender, clover, acacia, and marruca honeys) is characterized by emission spectra with a maximum emission around 330–360 nm (at λex = 280 nm);
- -
- the second group (sulla and heather honeys) presents similar emission spectra with a maximum emission around 410–440 nm (at λex = 280 nm);
- -
- the third group (arbutus, inula, alfalfa honey, and forest honeydew honeys) is characterized by different emission spectra (at λex = 280 nm), but similar emission spectra are obtained exciting the sample at λex = 340 nm, with a typical shoulder around 360–385 nm.
- -
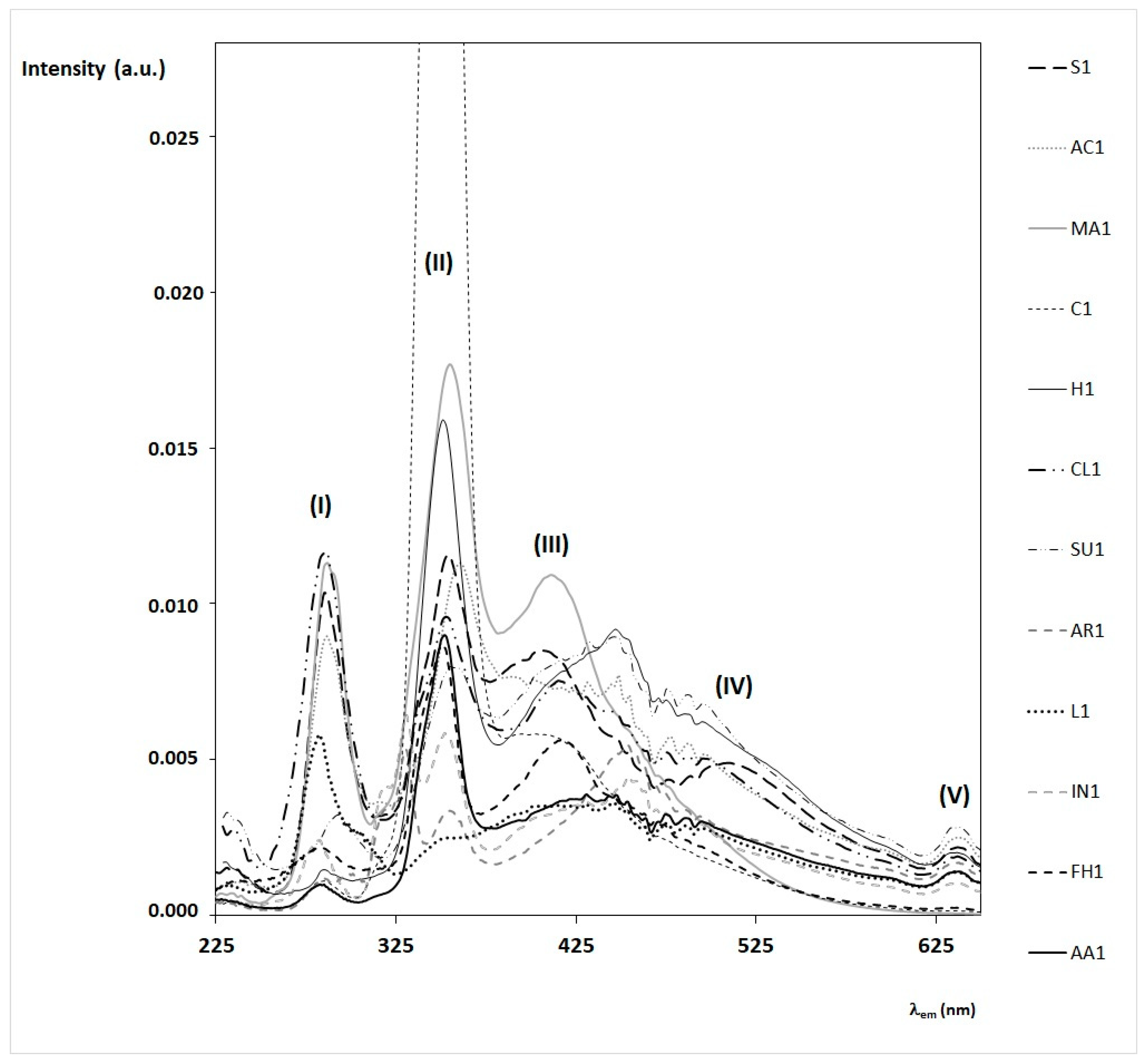
- the first intense band (I) has a maximum at λex = 280–290 nm, mostly due to amino acids (mainly tryptophan), proteins, and some phenolic acids;
- -
- the second intense band (II) has a maximum at λex = 340–350 nm, mostly due to other phenolic acids, flavonoids, and vitamins, such as vitamin B6 (see in particular, chestnut, marruca, heather, and sunflower honeys).
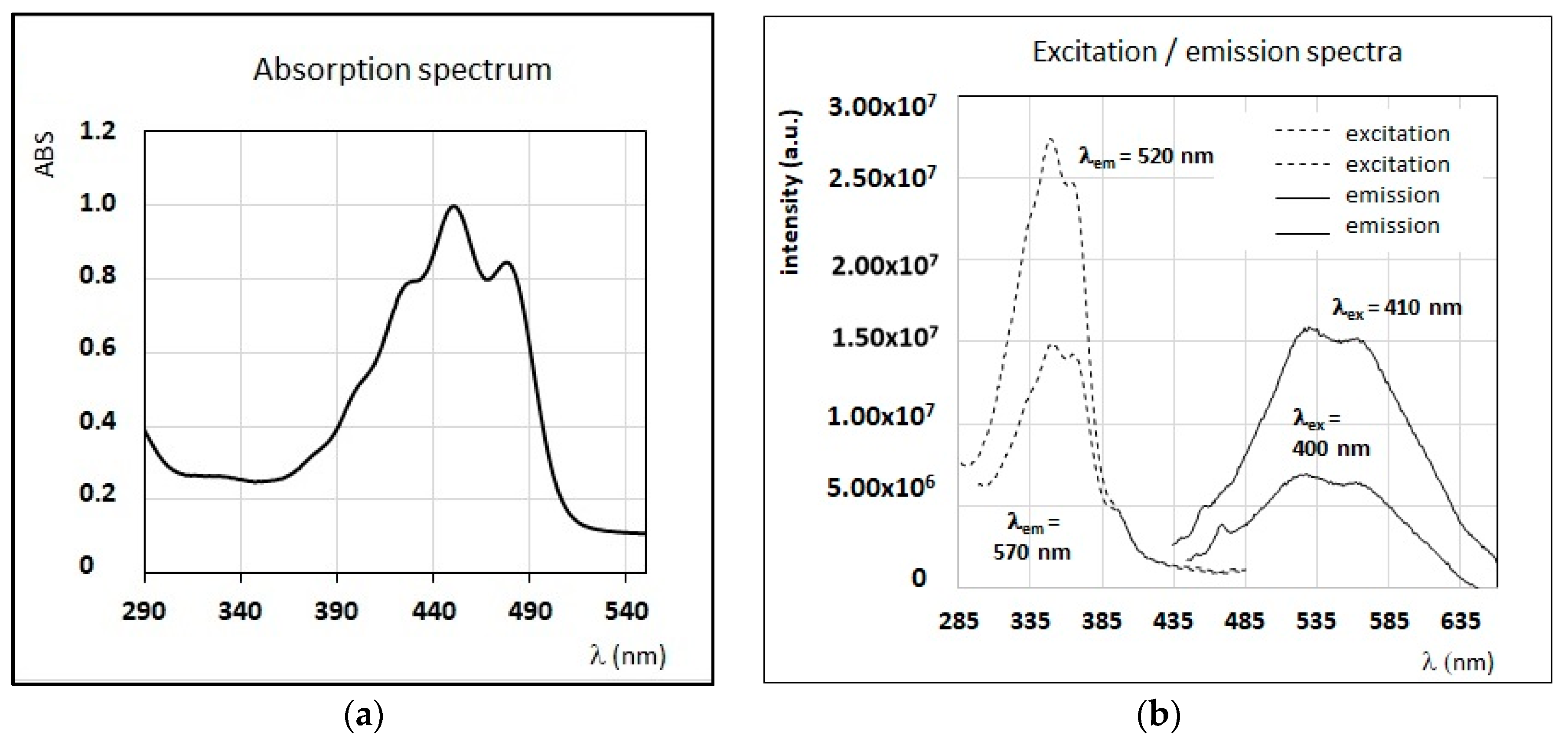
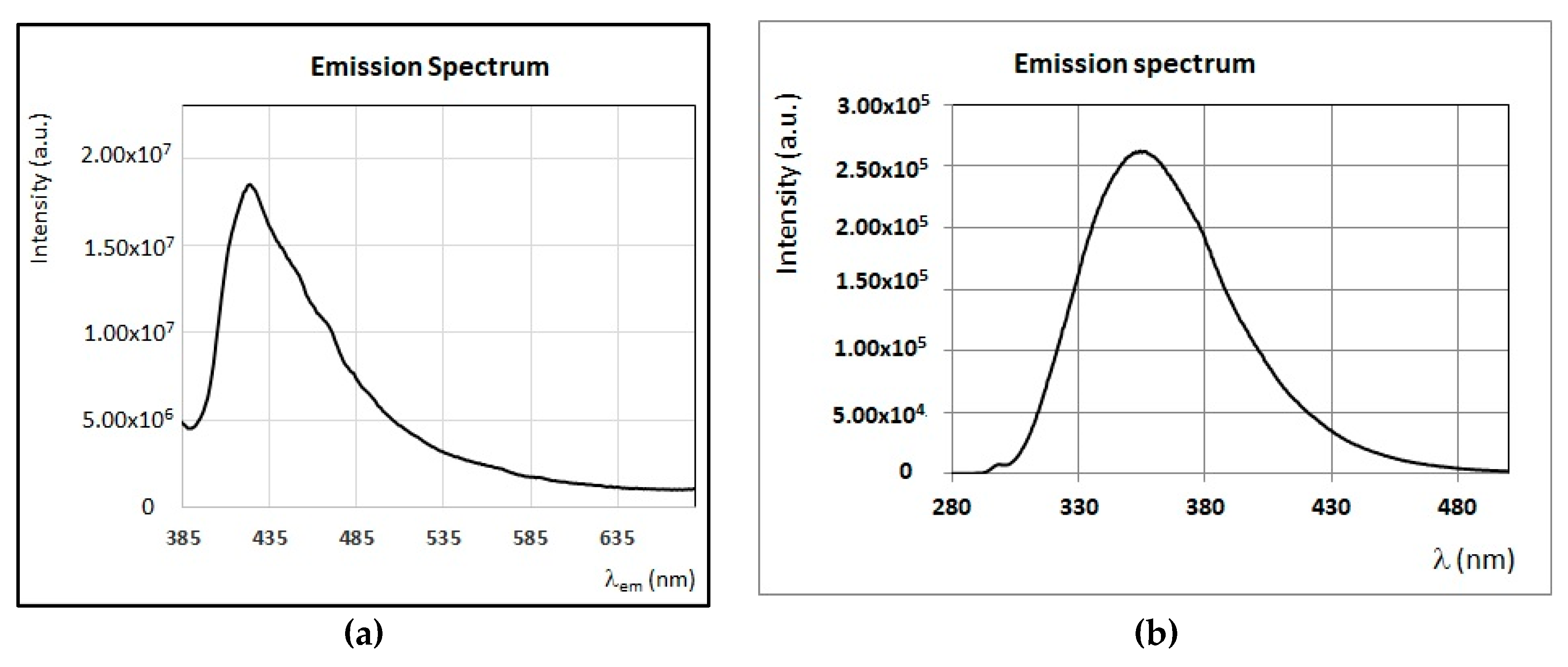
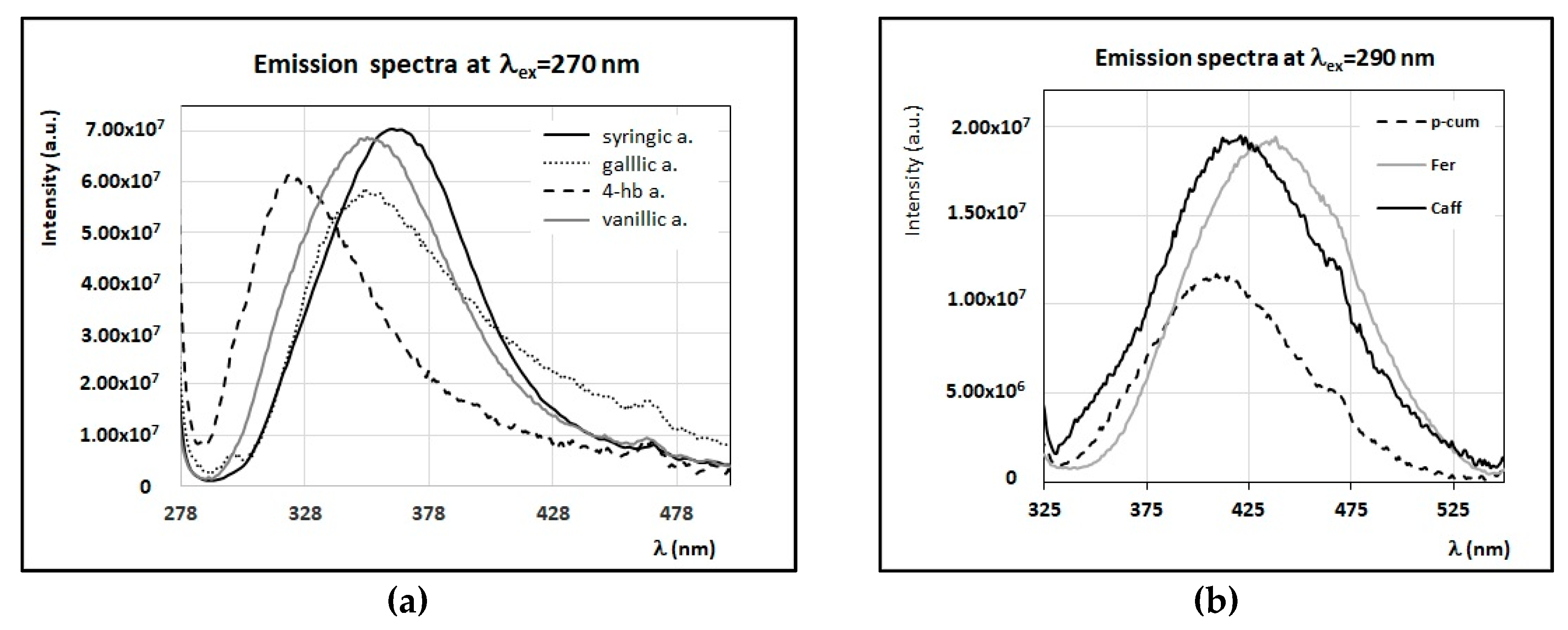
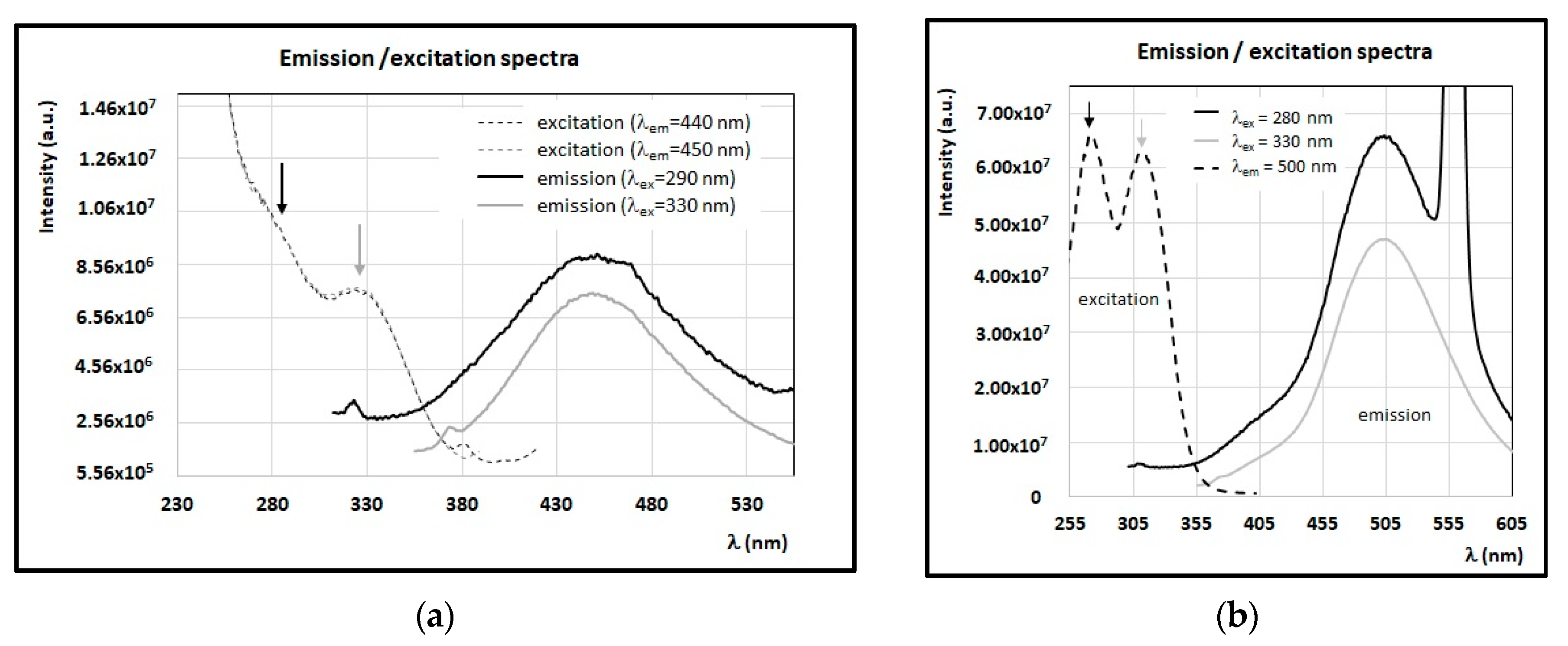
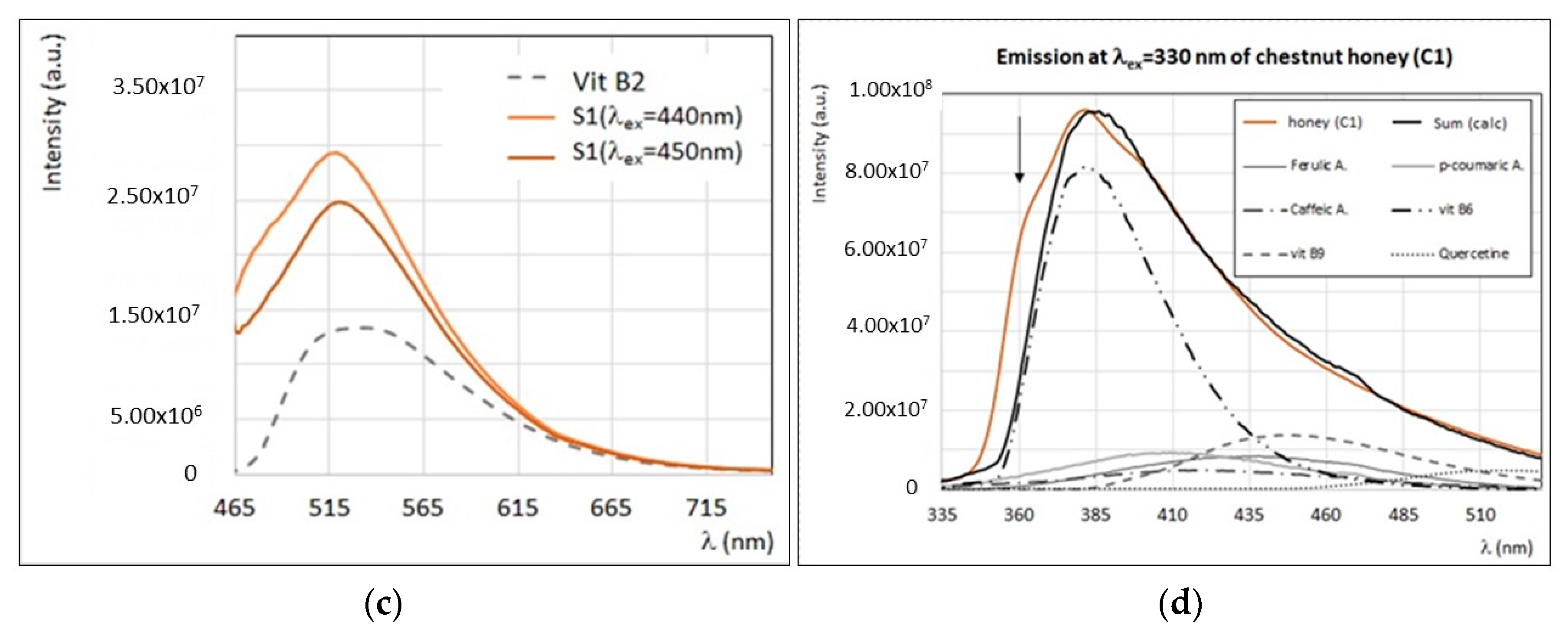
3.3. Fluorescence Properties of Minor Compounds Present in Honey
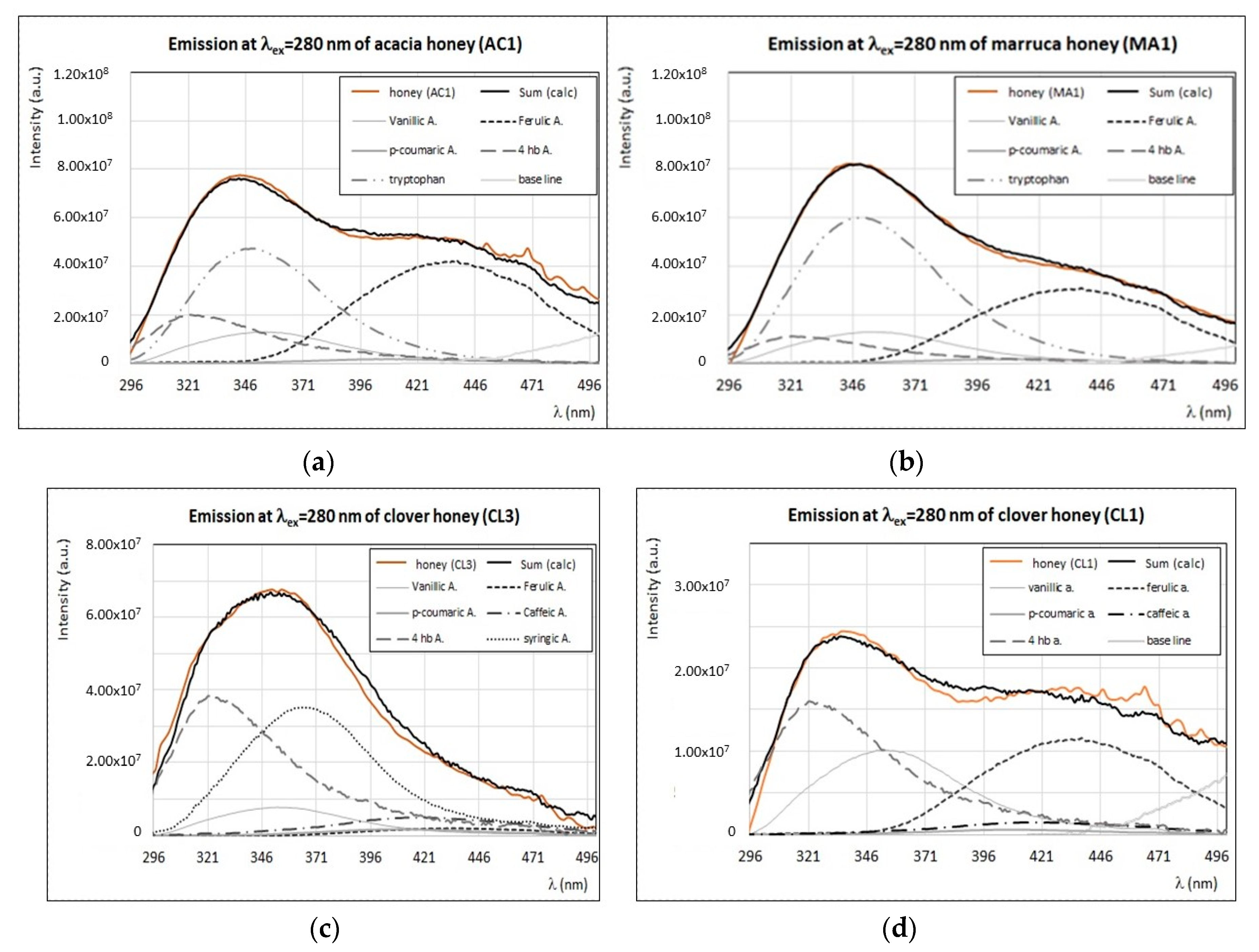
4. Discussion
4.1. Identification of Main Fluorophores in Sunflower and Chestnut Honey Samples
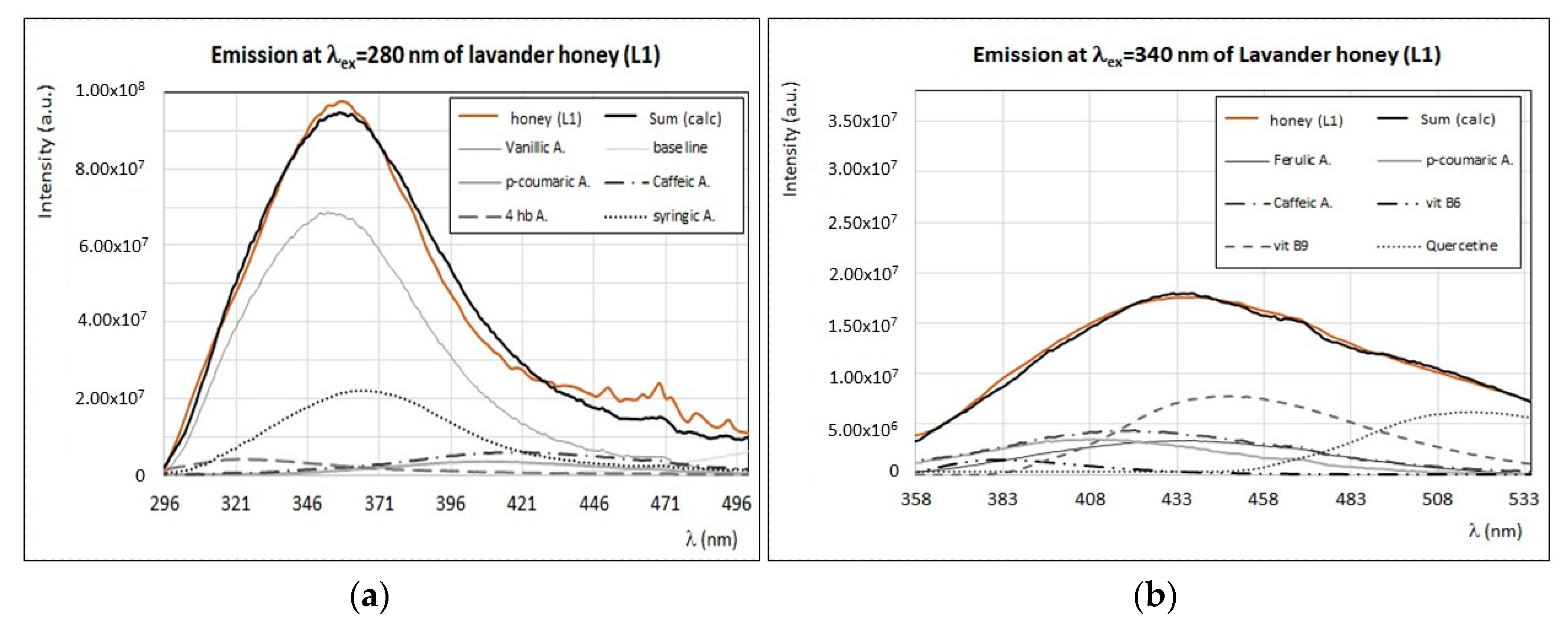
4.2. Identification of Main Fluorophores in Clover, Acacia, Marruca, and Lavender Honey
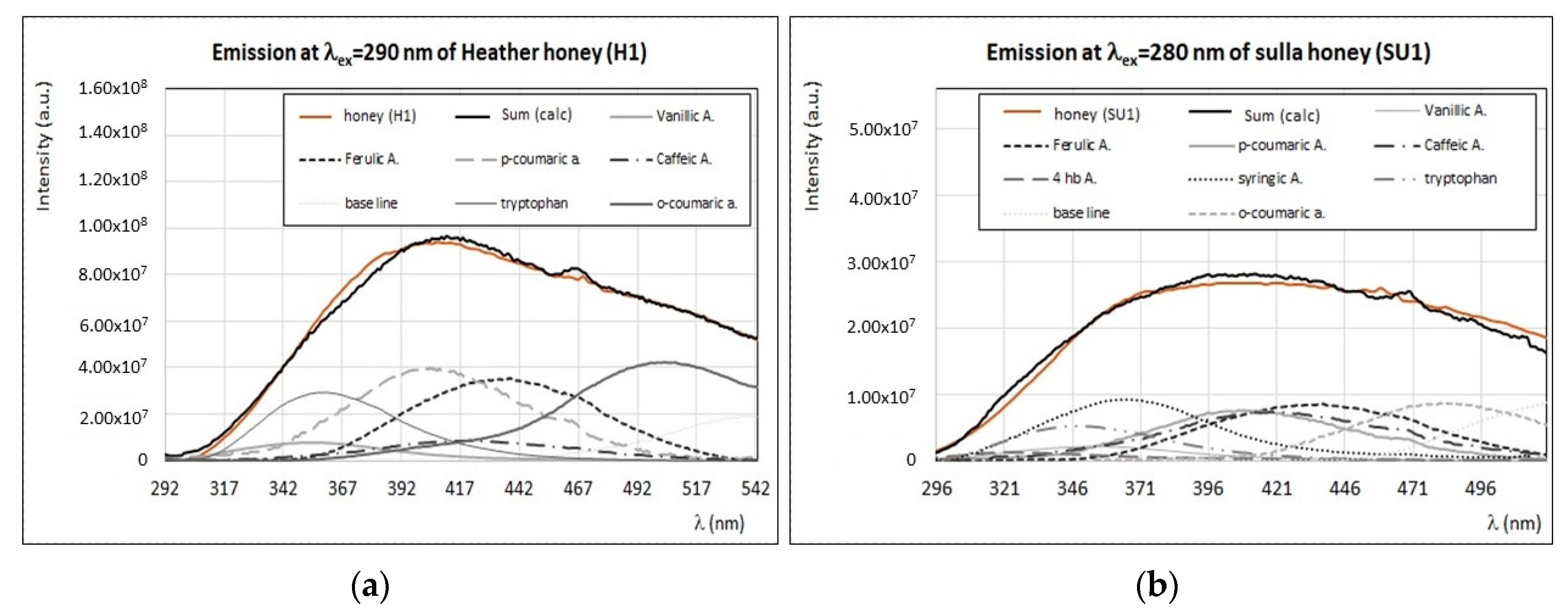
4.3. Identification of Main Fluorophores in Sulla and Heather Honey
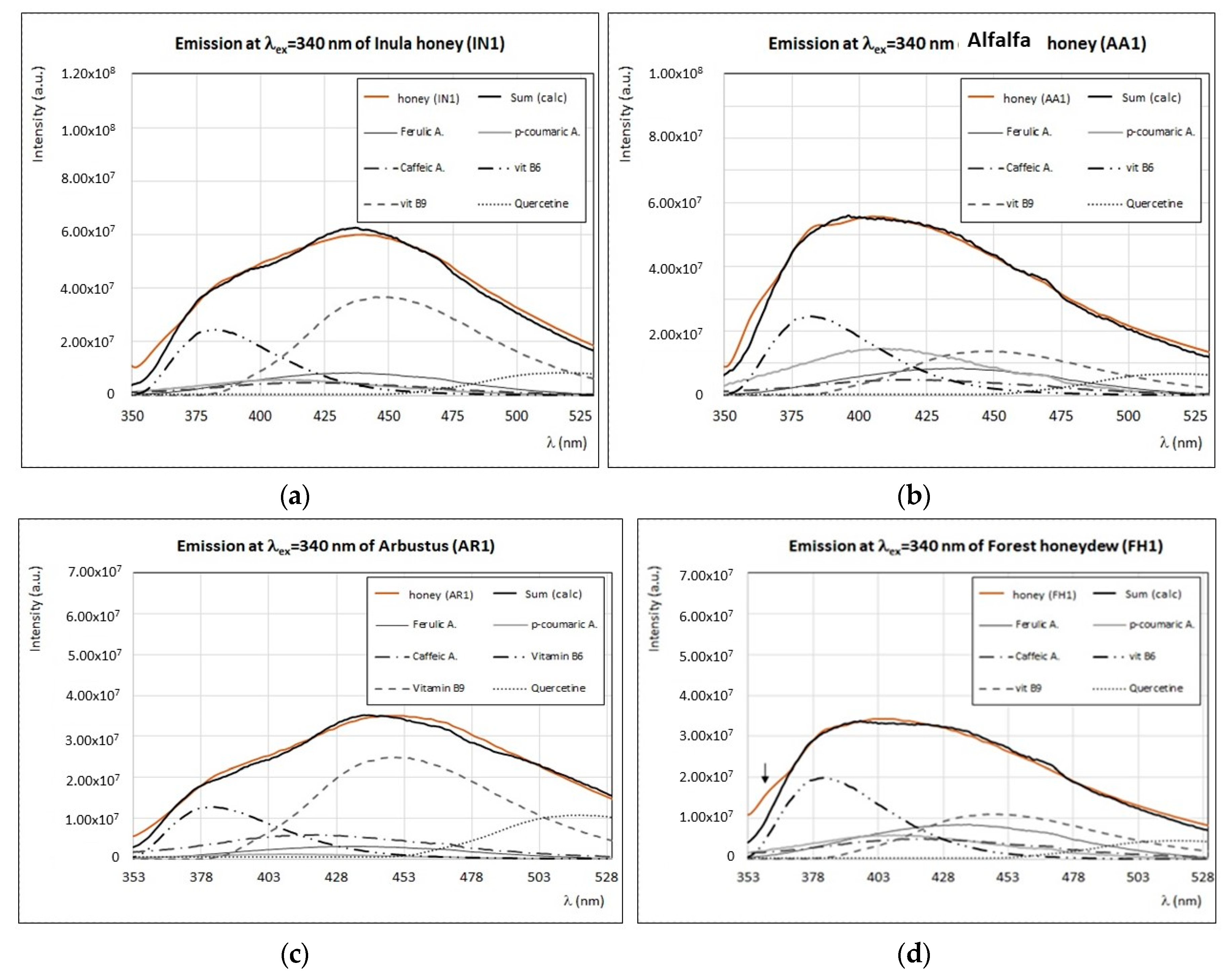
4.4. Identification of Main Fluorophores in Arbutus, Inula and Alfalfa Honey, and Forest Honeydew
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Crane, E. The Archaeology of Beekeeping, 1st ed.; Cornell University Press: Ithica, NY, USA, 1981. [Google Scholar]
- Ramli, N.Z.; Chin, K.Y.; Zarkasi, K.A.; Ahmad, F. A Review on the Protective Effects of Honey against Metabolic Syndrome. Nutrients 2018, 10, 1009. [Google Scholar] [CrossRef] [PubMed]
- Cianciosi, D.; Forbes-Hernandez, T.Y.; Yuliett, T.; Afrin, S.; Gasparrini, M.; Reboredo-Rodriguez, P.; Manna, P.P.; Zhang, J.J.; Lamas, L.B.; Florez, S.M.; et al. Phenolic Compounds in Honey and Their Associated Health Benefits: A Review. Molecules 2018, 23, 2322. [Google Scholar] [CrossRef] [PubMed]
- Codex Alimentarius Commission. Revised Codex Standards for Honey. Codex Standard 12-1981, Rev. 2; Codex Alimentarius Commission: Rome, Italy, 2001. [Google Scholar]
- Kaskoniene, V.; Venskutonis, P. Floral markers in honey of various botanical and geographical origins: A review. Comp. Rev. Food Sci. Food Saf. 2010, 9, 620–634. [Google Scholar] [CrossRef]
- Louveaux, J.; Maurizio, A.; Vorwohl, G. Methods of melissopalynology. BEE World 1978, 59, 139–157. [Google Scholar] [CrossRef]
- Von der Ohe, W.; Persano Oddo, L.; Piana, M.L.; Morlot, M.; Martin, P. Harmonized methods of melissopalynology. Apidologie 2004, 35, 18–25. [Google Scholar] [CrossRef]
- Anklam, E. A review of the analytical methods to determine the geographical and botanical origin of honey. Food Chem. 1998, 63, 549–562. [Google Scholar] [CrossRef]
- Serrano, S.; Villarejo, M.; Espejo, R.; Jodral, M. Chemical and physical parameters of Andalusian honey: Classification of Citrus and Eucalyptus honeys by discriminant analysis. Food Chem. 2004, 87, 619–625. [Google Scholar] [CrossRef]
- Moralesa, V.; Corzoa, N.; Sanzb, M.L. HPAEC-PAD oligosaccharide analysis to detect adulterations of honey with sugar syrups. Food Chem. 2008, 107, 922–928. [Google Scholar] [CrossRef]
- Padovan, G.J.; De Jong, D.; Rodrigues, L.P.; Marchini, J.S. Detection of adulteration of commercial honey samples by the 13C/12C isotopic ratio. Food Chem. 2003, 82, 633–636. [Google Scholar] [CrossRef]
- Persano Oddo, L.; Piazza, M.G.; Sabatini, A.G.; Accorti, M. Characterisation of unifloral honeys. Apidologie 1995, 26, 453–465. [Google Scholar] [CrossRef]
- Patrignani, M.; Fagundez, G.A.; Tananaki, C.; Thrasyvoulou, A.; Lupano, C.E. Volatile compounds of Argentinean honeys: Correlation with floral and geographical origin. Food Chem. 2018, 246, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Amiot, M.J.; Aubert, S.; Gonnet, M.; Tacchini, M. Les composés phénoliques des miels: Étude préliminaire sur l’identification et la quantification par familles. Apidologie 1989, 20, 115–125. [Google Scholar] [CrossRef]
- Martos, I.; Ferreres, F.; Yao, L.; Darcy, B.; Caffin, N.; Tomas-Barberan, F.A. Flavonoids in monospecific Eucalyptus honeys from Australia. J. Agric. Food Chem. 2000, 48, 4744–4748. [Google Scholar] [CrossRef] [PubMed]
- Ramanauskiene, K.; Stelmakiene, A.; Briedis, V.; Ivanauskas, L.; Jakštas, V. The quantitative analysis of biologically active compounds in Lithuanian honey. Food Chem. 2012, 132, 1544–1548. [Google Scholar] [CrossRef] [PubMed]
- Mattonai, M.; Parri, E.; Querci, D.; Degano, I.; Ribechini, E. Development and validation of an HPLC-DAD and HPLC/ESI-MS2 method for the determination of polyphenols in monofloral honeys from Tuscany (Italy). Microchem. J. 2016, 126, 220–229. [Google Scholar] [CrossRef]
- Özcan, M.M.; Ölmez, C. Some qualitative properties of different monofloral honeys. Food Chem. 2014, 163, 212–218. [Google Scholar] [CrossRef]
- Karabagias, I.K.; Vavoura, M.V.; Nikolaou, C.; Badeka, A.V.; Kontakos, S.; Kontominas, M.G. Floral authentication of Greek unifloral honeys based on the combination of phenolic compounds, physicochemical parameters and chemometrics. Food Res. Int. 2014, 62, 753–760. [Google Scholar] [CrossRef]
- Pichichero, E.; Canuti, L.; Canini, A. Characterisation of the phenolic and flavonoid fractions and antioxidant power of Italian honeys of different botanical origin. J. Sci. Food Agric. 2009, 89, 609–616. [Google Scholar] [CrossRef]
- Frausto-Reyes, C.; Casillas-Peñuelas, R.; Quintanar-Stephano, J.L.; Macías-López, E.; Bujdud-Pérez, J.M.; Medina-Ramírez, I. Spectroscopic study of honey from Apis mellifera from different regions in Mexico. Spectrosc. Acta Part A Mol. Biomol. Spectrosc. 2017, 178, 212–217. [Google Scholar] [CrossRef]
- Mignani, A.G.; Ciaccheri, L.; Mencaglia, A.A.; Di Sanzo, R.; Carabetta, S.; Russo, M.T. Dispersive Raman spectroscopy excited at 1064 nm to classify the botanic origin of honeys from Calabria and quantify the sugar profile. In Advanced Environmental, Chemical, and Biological Sensing Technologies XII; VoDinh, T., Lieberman, R.A., Gauglitz, G.G., Eds.; Book Series: Proceedings of SPIE; SPIE-INT Soc. Optical Engineering Publisher: Bellingham, WA, USA, 2015; Volume 9486, p. 94860E. [Google Scholar]
- Ghosh, N.; Verma, Y.; Majumder, S.K.; Gupta, P.K. A Fluorescence Spectroscopic Study of Honey and Cane Sugar Syrup. Food Sci. Technol. Res. 2005, 11, 59–62. [Google Scholar] [CrossRef]
- Ruoff, K.; Luginby, W.; Kunzlis, K.; Bogdanov, S.; Bosset, J.-O.; Von der Ohe, K.; Von der Ohe, W.; Amado, R. Authentication of the Botanical and Geographical Origin of Honey by Front-Face Fluorescence Spectroscopy. J. Agric. Food Chem. 2006, 54, 6858–6866. [Google Scholar] [CrossRef] [PubMed]
- Lenhardt, L.; Bro, R.; Zekovic, I.; Dramicanin, T.; Dramicanin, M.D. Fluorescence spectroscopy coupled with PARAFAC and PLS DA for characterization and classification of honey. Food Chem. 2015, 175, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Bong, J.; Loomes, K.M.; Lin, B.; Stephens, J.M. New approach: Chemical and fluorescence profiling of NZ honeys. Food Chem. 2018, 267, 355–367. [Google Scholar] [CrossRef]
- Lastra-Mejíasa, M.; Torreblanca-Zanca, A.; Aroca-Santosa, R.; Cancilla, J.C.; Izquierdo, J.C.; Torrecilla, J.S. Characterization of an array of honeys of different types and botanical origins through fluorescence emission based on LEDs. Talanta 2018, 185, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Strelec, T.; Brodar, L.; Flanjak, I.; Kenjerić, F.C.; Kovač, T.; Kenjerić, D.C.; Primorac, L. Characterization of Croatian Honeys by Right-Angle Fluorescence Spectroscopy and Chemometrics. Food Anal. Methods 2018, 11, 824–838. [Google Scholar] [CrossRef]
- Karoui, R.; Blecker, C. Fluorescence Spectroscopy Measurements for quality assessment of food systems—A review. Food Bioprocess Technol. 2011, 4, 364–386. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2007; Chapter 3. [Google Scholar]
- Biluca, F.C.; Bernal, J.; Valverde, S.; Ares, A.M.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. Determination of Free Amino Acids in Stingless Bee (Meliponinae) Honey. Food Anal. Methods 2019, 12, 902–907. [Google Scholar] [CrossRef]
- Zandomeneghi, M.; Carbonaro, L.; Caffarata, C. Fluorescence of vegetable oils: Olive oils. J. Agric. Food Chem. 2005, 53, 759–766. [Google Scholar] [CrossRef]
- Gabriele, M.; Parri, E.; Felicioli, A.; Sagona, S.; Pozzo, L.; Biondi, C.; Domenici, V.; Pucci, L. Phytochemical composition and antioxidant activity of Tuscan bee pollen of different botanic origins. Ital. J. Food Sci. 2015, 27, 248–259. [Google Scholar]
- Gabriele, M.; Gerardi, C.; Lucejko, J.J.; Longo, V.; Pucci, L.; Domenici, V. Effects of low sulfur dioxide concentrations on bioactive compounds and antioxidant properties of Aglianico red wine. Food Chem. 2018, 245, 1105–1112. [Google Scholar] [CrossRef]
- Sergiel, I.; Pohl, P.; Biesaga, M.; Mironczyk, A. Suitability of three-dimensional synchronous fluorescence spectroscopy for fingerprint analysis of honey samples with reference to their phenolic profiles. Food Chem. 2014, 145, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Karoui, R.; Dufour, E.; Bosset, J.-O.; De Baerdemaeker, J. The use of front face fluorescence spectroscopy to classify the botanical origin of honey samples produced in Switzerland. Food Chem. 2007, 101, 314–323. [Google Scholar] [CrossRef]
- Mehretie, S.; Al Riza, D.F.; Yoshito, S.; Kondo, N. Classification of raw Ethiopian honeys using front face fluorescence spectra with multivariate analysis. Food Control 2018, 84, 83–88. [Google Scholar] [CrossRef]
- Stanković, M.; Bartolić, D.; Šikoparija, B.; Spasojević, D.; Mutavdžić, D.; Natić, M.; Radotić, K. Variability estimation of the protein and phenol total content in honey using front-face fluorescence spectroscopy coupled with MCR-ALS analysis. J. App. Spectrosc. 2019, 86, 256–263. [Google Scholar] [CrossRef]
- Dramicanin, T.; Ackovic, L.L.; Zekovic, I.; Dramicanin, M.D. Detection of Adulterated Honey by Fluorescence Excitation-Emission Matrices. J. Spectrosc. 2018, 8395212. [Google Scholar] [CrossRef]
- Bong, J.; Loomes, K.M.; Schlothauer, R.C.; Stephens, J.M. Fluorescence markers in some New Zealand honeys. Food Chem. 2016, 192, 1006–1014. [Google Scholar] [CrossRef]
- Chen, Q.; Qi, S.; Li, H.; Han, X.; Ouyang, Q.; Zhao, J. Determination of rice syrup adulterant concentration in honey using three-dimensional fluorescence spectra and multivariate calibrations. Spectrosc. Acta Part A Mol. Biomol. Spectrosc. 2014, 131, 177–182. [Google Scholar] [CrossRef]
- Di Marco, G.; Manfredini, A.; Leonardi, D.; Canuti, L.; Impei, S.; Gismondi, T.; Canini, A. Geographical, botanical and chemical profile of monofloral Italian honeys as food quality guarantee and territory brand. Plant Biosyst. 2017, 151, 450–463. [Google Scholar] [CrossRef]
- Ballabio, D.; Robotti, E.; Grisoni, F.; Quasso, F.; Bobb, M.; Vercelli, S.; Gosetti, F.; Calabrese, G.; Sangiorgi, E.; Orlandi, M.; et al. Chemical profiling and multivariate data fusion methods for the identification of the botanical origin of honey. Food Chem. 2018, 266, 79–89. [Google Scholar] [CrossRef]
- Mannina, L.; Sobolev, A.P.; Anatoly, P.; Di Lorenzo, A.; Vista, S.; Tenore, G.C.; Daglia, M. Chemical Composition of Different Botanical Origin Honeys Produced by Sicilian Black Honeybees (Apis mellifera ssp sicula). J. Agric. Food Chem. 2015, 63, 5864–5874. [Google Scholar] [CrossRef]
- Scanu, R.; Spano, N.; Panzanelli, A.; Pilo, M.I.; Piu, P.C.; Sanna, G.; Tapparo, A. Direct chromatographic methods for the rapid determination of homogentisic acid in strawberry tree (Arbutus unedo L.) honey. J. Chrom. A 2005, 1090, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Spettoli, P.; Bottacin, A.; Pescia, P.; Girolami, V. Physical-chemical characteristics of Tuscan honeys from Erica-Arborea. Ind. Aliment. 1982, 21, 617–620. [Google Scholar]
- Parri, E. Analisi di Component Minoritari in Mieli Uniflorali Toscani Mediante Tecniche Cromatografiche e Spettroscopiche. Master’s Thesis, University of Pisa, Pisa, Italy, 2014. [Google Scholar]
- Parri, E.; Lenzi, A.; Cifelli, M.; Restivo, A.; Degano, I.; Ribechini, E.; Zandomeneghi, M.; Domenici, V. Studio di mieli toscani monoflorali mediante tecniche chimiche cromatografiche e spettroscopiche. In Codice Armonico 2014 Quinto Congresso di Scienze Naturali Ambiente Toscano; Edizioni ETS: Pisa, Italy, 2014; pp. 159–169. ISBN 9788846738899. [Google Scholar]
- Zandomeneghi, M. Fluorescence of Cereal Flours. J. Agric. Food Chem. 1999, 47, 878–882. [Google Scholar] [CrossRef] [PubMed]
- Vo-Dinh, T. Synchronous luminescence spectroscopy. In Modern Fluorescence Spectroscopy; Wehry, E.L., Ed.; Plenum Press: New York, NY, USA; London, UK, 1981; pp. 169–191. [Google Scholar]
- Domenici, V.; Ancora, D.; Cifelli, M.; Serani, A.; Veracini, C.A.; Zandomeneghi, M. Extraction of Pigment Information from Near-UV Vis Absorption Spectra of Extra Virgin Olive Oils. J. Agric. Food Chem. 2014, 62, 9317–9325. [Google Scholar] [CrossRef]
- Dreuw, A.; Fleming, G.R.; Head-Gordon, M. Role of electron-transfer quenching of chlorophyll fluorescence by carotenoids in non-photochemical quenching of green plants. Biochem. Soc. Trans. 2005, 33, 858–862. [Google Scholar] [CrossRef] [PubMed]
- Krause, G.H.; Weis, E. Chlorophyll Fluorescence and Photosynthesis: The Basics. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1991, 42, 313–349. [Google Scholar] [CrossRef]
- Jasicka-Misiak, I.; Gruyaert, S.; Poliwoda, A.; Kafarski, P. Chemical Profiling of Polyfloral Belgian Honey: Ellagic Acid and Pinocembrin as Antioxidants and Chemical Markers. J. Chem. 2017, 2017, 5393158. [Google Scholar] [CrossRef]
- Cheung, Y.; Meenu, M.; Yu, X.; Xu, B. Phenolic acids and flavonoids profiles of commercial honey from different floral sources and geographic sources. Int. J. Food Prop. 2019, 22, 290–308. [Google Scholar] [CrossRef]
- Leon-Ruiz, V.; Vera, S.; Gonzalez-Porto, A.V.; San Andres, M. Analysis of Water-Soluble Vitamins in Honey by Isocratic RP-HPLC. Food Anal. Meth. 2013, 6, 488–496. [Google Scholar] [CrossRef]
- Kivrak, S. Determination of B-group vitamins in Turkish honey using ultra-performance liquid chromatography with electrospray ionization coupled to tandem mass spectrometry. J. Liq. Chrom. Rel. Technol. 2016, 39, 847–851. [Google Scholar] [CrossRef]




 (a) |  (b) |  (c) |  (d) |
 (e) |  (f) |  (g) |  (h) |
 (i) |  (l) |  (m) |  (n) |
| Label | Botanic Origin | Geographic Area of Flowers | Year of Honey Production | Sample Photo | Label | Botanic Origin | Geographic Area of Flowers | Year of Honey Production | Sample Photo |
|---|---|---|---|---|---|---|---|---|---|
| AC1 | Acacia | Lucca (LU) | 2013 |  | L1 | Lavender | Grosseto (GR) | 2016 |  |
| SU1 | Sulla | Grosseto (GR) | 2013 |  | AR1 | Arbutus | Lucca (LU) | 2016 |  |
| CL1 | Clover | Pisa (PI) | 2013 |  | IN1 | Inula | Grosseto (GR) | 2016 |  |
| CL2 | Clover | Grosseto (GR) | 2014 |  | FH1 | Forest Honeydew | Siena (SI) | 2015 |  |
| CL3 | Clover | Siena (SI) | 2014 |  | AA1 | Alfalfa | Grosseto (GR) | 2015 |  |
| MA1 | Marruca | Livorno (LI) | 2013 |  | C1 | Chestnut | Lucca (LU) | 2013 |  |
| MA2 | Marruca | Grosseto (GR) | 2013 |  | H1 | Heather | Lucca (LU) | 2013 |  |
| MA3 | Marruca | Grosseto (GR) | 2014 |  | S1 | Sunflower | Grosseto (GR) | 2014 |  |
| Honey Sample Label* | λmax (nm) |
|---|---|
| AC1 | 270 (326) |
| SU1 | 286 (451, 690) |
| CL1 | 277 (326) |
| CL2 | 277 (325) |
| CL3 | 276 (326) |
| MA1 | 276 (329) |
| MA2 | 275 (330) |
| MA3 | 275 (329) |
| L1 | 282 (327) |
| AR1 | 285 (335) |
| IN1 | 282 (326) |
| FH1 | 278 (325) |
| AA1 | 277 (334) |
| C1 | 243 (318–355, 607) |
| H1 | 272 (334) |
| S1 | 269 (425–450–485, 692) |
| Honey Sample Label | λmax (nm) at λex = 280 nm | λmax (nm) at λex = 340 nm | λmax (nm) at λex = 420 nm |
|---|---|---|---|
| AC1 | 345 (440 *) | 420 | 483 |
| SU1 | 420 (490 *) | 435 | 495 |
| CL1 | 340 (320 **) | 430 | 502 |
| CL2 | 335 (320 **), 440 | 433 | 500 |
| CL3 | 340 (320 **, 440 *) | 430 | 502 |
| MA1 | 345 (450 **) | 415 | 487 |
| MA2 | 345 (450 **) | 427 | 485 |
| MA3 | 340 (450 **) | 435 (400 **) | 487 |
| L1 | 360 | 440 | 503 (475 **) |
| AR1 | 470 (340 *) | 445 (385 **, 502 **) | 491 |
| IN1 | 373 (470 *) | 443 (362 **, 385 **, 503 **) | 500 (475 **) |
| FH1 | 410 *** | 405 (362 **, 385 *) | 483 |
| AA1 | 470 (361 **, 385 **) | 407 (362 **, 385 *, 470 **) | 497 |
| C1 | 385 (403 **, 463 **) | 385 (362 **, 400 **) | 487 |
| H1 | 410 (500 **) | 413 | 500 |
| S1 | 355 (420 *) | 430 (530 **) | 515 (475 *) |
| Honey Sample Label | Group | Main Fluorophores Contributing to Fluorescence Emission (λex, nm) |
|---|---|---|
| S1 | 1 | Vanillic, ferulic, p-coumaric, 4-hydroxybenzoic, and caffeic acids (290); p-coumaric and caffeic acids (330); vitamin B9 and quercetin (330–340); vitamin B2 (430); carotenoids (400–500); chlorophylls (670). |
| C1 | Caffeic, p-coumaric, ferulic acids (270–290) and unknown fluorophore X (270–340); vitamin B6; vitamin B9 (330–340). | |
| CL1 | 2 | 4-hydroxybenzoic, caffeic, vanillic, ferulic, syringic, and p-coumaric acids (280–290); quercetin (340); chlorophylls (670). |
| CL2 | 4-hydroxybenzoic, caffeic, vanillic, ferulic, syringic, and p-coumaric acids (280–290); quercetin (340); chlorophylls (670). | |
| CL3 | 4-hydroxybenzoic, caffeic, vanillic, ferulic, syringic and p-coumaric acids (280–290); quercetin (340); chlorophylls (670). | |
| MA1 | Tryptophan, 4-hydroxybenzoic, p-coumaric, vanillic acids, and ferulic acid/chlorogenic acid (280–290); vitamin B6, vitamin B9, and quercetin (330). | |
| MA2 | Tryptophan, 4-hydroxybenzoic, p-coumaric, vanillic acids, and ferulic acid/chlorogenic acid (280–290); vitamin B6, vitamin B9, and quercetin (330). | |
| MA3 | Tryptophan, 4-hydroxybenzoic, p-coumaric, vanillic acids, and ferulic acid/chlorogenic acid (280–290); vitamin B6, vitamin B9, and quercetin (330). | |
| L1 | Vanillic and syringic acids (280–290); vitamin B9 and quercetin (330–340); chlorophylls (670). | |
| AC1 | Tryptophan, 4-hydroxybenzoic, p-coumaric, vanillic acids, and ferulic acid/chlorogenic acid (280–290); vitamin B6, vitamin B9, and quercetin (330); chlorophylls (670). | |
| H1 | 3 | Tryptophan, vanillic, ferulic, syringic, p-coumaric, and o-coumaric acids (280–290); vitamin B6, vitamin B9, and quercetin (330); chlorophylls (670). |
| SU1 | Tryptophan, vanillic, ferulic, syringic, p-coumaric, and o-coumaric acids (280–290); vitamin B6, vitamin B9, and quercetin (330); carotenoids (400-500); chlorophylls (670). | |
| AA1 | 4 | Vanillic, p-coumaric, 4-hydroxybenzoic, ferulic, and caffeic acids (290); vitamin B9, vitamin B6, and quercetin (330–340); chlorophylls (670). |
| AR1 | Vanillic, p-coumaric, 4-hydroxybenzoic, ferulic, and caffeic acids (290); vitamin B9, vitamin B6, and quercetin (330–340); chlorophylls (670). | |
| FH1 | Low content of vanillic, ferulic, p-coumaric, 4-hydroxybenzoic, and caffeic acids (290); vitamin B9, vitamin B6, and quercetin (330–340). | |
| IN1 | Vanillic, p-coumaric, 4-hydroxybenzoic, ferulic, and caffeic acids (290); vitamin B9, vitamin B6, and quercetin (330–340); chlorophylls (670). |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parri, E.; Santinami, G.; Domenici, V. Front-Face Fluorescence of Honey of Different Botanic Origin: A Case Study from Tuscany (Italy). Appl. Sci. 2020, 10, 1776. https://doi.org/10.3390/app10051776
Parri E, Santinami G, Domenici V. Front-Face Fluorescence of Honey of Different Botanic Origin: A Case Study from Tuscany (Italy). Applied Sciences. 2020; 10(5):1776. https://doi.org/10.3390/app10051776
Chicago/Turabian StyleParri, Erica, Giulia Santinami, and Valentina Domenici. 2020. "Front-Face Fluorescence of Honey of Different Botanic Origin: A Case Study from Tuscany (Italy)" Applied Sciences 10, no. 5: 1776. https://doi.org/10.3390/app10051776
APA StyleParri, E., Santinami, G., & Domenici, V. (2020). Front-Face Fluorescence of Honey of Different Botanic Origin: A Case Study from Tuscany (Italy). Applied Sciences, 10(5), 1776. https://doi.org/10.3390/app10051776






